Veeda CR is one of the leading Independent clinical research companies in India with an in-depth clinical research knowledge in clinical trails, BA BE Studies, clinical phase CRO, full services CRO etc.
Don't wanna be here? Send us removal request.
Text
Best Digital Marketing Agency in London
Tools You Can Use to Check Traffic On a Website
Do you ever wonder about how your website is actually doing? Do you often think about how your competitor’s website is doing? In this blog, we will discuss the tools you can use to check traffic on any website.

Firstly, it is very important to know why you need to check your website traffic? When you check your website stats, you get an easy idea about how your website is performing. These stats can show you all the minute details like where your traffic is coming from, how are they engaging with the site and how your digital marketing strategies are working. This gives you a window to get better and create your strategies that can enhance your reach.
It is also important that you also check your competitors’ website stats to get a clear idea on what kind of content is bringing them more traffic, which keywords are helping your competitor rank and what channels are driving them traffic. You can use all this information to improve your content marketing strategy and enhance link building and keyword research process. This will enable you to target the same keywords and generate more traffic for your website.
There are a lot of tools that can help you analyse website traffic. Some of them are paid while some are unpaid. You can use these tools to fill in the gaps and get more traffic on your website.
Here are some of the tools you can use to check website traffic:
SEMRush: SEMRush is one of the widely used traffic analysis and competition research tool. You can use this tool for keyword research, for tracking keyword ranking, for analysing traffic levels of multiple websites at once and also get detailed analysis of website traffic. Using this tool to see important website metrics like the volume of monthly traffic over time, traffic distribution by country, the keywords that bring them the most traffic, and more. The tool can also help you check the number of unique visitors, total visits, average visit duration, number of page views, and the bounce rate the site receives.
SimilarWeb: This website traffic checker can give you an overview of your competitor’s traffic and which channels are the most valuable. Instead of just providing traffic from search, you’ll get detailed reports of a website’s entire traffic strategy. Once you run a website through the tool, you’ll get a breakdown of the overall traffic, including a country-by-country report. You’ll also get engagement metrics like bounce rate, number of pages viewed per visit, average visit duration, and the top traffic sources for that website. You’ll find what paid keywords bring the website traffic, what social channels are the most valuable, and the display networks the site uses to monetize.
Google Search Console: Google Search Console is a free tool by google that let you analyse your search engine traffic. It can give you a detailed picture of how your website is viewed by search engines. It shows the number of impressions, clicks, click-through rate (CTR), and the average position of your keywords in the search engines which can help you understand what keywords you’re ranking for and the search volume they’re bringing in. This information can help you improve your traffic.
Ubersuggest: Ubersuggest is a free and premium tool from Neil Patel. The tool offers a ton of useful website traffic data. It’s straightforward to use and the interface is built for beginners. This tool makes it incredibly simple to carryout keyword research and analyse traffic if you’ve never done it before. The traffic analyser feature gives you a detailed breakdown of your competitor’s traffic over time, the keywords bringing them the most traffic, and the most popular pages on the site. All you have to do is enter the website URL you want to analyse and click ‘Search’.
There are various other tools that you can use too. But these are among the most used tools to check the website stats. We hope that this article helps you find ways to reach a larger audience.
10 notes
·
View notes
Video
vimeo
Veeda is one of the leading Independent clinical research companies in India with In-depth Clinical research knowledge in Full Services CRO, clinical trials, CRO Services, BA BE studies, Clinical Trials CRO India, etc
#cro services#top cro services#best cro services#oncology services#clinical trials#clinical trials services
0 notes
Text
Contribution of Biopharmaceutics Services in Pharma
Biopharmaceutics is a major branch in pharmaceutical sciences that relates between the physicochemical properties of a drug in dosage form and the pharmacology, toxicology, or clinical response observed after its administration. The importance of the drug substance and the drug formulation on absorption is described as a sequence of events that precede the elicitation of a drug's therapeutic effect.

Drug efficacy and safety are dependent on the dosing regimen. The optimal dosage and dosing intervals can be quite different for different drugs. Moreover, for a single drug, the optimal dosage can be different widely between patients.
It is not sufficient to know what the drug does to the body; it is also crucial to know what the body does to the drug. The knowledge of the pharmacodynamics and pharmacokinetic properties of the drug and its metabolites in humans and animals is crucial to understand its different effects among species and for adjusting drug dosing. The plasma concentration in Biopharmaceutics of the drug is the basic concept of pharmacokinetics. Based on the protein binding of the drug, the concentration of free drug available in the circulation influences greatly the dose calculations. The concentration of drug in the plasma is in equilibrium with some tissues in the body.
Biopharmaceutics has evolved into a broad-based discipline that encompasses fundamental principles from basic scientific and related disciplines, including chemistry, physiology, physics, statistics, engineering, mathematics, microbiology, enzymology, and cell biology. The biopharmaceutical scientist, therefore, must have a sufficient understanding of all of these scientific fields in order to be most effective in a drug development role. For the subsequent discussion, we will look broadly at the areas of physical pharmacy (pharmaceutics) and PK and their roles and interdependencies in the drug development process.
#CRO in India#Clinical Research Organization#Clinical Trial#Clinical Research#CRO Services#Full Service CRO
2 notes
·
View notes
Text
Early Phase Clinical Trials Research Organization
Early phase Clinical trials to test new treatments involve a series of steps called phases. Early phase clinical trials (phase I and phase II) help observer to understand how a new drug or other treatment works within a patient. If a new treatment is successful in one phase, it proceeds in next part of early phase clinical trials for testing.

In early phase clinical trials researcher observed that a new treatment is safe, it has side effects or not and if have how much that drug effected the patient and in which way and also they make sure about the dose they have given to the patient.In general, early phase clinical trials are for patients who are brawling diseases that are no longer acknowledging to standard therapies or patients who have diseases that do not have a worthy treatment. Early phase clinical trials may offer another choice and the opportunity to make a positive impact on future generations of cancer patients.
Through the early phases (phases I and II), researchers determine:
Whether a new treatment is safe
What its side effects are
The best dose of the new treatment
Benefit of Early Phase Clinical Trials
In general, early phase studies are for patients who are struggling diseases that are no longer answering to standard therapies or patients who have infections that do not have a standard treatment. For these patients, early phase study may offer another option and the chance to make a positive effect on future generations of cancer patients.
Types of Early Phase Trials
Targeted therapies
Immune-based therapies
Nanotechnology
Novel combinations of chemotherapeutic drugs
2 notes
·
View notes
Text
CRO Services Play a Major Roles in Drug Development
A best CRO (Contract research organization) is a company that supports the biotechnology, pharmaceutical and medical devices firms in form of clinical research and clinical trials services outsourced on some contract basis. Which provides services as preclinical research, Pharmacovigilance, clinical trial management, clinical research and biopharmaceutical development.

Most of the pharma firm developing medicines and drugs in niche market outsourced the services provided by CRO to reduce cost.
The best CRO services are the ones which provided by any CRO to the contracted company in desired timeline and cost effective. Best CRO services aims to simplify entry into drug market and development as well. Because everything doing in house now inessential for large pharmaceutical companies. Contract research organization provides clinical study and clinical trial support and it ranges from international, large full service organization to niche, small speciality group. CRO testing services are required throughout the development phase of any drug substance. Outsourcing CRO services helps pharmaceutical and biopharmaceutical firms to alleviate risks by avoiding large investments. It also helps pharmaceutical companies to focus on their core competencies. Thus, the development of new drug molecules is expected to drive the demand for CRO services.
Some CROs manage almost all features of a clinical trial, from position of selection and patient enrolment through final regulatory approval from the Food and Drug Administration (FDA).
Although a trial promoter may transfer all trial functions to a third-party CRO, the promoter remains responsible for the honesty of the trial data and to ensure it is all factual and backed by good science.
Types of Services Provided by CROs
Contract research organizations offer comprehensive services, including:
Project management
Database design & build
Data entry & validation
Clinical trial data management
Medicine and disease coding
Quality and metric reporting
Statistical analysis plans and reports
Validation programming
Safety and efficacy summaries
Final study report
#Best CRO Services#cro services#CRO in India#clinical trials#Clinical Research#biopharmaceutical development
4 notes
·
View notes
Text
Late Phase Clinical Trials Services
Late phase study is conducted to evaluate drugs long term effectiveness and impact on patient quality of life over a longer period.

These studies provide ongoing safety information through the long-term evaluation of patients in a real-world setting. Regulatory agencies may require additional data about the side effects of new compounds.
Late phase services include regulatory affairs, site qualification, site management, monitoring, data management, trial logistics, Pharmacovigilance, biostatistics, medical writing, and project management.
Late phase Contains phase II, Phase III and Phase IV.
Where in, In Phase II trials once a dose or range of doses is determined in Phase I, the next goal is to evaluate whether the drug has any biological activity or effect.
Phase III trials compare new treatments with the best currently available treatment with objectives of which treatment works better for a particular type of cancer, more about the side effects, how the treatment affects people’s quality of life.
In Phase IV trial, done after a drug has been shown to work and has been licensed and more concern about the effects and safety of the drug, what the long term risks and benefits are, how well the drug works when it’s used more widely.
#Clinical Trial Services#Phase 1 Clinical Trial#Phase 4 Clinical Trials#Medical Writing#Pharmacokinetic Trials#Phase 3 Studies#Patient Based PK Trials
4 notes
·
View notes
Text
In-house or Outsource Full Service CRO
Full-service CRO is Contract Research Organization is an organization contracted by other companies to manage, provide and lead clinical trials and other research support services for medical devices industries, biotechnology, Pharmaceuticals and also works with universities, government institutions, and foundations.

With this changing economy and innovation in the pharma industry, Pharma companies mostly outsource their critical functions including research and manufacturing. So, The Pharma companies mostly looking for full service CRO for their clinical trials and to develop new medication as well.
The company’s main focus is to reduce the time it takes to conduct a trial versus doing in house trials that render significant cost savings. Outsourcing these things will reduce costs like the company doesn’t have to build extra infrastructure, office space or use manpower to run these trials themselves. As there are many numerous and unidentified diseases acquiring big populations. This population desperately needs better medical care with the discovery of uncovering new medicines.
It’s better to start with some expertise rather than doing something about which you don’t know, the reason being this company contracts with full service CROs to acquire specific expertise without hiring permanent staff.
There are types of comprehensive services provided by CRO’s which includes Project management, Data entry and validation, Clinical trial data management, medicines, and design coding, quality and metric reporting, statistical analysis plans and reports, Validation programming, safety and efficacy summaries, final study report and database design and built, etc. to the company with the full service CRO contracted.
#Full Service CRO#Contract Research Organization#Clinical Trials CRO in India#CRO in India#CRO Services
3 notes
·
View notes
Text
Clinical Data Management Services
CDM (Clinical data management) services professionalized in clinical trial management services, more specifically management of the clinical trial process, patient recruitment resource management, including site performance, operational planning, contact information, and regulatory document recordkeeping.

Companies that conduct clinical trials, Medical device manufacturers, Pharmaceuticals and Biotechnology companies, and university or governmental research organization uses this kind of software system which is good at managing clinical data.
There are many clinical data management services like On-shore and Off-shore clinical data management, CRF/eCRF design and development, CRF annotation and reviews, Database build and design, Medical coding, Real-time data viewing, and reporting, etc.
The clinical data management systems must have below mentioned attributes and also FDA guidance contains these fundamental elements of data quality for both paper and electronic records.
It should be Attributable, Legible, Contemporaneous, Original, Accurate, Readily available, Transmissible, Storable and most importantly data must be complete and unbiased.
#RegulatoryClinicalDevelopment#ClinicalTrialManagement#ClinicalDataManagement#ClinicalDevelopment#Clinical studies#Clinical Development
3 notes
·
View notes
Text
Global Oncology Biosimilar Market
Global Oncology Biosimilar Treatment Market – Past, present, and way forward
Biosimilar or biogenerics are biotherapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.

There is a palpable sense of urgency among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that is easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively.
#Oncology CRO in India#Oncology CRO#Biosimilar Studies in India#Oncology Biosimilar#Biopharmaceutics Services India
3 notes
·
View notes
Text
Changing Landscape of Clinical Trials in India in Line With Improving Regulatory Environment
The clinical trial environment in India witnessed a downfall in 2013 following below-mentioned concerns around the conduct and continuation of clinical trials in India after a series of unfortunate incidents:

The death of 2,644 volunteers during clinical trials over the preceding seven years.
A Public Interest Litigation petition filed in 2012 on behalf of the NGO Swasthya Adhikar Manch (SAM) that sought justice for drug trial victims throughout the nation, asking for a ban the conduct of clinical trials in India for new products that will not be sold or marketed in India.
Consequently, halting of 162 trials from global pharma companies.
As a result, the Supreme Court asked the Drug Controller General of India (DCGI) for details on the mechanism adopted to approve the trials. This propelled the government to make some changes in the regulatory framework regarding clinical trials, such as the online review process was extended with added liability for sponsors along with constraints on trial numbers per investigator and required sizes of sites. With the improved regulations setting in to reduce approval time and increase harmonization, India could soon be able to encourage a patient-friendly environment for clinical trials in the near future.
Improvement in Indian Regulatory Environment Over the Years:
There have been constant updates to policy and guidelines governing the conduct of clinical research in India in the past few years. The fundamental objective of these updates was to give topmost priority to patient safety in an overall difficult regulatory environment. However, Indian regulations have seen positive development in the recent past to favorably support clinical research, while simultaneously focusing on patient safety. These regulatory changes will facilitate the entry of newer innovative medicines to Indian patients at the earliest:
Three tier review process for clinical trial applications (By SEC Meeting, Technical Committee, and Apex Committee)
Mandatory registration of ethics committees with DCGI
Compensation and free medical management (In case of trial-related injury or death)
Audio-Video recording of Informed Consent process
Tougher requirements for site selection
Regulatory inspections
A comprehensive policy and guidelines for all aspects related to approval of new drugs and clinical trials (originated as a result of a setback received by the industry in 2012-13)
In addition, other regulatory updates suggest that academicians conducting trials with 'new drugs' no longer need approval from the DCGI for the conduct of the trial and IEC approval would suffice. Also, customs authorities at the port of entry/exit shall permit the export of biological samples without prior approvals from any government agency, provided the concerned firm submits an undertaking confirming adherence to rules for safe transfer, and disposal of biological samples.
The rising number of clinical trials post new regulations in 2014
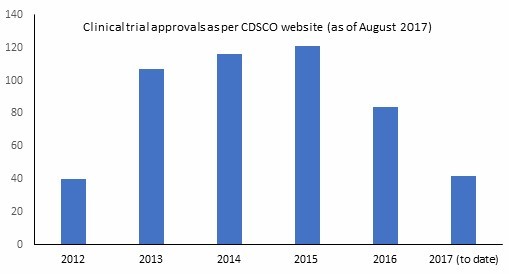
The number of drug approvals has been observed to increase in 2015 after three-tier reviews based on the cases. Clearly, the rising number of clinical trials being approved in India since 2014 is evident from the above figure. Moreover, from 2016 onward, slow revival has been reported, with Phase IV trials seeing an increase by 10 to 14 percent from 2015.
As we can see from the latest available data, the Indian environment is again becoming conducive to clinical trials owing to the refined regulations and reduced approval times as well as the overall improved and more structured regulatory framework. Conducting trials in an emerging market like India, where expenses are lesser and the patient pool is bigger, can significantly benefit sponsor pharmaceutical companies, while also helping the local healthcare system and the economy of the developing country. With not only the huge population but also ever improving socioeconomic status; India is one of the rapidly growing destinations for both the conduct of the clinical studies as well as the marketing of new drugs. With GCP and investigators at par with the global standards, supported by a large patient base, Indian clinical trials could be the solution that the industry is seeking for high-quality data, expeditious study enrolments, and low cost.
Veeda Clinical Research is a rapidly growing CRO in India, where we strive to keep up with the current regulatory updates and provide our customers with comprehensive services, such as Phase II-III clinical trials, feasibility studies, end-to-end regulatory submissions, comprehensive project management, data management and statistics solutions among others.
#CRO in India#Clinical Trials in India#Data Management#Clinical Trials#Phase 2 Clinical Trials#Phase 3 Clinical Trials
5 notes
·
View notes