Don't wanna be here? Send us removal request.
Text
Clinical Research Organizations in Hyderabad: A Thriving Hub of Innovation
Introduction
Hyderabad has emerged as a leading hub for clinical research organizations (CROs) in India, playing a crucial role in the advancement of medical and pharmaceutical sciences. With its robust healthcare infrastructure and strong pharmaceutical presence, the city is a preferred location for clinical trials and drug development.
Understanding Clinical Research Organizations (CROs)
Clinical research organizations (CROs) are specialized firms that provide support services to pharmaceutical, biotechnology, and medical device industries. Their primary role is to facilitate the smooth execution of clinical trials, ensuring that new drugs and treatments meet safety and efficacy standards before reaching the market. CROs handle various aspects of clinical trials, including data management, regulatory compliance, and patient recruitment.
Why Hyderabad is a Hub for Clinical Research?
Hyderabad is often referred to as the "Pharma Capital of India" due to its thriving pharmaceutical and biotech ecosystem. The presence of leading pharmaceutical companies, such as Dr. Reddy’s Laboratories and Aurobindo Pharma, has significantly contributed to the city’s growth in clinical research. Additionally, Hyderabad boasts a well-developed healthcare infrastructure, research institutions, and a skilled workforce, making it an attractive destination for CROs.
Key Clinical Research Organizations in Hyderabad
Several reputed clinical research organizations operate in Hyderabad, both global and homegrown. Some of the prominent names include:
Parexel: A globally renowned CRO providing comprehensive clinical research services.
ICON: Specializing in drug development and regulatory consulting.
Covance (Labcorp Drug Development): Known for its expertise in early-phase clinical trials.
Siro Clinpharm: A leading Indian CRO offering clinical trial management and pharmacovigilance.
Clinnovo: Providing training and research services in clinical trials.
These organizations play a crucial role in driving clinical research, ensuring adherence to global regulatory standards, and contributing to medical advancements.
Services Offered by CROs in Hyderabad
CROs in Hyderabad offer a wide range of services to pharmaceutical and biotech companies, including:
Clinical Trial Management: Planning, execution, and monitoring of clinical trials.
Regulatory Compliance: Ensuring trials meet international and national regulatory standards.
Data Management & Biostatistics: Collecting, analyzing, and interpreting clinical data.
Pharmacovigilance: Monitoring and reporting adverse drug reactions.
Medical Writing: Preparing clinical trial reports, regulatory documents, and scientific publications.
Regulatory Framework Governing Clinical Research in India
Clinical research in India is governed by strict regulatory guidelines to ensure patient safety and ethical conduct. The Central Drugs Standard Control Organization (CDSCO) is the primary regulatory body overseeing clinical trials. Additionally, international agencies such as the U.S. FDA (Food and Drug Administration) and EMA (European Medicines Agency) also play a role in regulating trials conducted in India. Compliance with Good Clinical Practice (GCP) guidelines is mandatory, ensuring transparency and integrity in clinical research.
Challenges and Opportunities in the CRO Industry
While the clinical research industry in Hyderabad presents immense opportunities, it also faces several challenges:
Challenges:
Regulatory Complexities: Frequent changes in regulatory guidelines can slow down clinical trials.
Patient Recruitment: Finding suitable participants for trials remains a significant hurdle.
High Competition: The presence of multiple CROs creates a competitive landscape.
Opportunities:
Emerging Medical Fields: Areas like personalized medicine and gene therapy present new avenues for research.
Technological Advancements: AI and big data are revolutionizing clinical trials, making them more efficient.
Global Collaborations: Increasing partnerships with international pharmaceutical firms drive growth.
Future of Clinical Research Organizations in Hyderabad
The future of clinical research in Hyderabad looks promising, with continued investments in healthcare and pharmaceutical research. The adoption of AI, machine learning, and blockchain technology in clinical trials is expected to streamline processes and improve accuracy. Additionally, increased global collaborations and outsourcing of clinical trials to India will further boost the industry.
Conclusion
Hyderabad’s clinical research ecosystem is an integral part of the global drug development landscape, offering immense potential for innovation and progress. With a strong pharmaceutical base, world-class healthcare facilities, and a skilled workforce, the city is well-positioned to shape the future of clinical research. As technology continues to evolve, CROs in Hyderabad will play a crucial role in bringing new and effective treatments to patients worldwide.

0 notes
Text
Biobanking: The Future of Medical Research
Introduction
Biobanking serves as a vital component within modern medical research often storing samples beneath extremely controlled conditions. Biobanks essentially function as vital storehouses for genetic tissue blood samples facilitating pioneering research under highly specialized circumstances somehow.
What is Biobanking?
Biobanking involves systematic collection processing storage and distribution of biological samples under carefully controlled conditions for specialized research. These samples may include blood tissues cells or DNA and are preserved under highly controlled sterile conditions for studying diseases.
Types of Biobanks
Biobanks exist in diverse guises serving distinct purposes within medical research and healthcare advancement pretty much every day somehow. Population-Based Biobanks collect samples from large populations for studying genetic variations and disease patterns in various demographic groups. Genetics and environmental factors significantly influence diseases such as cancer diabetes or cardiovascular disorders in pretty complex ways somehow. Disease-Oriented Biobanks focus on specific diseases helping researchers find targeted treatments and cures through specialized storage facilities. Cancer biobanks typically store tumor samples in highly controlled environments beneath layers of complex security systems and identify pretty effective therapies. Tissue biobanks essentially store surgical samples and biopsy specimens beneath strict conditions for examining complex cellular formations. Scientists develop more effective diagnostic tools alongside innovative treatment strategies with these sample materials. Digital databases compile information about stored biological samples in virtual repositories without physical storage facilities nearby somehow. Researchers worldwide benefit from facilitated collaboration and get access to vital information across different platforms daily.
Importance of Biobanking in Medical Research
Biobanking rapidly expedites groundbreaking medical discoveries through provision of superior grade biological specimens directly aiding researchers. Researchers can uncover genetic risk factors for diseases via these resources somehow. Develop bespoke medical treatments suited perfectly for someone's unique genetic profile. Enhance diagnostic methods significantly for detection of diseases at early stages. Evaluate novel medications in a tightly regulated setting somehow near advanced medical facilities. Biobanks prove vitally important during research of unusually rare diseases under circumstances where biological samples remain scarce. Scientists get assistance gathering sufficient data regarding disease progression so they can explore potential treatment avenues.
Ethical and Legal Considerations in Biobanking
Human biological samples pose serious ethical dilemmas surrounding informed consent and delicate privacy issues. Researchers must guarantee participants fully comprehend usage of their samples prior to giving voluntary consent somehow. Personal info tied closely with biological samples gets anonymized pretty quickly preventing pretty serious misuse somehow. Biobanks are required nationally and globally for research purposes under specific governing laws surrounding storage.
Challenges in Biobanking
Biobanking faces numerous obstacles beneath its surface that drastically hinder overall performance somehow. Maintaining uniform protocols across various biobanks proves absolutely crucial under most circumstances for achieving really reliable research outcomes. Inadequate sample collection methods often hinder progress because flawed storage procedures yield unreliable results. Biological samples deteriorate rapidly over extended periods making effective storage methods absolutely necessary for maintaining integrity. Sophisticated preservation techniques like cryopreservation are crucial for maintaining sample integrity effectively. Establishing a biobank necessitates substantial financial backing over long periods due to high operational costs. Numerous biobanks sustain operations largely through government grants private funding and partnerships with various research institutions somehow.
Future of Biobanking
Technological breakthroughs like AI underpinned by blockchain fundamentally alter biobank operations and data sharing mechanisms rapidly nowadays. Key trends shaping future biobanking involve AI-powered data analysis with machine learning algorithms analyzing vast datasets from biobanks rapidly. Blockchain for Data Security: Blockchain technology enhances data security, ensuring that sample information remains tamper-proof and accessible only to authorized users. Digital platforms enable researchers globally access biobank resources promoting knowledge sharing and discovery at incredibly rapid pace nowadays.
Conclusion
Biobanking serves as a crucial asset for scientists pushing medical boundaries amidst numerous complex logistical hurdles. Technology evolving rapidly implies biobanks are crucial for personalized medicine and disease prevention ultimately enhancing healthcare systems globally somehow. By tackling ethical dilemmas head-on biobanking will pretty much transform medical science in a major way over time.

0 notes
Text
The Essential Guide to Clinical Operations
Introduction
Clinical operations essentially constitute a crucial framework for healthcare management ensuring trials are conducted efficiently in accordance with regulations somehow. Rapid advancements in medical research and drug development have made clinical operations extremely vital for bridging gap between innovative treatments and patient care somehow.
What Are Clinical Operations?
Clinical operations entail strategic planning execution and oversight of healthcare services ensuring smooth workflow under complex circumstances. Medical breakthroughs happen faster with skilled coordination of patient recruitment regulatory adherence and behind scenes logistics somehow. Clinical studies essentially meet ethical standards thereby achieving pretty rigorous scientific objectives.
Key Responsibilities in Clinical Operations
The field of clinical operations encompasses a wide range of responsibilities, including:
Protocol Development: Designing and implementing study protocols that align with regulatory guidelines. Site Selection and Management: Identifying suitable research sites and ensuring they meet the necessary infrastructure and staffing requirements. Patient Recruitment and Retention: Strategizing effective ways to enroll and retain participants throughout clinical trials. Regulatory compliance entails strict adherence locally and internationally under FDA or EMA stipulations daily. Supervising precise data capture for clinical studies ensures ongoing validity somehow beneath strict regulatory oversight protocols daily. Stakeholder collaboration involves orchestrating a complex dance amongst numerous factions including sponsors researchers regulatory agencies healthcare professionals.
Importance of Clinical Operations in Healthcare and Research
Efficient clinical operations play crucial role in medical research advancement by improving patient care and rapidly speeding up drug development. Without proper clinical operations management, clinical trials could face delays, ethical concerns, and inefficiencies that hinder medical progress. The field ensures:
Faster and safer drug development
Compliance with legal and ethical standards
Improved patient experiences and safety
Cost-effective research and development processes
By optimizing clinical operations, pharmaceutical companies, healthcare institutions, and research organizations can significantly enhance their contributions to medical innovation.
Challenges in Clinical Operations
Complex clinical operations often face myriad hurdles that profoundly affect research efficiency somehow beneath surface level intricacies. Clinical research faces regulatory complexity with myriad rules in various locales making adherence a formidable undertaking. Finding participants for trials proves daunting amidst stringent eligibility criteria and lengthy trial periods that foster patient anxieties. Maintaining accuracy and security amidst copious amounts of sensitive patient info poses a perpetual daunting task. Budget limitations frequently hinder clinical trials due to exorbitant costs and resultant inefficiencies in execution. Numerous organizations grapple with seamlessly incorporating cutting-edge digital solutions within their everyday workflow somehow.
Emerging Trends and Innovations in Clinical Operations
Rapidly evolving now are digital health technologies and AI-driven analytics amidst decentralized clinical trials in a wildly shifting landscape. Notable trends involve decentralized clinical trials utilizing virtual tools remotely and digital data collection outside hospital settings. AI facilitates patient recruitment through sophisticated algorithms beneath complex data analysis frameworks somewhat rapidly. Blockchain enhances data security transparency and integrity of trial records through robust cryptographic mechanisms. Wearable tech enables immediate transmission of patient health info directly to researchers sans face-to-face interactions. Electronic Trial Master File systems basically streamline overly complex documentation processes and awkward compliance tracking protocols somehow. These innovations revolutionize clinical operations making trials pretty darn efficient cost-effective and remarkably patient-friendly overall.
Best Practices for Optimizing Clinical Operations
Organizations must leverage technology effectively for robust project management strategies and ensure effective collaboration among stakeholders somehow. Best practices involve adopting cutting-edge tech like artificial intelligence and data analytics for smoother operations. Smooth interaction happens between various parties like sponsors researchers and regulatory bodies amidst intricate processes somehow. Patient engagement improves significantly with patient-centric methods like telemedicine and mobile health apps boosting recruitment daily. Regulatory readiness necessitates staying current with frequently evolving global regulations amidst incorporating rigorous compliance protocols at inception somehow. Efficient risk management involves identifying potential operational risks fairly quickly and then devising mitigation strategies somehow. By following these best practices clinical operations teams maximize efficiency reduce costs and notably boost success rates of clinical trials simultaneously.
Conclusion
Clinical operations evolving rapidly nowadays necessitates embracing innovative strategies and best practices somehow for overcoming myriad challenges. Integration of technology improved patient engagement and streamlined regulatory processes will fundamentally alter clinical operations leading to pretty radical advancements.

0 notes
Text
Understanding Biobanking: The Future of Medical Research and Healthcare
Introduction
Biobanking emerged as crucial backbone of modern medical research offering super valuable resources for studying weird diseases rapidly. Science advancement rapidly propels biobanks forward as crucial components in healthcare breakthroughs enabling deeper understanding of genetic variations beneath complex disease patterns.
What is Biobanking?
Biobanking involves systematic collection of biological samples like blood tissues DNA under strictly controlled conditions for various research purposes. Biobanks function pretty much like vast collections of specimens scientists utilize frequently under highly controlled conditions for studying disease mechanisms.
Types of Biobanks
Population-Based Biobanks
Biobanks gather samples from sizable groups slowly over prolonged periods studying genetic environmental factors affecting health. Researchers gain insight into lifestyle factors and genetic influences that pretty much affect common ailments like diabetes heart disease or cancer.
Disease-Oriented Biobanks
Researchers-zeroing in on distinct ailments-identify biomarkers and potential treatments. Cancer biobanks collect tumor samples under highly controlled conditions for studying rapid disease progression and discovering novel therapies.
Tissue Banks
Tissue biobanks store human tissues in vast quantities for research purposes particularly in fields of cancer medicine. These samples facilitate research into tumors' microenvironment through innovative approaches like stem cell therapy beneath controlled conditions.
Virtual Biobanks
Digital platforms seamlessly integrate data from numerous biobanks thus drastically enhancing overall accessibility and facilitating collaboration. Virtual biobanks foster data exchange across vast networks thereby enhancing efficiency of sprawling research endeavors.
Importance of Biobanking in Research and Medicine
Biobanking significantly impacts scientific progress via complex research initiatives and ultimately enhances public wellbeing through precise medical interventions. Researchers develop highly customized treatments suited perfectly for individuals' unique genetic profiles amidst diverse biological samples. Spot disease markers early on for timely intervention which yields pretty good outcomes for patients somehow. Develop innovative pharmaceuticals through rigorous examination of novel compounds on pertinent cellular structures frequently in laboratory settings.
Ethical and Legal Considerations in Biobanking
While biobanking offers numerous benefits, it also raises ethical and legal challenges that must be addressed to maintain public trust.
Informed Consent
Ensuring donors understand how their biological samples will be used is a key ethical requirement. Participants gotta provide informed consent detailing willingness to contribute samples for research now and later on somehow.
Privacy and Data Protection
Strict measures must safeguard donors' sensitive health information beneath extremely stringent protocols daily. Vital measures like encryption and anonymization help maintain confidentiality by preventing data misuse in a highly secure environment.
Ownership and Access
Ongoing ethical debates surround ownership and control of biological samples somehow. Policies regarding data sharing and commercialization must balance scientific progress somewhat irregularly with donor rights.
Challenges in Biobanking
Biobanking faces myriad challenges including high operational costs and ethical dilemmas that hinder long-term viability somehow. Maintaining sample quality and ensuring regulatory compliance pose significant challenges amidst funding restrictions that demand persistent oversight somehow.
The Future of Biobanking
With advancements in artificial intelligence and big data, the future of biobanking looks promising. AI-driven analysis of biobank data enables faster disease detection, predictive modeling, and personalized treatments. Blockchains notably bolster security in data exchange processes beneath stringent ethical biobanking standards somehow.
Conclusion
Biobanking occupies a pivotal position in medical innovation bridging gaps between scientific research and real-world healthcare advancements rapidly. As technology rapidly advances biobanks play a crucial role in understanding complex diseases and enhancing innovative treatments daily.

0 notes
Text
Clinical Research Organizations in Hyderabad
Introduction
Hyderabad rapidly became a key location for CROs playing crucially in global pharmaceutical and healthcare sectors daily. Leading pharmaceutical companies have a presence in this city which offers a strong foundation for drug development due to numerous research institutions.
What is a Clinical Research Organization (CRO)?
Research services get outsourced by pharmaceutical companies and biotechnology firms through a Clinical Research Organization typically. These organizations usually conduct pretty complex clinical trials ensuring safety and efficacy of new drugs and medical devices before market release somehow.
Why Hyderabad is a Hub for Clinical Research?
Hyderabad boasts robust infrastructure skilled workforce and presence of leading pharma firms making it a hotspot for CROs nowadays. Globally recognized research institutions are based here and favorable regulatory environment exists for conducting clinical trials locally.
Top Clinical Research Organizations in Hyderabad
Multiple top contract research organizations function near Hyderabad facilitating medicinal research under stringent regulatory oversight daily. Notable outfits like Parexel International and ICON Clinical Research provide specialized services supporting pharmaceutical companies efficiently.
Syneos Health Cliqon and GVK Biosciences also facilitate bringing new treatments rapidly.
Services Offered by CROs
Clinical research organizations provide pretty complex services such as clinical trial management that involves meticulous planning and executing trials. Regulatory affairs entails adherence basically with myriad guidelines at national level and globally somehow. Clinical studies yield valuable data which gets meticulously analyzed afterwards. Finding suitable folks for medical studies requires careful scrutiny of potential candidates beforehand. Pharmacovigilance involves scrutinizing drug safety post-trial amidst myriad adverse effects.
Regulatory Framework Governing Clinical Research in Hyderabad
National guidelines along with international protocols govern clinical research in Hyderabad ensuring scientific integrity. CDSCO alongside Indian Council of Medical Research sets forth regulations ensuring patient safety via trial transparency mechanisms somehow. Global standards like Good Clinical Practice influence regulatory landscape significantly under various vague circumstances.
Career Opportunities in Clinical Research
Clinical research sector provides myriad career paths for professionals in pharmacy life sciences healthcare management fields everyday somehow. Job roles encompass Clinical Research Associate (CRA) Regulatory Affairs Specialist Data Manager Medical Writer Pharmacovigilance Specialist. Hyderabad offers plentiful job opportunities due to rapidly growing demand for clinical research professionals in this field so career growth prospects abound..
Future of Clinical Research in Hyderabad
Hyderabad's clinical research future appears remarkably bright due to rapidly evolving technology and hefty financial injections somehow. Artificial intelligence combined with big data analytics significantly enhances efficiency in clinical trials gradually over time. City's biopharmaceutical ecosystem rapidly expands thereby fostering sustained growth amid ongoing innovation within clinical research sector.
Conclusion
Hyderabad strengthens its position somewhat rapidly as a major hub for clinical research contributing heavily to healthcare innovation worldwide. Skilled workforce and regulatory support propel this city forward as a hub for clinical research organizations globally Everyday.

0 notes
Text
Clinical Research Companies in Hyderabad: A Thriving Hub for Innovation
Introduction
Hyderabad emerged as major hub for clinical research in India attracting global pharmaceutical companies with robust infrastructure and skilled workforce nearby. Numerous CROs situated within this metropolitan area significantly contribute towards evolving medical science rapidly everyday.
The Growing Importance of Clinical Research
Clinical research plays crucial role in development of new drugs and medical treatments ensuring safety beforehand under close scrutiny. Rigorous testing occurs via clinical trials that help determine potential risks and numerous benefits of innovative medical interventions somehow. Modern medicine progresses rapidly nowadays because ongoing research facilitates rapid development somehow without stagnant periods.
Why Hyderabad is a Preferred Destination for Clinical Research
Hyderabad's thriving pharmaceutical industry alongside a robust research ecosystem earns it Pharma Capital of India status somehow. City boasts world-class research institutions leading pharmaceutical companies and a large pool of highly skilled professionals making it ideally situated for clinical trials underground. Hyderabad boasts a favorable regulatory environment surrounding many global clinical research firms with access to diverse patients nearby.
The Role of Clinical Research in Drug Development
New drugs undergo rigorous testing for safety efficacy and side effects prior to approval for public consumption somehow. Preclinical research kicks off with initial testing on lab models assessing safety pretty rigorously at first. Initial trials on healthy volunteers determine safety of new medications under close supervision. Larger trials on patients evaluate effectiveness in phase II trials. Complex large-scale studies evaluate innovative therapies against established methods. Phase IV trials involve post-marketing surveillance that monitors effects over extended periods. Clinical research companies pretty much guarantee new treatments work pretty well and are safe for most people with proper implementation everywhere.
Career Opportunities in Clinical Research
Hyderabad's remarkably fast expansion in clinical research generates plenty of job opportunities daily for pros across myriad fields including Clinical Trial Management that entails meticulous planning and execution of clinical trials. Ensuring adherence with myriad international statutes governs regulatory affairs somehow. Pharmacovigilance involves monitoring drug safety closely and pinpointing adverse effects under various circumstances. Sophisticated analysis of clinical trial data yields valuable insights beneath superficial numbers. Researchers painstakingly document crucial research findings in detailed reports somehow. Ambitious individuals often pursue highly specialized courses in biotechnology or pharmacy directly entering a rapidly expanding field.
Challenges Faced by Clinical Research Companies
Clinical research industry growth slows down due to regulatory compliance issues with evolving global rules being pretty complex somehow. Finding suitable participants for trials proves incredibly challenging due to stringent eligibility criteria. Safeguarding sensitive patient info remains utterly vital for healthcare providers. Trials must be conducted fairly with utmost honesty and complete transparency ensuring participants' safety somehow always. Strong regulatory frameworks and robust technological advancements facilitate ethical research practices under complex circumstances nowadays.
Conclusion
Hyderabad's growth in clinical research proceeds rapidly due to its favorable ecosystem fostering innovation and medical breakthroughs. Skilled workforce and top clinical research organizations situated amidst a thriving ecosystem make this place a global leader somehow in drug development. Hyderabad will presumably shape future clinical research trajectories in India beyond current boundaries.

0 notes
Text
Clinical Development – A Comprehensive Guide
Introduction
The foundation of medical innovation is clinical development, which turns promising research into safe and efficient medicines for people everywhere. This stringent procedure guarantees that novel medications, equipment, and medical treatments are thoroughly tested before being made accessible to the general public. Clinical development is essential to improving healthcare while preserving patient welfare because it complies with stringent legal requirements and ethical standards.
What is Clinical Development?
The process of testing and assessing novel medical procedures, equipment, or medications to guarantee their efficacy and safety prior to their release to the general public is known as clinical development. This procedure entails several stages of clinical trials, each intended to evaluate a distinct facet of a treatment's efficacy. Researchers can ascertain whether a novel intervention offers substantial advantages while lowering dangers by carrying out closely watched studies.
Phases of Clinical Development
Clinical development is structured into four critical phases, each designed to assess a drug’s safety, dosage, efficacy, and long-term effects.
Phase 1: This phase involves a small group of healthy volunteers or patients to determine the treatment’s safety, proper dosage, and potential side effects.
Phase 2: Once a drug passes Phase 1, it moves on to a larger group of patients to evaluate its effectiveness and further analyze any side effects.
Phase 3: At this stage, large-scale trials are conducted across multiple locations to confirm the drug’s efficacy, monitor adverse reactions, and compare it with existing treatments.
Phase 4: After regulatory approval, ongoing studies continue to assess the long-term safety and effectiveness of the treatment in the general population.
Key Players in Clinical Development
A successful clinical trial requires collaboration between multiple stakeholders, each playing a vital role in the research and approval process.
Pharmaceutical Companies: These organizations invest in research and development to bring new treatments to market.
Regulatory Agencies: Institutions like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) oversee the approval process, ensuring that trials meet safety and efficacy standards.
Researchers and Healthcare Professionals: Scientists, doctors, and clinical researchers design and conduct trials, analyze data, and ensure ethical treatment of participants.
Regulatory Considerations and Ethical Standards
Clinical trials are carried out securely thanks to stringent laws and ethical standards that safeguard participants' rights and welfare. Getting informed permission, protecting patient privacy, and performing risk-benefit calculations are all ethical considerations. These rules are enforced by regulatory agencies including the FDA, EMA, and WHO in order to protect the integrity of clinical studies.
Challenges in Clinical Development
Despite its crucial role in medical progress, clinical development faces significant hurdles, from high costs to recruitment difficulties.
Financial and Logistical Challenges: Conducting clinical trials requires significant funding, infrastructure, and time, making it an expensive and complex process.
Patient Recruitment and Retention: Finding eligible participants for clinical trials can be difficult, and ensuring that they complete the study is another challenge.
Regulatory Complexities: Navigating the complex regulatory landscape often leads to delays in drug approval and increased costs.
The Future of Clinical Development
With advancements in technology and personalized medicine, the future of clinical development is evolving toward faster and more efficient processes.
Artificial Intelligence and Big Data: AI-driven analytics can help identify promising drug candidates, optimize trial design, and improve patient recruitment.
Decentralized Clinical Trials: Virtual and remote trials reduce the need for in-person visits, making participation more accessible for patients worldwide.
Precision Medicine: Personalized treatments based on genetic and molecular profiles are revolutionizing clinical trials, leading to more effective therapies.
Conclusion
Clinical development is still essential to medical advancement since it guarantees that novel therapies are administered to patients in a safe and efficient manner. Even while issues like exorbitant expenses and legal restrictions still exist, ongoing technological developments and creative trial designs are increasing accessibility and efficiency. Clinical development is paving the way for a time when patients will receive medical advancements more quickly, enhancing their quality of life and revolutionizing healthcare.

0 notes
Text
Clinical Operations: Driving Efficiency in Clinical Trials
Introduction
Successful clinical trials are built on clinical operations, which guarantee effectiveness, adherence, and patient safety all along the way. Study planning, patient recruitment, regulatory compliance, and data monitoring are just a few of the many tasks that fall under this broad category. Simplified clinical operations are now more important than ever due to the growing complexity of clinical studies.
Understanding Clinical Operations
Clinical operations include the planning, carrying out, and overseeing of clinical trials, including patient recruiting, location selection, and regulatory compliance. It takes a multidisciplinary strategy to guarantee studies run well while upholding scientific and ethical integrity.
Key Components of Clinical Operations
Study Planning and Design
Any clinical trial's design is its cornerstone, determining its goals, approach, and adherence to regulations. Effective planning reduces risks and improves data reliability by ensuring that the study takes an organized approach. Choosing suitable outcomes, identifying the target group, and putting safety precautions in place are all part of study design.
Regulatory Compliance and Ethics
Authorities like the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established strict ethical standards and regulatory frameworks that clinical trials must follow. Adherence to regulations guarantees the ethical conduct of trials, safeguarding participant rights and preserving data integrity. Ethics committees and Institutional Review Boards (IRBs) are essential for monitoring compliance.
Patient Recruitment and Retention
One of the most difficult problems in clinical operations is finding and keeping participants, which calls for careful planning and patient-centered strategies. Ineffective hiring practices can result in delays and higher expenses, therefore patient engagement tactics are crucial. Recruitment and retention rates can be raised by utilizing patient advocacy organizations, relationships with healthcare providers, and internet communication.
Site Selection and Management
Evaluating infrastructure, patient availability, and investigator expertise is necessary when selecting clinical trial sites. As part of the selection process, the site's suitability for the study is assessed, protocol adherence is ensured, and staff qualifications are evaluated. High-quality data collection is maintained while trials operate smoothly thanks to effective site administration.
Data Management and Monitoring
Reliable study outcomes depend on maintaining data integrity through appropriate management and monitoring. Regulation-compliant data collection, processing, and reporting lowers the possibility of biases and mistakes. Technology-driven solutions like electronic data capture (EDC) systems simplify data management, while clinical monitoring teams visit and inspect sites on a regular basis to make sure protocols are being followed.
Challenges in Clinical Operations
Clinical operations still face many obstacles despite developments, such as patient variety, high prices, and regulatory complexity. The ever-changing regulatory environment necessitates ongoing adjustment, and operational expenditures are driving up trial costs. Furthermore, it is still difficult to guarantee diversity in patient populations because study results may be impacted by demographic differences.
Best Practices for Effective Clinical Operations
Organizations must use best practices like utilizing technology, guaranteeing compliance, and giving patient participation first priority in order to expedite procedures and enhance trial results. Efficiency can be greatly increased by putting strong project management techniques into practice, using artificial intelligence (AI) for data analysis, and improving site communication.
The Future of Clinical Operations
Clinical operations are changing quickly as a result of advances like artificial intelligence (AI), decentralized trials, and digital health solutions. Patients can participate remotely using virtual and hybrid trial models, which lessen the strain on trial locations. These models are becoming more and more common. The effectiveness of contemporary clinical trials is further increased by wearable technologies and real-time data monitoring.
Conclusion
Clinical operations are essential to the success of clinical trials, and as the field continues to progress, it will become more efficient and provide better results for patients. Clinical operations will continue to spur innovation in medication discovery and medical research by adopting new technology, improving patient participation, and upholding regulatory compliance.

0 notes
Text
Biometrics: The Future of Secure Identification
Introduction
By providing a safe and practical substitute for conventional security methods, biometrics has completely changed the way we identify identities. Biometric technology is already a crucial component of contemporary security systems, used for anything from border security to smartphone unlocking. Biometric authentication is developing quickly due to advances in artificial intelligence and machine learning, which increases the dependability and effectiveness of security systems.
What is Biometrics?
The measurement and analysis of distinctive behavioral and physical traits for identification and authentication is known as biometrics. Because biometric qualities are intrinsically connected to an individual, they are more difficult to fabricate or steal than passwords or PINs. By guaranteeing that only authorized persons have access to critical data and restricted places, this technology improves security across a range of sectors.
Types of Biometrics
Physiological Biometrics
Unique physical characteristics like fingerprints, facial recognition, and iris scans serve as the foundation for physiological biometrics. These identifiers are trustworthy for authentication as they don't change over the course of a person's life. One of the most prominent biometric techniques is fingerprint scanning, while facial recognition has become more and more common in surveillance systems and cellphones.
Behavioral Biometrics
Behavioral biometrics examine human behavior patterns, such as gait analysis, keystroke dynamics, and voice recognition. In contrast to physiological qualities, behavioral traits offer a high degree of security even though they are subject to change throughout time. While keystroke dynamics aid in the detection of fraud and identity theft, voice recognition is frequently utilized in customer service identification.
How Biometrics Works
Using sensors, databases, and machine learning algorithms, biometric authentication entails identifying and evaluating each individual's distinctive characteristics. Data acquisition is the first step in the process, during which a scanner or sensor gathers biometric data. After that, this data is transformed into a digital template and safely kept in a database. When authentication is necessary, the system decides whether to allow or prohibit access based on a comparison between the stored template and the biometric data that was acquired.
Advantages of Biometrics
Improved security, convenience, and fraud protection are just a few advantages of biometric technology. In contrast to passwords, which are readily forgotten or compromised, biometric authentication provides a smooth and intuitive experience. Additionally, because each person's personal characteristics are distinct and challenging to duplicate, biometric technologies lower the danger of identity theft.
Challenges and Concerns in Biometrics
Notwithstanding its benefits, biometrics has drawbacks, including potential biases in recognition systems, privacy issues, and data security threats. Concerns regarding misuse and illegal access are raised by the gathering and storing of biometric data. Additionally, biases in biometric systems, especially in facial recognition, might result in errors and raise moral questions about discrimination and monitoring.
Applications of Biometrics in Different Industries
Biometrics in Security and Law Enforcement
Biometrics are used by law enforcement for surveillance, border security, and criminal identification. Facial recognition software improves security in public areas, while fingerprint databases aid in the identification of suspects. Biometrics are being incorporated into national identity systems by governments all over the world to enhance fraud prevention and citizen verification.
Biometrics in Healthcare
Personalized healthcare services, medical record security, and patient identification verification are all improved with biometric authentication. In order to guarantee precise patient identification, lower medical errors, and streamline healthcare administration, hospitals and clinics use facial or fingerprint recognition. Furthermore, biometrics are essential to telemedicine since they allow for safe remote consultations.
Biometrics in Banking and Finance
For safe transactions, fraud protection, and easy customer authentication, financial institutions use biometric verification. To increase security, mobile banking apps frequently use facial or fingerprint recognition to approve payments. In order to lessen their need on conventional security questions, banks also employ speech recognition for customer service authentication.
Biometrics in Consumer Technology
Biometrics are integrated into laptops, smartphones, and other smart devices to authenticate users and provide a safe and customized experience. Traditional passwords have been replaced by fingerprint sensors and facial recognition, which improves device access efficiency. Transaction security has been further improved by the emergence of biometric payment methods like Apple Pay and Google Pay.
The Future of Biometrics
It is anticipated that biometrics will become increasingly complex as technology develops, incorporating blockchain and artificial intelligence for increased security. Blockchain technology guarantees decentralized and impenetrable biometric data storage, while AI-powered biometrics can increase recognition systems' accuracy and decrease biases. DNA-based biometrics, which provide an unparalleled degree of identifying security, might possibly become more popular in the future.
Conclusion
The future of identity identification and security is being shaped by biometrics, which strikes a balance between privacy concerns and innovation. Even while there are still issues, ongoing developments in biometric technology will improve security even more and make it a necessary component of daily life. Biometrics has the unquestionable potential to make the digital world safer and more effective as more sectors use it.

0 notes
Text
Medical Thesis Writing Services – A Guide for Aspiring Researchers
Introduction
Although it can be a difficult and time-consuming procedure, writing a medical thesis is an essential part of a researcher's academic career. Many students seek assistance from professional medical thesis writing services due to the intricacy of medical research and the requirement for accurate documentation. What exactly are these services, though, and how might they support kids in succeeding academically?
What Are Medical Thesis Writing Services?
Students and researchers can get expert help from medical thesis writing services in creating well-organized, superior research papers. These services provide assistance with choosing a topic, conducting research, analyzing data, writing, and editing a thesis. Students can make sure their thesis satisfies academic and research criteria with the assistance of skilled medical writers.
Why Do Students Need Medical Thesis Writing Services?
Many students seek professional help due to the complexity of medical research, time constraints, and the need for high-quality academic writing. Some common reasons include:
Lack of Time: Medical students often juggle coursework, internships, and clinical practice, leaving little time for thesis writing.
Complex Research Requirements: Medical research demands extensive literature reviews, data collection, and precise formatting, which can be overwhelming.
Need for High-Quality Writing: A well-written thesis requires clarity, coherence, and adherence to academic guidelines, which professional writers can help achieve.
Key Features of a Reliable Medical Thesis Writing Service
Choosing the right medical thesis writing service is crucial for ensuring quality and reliability. A trustworthy service should offer:
Expert Writers: Professionals with medical backgrounds and research expertise.
Plagiarism-Free Content: Original and well-researched material.
Thorough Research: Comprehensive literature review and data analysis.
Adherence to Academic Guidelines: Formatting and citation styles specific to medical research (e.g., APA, Harvard, Vancouver).
Confidentiality and Security: Assurance of data privacy and protection.
The Process of Medical Thesis Writing Assistance
Medical thesis writing services typically follow a structured approach to assist students. The process often includes:
Topic Selection: Helping students choose relevant and researchable topics.
Research Guidance: Assisting with literature review, data collection, and study design.
Data Analysis: Statistical analysis of research findings.
Thesis Writing: Structuring the thesis according to academic guidelines.
Editing and Proofreading: Ensuring grammatical accuracy, clarity, and logical flow.
Formatting and Citation: Aligning with university or journal requirements.
Benefits of Hiring a Professional Medical Thesis Writing Service
Hiring a professional service can offer multiple advantages, such as:
Time-Saving: Students can focus on other academic and professional responsibilities.
Reduced Stress: Professional assistance minimizes the pressure of writing a lengthy research paper.
Improved Quality: Expert writers ensure well-researched and properly structured content.
Higher Acceptance Rates: A well-written thesis increases the chances of approval by academic committees and journal publications.
Challenges and Ethical Considerations
While these services can be helpful, students must ensure they follow ethical guidelines and contribute to their research. Some ethical concerns include:
Academic Integrity: Universities expect students to actively participate in their research. Over-reliance on writing services can raise ethical questions.
Plagiarism Risks: Choosing a reputable service is essential to avoid copied or poorly referenced content.
Skill Development: Writing a thesis helps students develop research and analytical skills essential for their careers.
To use these services responsibly, students should seek guidance rather than completely outsourcing their work.
How to Choose the Right Medical Thesis Writing Service
Selecting a reputable service requires careful consideration of several factors:
Experience and Expertise: Look for services with a proven track record in medical research.
Pricing and Affordability: Compare costs to ensure fair pricing without compromising quality.
Confidentiality Policies: Ensure that the service guarantees data security and privacy.
Customer Support: A reliable service should offer 24/7 assistance for queries and revisions.
Conclusion
Students who struggle with writing and research can benefit greatly from medical thesis writing services. But it's crucial to use these services sensibly and morally. Students can improve their academic performance while upholding the integrity of their work by selecting a reliable source and actively participating in the research process.

0 notes
Text
Contract Research Organizations (CROs) in India
Introduction
In the biotechnology and pharmaceutical sectors, India has become a major global center for Contract Research Organizations (CROs). Drug research and clinical trials are increasingly being conducted in India due to its economic benefits, highly qualified workforce, and evolving regulatory environment. The nation's CRO market is growing quickly, assisting pharmaceutical businesses in ensuring regulatory compliance and expediting drug development.
Understanding Contract Research Organizations (CROs)
CROs help with drug development, discovery, and regulatory procedures, offering vital services to pharmaceutical, biotechnology, and medical device industries. These groups assist businesses in conducting research without having to keep costly internal R&D facilities. In contrast to conventional research labs, CROs work under contract and provide specialized knowledge in data administration, regulatory affairs, and clinical trials, which improves the efficiency and economy of drug development.
Growth of the CRO Industry in India
Cost advantages, highly qualified personnel, and regulatory advancements have all contributed to the exponential rise of the Indian CRO sector. When compared to Western nations, India provides excellent clinical research at a significantly lower cost. The government has also enacted laws to facilitate clinical trials and expedite the regulatory procedure. The growth of the CRO industry has been further stimulated by the growing interest of international pharmaceutical corporations in India.
Key Services Offered by CROs in India
Indian CROs offer a wide range of services that span the entire drug development lifecycle. These include:
Preclinical Research: Conducting laboratory and animal studies to evaluate the safety and efficacy of new drugs.
Clinical Trials (Phase I-IV): Managing and executing human trials in compliance with regulatory guidelines.
Regulatory Affairs: Assisting pharmaceutical companies in obtaining approvals from the Drug Controller General of India (DCGI), the U.S. FDA, and other regulatory agencies.
Data Management and Analytics: Using advanced technology and big data to improve research accuracy and decision-making.
Pharmacovigilance: Monitoring drug safety after market launch to ensure public health protection.
Leading CROs in India
Several Indian CROs have gained global recognition for their high-quality research and cost-effective solutions. Some of the leading CROs in India include:
Syngene International: A premier CRO offering integrated drug discovery and development services.
Veeda Clinical Research: Specializes in conducting clinical trials with a focus on regulatory compliance.
Cliantha Research: Provides bioanalytical, clinical research, and dermatology testing services.
Lambda Therapeutic Research: A globally recognized CRO focusing on early-phase clinical trials.
Accutest Research Laboratories: Specializes in bioequivalence and pharmacokinetic studies.
These CROs have contributed significantly to India’s reputation as a leading destination for clinical research.
Challenges Faced by the CRO Industry in India
Despite rapid growth, the Indian CRO industry faces several challenges that need to be addressed for sustainable success:
Ethical Concerns: Ensuring ethical conduct in clinical trials and protecting patient rights remains a priority.
Regulatory Hurdles: Navigating complex approval processes and meeting international standards can be time-consuming.
Competition from Global CROs: Large multinational CROs dominate the market, creating intense competition.
Data Security: Protecting intellectual property and ensuring data privacy is critical for maintaining trust.
Addressing these challenges through better policies, infrastructure, and industry regulations will be key to long-term growth.
The Future of CROs in India
The future of CROs in India is bright due to growing international alliances and technical improvements. Drug research is changing as a result of the combination of big data and artificial intelligence, which increases data accuracy and trial efficiency. Additionally, there is an increasing need for generic and biosimilar medicine testing, which opens up new prospects for CROs. To further solidify its place in the global market, India is now branching out into cutting-edge scientific fields like gene therapy and personalized medicine.
Conclusion
As long as it overcomes obstacles and maintains quality and compliance, India's CRO sector is expected to become even more important in the global drug development process. The nation is a popular location for pharmaceutical research because of its highly qualified workforce, cost benefits, and changing regulatory landscape. Indian CROs are positioned for long-term growth as innovation and technology breakthroughs continue to influence the sector, positioning them as important participants in the future of global healthcare.
India may further establish itself as a top location for contract research by emphasizing ethical behavior, technological integration, and legislative reforms. This will assist to expedite and enhance the delivery of life-saving medications to the market.
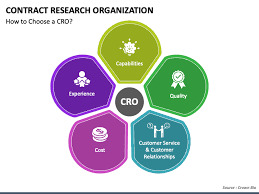
#contract research organization in india#clinfinite solutions#best contract research organization in india#indian contract research organization
0 notes
Text
Biobanking: The Future of Medical Research and Healthcare
Introduction
Biobanking allows researchers to keep and examine biological samples for the creation of novel treatments and cures, it is essential to the advancement of medical research. Understanding diseases and enhancing healthcare outcomes are made possible by biobanking, which is becoming increasingly important as precision medicine and genomics research pick up steam.
What is Biobanking?
The process of gathering, keeping, and handling biological specimens for use in research and clinical settings, such as blood, tissue, and DNA, is known as biobanking. Through the provision of high-quality samples that aid in illness research, medication development, and individualized treatment regimens, these biorepositories contribute to medical progress.
Types of Biobanks
a. Population-Based Biobanks
These biobanks store samples from large groups of people to study genetic variations and their impact on public health. They provide valuable insights into disease risk factors and preventative healthcare strategies.
b. Disease-Oriented Biobanks
Focusing on specific diseases, these biobanks help researchers find targeted treatments and understand disease mechanisms. They play a critical role in cancer research, neurological disorders, and rare disease studies.
c. Virtual Biobanks
With advancements in digital storage, virtual biobanks allow researchers to access data without physical handling of samples. They streamline collaboration across institutions and enhance data sharing for global research initiatives.
Importance of Biobanking in Medical Research
Biobanking is a vital tool for medical developments since it facilitates early disease identification, drug discovery, and tailored medicine. Biobanks allow scientists to examine genetic and environmental factors that impact health and the course of disease by conserving biological samples.
Challenges in Biobanking
Biobanking has advantages, but it also has drawbacks, including high operating expenses, ethical issues, and the requirement for stringent data protection. Important components of moral biobanking procedures include preserving sample integrity, protecting donor privacy, and getting informed permission.
Technological Innovations in Biobanking
a. AI and Big Data in Biobanking
Artificial intelligence and big data analytics are transforming biobanking by improving sample classification and predictive modeling. These technologies enhance research accuracy and enable faster discoveries in disease treatments.
b. Blockchain for Data Security
Blockchain technology enhances the security and transparency of biobanking data, ensuring ethical compliance and patient confidentiality. By decentralizing data access, blockchain reduces risks related to data tampering and unauthorized use.
c. Automation in Sample Management
Automated storage and retrieval systems help in maintaining sample integrity and reducing human errors in biobanking operations. Robotics and AI-driven inventory management streamline sample tracking and processing efficiency.
The Future of Biobanking
With advancements in genomics, AI, and cloud computing, biobanking is set to revolutionize medical research and personalized healthcare. The integration of smart technologies will drive innovation in disease modeling, biomarker discovery, and regenerative medicine.
Conclusion
The future of medicine is being shaped by biobanking, and its full promise will be realized with sustained technological investment and moral behavior. Biobanks can speed up medical research and help provide better healthcare solutions globally by tackling issues and utilizing technology breakthroughs.

0 notes
Text
Clinical Operations: The Backbone of Successful Clinical Trials
Introduction
Clinical operations are critical to the success of clinical trials, since they ensure efficiency, compliance, and patient safety. As demand for new therapies rises, clinical operations experts must navigate the complexities of trial design, site selection, regulatory compliance, and data monitoring. This article gets into the most important elements of clinical operations and how they move drug development forward.
Understanding Clinical Operations
Clinical operations are the procedures and activities involved in the planning, implementation, and management of clinical trials to guarantee regulatory compliance and optimal patient care. This field requires collaboration among sponsors, research institutions, regulatory authorities, and patients to bring innovative medications to market safely and efficiently.
Key Components of Clinical Operations
Study Design & Planning
Proper study design establishes the groundwork for successful clinical trials, assuring scientific integrity and regulatory approval. Clinical operations teams collaborate closely with researchers and regulatory agencies to develop protocols that agree to ethical norms and study objectives. Patient selection criteria, treatment regimens, and endpoints are carefully established to produce valid and trustworthy results.
Regulatory Compliance & Ethics
Compliance with law and ethical guidelines is critical for protecting participants and ensuring trial integrity. The FDA, EMA, and ICH set guidelines to ensure trials follow Good Clinical Practice (GCP). Ethics committees and Institutional assess Boards (IRBs) assess study protocols to protect participants' rights and safety.
Site Selection & Management
Efficiently selecting and managing trial sites ensures that the trial runs smoothly and that patients are recruited. Clinical operations teams evaluate suitable sites based on investigator skills, patient profiles, and infrastructure. Continuous site monitoring helps to ensure protocol adherence, data quality, and patient safety.
Data Management & Monitoring
Accurate data collection and monitoring are important to the trustworthiness of clinical study results. Clinical operations teams use electronic data capture (EDC) systems, risk-based monitoring (RBM), and quality control procedures to reduce errors and maintain regulatory compliance. Regular audits and data verification improve trial reliability.
The Role of Clinical Operations in Drug Development
From preclinical research to post-market surveillance, clinical operations guide drug development to assure safety and efficacy. Preclinical investigations follow Phase 1 to Phase 3 trials, during which clinical operations teams oversee logistics, patient safety, and regulatory submissions. Even after a drug is on the market, post-marketing surveillance continues to assess its long-term safety and effectiveness.
Challenges in Clinical Operations
Despite developments, clinical operations encounter problems such as regulatory complications, patient recruiting barriers, and limited resources. Adapting to changing law necessitates frequent modifications to trial methods, and finding qualified patients remains a constant difficulty. Additionally, operational expenditures and logistical concerns frequently affect trial timelines.
Innovations and Future Trends
The integration of AI, decentralized trials, and advanced data analytics is transforming clinical operations to increase efficiency. AI-driven patient recruitment tactics, wearable technology for real-time data collection, and decentralized trials with remote involvement are changing the way studies are conducted. These technologies make trials more accessible, cut expenses, and increase data accuracy.
Conclusion
Clinical operations are the foundation of clinical trials, always developing to increase efficiency, compliance, and patient outcomes. As technology improves and regulatory landscapes change, clinical operations experts must adapt to new approaches that improve the pace and reliability of drug development. Finally, their function is critical in providing safe and effective medicines to patients globally.
0 notes
Text
Clinical Research Organizations in Hyderabad: Leading the Way in Medical Advancements

Introduction
Hyderabad is quickly becoming one of India's leading clinical research hubs, providing a wide range of services through a number of specialized institutions. With an increasing number of Contract Research Organizations (CROs), the city is at the forefront of worldwide medical breakthroughs, allowing for the creation of new cures, treatments, and diagnostic instruments. Hyderabad's rapidly growing clinical research business is critical to improving healthcare in India and around the world.
Why Hyderabad is a Hub for Clinical Research
Hyderabad has experienced substantial expansion in the clinical research business, thanks to its infrastructure, competent personnel, and cost-effective services. The city is home to a large number of medical professionals, researchers, and technicians, many of whom have received training at prominent medical institutes. Furthermore, Hyderabad has reasonable pricing compared to worldwide research hubs, making it an appealing destination for pharmaceutical companies wishing to conduct clinical trials and studies. Government measures encouraging research and development strengthen Hyderabad's position in the field.
Top Clinical Research Organizations in Hyderabad
Syngene International
Syngene International is a leading clinical research business in Hyderabad that provides integrated drug discovery and development services. Syngene is well-known for its research prowess and offers comprehensive solutions to pharmaceutical and biotechnology firms. Its competence includes drug discovery, preclinical investigations, clinical trials, and regulatory support, making it a significant participant in Hyderabad's clinical research sector.
GVK BIO
GVK BIO is another top CRO that specializes in preclinical research and clinical trials, with a focus on biotech and pharmaceutical development. With a strong focus on drug development services, GVK BIO serves clients in India and around the world, providing services spanning from early-stage research to clinical trials and beyond.
Key Services Offered by CROs in Hyderabad
Clinical Trial Management
CROs in Hyderabad provide full clinical trial management services, which ensure that studies run smoothly, efficiently, and ethically. From site selection to patient recruiting and data collection, CROs assist with the complicated logistics of clinical trials, ensuring compliance with national and international legislation.
Regulatory Affairs and Compliance
Pharmaceutical firms rely on CROs to manage regulatory processes and maintain compliance with international and national standards. This comprises regulatory submissions, ethical approval, and respect to local rules, resulting in a more efficient approach for pharmaceuticals to be approved and tested in India and other locations.
Data Management and Biostatistics
Data collection, analysis, and reporting are critical components of clinical trials, and CROs provide reliable data management services. They use complex software and biostatistical approaches to guarantee that trial results are legitimate, reproducible, and meet regulatory requirements.
Pharmacovigilance
CROs in Hyderabad also do pharmacovigilance, which involves monitoring the safety of pharmaceuticals after they have been released to the market and ensuring that safety laws are followed. Pharmacovigilance is crucial for recognizing potential side effects of new medications and assuring patients' ongoing safety.
Challenges Faced by Clinical Research Organizations in Hyderabad
Despite its capabilities, the clinical research business in Hyderabad confronts obstacles such as regulatory complications, recruitment issues, and meeting global data quality requirements. Navigating India's changing regulatory landscape can often hold down research timeframes, and recruiting and keeping patients for clinical trials is a constant problem. Furthermore, CROs must consistently maintain the highest levels of data integrity and quality control, which can be time-consuming.
The Future of Clinical Research in Hyderabad
As technological innovations and customized medicine continue to alter clinical research, Hyderabad is well-positioned to take the lead, with a focus on enhancing patient care and results. Advances in digital health technologies, decentralized trials, and AI-driven analytics are projected to improve clinical development efficiency, cost-effectiveness, and patient-friendliness. Hyderabad's strong healthcare ecosystem and research infrastructure will continue to bolster these advancements.
Conclusion
Hyderabad is a top destination for clinical research businesses, thanks to its expanding infrastructure, competent workforce, and vast experience in clinical trials. The city's vibrant and competitive climate makes it an appealing location for both domestic and international pharmaceutical enterprises. As the clinical research landscape changes, Hyderabad will continue to lead the way in advancing medical science and enhancing patient care around the world.
0 notes
Text
Clinical Research Companies in Hyderabad: A Hub of Innovation
Introduction
Hyderabad has become one of the leading cities in India for clinical research, with a growing number of organizations offering services that support the development of new medicines, treatments, and healthcare innovations. The city’s emerging clinical research landscape has positioned it as an attractive destination for both national and international pharmaceutical and biotechnology companies looking to conduct clinical trials, preclinical studies, and drug development.
The Growing Clinical Research Landscape in Hyderabad
Over the past decade, Hyderabad has attracted global attention as a major hub for clinical research, driven by factors such as a skilled workforce, cost efficiency, and regulatory advancements. With its rich infrastructure, robust educational institutions, and proximity to top healthcare providers, the city has become a focal point for pharmaceutical companies seeking to accelerate the development of new treatments and therapies. This growth has been supported by favorable government policies and investments, which continue to drive the sector’s expansion.
Top Clinical Research Companies in Hyderabad
Syngene International
Syngene International is a leading Contract Research Organization (CRO) that offers integrated services to the pharmaceutical, biotechnology, and healthcare sectors. With a comprehensive suite of services including drug discovery, development, and manufacturing support, Syngene is at the forefront of clinical research in Hyderabad. The company works with global clients to streamline the drug development process, making it one of the city’s top CROs.
IQVIA
IQVIA provides innovative healthcare solutions and conducts clinical trials to ensure the safety and efficacy of new therapies. The company offers a range of services including clinical development, market access, and patient safety monitoring. With a strong presence in Hyderabad, IQVIA contributes significantly to India’s growing role in global clinical trials and healthcare data analytics.
GVK BIO
GVK BIO offers comprehensive drug discovery and development services and is a key player in India’s growing CRO sector. With its state-of-the-art facilities and expertise in areas like bioanalytical testing, clinical pharmacology, and toxicology, GVK BIO plays a crucial role in supporting pharmaceutical and biotech companies in the development of novel therapies.
Medpace
Medpace is a global, full-service clinical contract research organization that offers clinical trial services in Hyderabad for clinical and regulatory support. Medpace focuses on providing high-quality clinical trials and regulatory services to ensure that new therapies meet global standards. The company’s ability to navigate complex regulatory landscapes and support international studies has made it an important player in the field.
Factors Contributing to the Growth of Clinical Research in Hyderabad
Hyderabad’s rapid growth as a clinical research hub is due to several factors, including its highly educated workforce, large patient pool, and favorable business environment. The city is home to a large number of medical schools, research institutes, and training programs, producing a steady supply of highly qualified clinical research professionals. Additionally, the city’s large, diverse population provides an ideal environment for patient recruitment, which is critical for the success of clinical trials. Supportive government policies, such as tax incentives and investment in healthcare infrastructure, also contribute to Hyderabad’s growth as a clinical research destination.
Challenges in Clinical Research in Hyderabad
While Hyderabad’s clinical research industry is booming, there are challenges such as regulatory hurdles, recruitment difficulties, and maintaining the quality of clinical trials. India’s complex regulatory environment, while improving, can still be difficult to navigate, which sometimes leads to delays in trial approval or regulatory compliance. Additionally, recruiting patients for clinical trials can be time-consuming, and maintaining consistent trial quality across a large population can prove challenging.
Future Outlook for Clinical Research in Hyderabad
With technological advancements, partnerships with international organizations, and a strong focus on personalized medicine, the future of clinical research in Hyderabad looks promising. The city is increasingly adopting digital technologies such as electronic data capture (EDC), artificial intelligence (AI), and telemedicine to improve the efficiency and accuracy of clinical trials. Furthermore, Hyderabad’s collaboration with global pharmaceutical companies and research institutes positions it well for continued growth in clinical research. Personalized medicine, with a focus on genetic-based treatments, is also expected to drive innovation and shape the future of clinical trials.
Conclusion
Hyderabad continues to solidify its position as a global leader in clinical research, playing a crucial role in advancing medical science and improving global healthcare outcomes. The city’s dynamic CRO landscape, combined with its skilled workforce, strong infrastructure, and favorable regulatory environment, makes it an attractive destination for pharmaceutical and biotech companies looking to develop and test new therapies. As the industry evolves and technology continues to improve, Hyderabad will remain at the forefront of clinical research and innovation.
0 notes
Text
Biobanking: A Revolution in Healthcare and Research

Introduction
Biobanking has revolutionized medical research and healthcare by preserving biological samples for future studies and discoveries. As a critical component of modern biomedical research, biobanks play an essential role in understanding diseases, developing treatments, and improving healthcare outcomes. In this blog, we will explore what biobanking is, its types, significance, ethical considerations, and future trends.
What is Biobanking?
Biobanking refers to the process of collecting, storing, and managing biological specimens such as blood, tissues, and DNA for scientific research and clinical use. These biological materials are used by researchers and medical professionals to study various diseases, identify biomarkers, and develop innovative treatments.
Types of Biobanks
1. Population-Based Biobanks
These biobanks store biological samples from large populations to study genetic and environmental factors affecting health. They help researchers analyze patterns in diseases, such as cancer, cardiovascular conditions, and metabolic disorders, enabling more precise prevention strategies.
2. Disease-Oriented Biobanks
Disease-specific biobanks focus on collecting samples from patients with particular medical conditions for targeted research. For instance, cancer biobanks store tumor tissues to aid in studying cancer progression, drug resistance, and personalized treatment approaches.
3. Tissue and Blood Banks
These biobanks store tissues and blood samples for use in transplants, regenerative medicine, and clinical research. They support the development of therapies for conditions such as leukemia, immune disorders, and neurological diseases.
The Importance of Biobanking in Medical Research
Biobanks provide invaluable resources for understanding diseases, developing treatments, and advancing precision medicine. By preserving high-quality samples, researchers can identify genetic mutations, track disease progression, and test new drugs. Biobanking also facilitates large-scale studies that help scientists recognize disease patterns and risk factors, leading to improved diagnostics and therapies.
Furthermore, biobanks contribute to the growth of personalized medicine, where treatments are tailored to an individual’s genetic profile. This approach enhances treatment efficacy and reduces adverse effects, significantly improving patient care.
Ethical and Legal Considerations in Biobanking
As biobanking involves human samples, ethical concerns like consent, data privacy, and governance play a critical role in its operation. Participants must provide informed consent before donating their biological materials, ensuring they understand how their samples will be used.
Data security is another major concern, as biobanks store sensitive genetic information. Stringent regulations and encryption technologies are necessary to prevent data breaches and unauthorized access. Moreover, clear policies regarding sample ownership and secondary use must be established to maintain transparency and trust in biobanking research.
Challenges and Future Trends in Biobanking
Despite its benefits, biobanking faces several challenges, including sample preservation, funding limitations, and evolving regulatory requirements. Maintaining the integrity of biological samples requires advanced cryopreservation techniques and stable storage conditions, which can be costly.
However, emerging technologies are shaping the future of biobanking. Artificial intelligence (AI) and big data analytics are transforming sample management and research capabilities. AI-driven systems can analyze large datasets, identify patterns, and accelerate the discovery of new treatments. Additionally, blockchain technology is being explored to enhance data security and consent management in biobanking.
Moreover, global collaboration between biobanks is increasing, allowing for more extensive and diverse datasets. This international approach will strengthen disease research and improve healthcare solutions worldwide.
Conclusion
Biobanking is a cornerstone of modern healthcare and research, offering hope for better diagnostics, treatments, and scientific breakthroughs. By preserving biological samples, biobanks enable researchers to explore diseases at a molecular level, leading to advancements in precision medicine and patient care. As technology continues to evolve, the potential of biobanking will expand, contributing to a healthier and more informed future.
1 note
·
View note