#ionic dissociation
Explore tagged Tumblr posts
Text
This question was first addressed by Svante Arrhenius (Nobel Prize in chemistry, 1903) in the 1880s (figure 15.13).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#questions#answers#svante arrhenius#nobel prize#00s#1900s#20th century#80s#1880s#19th century#physical chemistry#ionic dissociation
1 note
·
View note
Text
When both cation and anion are doubly charged, the improvement in % dissociation with dilution is even greater.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
1 note
·
View note
Text
yesterday, one of my elementary school students, whom i teach english to as a second language, explained ionic dissociation of acids to me. like, she walked me through the equations and everything. in english. all by herself. i know the elements of the periodic table in english and a few basic chemistry terms from school, but that’s about it. she went on to explain the whole dissociation process, and whenever we hit a word neither of us knew, she just looked it up in the dictionary and kept going, immediately using the new vocab. keep in mind, she’s technically supposed to be in a lower grade but started school a year early. kids her age usually have A2-level english (god i wish) and don’t know a word outside their textbooks. they wouldn’t even attempt to tell me what they did over the weekend if it meant using vocabulary that wasn’t taught in school. then, after the lesson, she texts me this:
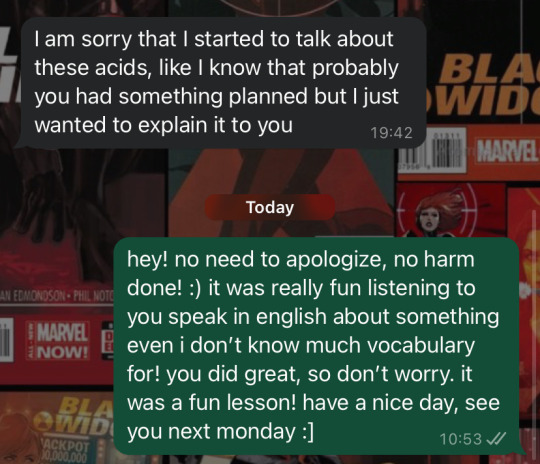
WHY ARE YOU APOLOGIZING FOR BLOWING MY MIND!!!! YOU’RE LITERALLY LINGUISTICALLY GIFTED!!!!
#NEVER apologize to me for stuff like that!!!!#agh!!!!#after the lesson i talked to her mom bc there was no way i was going to let it slide#also i might have told the mom to get her checked for adhd#but i’ve been saying this for years now#anyway#before i had a lesson with this student i had a tragic class with another student of mine#it was sooooo bad i wanted to scream#had to do a pep talk by the end of the lesson (hated doing that)#but then i taught the student from the post#and it was just#wow#felt like i wasn’t even working tbh#english teacher#teaching#teachers of tumblr#english#languages#moira speaks
10 notes
·
View notes
Text
call me an ionic compound dissolving in water the way im dissociating
2 notes
·
View notes
Note
Hello !
I love your account so much I love the subject but I am unable to score well which has lead to develop some negative feelings sooo
I just wanted to ask if you will be able to provide some resource / video / reference anything to understand these two topics:
Equilibrium in specific Ionic equilibrium ( ionisation constants , buffer solution , common ion effect in focus )
Thermodynamics i.e the different kinds of bond dissociation energies
its not like I don't get the topics but more like I am not crystal clear with them which leads to not being able to solve questions
Hello! Let's see what I can do.
Chemistry: Molecules, Matter, and Change (Jones, Atkins) has a whole chapter on solution chemistry. It's a gen chem textbook intended for uni first-years and if you can get your hands on it, I recommend it for studying chemistry in general. It has practice problems at the end of every chapter too which is very helpful when there's an issue with solving questions.
Online textbook (namely chem.libretexts which is like a library of chemistry textbooks and which I also recommend as I use it myself!):
Ionization Constants
Aqueous Ionic Equilibrium
Videos:
Buffer solutions
Common ion effect and buffers just in case you haven't raided Khan Academy's channel yet
The acids and bases playlist (ditto; these are pretty old videos jsyk but I think the explanations are good)
And if I may offer a piece of advice as well: sometimes it just so happens that we read a chapter, think, "Okay, I get it!", move on to solving problems and realize we actually don't really get it. And sometimes, in this situation, it's helpful to just leave the topic whatsoever for a day or two (but not longer!), study something else in the meantime, then return to the problematic topic with a fresh outlook. Because either that new information "settled" in our brain and suddenly we find ourselves with a much better understanding of the topic OR we can now clearly identify our weak points where our knowledge is lacking and therefore holding us back. Oftentimes, in this case, working through the chapter in question once more can be enough.
If that doesn't work for you, I hope these resources will. If you have any specific questions about these topics, also feel free to hmu again!
And please, don't be too hard on yourself. I know it's easier said than done, but focusing on grades more than on the joy of studying an interesting subject is a self-esteem killer. You'll get to where you need to be. Struggling sometimes says nothing about your capabilities!
5 notes
·
View notes
Note
pls tell us your acetate salt wisdom :3
"acetate (CH3COO-) anions are always soluble" is one of those rules you learn in general chemistry that doesn't ever quite make intuitive sense. All the other anions which strongly suggest solubility are the conjugate bases of strong acids, whereas acetic acid is (for these purposes) a weak acid. Moreover, they're either highly electronegative halide ions, or in the case of sulfate and nitrate, polyatomic atoms situated around a single nonmetal with 3-4 oxygen atoms greatly inducing a dipole moment with very ionic tendencies. So, why does the relatively complex acetate ion dissociate so strongly, especially when the methyl (CH3) group complicates the dipole moment?
In short, the acetate ion is in a unique position. Organic groups (parts of the ion where carbon bonds to hydrogen) on an ion do two things: reduce its overall ionic character, making it more difficult to dissociate in water, and reduce the strength of the crystalline lattice of its ionic structure, which makes it easier to dissociate its structure. Acetate ions are sort of the last stop for organic salts where the reduction in their propensity for forming lattices is more than the overall reduction in their ionic character, making its water solubility notable.
The thing obsessing me now is why we talk about acetate in those terms and not formate (HCOO-), which by all accounts is also very reliably ionic and is closer in character to the other strongly dissociative polyatomic ions. Is it just because acetate is more prevalent in undergraduate chemistry than formate?
11 notes
·
View notes
Text
Dissociation is ionic compound turn into ions
#incorrect qoute#ask away!#incorrect quotes#jade/eclipse#family memes#memes#funny memes#daily memes#jade eclipse li#chem#chemistry
3 notes
·
View notes
Text
Exploring the Diverse World of Inorganic Chemicals: Types, Applications, and Market Trends
The world of inorganic chemicals is vast and diverse, encompassing a wide range of compounds that play crucial roles in various industries. From the production of fertilizers and pigments to the manufacturing of electronics and pharmaceuticals, inorganic chemicals are essential building blocks for modern society. Types of Inorganic Chemicals: Inorganic chemicals can be broadly classified into several categories based on their composition and properties. Some of the major types include: 1. Acids and Bases: These compounds are characterized by their ability to donate or accept protons. Examples include sulfuric acid, hydrochloric acid, and sodium hydroxide. 2. Salts: Formed by the reaction between acids and bases, salts are ionic compounds that dissociate in water. Common examples include sodium chloride, potassium nitrate, and calcium carbonate. 3. Oxides: Inorganic oxides are compounds composed of oxygen and another element. They can be classified as acidic, basic, or amphoteric. Examples include silicon dioxide, aluminum oxide, and iron oxide. 4. Metals and Alloys: Metals and their alloys are widely used in various applications due to their unique properties. Examples include steel, aluminum, copper, and titanium. Applications of Inorganic Chemicals: Inorganic Chemicals find applications in a wide range of industries, contributing to the production of essential products and materials. Some key applications include: 1. Agriculture: Inorganic chemicals are used in the production of fertilizers, pesticides, and herbicides. They help in enhancing crop yield, controlling pests, and promoting plant growth. 2. Construction: Inorganic compounds such as cement, glass, and ceramics are integral to the construction industry. They are used in the manufacturing of buildings, roads, and infrastructure. 3. Electronics: Inorganic materials, including semiconductors and superconductors, are crucial components in electronic devices. They are used in the production of integrated circuits, solar cells, and display technologies. 4. Water Treatment: Inorganic chemicals play a vital role in water treatment processes. Compounds such as chlorine, alum, and activated carbon are used for disinfection, coagulation, and adsorption of impurities. 5. Pharmaceuticals: Inorganic compounds are used as active ingredients, excipients, and catalysts in the pharmaceutical industry. They are essential for the synthesis and formulation of various drugs and medications.
Get More Insights on Inorganic Chemicals

#Inorganic Chemicals#Coherent Market Insights#oxides#metals#chlor-alkali#corrosion inhibitors#semiconductor#microreactor systems
0 notes
Text
I'd like to note that Arrhenius was not just Some Guy. He was a Nobel Laureate in Chemistry, one of the founders of the entire field of physical chemistry, the guy who came up with (the first correct) theory of acid-base interaction, and the guy who came up with the concepts of ionic dissociation (salt dissolves in water by turning to Na+ and Cl-) and temperature dependence of reaction rates (i.e. that reactions go faster when it's hotter).
This is roughly equivalent to the Roosevelt administration getting that letter on nuclear fission bombs from Einstein and going "Ehh, sounds like a problem for future generations. The Germans will never figure it out." And then people in 2023 having lively arguments on YouTube about whether nuclear fission chain reactions are actually possible. "The demon core was faked." Etc.

#the overall effects of global warming are still fairly argued over#but generally it's considered fairly likely that there will be more catastrophic weather events and ocean acidification#the water level rise is surprisingly one of the least sure bits of it#which is awkward because it's the easiest to explain to laymen#oh and of course. reactions go faster when it's hotter. as per arrhenius#physical chemistry#ig
89K notes
·
View notes
Text
Edi Ultrapure Water System Market Industry Growth Forecast: Key Drivers and Market Trends to 2033
Edi Ultrapure Water System Market : Comprehensive Analysis and Growth Forecast (2025-2033)
Global EDI Ultrapure Water System Market Size is expected to grow with a CAGR of 6.1% during the forecast period. North America region has the largest Market.
Market Overview
The Edi Ultrapure Water System Market Report delivers a detailed exploration of a dynamic market cutting across multiple industries. Forecasts, trend analyses, and practical insights covering the years 2024–2033 are all included in this thorough study. The research explores important aspects such product innovation, adoption patterns, pricing tactics, and regional penetration by fusing quantitative data with professional comments.
According to Market Strides the Global EDI Ultrapure Water System Market Size will approximately grow at a CAGR of 6.1% during the forecast period.
EDI stands for Electro Deionization Ultrapure Water System. The Electro Deionization process uses ion exchange resins, ion-selective membranes, and an applied DC electrical current to remove ions, such as salts, acids, and bases. Weakly ionized materials, like dissolved silica, carbon dioxide, boron, and some organics, are also removed. EDI modules are built with membranes permeable to either cations or anions in an alternating sequence. The spaces between them act as liquid flow compartments, each with an inlet and an outlet. Dependent on the polarity of the ion exchange membrane and its orientation to a cathode or anode, an individual compartment depletes the water of ions (product water) or accumulates ions (reject water).
The dilute and concentrate compartments are filled with ion exchange resins to transfer in low ionic strength solutions. In this EDI module, approximately 5% of the volume of feed flow continuously washes out the ions from the concentrate compartments. This concentrate outlet, the reject stream, is recirculated upstream of the RO system to be recovered, while the dilute compartments deliver purified product water. Unlike traditional Ion Exchange resin systems, EDI needs no chemical regeneration. All EDI devices producing ultrapure water operate in electro-regeneration mode, in which the resins are continuously regenerated by H+ and OH- ions released by the electrically-induced dissociation of water molecules.
Technological advancements in EDI systems are helping in improved efficiency and enhanced performance. They have advanced monitoring and control capabilities, improved resin design, and innovative electrode configurations, which allow efficient water treatment processes.
Industries such as Pharmaceuticals, Electronics, and Manufacturing mostly need EDI systems as they must follow strict water quality standards and regulations. Additionally, it considers macroeconomic indicators like GDP growth and socio-economic trends to contextualize market dynamics effectively, enabling stakeholders to make informed decisions.
Get Sample Research Report: https://marketstrides.com/request-sample/edi-ultrapure-water-system-market
Key Focus Areas
Sectors Utilizing Edi Ultrapure Water System Market Products/Services: A detailed examination of industries leveraging the offerings
Market Leaders and Consumer Preferences: Insights into leading participants and evolving trends in customer behavior.
Competitive Landscape: An analysis of competitive positioning, regulatory influences, and emerging technologies shaping the market.
Organized into well-structured segments, the Edi Ultrapure Water System Market report fosters a multi-dimensional understanding of the industry, ensuring actionable insights across economic, political, and cultural contexts.
Growth and Emerging Trends
The Edi Ultrapure Water System Market industry is undergoing transformative changes driven by pivotal trends, which include:
Digital transformation: is the use of cutting-edge technologies to improve customer engagement and expedite processes through data-driven solutions.
Consumer-Centric Innovation: Using customized products to meet the increasing need for ease and personalization.
Regulatory Evolution: To stay competitive, quickly adjust to new regulations and more stringent compliance standards.
Top Key Players in Edi Ultrapure Water System Market
Veolia
Suez
Ovivo
Hitachi
Evoqua
Rightleder
Hyflux
Pure Water No.1
Hongsen Huanbao
Mar-Cor Purification
Nalco
Hongsen Huanbao
Beijing Related
This section provides a SWOT analysis of the top players, focusing on their strategies, opportunities, and challenges. Highlights include:
Top 3-5 Companies: Comprehensive profiles and analysis of key strengths, weaknesses, and growth strategies.
Competitive Landscape: Insights into recent developments, such as partnerships, mergers, acquisitions, and product launches.
Regional Influence: Assessment of regional presence and contributions using the Ace matrix criteria to evaluate market share and growth potential.
Edi Ultrapure Water System Market Segmentation
Segment by Type
0-10 m3/h
10-30 m3/h
30+ m3/h
Segment by Application
Electronics
Pharmaceuticals
Power and Energy
allowing stakeholders to identify specific opportunities and tailor strategies effectively.
Browse Details of Edi Ultrapure Water System Market with TOC: https://marketstrides.com/report/edi-ultrapure-water-system-market
Research Methodology
The report is backed by a meticulous research approach:
Primary Research: In-depth interviews, surveys, and consultations with industry experts, supported by corporate press releases, annual reports, and government publications.
Secondary Research: Extensive analysis of market drivers using industry reports, trade publications, and academic research.
Data Validation: Rigorous cross-verification with expert input to ensure accuracy and credibility.This methodology guarantees a reliable and actionable market perspective, empowering stakeholders with informed decision-making tools.
Regional Analysis Edi Ultrapure Water System Market
The Edi Ultrapure Water System Market report provides an in-depth regional breakdown, offering insights into unique opportunities and characteristics across
North America
Europe
Asia-Pacific
Latin America
The Middle East and Africa
Our Reports Empower Clients Through:
Market sizing and competitive analysis
Strategic guidance for due diligence
product expansion
plant setup
acquisition intelligence
Buy Now:https://marketstrides.com/buyNow/edi-ultrapure-water-system-market
About Us:
Market Strides is a Global aggregator and publisher of Market intelligence research reports, equity reports, database directories, and economic reports. Our repository is diverse, spanning virtually every industrial sector and even more every category and sub-category within the industry. Our market research reports provide market sizing analysis, insights on promising industry segments, competition, future outlook and growth drivers in the space. The company is engaged in data analytics and aids clients in due-diligence, product expansion, plant setup, acquisition intelligence to all the other gamut of objectives through our research focus.
Contact Us📧: [email protected]
#Edi Ultrapure Water System Market Size#Edi Ultrapure Water System Market Share#Edi Ultrapure Water System Market Growth#Edi Ultrapure Water System Market Trends#Edi Ultrapure Water System Market Players
0 notes
Text
D-PBS And Its Role In Cell-Cell Interactions
Dulbecco’s Phosphate Buffered Saline (D-PBS) is an essential component in cell culture and research laboratories across the globe. To the casual observer, this may seem like simply a rinsing solution, but it maintains physiological conditions and facilitates important cell-cell interactions.

Understanding D-PBS Composition
D-PBS composition has a very balanced salt solution especially constructed to emulate the physiological environment of mammalian cells. These are the main ingredients:
● Sodium chloride (NaCl): Osmotic balance, essential ions.
● Potassium chloride (KCl): Proper ion concentrations for cell function.
Sodium phosphate and potassium phosphate: Buffers with stable pH critical for optimal cell growth and activity.
Absence of Calcium and Magnesium
The important difference between D-PBS and standard PBS is the absence of calcium and magnesium ions. They are vital for many cellular processes, but they hamper certain experimental procedures:
● Cell dissociation: Calcium and magnesium ions encourage cell-cell adhesion. This is one factor that makes efficient dissociation of cells from culture surfaces or tissues problematic.
● Immunological studies: These ions may interfere with antibody binding, affecting the validity of immunostaining and flow cytometry experiments.
D-PBS and Cell-Cell Interactions
There are several ways in which D-PBS directly affects cell-cell interactions:
● Washing away growth factors and cytokines: These extracellular signaling molecules are known to influence different aspects of cell behavior-including proliferation, differentiation, and migration-when washed out using D-PBS potentially provide the possibility of being more controlled over experimental conditions.
● Cell viability maintenance: A physiologically balanced surrounding created by D-PBS minimizes osmotic stress and protects cell viability during washing and handling.
D-PBS Applications in Cell-Cell Interaction Studies
The crucial role played by D-PBS in all these various experimental settings with respect to cell-cell interactions:
● Co-culture experiments: Preparation of washed cells to be co-cultured with another cell type ensures that residuals do not affect the co-culture.
● Cell migration assays: D-PBS suspension of cells will be prepared and then produced in concentration-defined gradients of chemotactic substances, which will allow cells to migrate through chemotaxis.
● Adhesion studies: D-PBS washes both the cells and substrates before assays in order to prevent any potential altering of attachment of the cells due to residual proteins or factors.
Conclusion
D-PBS regulates cell-cell interactions and, despite its plain and simple composition, offers many aspects in governing cell-cell interactions. Delbecco’s PBS perhaps holds a spectrum in a microscopically changed ionic environment and neutral influence of divalent cations on any cell-based experiment, starting with simple cell culture to the most integrated studies of cell communication and behavior. Visit PurMa Biologics LLC to get more information.
0 notes
Text
Call me an ionic compound the way I dissociate in water
1 note
·
View note
Text
Lithium Hydroxide Market Growth: Exploring Industry Dynamics
Introduction
Lithium hydroxide (LiOH) is an inorganic compound, specifically a salt, that is a white crystalline solid when anhydrous but often encountered as a monohydrate (LiOH⋅H2O). It consists of lithium cations (Li+) and hydroxide anions (OH-). LiOH is highly soluble in water and is commonly encountered as a solution. Chemical Properties
As an ionic compound composed of lithium and hydroxide ions, LiOH shares many typical properties of salts. It dissolves readily in water to form an alkaline solution. When dissolved in water, LiOH ionizes into lithium cations and hydroxide anions according to the dissociation reaction: LiOH (s) → Li+ (aq) + OH- (aq) The resulting solutions are strongly alkaline, or basic, with a pH ranging from 11-12.5 depending on concentration. This solubility and basicity is indicative of the ionic character of the lithium-oxygen and hydrogen bonds in the solid. LiOH also has a high melting point of approximately 550°C, which reflects the strong electrostatic interactions between ions. Uses and Applications
As an alkali metal hydroxide, LiOH has various industrial uses that exploit its strong basic nature. One major application is in lithium batteries, where it is used as an electrolyte. The high solubility of LiOH allows it to readily dissolve lithium salts and conduct lithium ions between the battery electrodes. LiOH is also used in the manufacture of lithium greases and as a desiccant to absorb water and carbon dioxide from air or gases. In the glass industry, LiOH is added in small amounts to glass compositions. The lithium ions strengthen the glass by becoming part of the silicate network structure. Lithium-containing glasses have increased durability and improved chemical resistance. Pharmaceutical applications also exist, as LiOH is sometimes used as an ingredient in antacids and other medicines due to its alkalinizing properties. Physical Properties
Beyond its chemical makeup and strong basicity in aqueous solution, LiOH has notable physical properties as well. As mentioned previously, its melting point is approximately 550°C, reflecting the strength of ionic bonding. Like other ionic compounds, it is generally hard and brittle in solid form. Anhydrous LiOH appears as white crystals or a powder, while the monohydrate exists as colorless crystals. The density of LiOH is approximately 2.00 g/cm3. In terms of solubility, it exhibits high solubility in water, with a maximum solubility of 57g/100mL at 0°C. This enables it to act as a strong base when dissolved and essentially complete dissociation into its constituent ions. The highly soluble nature derives from the ability of waters’ polarity to strongly solvate both the lithium and hydroxide ions. Environmental and Safety Considerations
Being a strong base, lithium hydroxide presents certain hazards if not properly handled or contained. Direct contact with concentrated solutions or solid LiOH can cause severe irritation and chemical burns to skin and eyes on contact. When heated, it may emit toxic fumes of lithium oxides. For these reasons, it is recommended to use protective equipment like gloves, eye protection, and respiratory masks when working with this compound. Spills or releases of lithium hydroxide into the environment should also be avoided, as its high pH can raise the alkalinity of surrounding waters and soils. Solutions must be neutralized before disposal. However, as lithium is a relatively rare element, LiOH itself does not present significant environmental contamination concerns from a toxicity standpoint if carefully managed. Overall, through prudent handling and containment practices according to safety guidelines, LiOH can be utilized safely in industrial applications. Conclusion
In summary, lithium hydroxide is an important industrial compound with applications spanning batteries, glass, pharmaceuticals and other areas. Its wide use stems from versatile chemical properties including high solubility, strong basicity in solution, and ability to conduct lithium ions effectively. Though presenting hazards from its caustic nature, LiOH can be employed safely in manufacturing when appropriate precautions and personal protective equipment are followed. A thorough understanding of its chemical makeup, physical attributes and environmental/safety considerations provides necessary background for its utilization.
0 notes
Text
The Elemental Advantage: GenB_Magnesium Oxide Leadership

Magnesium Oxide: A Versatile and Essential Compound Introduction
Magnesium oxide, also known as magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium. It has a chemical formula of MgO and has important industrial and technical applications. Chemical Properties
Magnesium oxide has an empirical formula of MgO and a molecular weight of 40.304 g/mol. It has a melting point of 2852°C and a boiling point of 3600°C. Magnesium oxide is an ionic compound consisting of Mg2+ cations and O2- anions. It crystallizes in the cubic crystal structure known as the rock salt structure. Magnesium oxide is thermally stable up to about 3,000°C and is insoluble in water but soluble in acids and bases producing magnesium salts. Uses in Refractories and Metallurgy
Magnesium oxide is an important refractory material due its high thermal conductivity, high melting point and thermal stability. It is commonly used as a refractory lining material in kilns, furnaces, incinerators and fireplaces that operate at very high temperatures. When mixed with other oxides like aluminum oxide or calcium oxide, magnesium oxide is used to manufacture castable refractory concrete and mortars used to line industrial equipment handling molten metals. Its high refractoriness and thermal stability makes it ideal as a lining material that retains its strength and withstands heat shock. As a feedstock material, magnesium oxide is utilized in the production of magnesium metal which in turn finds applications across a variety of industries including aerospace, construction and automotive. Applications in Agriculture
Magnesium oxide is widely used as a fertilizer for magnesium deficient soils and crops. Magnesium is an essential plant nutrient required for photosynthesis, protein synthesis and activating enzymes. Magnesium oxide is oxidized by water and carbon dioxide to form magnesium hydroxide and bicarbonate which dissociate into bioavailable magnesium ions. Farmers commonly use magnesium oxide as an agricultural liming agent to raise soil pH and supply magnesium to crops like fruits, vegetables, nuts, corn and sugarcane. It is also added to fertilizers, manure and compost to prevent magnesium deficiencies in agricultural lands. Use as an Antacid and Laxative
Magnesium oxide has important medical uses due to its high magnesium content and alkaline nature. When taken orally, it passes through the gastrointestinal tract where it reacts with water to release magnesium hydroxide. This makes magnesium oxide a popular over-the-counter antacid used to relieve heartburn, acid indigestion and sour stomach. It works by neutralizing excess stomach acid. Magnesium oxide is also commonly used as a laxative due to its osmotic and lubricating effects in the intestines that promote regularity. As a nutritional supplement, magnesium oxide provides the essential mineral magnesium needed for optimal metabolic functioning in the body. Other Applications
In addition to the above major uses, magnesium oxide also finds applications as a smoke suppressant in fireworks and solid fuel rockets, an opacifying agent in paints and plastics, an absorbent for fluoride gases in air pollution control, a reflector in infrared mirrors and thermal insulation, and a filter medium used for purification processes like ultrapure water. Finely milled magnesium oxide is used as a refractory cement in bonding compounds for refractory bricks and concretes. Due to its alkaline nature, it is utilized as an additive in animal feeds to regulate pH and mitigate acidosis in cattle and poultry. Magnesium oxide is also used in desulfurization processes for removing sulfur from fuels like diesel and gasoline. Production and Supply
Magnesium oxide is commercially manufactured by calcination of magnesium hydroxide or carbonate obtained from seawater or mining magnesium rich brucite and serpentine rocks. The mined ores are crushed, roasted and ground to produce light burnt magnesia. The dead burned variety with higher purity levels is produced by heating magnesium oxide above 2800°C. China, Israel, Russia, Turkey and the US are among the major worldwide producers and suppliers of magnesium oxide with commercial products available in powder, granule and brick forms. Magnesia brick manufacture is an important industry for economic and employment generation. Conclusion In summary, magnesium oxide is a versatile, functional and widely utilized inorganic compound with important metallurgical, refractory, agricultural, medical and industrial relevance. Its high thermal stability, alkalinity and magnesium supplying properties have made it a valuable material across multiple applications and processing sectors. Advancements in mining, processing and manufacturing technologies are expected to further optimize magnesium oxide production and commercialization in the coming years.
___________________________________________________________________________
0 notes
Text
Empowering Energy Solutions: Global Perspectives on the Battery Electrolyte Market
An electrolyte serves as a solution facilitating the transfer of electrical charge between electrodes in an electrolytic cell or battery, existing in solid, liquid, or molten states. Formed by combining water with ionic compounds, especially salts, electrolytic solutions dissociate into cations and anions, acting as charge carriers. As a fundamental component of batteries, electrolytes play a crucial role in determining overall performance. Batteries find diverse applications across electrical appliances, with major end-users in the automobile and consumer electronics sectors.
Request Sample Report: https://www.alliedmarketresearch.com/request-toc-and-sample/10954
Market Scope and Structure Analysis:
Report Metrics:
Market size available for years: 2019–2027
Base year considered: 2019
Forecast period: 2020–2027
Forecast units: Value (USD)
Segments covered: Battery Type, Electrolyte Type, Industry Vertical, and Region
Regions covered: North America, Europe, Asia-Pacific, Latin America, and The Middle East and Africa
Key Companies: Mitsubishi Chemical Corporation, Shenzhen Capchem Technology Co. Ltd., Johnson Controls, BASF SE, LG Chem, Ube Industries, Guangzhou Tinci Materials Technology Co. Ltd., GS Yuasa Corporation, Advanced Electrolyte Technologies, and American Elements.
Request for Customization of This Report at: https://www.alliedmarketresearch.com/request-for-customization/10954
COVID-19 Scenario Analysis:
The global battery electrolyte market faced severe disruptions due to the COVID-19 pandemic:
The epicenter, China, experienced a complete shutdown, impacting manufacturing, imports, and exports, significantly affecting the battery electrolyte market.
Industrial demand for batteries plummeted as business activities halted globally.
The demand for electric vehicles, a major growth driver for the battery market, saw a drastic decrease.
Economic downturn led to a sharp decline in demand for electronics and automobiles, affecting the overall battery electrolyte market.
Top Impacting Factors: Market Scenario Analysis, Trends, Drivers, and Impact Analysis:
Rapid technological advancements, including Kyocera's introduction of semi-solid lithium-ion batteries, and increased applications of electrical appliances have been key growth drivers.
Lithium-ion batteries are widely used in electronic gadgets and the automobile industry, with the rise of electric vehicles contributing to increased demand.
Growing use of solar photovoltaic modules has positively impacted the battery electrolyte market, driven by consumer shifts towards renewable energy sources like solar power.
Environmental concerns and consumer preferences for renewable energy have increased battery demand.
Challenges include the high cost of electric vehicles and a lack of recycling technologies for battery materials.
Massive investments in the lithium-ion battery segment, especially in China, are expected to propel market growth.
E𝐧𝐪𝐮𝐢𝐫𝐲 𝐁𝐞𝐟𝐨𝐫𝐞 𝐁𝐮𝐲𝐢𝐧𝐠 : https://www.alliedmarketresearch.com/purchase-enquiry/10954
Regional Analysis:
Asia-Pacific leads the global battery electrolyte market, driven by a substantial electronics sector, particularly in China.
North America is poised for significant growth due to high demand for electronics and automobiles, coupled with increasing applications of renewable energy sources.
Key Segments Covered:
Segments:
Battery Type: Lead-acid, Lithium-ion, Nickel Metal, Others
Electrolyte Type: Sodium Chloride, Nitric Acid, Sulfuric Acid, Others
Industry Vertical: Industrial, Automobile, Energy Storage, Consumer Electronics, Others
Key Benefits of the Report:
Analytical depiction of the global battery electrolyte industry, presenting trends and future estimations.
Information on key drivers, restraints, and opportunities, along with detailed market share analysis.
Quantitative analysis of the market from 2020 to 2027, illustrating growth scenarios.
Porter’s five forces analysis showcasing buyer and supplier potency.
Detailed global battery electrolyte market analysis based on competitive intensity and future market dynamics.
Battery Electrolyte Market: Global Opportunity Analysis and Industry Forecast, 2020–2027 Report Highlights:
Aspects:
By Battery: Lead-acid, Lithium-ion, Nickel Metal, Others
By Region: North America, Europe, Asia-Pacific, LAMEA
By Electrolyte Type: Sodium Chloride, Nitric Acid, Sulfuric Acid, Others
By Industry Vertical: Industrial, Automobile, Energy Storage, Consumer Electronics, Others
Key Market Players: Mitsubishi Chemical Corporation, Shenzhen Capchem Technology Co. Ltd., Johnson Controls, GS Yuasa Corporation, Ube Industries, Guangzhou Tinci Materials Technology Co. Ltd., BASF SE, LG Chem, Advanced Electrolyte Technologies, and American Elements.
0 notes
Text
Role of Polyelectrolytes in Wastewater Treatment
Effluent Treatment or Industrial wastewater treatment consists of mechanisms and processes which are used to treat water that have been contaminated by anthropogenic, industrial or commercial activities prior to its release into the environment. Industrial wastewater serves as one of the important pollution sources that add up in polluting the water environment. The sewage sludge produced is subjected to sludge treatment. Many problems are associated with wastewater collection and treatment such as foaming, solid accumulation, high BOD, sludge de-watering, heavy metals and offensive odour. But such treatments are no more complicated processes, since there are chemicals that are applicable for the treatment. These chemicals follow various treatment mechanisms in various different standards, which include from chemically treating raw wastewater before discharging it into the environment, to recovering valuable resources from sludge treatment and wastewater.

Conventional treatment consists of a combination of physical, chemical, and biological processes for removal of suspended solids, organic & inorganic contaminants, germs and microbes from wastewater. When using total wastewater treatment solutions, these chemicals remove and eliminate harmful pathogens, expel hazardous chemicals, detergents and toxins, reduce odour and improve water colour, and separate and extract valuable substances and clean water from the wastewater.
Polyelectrolytes are regarded as polymer chains with an electrolyte group on every repeat unit. When dissolved in a polar solvent, these polymers are charged due to dissociation of small counter ions that leave behind a charged micro ion. These are formulated in a way so as to be used as coagulants and flocculants as well as a sludge dewatering agent in the waste water treatment plants. Flocculants are chemicals responsible for bringing about secondary settling and sludge dewatering. They carry active groups with a charge which helps in counterbalancing the charge of the particles. Coagulants are referred to as chemicals that are used to help in the removal of colour and turbidity present in untreated, raw water and are used for their attributes like quick flocculation, precise pH value, etc.
Based on electrolyte group present in the chain, polyelectrolytes are categorized into anionic, cationic and non – ionic species. Cationic polyelectrolytes have positively charged group for use in coagulation of negatively charged flocs through electrostatic interactions. They are useful in various process of effluent r wastewater treatment, depending upon their molecular weight and charge density. Anionic polyelectrolytes have negatively charged group on each repeating unit, acrylamide-based polymers being the most available type. They are widely useful as flocculants, rheology modifiers, and adhesives and immensely used in municipal wastewater and effluent treatment. Anionic flocculants are also used in enhanced oil recovery, decolouring, paper making, mineral processing, etc. Non – Ionic Polyelectrolytes are organic high molecular weight polymers, used in flocculating colloidal suspensions, water clarification, sludge dewatering, etc.
In general, aqueous solutions having pH value more than 4 have negative charged suspended particles whereas having pH less than 4 contains positively charged suspensions. This might help in analysing the type of polyelectrolyte to be used in the treatment.
Chemtex Speciality Limited is a well-known manufacturer and supplier of liquid and powder polyelectrolytes. For more information on our range of products, visit https://www.chemtexltd.com/products-and-solutions/water-treatment-chemicals/wastewater-treatment/s
0 notes