#vavaclasses
Explore tagged Tumblr posts
Text
Morphology of Flowering Plants Class 11 Notes
Morphology is the branch of biology that deals with the form and structure of organisms. In the context of flowering plants (angiosperms), morphology helps us understand the external features of plants which are crucial for identification, classification, and studying the relationship between different plants.

Root
Types of Roots
1. Tap Root System:
- Found in dicots.
- Consists of a primary root (tap root) that grows directly downward and gives rise to lateral roots.
- Example: Carrot, Radish.
2. Fibrous Root System:
- Found in monocots.
- Consists of numerous thin, fibrous roots that grow from the base of the stem.
- Example: Grass, Wheat.
3. Adventitious Root System:
- Roots that develop from parts of the plant other than the radicle.
- Example: Banyan tree (prop roots), Maize (stilt roots).
Modifications of Roots:
Roots may be modified for various functions like storage, support, and respiration.
1. Storage Roots:
- Swollen roots that store food.
- Example: Carrot, Sweet Potato.
2. Prop Roots:
- Arise from the branches and descend downwards to provide support.
- Example: Banyan tree.
3. Pneumatophores:
- Also known as respiratory roots, they grow upwards and help in gaseous exchange.
- Example: Mangrove plants.
Stem
Structure and Functions
The stem is the ascending part of the plant axis that bears branches, leaves, flowers, and fruits. It conducts water, minerals, and food to different parts of the plant.
Modifications of Stems:
Stems may be modified to perform various functions such as storage, support, and vegetative propagation.
1. Tuber:
- Swollen, underground stem that stores food.
- Example: Potato.
2. Rhizome:
- Horizontal, underground stem that stores food and helps in vegetative propagation.
- Example: Ginger, Turmeric.
3. Bulb:
- Shortened, underground stem with fleshy leaves storing food.
- Example: Onion, Garlic.
4. Runner:
- Horizontal stem that grows above ground and helps in vegetative propagation.
- Example: Strawberry.
5. Cladodes:
- Green, flattened stems that perform photosynthesis.
- Example: Asparagus.
Leaf:
Structure:
A typical leaf consists of three parts:
Leaf Base: The part that attaches the leaf to the stem.
Petiole: The stalk that connects the leaf blade to the stem.
Lamina (Leaf Blade): The flat, green part of the leaf where photosynthesis occurs.
Venation:
Venation is the arrangement of veins in a leaf. It can be:
1. Reticulate Venation:
- Veins form a network.
- Common in dicots.
- Example: Mango, Hibiscus.
2. Parallel Venation:
- Veins run parallel to each other.
- Common in monocots.
- Example: Grass, Banana.
Read Also: Detailed Study Notes for Class 12-Human Reproduction
Modifications of Leaves
Leaves may be modified to perform various functions such as support, storage, and protection.
1. Tendrils:
- Leaves or parts of leaves modified into slender, coiling structures for support.
- Example: Pea.
2. Spines:
- Leaves or parts of leaves modified into sharp structures for protection.
- Example: Cactus.
3. Succulent Leaves:
- Thick, fleshy leaves that store water.
- Example: Aloe Vera.
4. Insectivorous Leaves:
- Leaves modified to trap and digest insects.
- Example: Pitcher Plant, Venus Flytrap.
Flower
Structure:
A flower is the reproductive part of angiosperms. It typically consists of four whorls:
Calyx: The outermost whorl, consisting of sepals which protect the flower bud.
Corolla: The whorl of petals, often brightly colored to attract pollinators.
Androecium: The male reproductive whorl, consisting of stamens. Each stamen has an anther and a filament.
Gynoecium: The female reproductive whorl, consisting of one or more carpels. Each carpel has an ovary, style, and stigma.
Types of Flowers:
1. Complete Flowers:
- Have all four whorls (calyx, corolla, androecium, gynoecium).
- Example: Hibiscus.
2. Incomplete Flowers:
- Lacking one or more whorls.
- Example: Corn (female flowers lack stamens).
Flower Symmetry:
1. Actinomorphic (Radial Symmetry):
- Can be divided into two equal halves in multiple planes.
- Example: Mustard.
2. Zygomorphic (Bilateral Symmetry):
- Can be divided into two equal halves in only one plane.
- Example: Pea.
Fruit:
Types of Fruits:
1. Simple Fruits:
- Develop from a single ovary.
- Example: Mango, Tomato.
2. Aggregate Fruits:
- Develop from multiple ovaries of a single flower.
- Example: Strawberry, Raspberry.
3. Multiple Fruits:
- Develop from the ovaries of multiple flowers.
- Example: Pineapple, Mulberry.
Fruit Development:
Fruits develop from the ovary after fertilization. The ovary wall develops into the pericarp, which may be fleshy or dry. The seed(s) develop from the ovules.
Seed:
Structure:
A typical seed consists of:
Seed Coat: The protective outer layer.
Embryo: The young plant, consisting of the radicle (future root), plumule (future shoot), and cotyledons (seed leaves).
Endosperm: The nutritive tissue for the developing embryo (present in some seeds).
Types of Seeds
1. Monocotyledonous Seeds:
- Have one cotyledon.
- Example: Maize, Wheat.
2. Dicotyledonous Seeds:
- Have two cotyledons.
- Example: Pea, Bean.
Seed Germination:
Germination is the process by which a seed develops into a new plant. It requires favorable conditions such as water, oxygen, and a suitable temperature. The radicle emerges first, followed by the plumule.
Summary
Understanding the morphology of flowering plants involves studying their external structures, including roots, stems, leaves, flowers, fruits, and seeds. Each part has unique adaptations and modifications that help the plant survive, reproduce, and thrive in its environment. This knowledge is fundamental for plant identification, classification, and understanding the intricate relationships within the plant kingdom.
These notes provide a comprehensive overview of the morphology of flowering plants and should be useful for Class 11 Biology students. If you need any additional details or specific topics covered, feel free to ask!
#morphology of flowering plants class 11#class 11 biology#11thclass#science#biology#vavaclasses#morphology of flowering plants
1 note
·
View note
Text
Biotechnology and Its Applications Detailed Explanation Suitable for Class 12 Students
Biotechnology and Its Applications:
Introduction to Biotechnology:
Biotechnology involves using living organisms, cells, and biological systems to develop products and technologies for various applications. It merges biology with technology, providing innovative solutions in multiple fields such as agriculture, medicine, and environmental management.

1. Biotechnological Applications in Agriculture:
Biotechnology in agriculture aims to enhance food production and crop quality through several advanced techniques:
Agro-Chemical Based Agriculture: This method uses chemical fertilizers and pesticides to increase crop yields. However, it can have negative environmental impacts.
Organic Agriculture: Involves using natural methods and products for farming, promoting sustainability and reducing chemical residues in food.
Genetically Engineered Crops (GMOs): Crops are modified using genetic engineering to exhibit desirable traits such as pest resistance, drought tolerance, and improved nutritional content. Examples include Bt cotton, which produces Bt toxin to protect against specific pests, reducing the need for chemical insecticides.
Read Also: Biodiversity and Conservation - Class 12 Detailed Notes
Tissue Culture: This technique allows the growth of entire plants from small tissue samples (explants). It helps in producing a large number of genetically identical plants, known as some clones, which are beneficial for maintaining uniform crop quality.
Micro-Propagation: A form of tissue culture used to produce a large number of plants quickly. It is useful for propagating plants that do not produce viable seeds.
Somatic Hybridization: Combines different plant species at the cellular level to create new hybrid plants with desirable traits from both parent species.
2. Biotechnological Applications in Medicine:
Biotechnology has revolutionized medicine, providing advanced methods for diagnosing, treating, and preventing diseases:
Recombinant DNA Technology: Allows the production of therapeutic proteins and drugs in large quantities. For example, insulin used to treat diabetes is now produced using genetically engineered bacteria, making it safer and more effective than animal-derived insulin.
Gene Therapy: Involves inserting healthy genes into a patient's cells to treat genetic disorders. It offers potential cures for diseases like cystic fibrosis and certain types of cancer.
Vaccines: Biotechnology has enabled the development of new vaccines, such as the recombinant hepatitis B vaccine, which is produced using yeast cells.
3. Transgenic Animals:
Transgenic animals are genetically modified to carry genes from other species. They serve various purposes, including:
Research: Studying gene functions and disease mechanisms in transgenic animals helps scientists understand human diseases better.
Pharming: Producing valuable proteins and drugs in the milk, eggs, or blood of transgenic animals, which can then be harvested and purified for medical use.
Improving Livestock: Enhancing traits like growth rate, disease resistance, and milk production in farm animals.
4. Ethical Issues:
Biotechnology raises several ethical and moral concerns:
Safety of GMOs: There is ongoing debate about the potential health risks and environmental impacts of genetically modified crops and animals.
Genetic Discrimination: The use of genetic information by employers or insurance companies could lead to discrimination against individuals based on their genetic predisposition to certain diseases.
Moral Implications: Genetic modifications in humans, such as designer babies, spark ethical questions about the extent to which humans should interfere with natural processes.
Conclusion:
Biotechnology offers immense potential to solve some of the world's pressing challenges in food production, healthcare, and environmental conservation. However, it is crucial to address the ethical and safety issues associated with its applications to ensure responsible and sustainable use of this powerful technology.
This more detailed overview should help Class 12 students understand the scope and implications of biotechnology and its applications in a comprehensive yet accessible manner.
#biotechnology and its applications#applications of biotechnology#biotechnology and its applications notes#biotechnology and its applications class 12#biotechnology and its applications ppt#biology#vavaclasses#science#chemistry#11thclass#botany#class 8#foundation#9thclass#11th class#Class 12
1 note
·
View note
Text
Exploring Light: Reflection and Refraction - A Comprehensive Guide for Class 10 Students
Unlock the mysteries of light with our comprehensive guide on Light- Reflection and Refraction Class 10 Students. From understanding the laws governing reflection and refraction to exploring the fascinating world of mirrors, lenses, and prisms, this resource provides in-depth insights and practical applications, empowering students to master these fundamental concepts with clarity and confidence.

Introduction to Light:
Light is a form of energy that enables us to see objects around us. It travels in straight lines and at an incredible speed of approximately 3 × 10^8 meters per second in a vacuum.
Reflection of Light:
Reflection is the process where light bounces off a surface. The laws of reflection govern this phenomenon:
1. The incident ray, the reflected ray, and the normal (perpendicular line) to the surface at the point of incidence all lie in the same plane.
2. The angle of incidence is equal to the angle of reflection.
Types of Reflection:
1. Regular Reflection: When light falls on a smooth surface, like a mirror, the reflection is regular, and an image is formed.
2. Diffuse Reflection: When light falls on a rough surface, like paper or wall, the reflection is irregular, and no clear image is formed.
Reflection in Spherical Mirrors:
Spherical mirrors are of two types: concave and convex.
1. Concave Mirror:
A concave mirror is a mirror with a reflecting surface that curves inward.
It can form real or virtual images depending on the position of the object.
When the object is beyond the focus, a real and inverted image is formed between the focus and the mirror.
When the object is between the focus and the mirror, a virtual and erect image is formed beyond the focus.
2. Convex Mirror:
A convex mirror is a mirror with a reflecting surface that curves outward.
It always forms virtual and erect images.
The image formed is smaller in size compared to the object.
Refraction of Light:
Refraction is the bending of light as it passes from one medium to another. It occurs due to the change in speed of light when it moves from one medium to another.
Laws of Refraction:
1. The incident ray, the refracted ray, and the normal to the interface of two transparent media at the point of incidence, all lie in the same plane.
2. The ratio of the sine of the angle of incidence to the sine of the angle of refraction is constant, provided the surrounding medium remains the same. This is known as Snell's Law.
Refraction through a Rectangular Glass Slab:
When light passes through a rectangular glass slab, it undergoes refraction twice: once when entering the slab and once when exiting.
1. Incident ray: The ray of light entering the slab.
2. Emergent ray: The ray of light leaving the slab.
3. Refracted ray: The ray of light inside the slab.
Refraction through Lenses:
Lenses are transparent objects made of glass or transparent plastic. There are two main types of lenses: convex and concave.
1. Convex Lens:
Also known as converging lens.
It converges the incident light rays to a point on the other side of the lens called the focus.
It forms real and inverted images when the object is beyond the focus.
It forms virtual and erect images when the object is within the focus.
2. Concave Lens:
Also known as diverging lens.
It diverges the incident light rays.
It always forms virtual and erect images, regardless of the position of the object.
Lens Formula:
The relationship between the object distance (u), image distance (v), and focal length (f) of a lens is given by the lens formula:
1/f=1/u + 1/v
Where:
f = focal length of the lens
v = image distance
u = object distance
Magnification (m):
The magnification produced by a lens is the ratio of the height of the image to the height of the object.
m = h'/h= -v/u
Where:
m = magnification
h' = height of the image
h = height of the object
Applications of Reflection and Refraction:
1. Mirrors: Used in everyday life for grooming, in telescopes, microscopes, and vehicles.
2. Lenses: Utilized in glasses, cameras, projectors, and microscopes.
3. Prisms: Employed in spectacles, binoculars, and cameras for correcting vision and splitting light into its constituent colors.
Conclusion:
Understanding the principles of reflection and refraction is crucial in comprehending various optical phenomena in our daily lives. From mirrors to lenses, these concepts find applications in a wide range of fields, from astronomy to medicine. By grasping the fundamentals outlined in this guide, Class 10 students can gain a deeper insight into the behavior of light and its interactions with different media.
#class 10#science#11thclass#class 8#botany#chemistry#foundation#11th class#biology#vavaclasses#9thclass#mathematics#physics
1 note
·
View note
Text
Class 10 Science: The Human Eye and the Colorful World Notes
Explore the fascinating world of the human eye and the science of color perception with Class 10 Science: The Human Eye and the Colorful World. From the structure of the eye to optical phenomena like refraction and dispersion.
Introduction
In the realm of biology, the human eye is a marvel of nature's engineering, allowing us to perceive the world around us with clarity and detail. It is not only a sensory organ but also a gateway to understanding the physics of light and color. Class 10 Science introduces students to the intricacies of the human eye and its interaction with light, delving into topics such as refraction, dispersion, and the perception of color. In this detailed study guide, we will explore these concepts comprehensively to aid students in understanding this fascinating aspect of biology and physics.
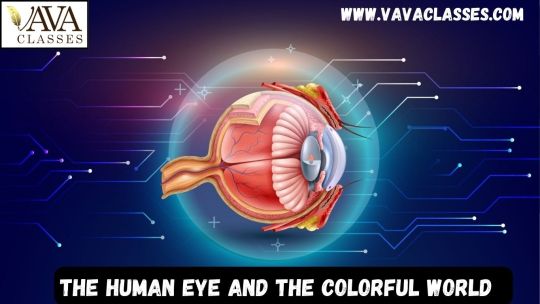
Structure of the Human Eye
The human eye is a complex optical instrument consisting of various components that work together to form visual images. At the forefront of this system is the cornea, a transparent covering that protects the eye and helps in focusing light. Behind the cornea lies the iris, a colored muscular structure that regulates the amount of light entering the eye by adjusting the size of the pupil.
The lens, located behind the iris, further refracts light to focus it onto the retina. The retina is a light-sensitive layer of tissue that contains photoreceptor cells called rods and cones. These cells convert light into electrical signals, which are then transmitted to the brain via the optic nerve for interpretation.
Optical Phenomena
Refraction: Refraction is the bending of light as it passes through different mediums of varying densities. In the context of the eye, refraction occurs primarily at the cornea and the lens, where light is bent to converge onto the retina, thus forming a clear image.
Dispersion: Dispersion is the separation of white light into its constituent colors, known as the spectrum. This phenomenon is observed when light passes through a prism or a refractive medium. In the eye, dispersion plays a crucial role in the perception of color, as it allows us to distinguish between different wavelengths of light.
Visual Acuity and Defects
Visual acuity refers to the clarity or sharpness of vision, which is dependent on the ability of the eye to focus light onto the retina. However, various factors can affect visual acuity, leading to common vision defects such as myopia (nearsightedness), hyperopia (farsightedness), and astigmatism.
Myopia occurs when the eyeball is too long or the cornea is too curved, causing light rays to focus in front of the retina instead of on it. Hyperopia, on the other hand, results from the eyeball being too short or the cornea being too flat, leading to the focal point falling behind the retina. Astigmatism is characterized by an irregular curvature of the cornea or lens, causing distorted vision at all distances.
Perception of Color
The perception of color is a fascinating aspect of human vision, governed by the interaction of light with the photoreceptor cells in the retina. These cells contain pigments that are sensitive to different wavelengths of light, allowing us to discern a wide spectrum of colors.
The three primary colors of light—red, green, and blue—combine in various proportions to produce the full range of hues that we perceive. This additive color mixing is the basis of how color displays such as televisions and computer monitors produce a vast array of colors using only three primary colors.
Class 10 Science: The Human Eye and the Colorful World - Questions and Answers
1. What is refraction? How does it occur in the human eye?
Answer: Refraction is the bending of light as it passes from one medium to another of different optical density. In the human eye, refraction primarily occurs at the cornea and the lens, where light is bent to converge onto the retina, enabling clear vision.
2. Describe the structure of the human eye and the function of each part.
Answer: The human eye consists of various components:
Cornea: Transparent covering that protects the eye and helps in focusing light.
Iris: Colored muscular structure that regulates the amount of light entering the eye by adjusting the size of the pupil.
Lens: Refracts light to focus it onto the retina.
Retina: Light-sensitive layer containing photoreceptor cells (rods and cones) that convert light into electrical signals.
Optic nerve: Transmits these signals to the brain for interpretation.
3. What is dispersion? How does it contribute to the perception of color?
Answer: Dispersion is the separation of white light into its constituent colors (spectrum) when passing through a prism or refractive medium. In the eye, dispersion allows us to distinguish between different wavelengths of light, contributing to the perception of color by enabling us to see a spectrum of hues.
4. Explain the common vision defects like myopia, hyperopia, and astigmatism.
Answer:
Myopia (nearsightedness) occurs when the eyeball is too long or the cornea is too curved, causing light rays to focus in front of the retina.
Hyperopia (farsightedness) results from the eyeball being too short or the cornea being too flat, leading to the focal point falling behind the retina.
Astigmatism is characterized by an irregular curvature of the cornea or lens, causing distorted vision at all distances.
5. How does the perception of color occur in the human eye?
Answer: Color perception in the human eye is governed by the interaction of light with photoreceptor cells (rods and cones) in the retina. These cells contain pigments sensitive to different wavelengths of light, allowing us to discern a wide spectrum of colors. The three primary colors of light—red, green, and blue—combine in various proportions to produce the full range of hues that we perceive.
6. What is visual acuity, and how is it affected by various factors?
Answer: Visual acuity refers to the clarity or sharpness of vision, which depends on the eye's ability to focus light onto the retina. Various factors such as the shape of the eyeball, irregularities in the cornea or lens, and age-related changes can affect visual acuity, leading to conditions like myopia, hyperopia, and astigmatism.
7. How do corrective lenses help in improving vision defects?
Answer: Corrective lenses, such as eyeglasses or contact lenses, alter the way light rays enter the eye, compensating for the irregularities in the eye's shape or structure. For example, concave lenses are used to correct myopia by diverging light rays before they enter the eye, allowing them to focus properly on the retina.
8. Explain the concept of additive color mixing.
Answer: Additive color mixing is the process of combining different colors of light to produce new colors. In this model, the three primary colors of light—red, green, and blue—are mixed in various proportions to create a wide range of hues. This concept is utilized in color displays such as televisions and computer monitors, where pixels emit different intensities of red, green, and blue light to generate the desired colors.
9. What role does the iris play in regulating the amount of light entering the eye?
Answer: The iris, a colored muscular structure, controls the size of the pupil, which is the aperture through which light enters the eye. By adjusting the size of the pupil, the iris regulates the amount of light entering the eye, thus ensuring optimal vision in varying lighting conditions.
10. How does the human eye adapt to changes in lighting conditions?
Answer: The human eye adapts to changes in lighting conditions through a process called pupil dilation or constriction. In bright light, the iris constricts the pupil to reduce the amount of light entering the eye, preventing glare and maintaining visual clarity. Conversely, in dim light, the iris dilates the pupil to allow more light to enter the eye, enhancing sensitivity to low-light environments.
Conclusion
In conclusion, the study of the human eye and the colorful world it perceives is a captivating journey into the realms of biology and physics. From the intricate structure of the eye to the complex phenomena of refraction, dispersion, and color perception, there is much to explore and understand. By grasping these concepts, students can gain a deeper appreciation for the wonders of vision and the role that light plays in shaping our perception of the world.
#class 10#science#notes#biology#chemistry#class 8#9thclass#foundation#vavaclasses#botany#11thclass#zoology#ecology
1 note
·
View note
Text
Important Notes for NEET and Board Biology: Biomolecules Class 11
Introduction:
Biomolecules are the building blocks of life, essential for the structure, function, and regulation of cells and organisms. Understanding biomolecules is crucial for both NEET and board examinations in biology.

1. Carbohydrates:
Function: Provide energy and serve as structural components in cells.
Types: Monosaccharides (e.g., glucose, fructose), disaccharides (e.g., sucrose, lactose), polysaccharides (e.g., starch, cellulose).
Examples: Glucose is the primary energy source for cells, while cellulose provides structural support in plant cell walls.
2. Proteins:
Function: Carry out various functions in cells, including enzyme catalysis, structural support, transport, and signaling.
Structure: Composed of amino acids linked by peptide bonds.
Types: Classified into primary, secondary, tertiary, and quaternary structures.
Examples: Enzymes (e.g., amylase, catalase), structural proteins (e.g., collagen, keratin).
3. Lipids:
Function: Serve as energy storage molecules, form cellular membranes, and act as signaling molecules.
Types: Fatty acids, triglycerides, phospholipids, steroids.
Examples: Fats and oils (triglycerides), phospholipids in cell membranes, cholesterol (a steroid).
4. Nucleic Acids:
Function: Store and transmit genetic information, as well as serve as templates for protein synthesis.
Types: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Structure: Composed of nucleotides, each consisting of a sugar, phosphate group, and nitrogenous base.
Examples: DNA stores genetic information, while RNA is involved in protein synthesis (mRNA), protein assembly (rRNA), and carrying amino acids (tRNA).
5. Enzymes:
Function: Act as biological catalysts, speeding up chemical reactions in cells.
Mechanism: Lower the activation energy required for reactions to occur.
Specificity: Highly specific to their substrates.
Examples: Amylase catalyzes the breakdown of starch into sugars, while catalase catalyzes the breakdown of hydrogen peroxide into water and oxygen.
Key Concepts to Remember:
Biomolecules exhibit structural diversity, allowing them to perform a wide range of functions in living organisms.
Understanding the structure and function of biomolecules is essential for understanding cellular processes and biochemical pathways.
Various factors, such as pH, temperature, and substrate concentration, can influence the activity of biomolecules.
Conclusion:
Biomolecules Class 11 biology are fundamental to life, playing crucial roles in maintaining the structure, function, and regulation of cells and organisms. A thorough understanding of biomolecules is essential for success in both NEET and board examinations in biology.
#class 11#biology#science#11thclass#chemistry#botany#vavaclasses#foundation#9thclass#ecology#zoology#conservation#taxonomy#animal behavior#class 8#11th class#neet2024#neet#neetpreparation#neetexam#exams#iit jee
1 note
·
View note
Text
NCERT Solutions for Moving Charges and Magnetism Class 12
Class 12 Physics typically covers the topic of moving charges and magnetism, which is an essential part of electromagnetism. Here's a brief overview of the key concepts:

1. Magnetic Field (B):
A magnetic field is a region around a magnet or a moving electric charge where magnetic forces are experienced.
The SI unit of magnetic field is the tesla (T).
2. Magnetic Force on a Moving Charge:
When a charged particle moves through a magnetic field, it experiences a magnetic force perpendicular to both its velocity and the magnetic field direction.
The magnitude of the force is given by the formula: F = qvBsinθ, where q is the charge of the particle, v is its velocity, B is the magnetic field strength, and θ is the angle between v and B.
3. Lorentz Force:
The total force experienced by a charged particle moving in an electric field (E) and a magnetic field (B) simultaneously is called the Lorentz force.
The formula for the Lorentz force is given by: F = q(E + v x B), where x represents the cross product.
4. Magnetic Field due to a Current-Carrying Conductor:
Ampere's law states that the magnetic field produced by a current-carrying conductor is directly proportional to the current and inversely proportional to the distance from the conductor.
The direction of the magnetic field can be determined using the right-hand rule.
5. Magnetic Force between Two Parallel Current-Carrying Conductors:
When two parallel current-carrying conductors are placed close to each other, they experience a magnetic force due to the interaction of their magnetic fields.
6. Magnetic Field due to a Circular Loop:
A circular loop carrying a current produces a magnetic field similar to that of a bar magnet. The field is stronger inside the loop and weaker outside.
7. Torque on a Current Loop in a Magnetic Field:
When a current-carrying loop is placed in a magnetic field, it experiences a torque, which tends to align the loop with the magnetic field.
These are some of the fundamental concepts covered in the topic of moving charges and magnetism in Class 12 Physics. Understanding these concepts is crucial for various applications, including the functioning of electric motors, generators, transformers, and many more.
#class 12#physics#moving charges and magnetism#chemistry#vavaclasses#11thclass#science#biology#foundation#11th class#botany#class 8#9thclass
1 note
·
View note
Text
NCERT Solutions- Structure of Atom: Discovery of Sub-Atomic Particles Class 11
In class 11 chemistry, students typically delve into the fascinating world of subatomic particles, the fundamental building blocks of matter. These particles, namely protons, neutrons, and electrons, constitute atoms, which are the smallest units of an element. Protons carry a positive charge, neutrons are neutral, and electrons bear a negative charge. They interact via electromagnetic forces and reside within the atom's nucleus (protons and neutrons) or in electron shells surrounding the nucleus (electrons). Understanding these subatomic particles and their arrangement within atoms is crucial for comprehending the behavior and properties of elements, paving the way for a deeper exploration of chemistry's intricacies.
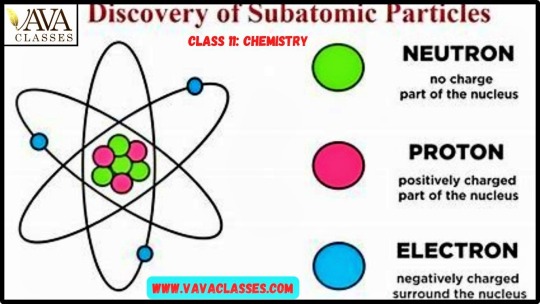
1. Introduction to Atoms: Atoms are the basic building blocks of matter. They consist of subatomic particles: protons, neutrons, and electrons.
2. Protons: Positively charged particles found in the nucleus of an atom. Protons have a relative charge of +1 and a mass of approximately 1 atomic mass unit (amu).
3. Neutrons: Neutral particles found in the nucleus of an atom. Neutrons have no charge (neutral) and a mass of approximately 1 amu.
4. Electrons: Negatively charged particles that orbit the nucleus of an atom in electron shells or energy levels. Electrons have a relative charge of -1 and a negligible mass compared to protons and neutrons.
5. Atomic Number (Z): The number of protons in the nucleus of an atom. It determines the identity of the element and its place in the periodic table.
6. Mass Number (A): The sum of the number of protons and neutrons in the nucleus of an atom. It represents the mass of the atom.
7. Isotopes: Atoms of the same element with different numbers of neutrons. Isotopes have the same atomic number but different mass numbers.
8. Atomic Mass Unit (amu): A unit of mass used to express atomic and molecular weights. 1 amu is approximately equal to the mass of a proton or neutron.
9. Relative Atomic Mass: The weighted average of the masses of the isotopes of an element, taking into account their relative abundances in nature.
10. Fundamental Forces: Subatomic particles interact through fundamental forces such as electromagnetic force, strong nuclear force, weak nuclear force, and gravitational force.
11. Subatomic Particle Arrangement: Electrons are arranged in electron shells or energy levels around the nucleus. The distribution of electrons follows specific rules such as the Aufbau principle, Pauli exclusion principle, and Hund's rule.
12. Quantum Numbers: Parameters that describe the properties of electrons in an atom including principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s).
In conclusion, the study of subatomic particles in class 11 chemistry provides a foundational understanding of the basic constituents of matter. Through the exploration of protons, neutrons, and electrons, students gain insight into the structure of atoms and the principles that govern their behavior. By comprehending the roles and interactions of these subatomic particles, learners lay the groundwork for more advanced topics in chemistry, enabling them to appreciate the complexity and diversity of the elements in the periodic table. Ultimately, the knowledge acquired about subatomic particles serves as a cornerstone for further exploration into the realms of chemical bonding, reactions, and the broader field of chemistry.
1 note
·
View note
Text
Notes on Class 12 Physics Semiconductor Electronics: Materials, Devices and Simple Circuits
Introduction to Semiconductor Electronics: Materials, Devices, and Simple Circuits
Semiconductor electronics forms the backbone of modern technology, revolutionizing communication, computing, and countless other fields. This branch of physics deals with the study of semiconductor materials, devices, and their applications in electronic circuits. Semiconductors, such as silicon and germanium, play a pivotal role due to their unique properties, which lie between those of conductors and insulators.
In this study, we explore the intricate workings of semiconductor devices like diodes, transistors, and integrated circuits. These devices enable the manipulation, amplification, and regulation of electronic signals, essential for powering electronic gadgets, processing information, and controlling machinery.
Through understanding semiconductor physics, device operation principles, and circuit design techniques, we embark on a journey to comprehend the intricate world of semiconductor electronics. This knowledge not only forms the foundation for advanced studies in electronics but also empowers us to innovate and contribute to the ever-evolving landscape of modern technology.
Here are some notes on Class 12 Physics Semiconductor Electronics: Materials, Devices, and Simple Circuits:
Introduction to Semiconductor Physics:
Semiconductors are materials with electrical conductivity between that of conductors and insulators. Examples include silicon (Si) and germanium (Ge).
Intrinsic semiconductors are pure semiconductors with equal numbers of electrons and holes.
2. Extrinsic Semiconductors:
Doping introduces impurities into semiconductor crystals to modify their conductivity.
N-type semiconductors are doped with materials that increase the number of free electrons.
P-type semiconductors are doped with materials that create electron-deficient holes.
3. PN Junction Diode:
A PN junction diode is formed by joining a P-type semiconductor with an N-type semiconductor.
It allows current to flow in one direction (forward-biased) and blocks it in the other direction (reverse-biased).
Characteristics include forward and reverse biasing, breakdown voltage, and diode equation.
4. Diode Applications:
Rectification: Converting AC to DC using diodes in half-wave or full-wave rectifier circuits.
Clipping and Clamping: Limiting voltage levels in electronic circuits using diodes.
Voltage Regulation: Stabilizing voltage levels using Zener diodes.
5. Transistors:
Bipolar Junction Transistor (BJT) and Field Effect Transistor (FET) are common types.
Transistors amplify or switch electronic signals and can be used as amplifiers, switches, or oscillators.
6. Transistor Configurations:
Common emitter, common base, and common collector configurations for BJTs.
Common source, common gate, and common drain configurations for FETs.
7. Transistor Amplifiers:
Amplifier circuits use transistors to increase the amplitude of electrical signals.
Common emitter and common collector configurations are commonly used for amplification.
8. Logic Gates:
Basic building blocks of digital circuits that perform logical operations (AND, OR, NOT, etc.).
Implemented using transistors to process binary inputs and produce binary outputs.
9. Integrated Circuits (ICs):
ICs are miniaturized electronic circuits fabricated on a single semiconductor chip.
Types include analog ICs (op-amps, voltage regulators) and digital ICs (microprocessors, memory chips).
10. Simple Circuits:
Basic electronic circuits incorporating semiconductor devices like diodes, transistors, and ICs.
Examples include amplifiers, oscillators, timers, and voltage regulators.
Understanding semiconductor electronics is essential for various applications in modern technology, including computers, telecommunications, and consumer electronics. These notes provide a foundational understanding of semiconductor materials, devices, and circuits, laying the groundwork for further exploration in the field of electronics.
1 note
·
View note
Text
Key Notes for NEET Biology- Principles of Inheritance and Variation.
Introduction to Principles of Inheritance and Variation in Genetics and Evolution:
The study of genetics and evolution unveils the intricate mechanisms governing the transmission of traits from one generation to the next, showcasing the principles of inheritance and variation. Inheritance refers to the passage of genetic information from parents to offspring, while variation encompasses the diversity observed among individuals within a population. These fundamental principles are central to our understanding of how living organisms evolve over time.
Genetics elucidates the molecular basis of inheritance, emphasizing the role of DNA, the genetic code, and the intricate processes of replication, transcription, and translation. Traits are inherited through genes, the units of heredity located on chromosomes, which determine an organism's characteristics. The principles of Mendelian inheritance, discovered by Gregor Mendel in the 19th century, lay the foundation for understanding how genes are transmitted through generations, defining the rules of dominance, segregation, and independent assortment.
Variation, on the other hand, is a cornerstone of evolution. Natural selection, proposed by Charles Darwin, operates on the variability present in populations. Genetic mutations, genetic recombination, and other mechanisms introduce novel variations, providing the raw material upon which natural selection acts. Those individuals possessing advantageous traits are more likely to survive and reproduce, passing on their beneficial genes to subsequent generations. Over time, this leads to the gradual accumulation of traits that enhance an organism's fitness and adaptation to its environment.
The principles of inheritance and variation intertwine to shape the genetic landscape of populations and drive the ongoing process of evolution. Understanding these principles is crucial for unraveling the complexities of life's diversity and the mechanisms underlying the perpetuation of genetic information across generations.
Below are condensed class 12 notes on the principles of inheritance and variation in genetics and evolution:
Mendelian Inheritance:
Gregor Mendel:
Father of genetics, conducted pea plant experiments.
Established three laws: Segregation, Independent Assortment, Dominance.
2. Law of Segregation:
Alleles segregate during gamete formation.
Each gamete receives one allele for each trait.
3. Law of Independent Assortment:
Alleles for different traits segregate independently during gamete formation.
4. Genetic Terminology:
Genes: Units on chromosomes carrying hereditary information.
Alleles: Different versions of a gene.
Genotype: Genetic makeup; Phenotype: Observable traits.
5. Punnett Squares:
Used to predict offspring genotypes and phenotypes in genetic crosses.
Non-Mendelian Inheritance:
1. Incomplete Dominance:
Heterozygotes exhibit an intermediate phenotype.
2. Codominance:
Both alleles in a heterozygote are expressed.
3. Multiple Alleles:
More than two alleles for a gene exist.
4. Polygenic Inheritance:
Traits influenced by multiple genes, leading to a range of phenotypes.
Chromosomal Basis of Inheritance:
1. Chromosomal Theory of Inheritance:
Genes are located on chromosomes.
2. Sex-Linked Inheritance:
Genes located on sex chromosomes (X and Y).
Hemophilia and color blindness are examples.
3. Linked Genes:
Genes located on the same chromosome tend to be inherited together.
Human Genetics:
1. Pedigree Analysis:
Charts representing family relationships and genetic traits.
Genetic Disorders:
Caused by mutations in genes.
Examples: Cystic fibrosis, Huntington's disease.
Molecular Basis of Inheritance:
1. DNA Structure:
Double helix composed of nucleotides.
2. DNA Replication:
Semi-conservative process ensuring genetic continuity.
3. Genetic Code and Protein Synthesis:
DNA codes for RNA, which codes for proteins.
Evolution:
1. Darwin's Theory:
Natural selection as the mechanism for evolution.
2. Evidence for Evolution:
Fossils, homologous structures, embryology, molecular biology.
3. Mechanisms of Evolution:
Natural selection, genetic drift, gene flow, mutation.
4. Speciation:
Formation of new species due to isolation and adaptation.
5. Hardy-Weinberg Equilibrium:
Conditions for a non-evolving population.
These class 12 notes provide a concise overview of the key principles in genetics and evolution. Further exploration and in-depth study can be done based on these foundational concepts.
#science#9thclass#11thclass#botany#biology#chemistry#class 8#foundation#11th class#vavaclasses#class 12#cbse#english
1 note
·
View note
Text
NCERT-Solutions Hydrocarbons class 11 Chemistry notes
In Class 11 Chemistry-hydrocarbons serve as a fundamental topic within organic chemistry. Hydrocarbons are organic compounds composed exclusively of carbon and hydrogen atoms. The class primarily focuses on four major types of hydrocarbons: alkanes, alkenes, alkynes, and aromatic hydrocarbons.

Classification of Hydrocarbons Class 11 Chemistry
Aliphatic Hydrocarbons:
1. Alkanes (Saturated Hydrocarbons):
Single-bonded carbon atoms.
General formula: CₙH₂ₙ₊₂.
Examples: Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈).
2. Alkenes (Unsaturated Hydrocarbons):
At least one carbon-carbon double bond.
General formula: CₙH₂ₙ.
Examples: Ethene (C₂H₄), Propene (C₃H₆), Butene (C₄H₈).
3. Alkynes (Unsaturated Hydrocarbons):
Contain at least one carbon-carbon triple bond.
General formula: CₙH₂ₙ₋₂.
Examples: Ethyne (C₂H₂), Propyne (C₃H₄), Butyne (C₄H₆).
Aromatic Hydrocarbons:
1. Benzene:
Consists of a six-carbon ring with alternating single and double bonds.
C₆H₆.
Exhibits resonance, making the bond lengths and strengths intermediate between single and double bonds.
2. Substituted Benzenes:
Benzene rings with one or more hydrogen atoms replaced by other functional groups (e.g., methyl, ethyl, nitro groups).
Nomenclature:
1. IUPAC Naming:
Follows the rules of the International Union of Pure and Applied Chemistry.
Prefixes indicate the number and arrangement of substituents, and the ending indicates the type of hydrocarbon (e.g., -ane for alkanes, -ene for alkenes).
Isomerism:
1. Structural Isomers:
Same molecular formula but different structural arrangements.
2. Stereoisomers:
Same structural formula but differ in the spatial arrangement of atoms.
Reactions:
1. Combustion:
Hydrocarbons react with oxygen to produce carbon dioxide and water.
2. Substitution and Addition Reactions:
Alkanes undergo substitution reactions.
Alkenes and alkynes undergo addition reactions.
3. Aromatic Reactions:
Aromatic hydrocarbons undergo electrophilic substitution reactions.
Important Terms:
1. Saturated vs. Unsaturated:
Saturated hydrocarbons have only single bonds, while unsaturated hydrocarbons have double or triple bonds.
2. Isomerization:
Process where one isomer can be converted into another.
These are just basic notes, and there's much more to explore within each category.
1 note
·
View note
Text
Understanding the Classification of Hydrocarbons: A Class 11 Overview
What are hydrocarbons? Hydrocarbons are organic compounds made up of hydrogen and carbon atoms. These compounds are the building blocks of organic chemistry and are fundamental to the study of organic compounds, which are essential in various biological, industrial, and environmental processes. Hydrocarbons can be classified into two main types: aliphatic and aromatic.
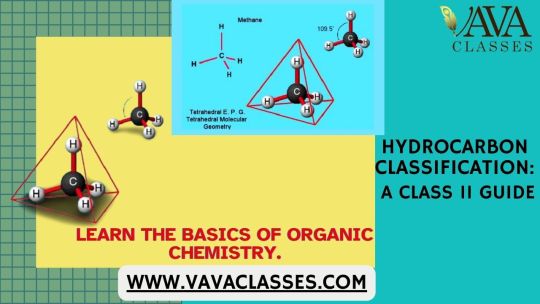
What are aliphatic hydrocarbons?
Aliphatic Hydrocarbons: Saturated Aliphatic Hydrocarbons (Alkanes): These hydrocarbons have single bonds between carbon atoms. The general formula is CnH2n+2. Methane (CH4), ethane (C2H6), and propane (C3H8) are examples.
Unsaturated Aliphatic Hydrocarbons (Alkenes and Alkynes): These hydrocarbons have at least one double (alkenes) or triple (alkynes) bond between carbon atoms. The general formulas are CnH2n (alkenes) and CnH2n-2 (alkynes). Ethene (C2H4) and ethyne (C2H2) are examples.
Aromatic Hydrocarbons:
These hydrocarbons contain a special type of ring known as a benzene ring. The simplest aromatic hydrocarbon is benzene (C6H6). Aromatic compounds can have additional substituents attached to the benzene ring.
Hydrocarbons are the primary components of fossil fuels, including coal, petroleum, and natural gas. They play a crucial role in the energy sector as fuel sources for various applications, such as transportation and electricity generation. Additionally, hydrocarbons are involved in the production of many industrial chemicals and materials. However, the combustion of hydrocarbons also contributes to environmental issues, such as air pollution and the release of greenhouse gases.
What are unsaturated hydrocarbons?
Unsaturated hydrocarbons are a category of organic compounds that contain one or more carbon-carbon double bonds (alkenes) or carbon-carbon triple bonds (alkynes). These double or triple bonds create a degree of unsaturation in the hydrocarbon molecule, meaning that not all carbon atoms are saturated with the maximum number of hydrogen atoms.
There are two main types of unsaturated hydrocarbons:
Alkenes (Olefins):
Alkenes have at least one carbon-carbon double bond.
The general formula for alkenes is CnH2n, where "n" represents the number of carbon atoms.
Example: Ethene (C2H4) is the simplest alkene. Example of a structural formula for an alkene: H \ C=C / H
Alkynes:
Alkynes have at least one carbon-carbon triple bond.
The general formula for alkynes is CnH2n-2, where "n" represents the number of carbon atoms.
Example: Ethyne (C2H2) is the simplest alkyne.
Example of a structural formula for an alkyne: H \ C≡C / H Unsaturated hydrocarbons are important in various industrial processes and in the synthesis of many organic compounds. They participate in reactions such as addition reactions, where atoms or groups are added to the carbon-carbon double or triple bonds, leading to the formation of new compounds. Additionally, unsaturated hydrocarbons are involved in the production of plastics, polymers, and various chemicals.
What are aromatic hydrocarbons?
Aromatic hydrocarbons are a type of hydrocarbon compound that contains a cyclic structure known as a benzene ring. These compounds exhibit special stability and unique bonding characteristics due to the presence of alternating single and double bonds within the ring. The most common and simplest aromatic hydrocarbon is benzene (C6H6).
Key features of aromatic hydrocarbons:
Benzene Ring:
The basic unit of aromatic hydrocarbons is the benzene ring, which consists of six carbon atoms arranged in a hexagonal ring with alternating single and double bonds.
The molecular formula for benzene is C6H6.
The structure of benzene can be represented as a resonance hybrid, indicating that the double bonds are not fixed in specific locations but are spread out over the entire ring.
Structural representation of benzene: H H \ / C=C=C=C / \ H H
Aromatic Compounds:
Aromatic hydrocarbons can have additional substituents attached to the benzene ring.
Aromatic compounds often exhibit distinct aromaticity, which imparts stability to the molecule.
Example of a substituted aromatic compound (toluene): H | CH3 | C6H5
Aromaticity:
Aromatic compounds follow Huckel's Rule, which states that a compound is aromatic if it is cyclic, planar, fully conjugated, and possesses 4n + 2π electrons (where "n" is an integer).
Reactivity:
Aromatic compounds often undergo substitution reactions rather than addition reactions. Common examples include electrophilic aromatic substitution reactions.
Aromatic hydrocarbons play a significant role in organic chemistry and industry. They are used as precursors in the production of a wide range of chemicals, including plastics, dyes, pharmaceuticals, and solvents. Examples of aromatic hydrocarbons include benzene, toluene, xylene, and naphthalene.
#biology#botany#11thclass#9thclass#science#class 8#chemistry#foundation#vavaclasses#hydrocarbons#classification of hydrocarbons#notes#ncertsolutions#ncertbooks#ncert textbooks#cbse#cbse school#students#cbse education#cbseboard#cbs elementary#essaywriter#online school#colleges#11th class
1 note
·
View note
Text
Difference between atomic mass and molecular mass class 9-Science
In Class 9 science, understanding the difference between atomic mass and molecular mass is fundamental in the context of atoms and molecules. Let's explore these concepts:
Atomic Mass: -Definition: Atomic mass refers to the mass of an individual atom of an element, usually expressed in atomic mass units (amu) or unified atomic mass units (u). -Unit: Atomic mass is measured in atomic mass units (amu) or unified atomic mass units (u). -Calculation: The atomic mass of an element is determined by considering the weighted average of the masses of its isotopes, taking into account their abundance in nature. The atomic mass is typically found on the periodic table. -Example: The atomic mass of carbon (C) is approximately 12.01 u, indicating the average mass of a carbon atom.
2. Molecular Mass: -Definition: Molecular mass refers to the sum of the atomic masses of all the atoms present in a molecule. It is expressed in atomic mass units (amu) or unified atomic mass units (u). -Unit: Molecular mass is measured in atomic mass units (amu) or unified atomic mass units (u).
Calculation: To find the molecular mass, add up the atomic masses of all the atoms in the molecule, as indicated by its chemical formula. For example, the molecular mass of water (H₂O) is calculated by adding the atomic masses of two hydrogen atoms and one oxygen atom. -Example: The molecular mass of water (H₂O) is approximately 18.02 u, calculated as 2(1.01 u) + 16.00 u.
Key Differences:
-Focus:
Atomic mass is concerned with the mass of an individual atom of an element.
Molecular mass deals with the combined mass of all atoms in a molecule.
-Unit:
Both atomic and molecular masses are expressed in atomic mass units (amu) or unified atomic mass units (u).
-Calculation:
Atomic mass is determined by considering the weighted average of the masses of isotopes.
Molecular mass is calculated by adding the atomic masses of all atoms in a molecule.
Example:
Atomic mass is associated with individual elements, such as the atomic mass of carbon.
Molecular mass is associated with compounds or molecules, such as the molecular mass of water.
Understanding these concepts is crucial for further studies in chemistry and provides a foundation for comprehending the quantitative aspects of chemical reactions and reactions' stoichiometry.
#science#9thclass#chemistry#class 8#11th class#botany#biology#foundation#vavaclasses#11thclass#atomic mass and molecular mass#atoms&molecules#atoms
1 note
·
View note
Text
Detailed Study Notes on Class 12 Chemistry: Proteins
Introduction:
Proteins are vital biological macromolecules essential for life. In Class 12 Chemistry (Chapter: Biomolecules), understanding proteins includes their structure, types, and functions. These notes help you grasp the key concepts easily for board exams and competitive preparation like NEET and JEE.

What Are Proteins?
Proteins are large, complex molecules made up of amino acids joined by peptide bonds. They play critical roles in almost all biological processes. Chemically, proteins are polymers of α-amino acids.
Amino Acids – Building Blocks of Proteins
Structure of α-Amino Acids: Each amino acid has a central α-carbon, an amino group (–NH₂), a carboxylic group (–COOH), a hydrogen atom, and a variable R group. General formula: NH₂–CH(R)–COOH Classification: - Essential Amino Acids (e.g., Valine, Leucine) - Non-Essential Amino Acids (e.g., Glycine, Alanine)
Formation of Peptides and Proteins
Peptide Bond: Formed when –COOH of one amino acid reacts with –NH₂ of another, releasing water. Types: - Dipeptide: 2 amino acids - Tripeptide: 3 amino acids - Polypeptide: Many amino acids - Protein: More than ~50 amino acids in a specific 3D structure
Structure of Proteins
1. Primary Structure: Linear sequence of amino acids. 2. Secondary Structure: α-Helix and β-Pleated Sheet (hydrogen bonds). 3. Tertiary Structure: 3D shape formed by R-group interactions. 4. Quaternary Structure: Multiple polypeptide chains (e.g., Hemoglobin).
Classification of Proteins
By Composition: - Simple Proteins: Only amino acids - Conjugated Proteins: With non-protein parts By Function: - Structural: Keratin, Collagen - Enzymatic: Pepsin, Amylase - Transport: Hemoglobin - Hormonal: Insulin
Denaturation of Proteins
Loss of structure and biological activity due to temperature, pH, or chemicals. Example: Cooking an egg – Albumin protein coagulates.
Functions of Proteins
- Structural: Build cells and tissues - Enzymatic: Catalyze reactions - Transport: Carry substances - Regulatory: Hormones - Protective: Antibodies
Protein Tests (Class 12 Practical Relevance)
Biuret Test: Purple color indicates proteins. Xanthoproteic Test: Yellow color shows aromatic amino acids.
Important Protein Examples for Board Exams
Hemoglobin – Oxygen transport Insulin – Blood sugar regulation Albumin – Osmotic pressure control Pepsin – Protein digestion
Conclusion
Understanding proteins in Class 12 Chemistry is crucial for exams and future studies. Focus on structure, classification, and functions. Use diagrams and tables for clarity.
#vavaclasses#11thclass#science#biology#chemistry#physics#class 12#iit jee#neet#class 11 physics notes
0 notes
Text
Carbohydrates Class 12 Chemistry Notes- Biomolecules Chapter Explained
Introduction to Carbohydrates
Carbohydrates are the most abundant organic compounds found in nature and are vital for life. They serve as a primary source of energy for living organisms. Chemically, carbohydrates are polyhydroxy aldehydes or ketones or compounds that yield them on hydrolysis. The general formula is Cn(H2O)n.

Classification of Carbohydrates
1. Monosaccharides
• Simplest form of carbohydrates. • Cannot be hydrolyzed further. • General formula: CnH2nOn • Examples: Glucose, Fructose
2. Oligosaccharides
• Contain 2–10 monosaccharide units. • Most important oligosaccharide: Disaccharides • Examples: Sucrose (Glucose + Fructose), Lactose (Glucose + Galactose), Maltose (Glucose + Glucose)
3. Polysaccharides
• Long chains of monosaccharide units. • Not sweet, insoluble in water. • Examples: Starch, Cellulose, Glycogen
Structure and Function of Monosaccharides
Monosaccharides are further classified based on: - Number of carbon atoms: Trioses, Tetroses, Pentoses, Hexoses - Functional group: • Aldoses (with –CHO group) • Ketoses (with –CO group) Glucose is an aldohexose with the formula C6H12O6. It exists in two cyclic forms: α-glucose and β-glucose.
Reducing and Non-Reducing Sugars
Reducing Sugars
• Can reduce Fehling’s or Tollen’s reagent. • Contain free aldehyde or ketone group. • Examples: Glucose, Maltose, Lactose
Non-Reducing Sugars
• Do not have a free –CHO or –CO group. • Example: Sucrose
Properties of Glucose
• Sweet, soluble in water • Undergoes oxidation and reduction • Does not react with Schiff’s reagent • Exists as α- and β- anomers in solution
Polysaccharides in Biological Systems
Starch
• Plant storage carbohydrate • Made of amylose and amylopectin
Glycogen
• Animal storage carbohydrate • Similar to amylopectin but more branched
Cellulose
• Structural polysaccharide in plants • Cannot be digested by humans
Tests for Carbohydrates
1. Molisch’s Test
• General test for carbohydrates (violet ring formation)
2. Benedict’s Test
• Detects reducing sugars (brick red precipitate)
3. Fehling’s Test
• Another test for reducing sugars (red ppt. of Cu2O)
Importance of Carbohydrates:
• Source of energy: 1 gram = 4 kcal • Storage: Glycogen in animals, starch in plants • Structural role: Cellulose in plant cell walls • Component of biomolecules: DNA, RNA (ribose, deoxyribose)
Conclusion
Carbohydrates are crucial biomolecules that play multiple roles in metabolism and structure. Understanding their classification, structure, and functions provides a strong foundation in organic chemistry and biology. In exams, focus on glucose structure, disaccharide linkages, and polysaccharide functions for scoring high.
#vavaclasses#11thclass#science#biology#chemistry#physics#class 12#iit jee#neet#class 11 physics notes
0 notes
Text
Integration by Parts – Class 12 Mathematics Notes
Introduction:
Integration by Parts is a crucial technique in integral calculus, especially useful when dealing with the integration of the product of two functions. It is derived from the product rule of differentiation and helps solve complex integrals that cannot be integrated directly. Understanding this method thoroughly will aid in solving various problems in CBSE Class 12 board exams and competitive exams like JEE Main.

Formula for Integration by Parts:
If u = f(x) and v = g(x), then:
∫ u·v dx = u ∫v dx - ∫ (du/dx · ∫v dx) dx
Or simply,
∫ u·v dx = uv - ∫ v·(du/dx) dx
Choosing u and v – ILATE Rule:
To select which function to differentiate and which to integrate, use the ILATE rule:
I: Inverse Trigonometric functions L: Logarithmic functions A: Algebraic functions T: Trigonometric functions E: Exponential functions
Solved Examples of Integration by Parts:
Evaluate ∫ x · e^x dx
Let u = x (Algebraic), dv = e^x dx Then, du = dx, and v = ∫ e^x dx = e^x Apply the formula: ∫ x·e^x dx = x·e^x - ∫ e^x dx = x·e^x - e^x + C Answer: ∫ x·e^x dx = e^x(x - 1) + C
Evaluate ∫ ln x dx
Let u = ln x, dv = dx Then, du = (1/x) dx, v = ∫ dx = x Apply the formula: ∫ ln x dx = x·ln x - ∫ x·(1/x) dx = x·ln x - ∫ 1 dx = x·ln x - x + C Answer: ∫ ln x dx = x(ln x - 1) + C
Evaluate ∫ x · sin x dx
Let u = x, dv = sin x dx Then, du = dx, v = ∫ sin x dx = -cos x Apply the formula: ∫ x·sin x dx = -x·cos x + ∫ cos x dx = -x·cos x + sin x + C Answer: ∫ x·sin x dx = -x·cos x + sin x + C
Special Cases and Tips:
Some integrals may require repeated application of the formula. For example: ∫ x^2 e^x dx
Practice Questions
1. ∫ x · cos x dx 2. ∫ x · ln x dx 3. ∫ x^2 · e^x dx 4. ∫ arctan x dx 5. ∫ ln x dx
Conclusion:
Integration by Parts is a powerful technique in calculus, especially when dealing with products of functions. Mastery of the ILATE rule and regular practice of varied problems ensures confidence and accuracy in the exams.
#vavaclasses#11thclass#science#biology#chemistry#physics#class 12#iit jee#neet#class 11 physics notes#Integration by Parts Class 12#Integration by Parts formula#Integration by Parts examples with solutions#Class 12 Maths Chapter Integrals#Integration methods for Class 12#CBSE Class 12 Integration notes
0 notes
Text
Method of Preparation of Diazonium Salts – Class 12 Chemistry Notes
Introduction:
Diazonium salts form an important category of organic compounds that are widely used in organic synthesis, especially in the manufacture of azo dyes, pharmaceuticals, and aromatic derivatives. These compounds are prepared primarily from aromatic amines and are characterized by the presence of the functional group –N₂⁺X⁻. In Class 12 Chemistry, the topic is covered under the chapter “Amines”, and it often carries significant weightage in CBSE Board exams and entrance exams like NEET and JEE.

What are Diazonium Salts?
Diazonium salts are organic compounds that contain a diazonium group (–N₂⁺) attached to an aryl group. The general formula is ArN₂⁺X⁻, where: - Ar = Aromatic group (usually derived from benzene) - X⁻ = Anion like Cl⁻, Br⁻, NO₃⁻, HSO₄⁻, etc.
Example: Benzenediazonium chloride (C₆H₅N₂⁺Cl⁻)
General Method of Preparation of Diazonium Salts
Diazonium salts are prepared by a chemical reaction known as diazotization, which involves the reaction of primary aromatic amines with nitrous acid (HNO₂) in an acidic medium under cold conditions (0–5°C).
General Reaction: ArNH₂ + HNO₂ + HX → ArN₂⁺X⁻ + 2H₂O
Example using Aniline: C₆H₅NH₂ + HNO₂ + HCl → C₆H₅N₂⁺Cl⁻ + 2H₂O
Laboratory Preparation of Diazonium Salts:
In the lab, nitrous acid is prepared in situ: NaNO₂ + HCl → HNO₂ + NaCl
Step-by-Step Procedure: 1. Prepare a cold solution of aniline in dilute HCl. 2. Cool to 0–5°C. 3. Add NaNO₂ solution slowly while stirring.
Conditions Required for Diazotization Reaction:
Temperature: 0°C to 5°C. Higher temperatures cause decomposition. Acidic Medium: Usually dilute HCl or H₂SO₄. Type of Amine: Only primary aromatic amines.
Mechanism of Diazotization Reaction:
Step 1: NaNO₂ + HCl → HNO₂ + NaCl Step 2: HNO₂ + H⁺ → NO⁺ + H₂O Step 3: ArNH₂ + NO⁺ → ArN₂⁺ + H₂O
Applications of Diazonium Salts:
1. Azo Dye Formation 2. Sandmeyer Reaction 3. Gattermann Reaction 4. Phenol Formation 5. Synthesis of Aromatic Compounds 6. Medicinal Chemistry Intermediates
Conclusion:
The preparation of diazonium salts via diazotization is a cornerstone topic in Class 12 Organic Chemistry. By understanding the conditions, mechanism, and practical importance of this reaction, students can master one of the most high-scoring sections of their board and entrance exams.
#vavaclasses#11thclass#science#biology#chemistry#physics#class 12#iit jee#neet#class 11 physics notes
0 notes