Don't wanna be here? Send us removal request.
Text
Dosage and usage adjustment of everolimus for different cancers 2

There may be differences in the usage and dosage of Everolimus in different dosage forms and specifications. Please read the specific drug instructions for use, or follow the doctor's advice.
Subependymal giant cell astrocytoma associated with tuberous sclerosis complex
1 Recommended dose
The recommended starting dose is 4.5 mg/m2 once daily. It should be used under the guidance of a specialist physician experienced in the treatment of tuberous sclerosis and its associated subependymal giant cell astrocytoma.
The dose is individualized according to body surface area (BSA, m2). The calculation of body surface area adopts the Dubois formula, where the unit of body weight (W) is kilograms, and the unit of height (H) is centimeters BSA=(W0.425×H0.725)× 0.007184
For patients with severe hepatic impairment (Child-Pugh class C) or who require concomitant use of moderate CYP3A4 and/or PgP inhibitors, the recommended starting dose is 2.5 mg/m2 once daily. For patients requiring concomitant use of a strong CPY3A4 inducer, the recommended starting dose is 9 mg/m2 once daily. Please round up the calculated dose to the nearest strength of this product.
Follow-up dosing is guided by therapeutic drug monitoring. If necessary, the dose can be adjusted after a 2-week interval to achieve a trough concentration of 5-15ng/ml.
Treatment was continued until disease progression or unacceptable toxicity. The optimal duration of treatment is not known.
2 Therapeutic drug monitoring
All patients should be routinely monitored for everolimus through blood concentration. If possible, the same analytical methods and laboratories should be used for therapeutic drug monitoring during treatment.
Trough concentrations should be assessed approximately 2 weeks after initiation of therapy, after a dose change, after initiation or adjustment of a co-administered CYP3A4 and/or PgP inducer or inhibitor, or approximately 2 weeks after a change in liver function. After reaching a stable dose, trough levels should be monitored every 3-6 months for patients with altered body surface area and every 6-12 months for patients with stable body surface area during treatment.
Dosage is adjusted to achieve trough concentrations of 5-15 ng/ml.
If the trough concentration is below 5ng/ml, increase the daily dose by 2.5mg.
If the trough concentration is greater than 15ng/ml, reduce the daily dose by 2.5mg.
If a dose reduction is required in patients receiving the lowest available strength dose, it should be administered every other day.
3 Dose adjustment
(1) Treatment of adverse reactions
In the event of severe and/or intolerable adverse reactions, dose reduction and/or discontinuation of this product is required. Reduce the dose of this product by approximately 50%. If a dose reduction is required in patients receiving the lowest available strength dose, it should be administered every other day.
(2) Impaired renal function
No clinical studies of this product have been conducted in patients with reduced renal function. Impaired renal function is not expected to affect drug exposure, and no dose adjustment of everolimus is recommended in patients with impaired renal function.
(3) Impaired liver function
For patients with subependymal giant cell astrocytoma with severe hepatic impairment (Child-Pugh grade C), the starting dose of ezetimibe should be reduced by approximately 50%. For patients with subependymal giant cell astrocytoma with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, no adjustment to the recommended starting dose may be necessary, but subsequent dosing should be based on treatment Drug monitoring.
Minbiotech is an Everolimus manufacturer and Everolimus company, Minbiotech CAS:159351-69-6, Everolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol has been listed on the drug introduction
Advantages of Bevacizumab
0 notes
Text
Dosage and usage adjustment of everolimus for different cancers 1
Everolimus is mainly used clinically to prevent rejection after kidney transplantation and heart transplantation. Its mechanism of action mainly includes immunosuppressive effects, anti-tumor effects, anti-viral effects, and vascular protection effects. It is often used in combination with other immunosuppressants such as cyclosporine to reduce toxicity.
There may be differences in the usage and dosage of Everolimus in different dosage forms and specifications. Please read the specific drug instructions for use, or follow the doctor's advice.
Advanced Renal Cell Carcinoma and Advanced Pancreatic Neuroendocrine Tumor
1 Recommended dose
(1) This product is administered orally once a day, at the same time every day, with or without food.
(2) Take this tablet whole with a glass of water and should not be chewed or crushed. For patients who cannot swallow the tablet, put the tablet in a glass of water (about 30ml) and gently stir it until it is completely dissolved (about 7 minutes) before administration and take it immediately. Rinse the glass with the same volume of water and take the entire amount of the cleaning solution to ensure the full dose is taken.
(3) Treatment should be continued as long as there is a clinical benefit, or until intolerable toxicity occurs.

2 Dosage adjustment
When dealing with severe and/or intolerable adverse reactions, it may be necessary to temporarily reduce the dose and/or interrupt the treatment of this product. If a dose reduction is required, the recommended dose is approximately half of the previously administered dose. If the dose is reduced below the lowest available tablet size, dosing every other day should be considered. Table 1 summarizes the recommendations for dose reduction, interruption, or discontinuation of treatment in patients treated with this product in the event of adverse reactions, and recommendations for routine management are also made. Management should be guided by the benefit/risk assessment of the individual patient and the clinical judgment of the treating physician.
(1) Severity classification: grade 1 = mild symptoms; grade 2 = moderate symptoms; grade 4 = life-threatening symptoms. If a dose reduction is required, the recommended dose is approximately 50% of the previously given dose.
(2) Daily Life (ADL): Avoid products containing hydrogen peroxide, iodine, and thyme derivatives when dealing with stomatitis, as these ingredients may worsen canker sores.
(3) Impaired renal function: no clinical study of this product has been conducted in patients with reduced renal function. Impaired renal function is not expected to affect drug exposure, and no dose adjustment of everolimus is recommended in patients with impaired renal function.
(4) Impaired liver function: Impaired liver function will increase the exposure of everolimus. Dosing adjustments are made as follows:
Mild hepatic impairment (Child-Pugh A): The recommended dose is 7.5 mg/day; if not well tolerated, the dose can be reduced to 5 mg/day.
Moderate hepatic impairment (Child-Pugh class B): The recommended dose is 5 mg/day; if not well tolerated, the dose can be reduced to 2.5 mg/day.
Severe hepatic impairment (Child-Pugh C): If the expected benefit outweighs the risk, 2.5 mg once daily may be used, but this dose should not be exceeded.
(5) During treatment, if the patient's liver function (Child-Pugh classification) status changes, the dose should be adjusted.
Minbiotech is an Everolimus manufacturer and Everolimus company, Minbiotech CAS:159351-69-6, Everolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol has been listed on the drug introduction
Advantages of Bevacizumab
0 notes
Text
Some precautions should be taken while using adalimumab 2

Before adalimumab treatment, patients must be closely monitored for infection and tuberculosis, and treatment can only be started after the infection is under control. In the event of a new serious infection or reactivation of hepatitis B in a patient during treatment, treatment with this product should be interrupted until the infection is controlled.
1. Tuberculosis
There have been reports of tuberculosis in patients treated with this product, and it is worth noting that in the vast majority of reports, the tuberculosis present was of the extrapulmonary type, i.e. disseminated.
All patients must be evaluated for active and inactive (latent) tuberculosis infection prior to initiation of therapy with this product. This assessment should include the patient's detailed TB history, as well as previous contact with patients with active TB, and/or current immunosuppressive therapy. Appropriate screening tests, namely the tuberculin skin test and chest X-ray (which should be in accordance with local prevention and treatment guidelines), must be performed on all patients. It is also recommended that test results be recorded in the patient's medical history. Prescribing physicians should consider the possibility of a false-negative tuberculin skin test, especially in patients with severe medical conditions or on immunosuppressive agents.
Treatment with this product is contraindicated in patients diagnosed with active tuberculosis.
If latent tuberculosis infection is suspected, a physician with experience in tuberculosis treatment must be consulted. Under the conditions described below, physicians must carefully weigh the benefits and risks of treatment.
If inactive (latent) tuberculosis is diagnosed, appropriate tuberculosis prophylaxis must be administered for latent tuberculosis according to local treatment measures before treatment with this product.
For those patients with several or significant risk factors for TB who screen negative for latent TB, anti-TB therapy should be considered prior to treatment with this product.
For those patients with a history of latent and active tuberculosis infection who are uncertain of an adequate course of treatment, appropriate anti-tuberculosis therapy should be considered prior to treatment with this product. Because during the treatment of this product, some patients with latent and active tuberculosis who have received treatment have re-converted to active tuberculosis.
Patients should be advised to seek immediate medical attention if they develop signs/symptoms of tuberculosis infection (eg, persistent cough, wasting constitution/weight loss, low-grade fever) during or after treatment with this product.
2. Other opportunistic infections
Opportunistic infections, including invasive fungal infections, have been observed in patients receiving this product. Delays in appropriate treatment because such infections are not recognized in patients previously treated with TNF-antagonists can have fatal consequences.
Invasive fungal infection should be suspected in patients presenting with signs or symptoms of fever, malaise, weight loss, sweating, cough, dyspnea, and/or pulmonary infiltrates or another severe systemic disease (with or without accompanying shock), and stop using this product immediately. These patients should be diagnosed and antifungal therapy is administered in consultation with a physician experienced in the diagnosis and management of invasive fungal infections.
Minbiotech is a adalimumab manufacturer and adalimumab company, Minbiotech CAS:331731-18-1, adalimumab supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol has been listed on the drug introduction
Advantages of Bevacizumab
0 notes
Text
Some precautions should be taken while using adalimumab 1

Before adalimumab treatment, patients must be closely monitored for infection and tuberculosis, and treatment can only be started after the infection is under control. In the event of a new serious infection or reactivation of hepatitis B in a patient during treatment, adalimumab treatment with this product should be interrupted until the infection is controlled.
It should be used with caution in patients with a history of recurrent infection or conditions predisposing to infection, central nervous system demyelinating disorders, malignant disease, and mild heart failure. Patients with hematological abnormalities, symptoms of a lupus syndrome, and positive double-stranded DNA antibodies during treatment should be discontinued immediately. This product is not recommended for children or pregnant or breastfeeding women. Women of childbearing age should use contraception during the medication period to at least 5 months after the end of treatment. Breastfeeding women should not breastfeed.
1. Infection
Serious infections were more likely to occur in patients on TNF antagonists. Impaired lung function may increase the risk of infection.
Patients must be closely monitored for infections, including tuberculosis, before, during, and after the use of this product. Since clearance of adalimumab can be as long as 4 months, monitoring should be continued during this period.
Neither chronic active nor focal active infection should be initiated until the infection is under control. Treatment should be initiated before initiation of therapy in patients with a history of tuberculosis exposure and patients traveling in areas at high risk for tuberculosis or endemic mycosis (eg, histoplasmosis, coccidioidomycosis, or blastomycosis). risks and benefits are assessed. Patients who develop an infection during treatment should be closely monitored and undergo a comprehensive diagnostic evaluation. When a patient develops a new serious infection or sepsis, treatment with this product should be interrupted and an appropriate antibacterial or antifungal drug should be administered until the infection is controlled. Physicians should exercise caution when considering the use of this product in patients with a history of recurrent infections, or conditions that predispose them to infection, including those on immunosuppressive agents.
2. Serious infection
Data from clinical studies suggest that treatment with this product increases the risk of serious infections, including sepsis or other opportunistic infections caused by bacteria, mycobacteria, invasive fungi, parasites, and viruses, such as Listeria and Pneumocystis.
Other serious infections have been identified in clinical trials, including pneumonia, pyelonephritis, septic arthritis, and sepsis. There have also been reports of hospitalizations or fatalities resulting from the infection.
Minbiotech is a adalimumab manufacturer and adalimumab company, Minbiotech CAS:331731-18-1, adalimumab supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol has been listed on the drug introduction
Advantages of Bevacizumab
0 notes
Text
What is adalimumab? What conditions is adalimumab suitable for?

Adalimumab (Humira) is a self-injectable biotherapeutic drug. It has been approved by the State Food and Drug Administration (CFDA) for two indications, namely rheumatoid arthritis and ankylosing spine. inflammation.
In 2013, through the study of adverse events in clinical trials of adalimumab for various indications in the past 12 years, no new adverse reaction safety signals beyond previous experience were found. In adalimumab-treated patients, the overall malignancy incidence was as expected in the general population. This drug is usually used alone or in combination with methotrexate.
Adalimumab has been approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, juvenile idiopathic arthritis, and Crohn's disease in the United States and the European Union. To a large extent relieve the patient's symptoms. The launch of adalimumab in China brings a new dawn to the treatment of rheumatoid arthritis patients in China and opens a new era of rheumatoid immune disease treatment.
Rheumatoid Arthritis
Rheumatoid Arthritis (RA) is a chronic autoimmune disease characterized by symmetry, multiple joints, and small joints. It is known as "immortal cancer". The immune system of the patient will destroy healthy joints and cause joint damage. Pain, swelling, stiffness, symptoms of fatigue and weakness, late stiffness and deformity, severely impaired function, and ultimately loss of joint function.
Humira in combination with methotrexate for the treatment of Adult patients with moderately to severely active rheumatoid arthritis who have not responded well to disease-modifying antirheumatic drugs (DMARDs), including methotrexate. The combination of Humira and methotrexate slowed the progression of joint damage (shown on X-rays) and improved physical function. Compared with traditional drugs, these drugs have strong and long-lasting efficacy, can effectively relieve disease symptoms, imaging progress, and functional level, and have good safety, and are suitable for various rheumatoid arthritis patients.
Ankylosing spondylitis
Ankylosing spondylitis (AS) is a disease characterized by inflammation of the sacroiliac joints and spinal attachment points. Strong association with HLA-B27. Certain microorganisms, such as Klebsiella, share antigens with susceptible individuals' own tissues, which can trigger abnormal immune responses. Ankylosing spondylitis is a chronic inflammatory disease characterized by fibrosis and ossification of the large joints of the limbs, as well as the annulus fibrosus of the intervertebral disc and its adjacent connective tissue, as well as joint stiffness.
Humira is used in adult patients with severe active ankylosing spondylitis who have not responded well to conventional therapy. It has the characteristics of fast onset and good curative effect. The condition of most patients can be rapidly and significantly improved, such as morning stiffness, low back pain, peripheral arthritis, tendonitis, chest expansion, ESR and CRP, etc. After a period of application, the patient's physical function and health-related quality of life Significant improvement, in particular, can restore some newly emerged spinal mobility dysfunction.
Minbiotech is a adalimumabb manufacturer and adalimumabb factory, Minbiotech CAS:331731-18-1, adalimumabb supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol properties, uses and production process Maytansinol is the common pa
Specific contraindications for the use of Simorox tablets
0 notes
Text
What is paclitaxel? How to find out? Where does it come from?
Plitidepsin, alias paclitaxel, has been widely used in the clinical treatment of breast cancer, ovarian cancer, and some head and neck cancers and lung cancers, and is the best-known natural anticancer drug.
As a diterpene alkaloid compound with anticancer activity, paclitaxel has been favored by botanists and chemists due to its novel and complex chemical structure, extensive and significant biological activities, new and unique mechanism of action, and lack of natural resources, pharmacologists and molecular biologists making it a worldwide anti-cancer star and research focus in the second half of the 20th century.

Discovery
In 1963, American chemists M.C.Wani and MonreE. Wall first isolated the crude extract of paclitaxel from the bark and wood of a species of Pacific Yew growing in the great forests of the western United States.
In the screening experiment, Wani and Wall found that the crude extract of paclitaxel had high activity on mouse tumor cells cultured in vitro, and began to isolate this active ingredient. Because the active ingredient is found at very low levels in plants, it was not until 1971 that they worked with Duke University chemistry professor Andre T. McPhail to determine the active ingredient's level by X-ray analysis. Chemical structure, a tricyclic diterpenoid, and named it taxol.
Source
Paclitaxel is a natural secondary metabolite isolated and purified from the bark of the gymnosperm Taxus Chinensis. It has been clinically proven to have a good anti-tumor effect, especially for ovarian cancer, uterine cancer, and breast cancer with a high incidence of cancer. There are special effects. Paclitaxel is the most popular anticancer drug on the international market in recent years and is considered to be one of the most effective anticancer drugs for mankind in the next 20 years.
The paclitaxel required for clinical and scientific research is mainly directly extracted from Taxus Chinensis. The content of Taxol in the plant body is quite low. The bark of Taxus Brevis, which is recognized as having the highest content, is only 0.069%, and about 13.6kg of bark can be used. It is proposed that 1g of paclitaxel will require 3-12 yew trees over 100 years old to treat an ovarian cancer patient.

As a result, the yew has been cut down in large numbers, and this precious tree species are on the verge of extinction. Coupled with the scarcity of yew and the slow growth of yew plants, it is very difficult for the further development and utilization of paclitaxel.
Although chemical synthesis has been completed, it has no industrial significance due to strict requirements, low yield, and high cost. Now the semi-synthetic method of paclitaxel is relatively mature, and it is considered to be an effective way to expand the source of paclitaxel besides artificial cultivation.
The semi-synthetic method can maximize the use of plant resources, but it is not fundamentally different from the method of directly extracting paclitaxel. It needs to consume a lot of yew trees, and still cannot fundamentally solve the problem of lack of plant sources. Obviously, the extraction of paclitaxel from Taxus Chinensis plant tissue is extremely limited, and it is of great significance to find a new way to obtain paclitaxel.
Minbiotech is a plitidepsin manufacturer and plitidepsin factory, Minbiotech CAS: 137219-37-5, plitidepsin supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Maytansinol has been listed on the drug introduction
Advantages of Bevacizumab
1 note
·
View note
Text
Overview, precautions, and safe use of atezolizumab
Atezolizumab is a monoclonal antibody that binds to PD-L1 and blocks its interaction with the PD-1 receptor. By inhibiting PD-L1, it can activate T cells Destroy tumor cells.
Efficacy
1. This product is suitable for the treatment of locally advanced or metastatic urothelial bladder cancer.
2. Patients whose disease is still progressing during or after treatment with platinum-based chemotherapy regimens.
3. Patients whose disease progresses within 12 months of receiving platinum-containing adjuvant therapy.
Dosage
1. There may be differences in the usage and dosage of this product in different dosage forms and specifications. Please read the specific drug instructions for use, or follow the doctor's advice.
2. The recommended dose of this product is 1200 mg, diluted to 250 ml of 0.9% sodium chloride injection, intravenous drip for more than 60 minutes, and administered once every 3 weeks until the disease deteriorates or unacceptable toxicity occurs.

Precautions
1. Carefully check the drug solution for particles and discoloration before taking the medicine. If there is turbidity, discoloration, or particles, it should not be used.
2. This product must be diluted before use. When configuring, gently invert it to mix evenly. Do not shake it.
3. This product can only be diluted with a 9% sodium chloride injection.
4. Please use this product immediately after dilution. From the start of dilution to the completion of instillation, this product can be stored for a maximum of 6 hours at room temperature and 24 hours at a maximum of 24 hours.
Contraindications
1. According to its mechanism of action, this product may be harmful to the fetus, and animal studies have shown that inhibition of the PDL/PD1 pathway may lead to an increased risk of immune-related rejection in the developing fetus, resulting in fetal death. Women of childbearing age...
2. It is not clear whether this product is excreted in breast milk because human IgG can be excreted in breast milk. Considering that this product may cause serious adverse reactions in breastfed infants, it is recommended that breastfeeding women be treated during treatment and after the last dose. ...
3. The safety and efficacy of the drug for children have not been established.
4. There is no safety data on the use of this product in patients with moderate or severe hepatic insufficiency.
Adverse reactions
1. Serious adverse reactions include immune-related pneumonia, immune-related hepatitis, immune-related colitis, immune-related endocrine diseases, other immune-related diseases, infections, and infusion reactions.
2. Adverse reactions found in clinical trials include nausea, abdominal pain, constipation, abdominal pain, diarrhea, fatigue, fever, peripheral edema, urinary tract infection, decreased appetite, low back pain, arthralgia, hematuria, dyspnea, cough, rash...
3. Common laboratory abnormalities include lymphocyte reduction, hyponatremia, anemia, elevated ALP, elevated creatinine, elevated ALT and AST, and hypoalbuminemia.
Minbiotech is a atezolizumab manufacturer and atezolizumab factory, Minbiotech CAS:1380723-44-3 sale, atezolizumab supplier,Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Specific contraindications for the use of Simorox tablets
Maytansinol properties use and production process Maytansinol is the common pa
0 notes
Text
Aureobasidin A specific instructions for use
Aureobasidin A (AbA) is a cyclic lipopeptide antibiotic isolated from the filamentous fungus Aureobasidiumpullulans No. R106, which has a strong antifungal ability.
The mechanism of action of AbA is to inhibit the activity of the inositol phosphoryl ceramide (IPC) synthase encoded by the AUR1 gene (1,2) in yeast, interfering with sphingolipid synthesis, thereby killing the strain.
AbA can be toxic to yeast at low concentrations (0.1-0.5μg/mL). The AUR1 gene from Saccharomyces cerevisiae and the AURA gene from Aspergillus nidulans, both of which have homology, have been studied more for the genes encoding IPC synthase. By mutating the gene encoding AUR1-C, the strain can be resistant to AbA, which can be used as a reporter gene for protein interaction research.

AbA screening facilitates the growth and identification of positive clones because AbA kills sensitive yeast strains directly, rather than delaying strain growth. Compared to low stringency primary screening for nutritional reporter genes such as HIS3, AbA screening greatly eliminates background clones.
In practice, more clones will be obtained during primary screening with AbA, followed by high stringency secondary screening with 4 reporters (AUR1-C, HIS3, ADE2, and MEL1) to verify positive clones.
AbA is ideal for use as a drug selectable marker for screening positive clones; it is also an ideal reporter for yeast one/two-hybrid studies, simplifying yeast two-hybrid library screening. MEL1 and lacZ encode α-galactosidase and β-galactosidase, respectively, and can act on the corresponding substrates X-α-Gal and X-Gal to turn yeast plaques or yeast protein extracts blue.
Among them, α-galactosidase is an exocrine enzyme, which can be directly detected on the surface of yeast; β-galactosidase is an endocrine enzyme, which can only be detected after the yeast is broken. The method of displaying the interaction of two proteins in yeast cells with locus coeruleus not only has high sensitivity, but the depth of the locus coeruleus can also reflect the strength of the interaction between the two proteins.
Storage conditions: Store at 2-8°C, the validity period of the unopened package is 24 months (if frozen, it should be shaken and mixed well before use).
Instructions:
Two-hybrid screening applications
1. For self-activation detection. The bait transformation product was plated on AbA-containing deficient medium. The routine use concentration is 200ng/mL. If the inhibitory effect is too strong, the working concentration can be reduced to 125-150ng/mL; if the self-activation is too strong, the working concentration can be increased, but cannot exceed 1μg/mL.
2. For interactive screening. Adding the above concentration of AbA to the interaction screening medium, and coating the co-transformed or fusion product on the screening medium containing AbA can effectively reduce false positives.
Note: Due to the influence of transformation or fusion efficiency in the double hybrid, the transformation product or fusion product can be directly plated without dilution; if the selected colonies are shaken and then plated or plated, due to the excessive number of colonies per unit area, AbA inhibition effect will be greatly reduced.
One-hybrid screening application (pAbAi system)
Single hybrid principle: The bait sequence is cloned into the pAbAi vector to form pBait-AbAi, which is integrated into the Y1Hgold genome through homologous recombination. Basidin produces resistance to screen for interacting proteins.
Self-activation inhibition: Recombinant yeast will have low background AbAr expression, and the degree of expression of different bait is different. Generally, about 2000 cells will be screened with 100ng/mL, 150ng/mL, and 200ng/mL of aurabasidin (the OD600 value is diluted to 0.002, and 100μL of the plate is taken), and the growth of the colonies is inhibited by the gradient of aurabasidin, and the lowest The concentration of aurabasidin was used as the concentration for subsequent interaction screening.
Note: The effective concentration of aureobasidin is positively correlated with the unit concentration of yeast. If the concentration and volume of the bacterial solution are too large, the colonies on the plate will be connected into pieces, and aureobasidin cannot play an inhibitory effect. Under the premise that the concentration and volume of the bacterial solution are suitable, if the concentration of aureobasidin needs to exceed 1 μg/mL to be effectively inhibited, then the bait is not suitable for the interaction experiment of aurabasidin screening. During self-activation detection, it is recommended to dilute the bacterial solution to an OD value of ≈ 0.002 before coating the plate.
Minbiotech is an Aureobasidin A manufacturer and factory, Minbiotech CAS: 127785-64-2, Aureobasidin A supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Specific contraindications for the use of Simorox tablets
Maytansinol properties use and production process Maytansinol is the common pa
0 notes
Text
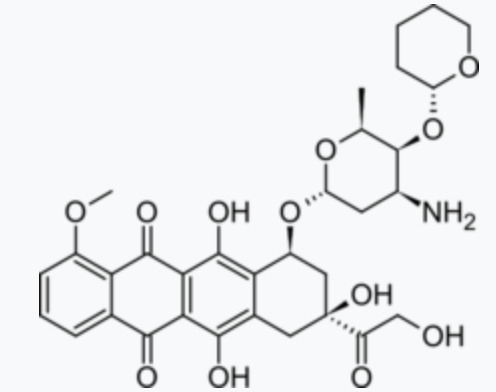
Dosage, adverse reactions, and contraindications of pirarubicin
Pirarubicinis an anthracycline cell cycle non-specific antineoplastic drug synthesized from doxorubicin and dihydropyran. It is suitable for the treatment of breast cancer. It is also effective in patients with drug resistance.
The mechanism of action of the drug is that it can directly intercalate between DNA double strands, inhibit DNA polymerase, prevent nucleic acid synthesis, and prevent cells from dividing in the G2 phase, resulting in tumor cell death.
Dosage
Dissolve this product in 10ml of 5% glucose injection or water for injection. Intravenous, arterial, and intravesical infusion.
Intravenous administration: generally 25-40 mg/m2 per body surface area. For breast cancer, the recommended combination is 40-50 mg/m2 each time. It is administered on the first day of each course of treatment and can be repeated at 21-day intervals according to the patient's blood picture. For acute leukemia, the adult dose is 25 mg/m2 once based on body surface area.
Arterial administration: For head and neck cancer, 7-20 mg/m2 per body surface area, once a day, for 5-7 days, or 14-25 mg/m2 each time, once a week.
Intravesical administration: for the prevention of postoperative recurrence of superficial bladder cancer. According to the body surface area, 15~30mg/m2 once, diluted to 500~1000μg/ml concentration, injected into the bladder cavity for 0.5h, once a week, 4~8 times in a row; then once a month, a total of 1 year.
Doctors can adjust the time, dosage, and frequency of administration according to the patient's condition.
Adverse reactions
1. Myelosuppression is dose-limiting toxicity, mainly neutropenia, with an average minimum value of 14 days and recovery on the 21st day, anemia and thrombocytopenia are rare;
2. Cardiotoxicity is lower than doxorubicin, acute cardiotoxicity is mainly reversible ECG changes, such as arrhythmia or non-specific ST-T abnormalities, and chronic cardiotoxicity is dose-accumulating. The incidence of acute and chronic cardiotoxicity of this product is about 1/7 and 1/4 of doxorubicin.
3. Hair loss: The overall incidence of hair loss with this product is about 40%, which is significantly lower than that of doxorubicin (80%); the incidence of severe hair loss is about 20%, which is significantly lower than that of doxorubicin (60%).
4. Gastrointestinal reactions: nausea, vomiting, loss of appetite, oral mucositis, and sometimes diarrhea;
5. Others: abnormal liver and kidney function, skin pigmentation, etc., occasional rash. The intravesical injection can cause bladder irritation symptoms such as frequent urination, dysuria, occasional hematuria, and rarely bladder atrophy.
Taboo
1. Patients with obvious bone marrow suppression due to chemotherapy or radiotherapy are contraindicated;
2. Patients with severe structural heart disease or abnormal cardiac function and those who are allergic to this product are prohibited;
3. Patients who have used high-dose anthracyclines (such as doxorubicin or daunorubicin) are contraindicated;
4. Women of pregnancy, lactation, and childbearing age are prohibited.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Where to buy sirolimus cheap
Introduction to Biosimilars
0 notes
Text
Instructions for use of sirolimus capsules and oral solution

Sirolimus Capsules:
This product should only be used by physicians experienced in immunosuppressive therapy and the management of kidney transplant patients. Patients receiving this drug should be treated in an institution equipped with appropriate laboratory and auxiliary medical facilities and personnel. Physicians responsible for maintenance therapy should have complete information necessary for patient follow-up. It is recommended that this product be used in combination with cyclostatin and corticosteroids. For other details, please refer to the manual.
Sirolimus oral solution:
1. It is recommended to use sirolimus oral solution in combination with cyclosporine and corticosteroids. A single loading dose of 6 mg on the first day, 2 mg/day within 2 weeks (the dose can be reduced if adverse reactions occur), and 1-2 mg/day after 2 weeks.
2. To minimize differences in the absorption of this medicine, this medicine should be taken constantly with or without food. Grapefruit juice slows the metabolism of sirolimus regulated by CYP3A4 and should not be used for oral administration or dilution of sirolimus oral solution.
3. It is recommended to take sirolimus 4 hours after taking cyclosporine oral solution (microemulsion) and/or cyclosporine capsule (microemulsion).
4. Dose adjustment:
(1) For patients over 13 years old but weighing less than 40kg, the starting dose should be adjusted by 1 mg/m2/day according to the body surface area, and the loading dose should be 3 mg/m2. It is recommended that the maintenance dose of this drug be reduced by about 1/3 in patients with hepatic impairment, but no loading dose adjustment is required. The pharmacokinetics of sirolimus has not been studied in patients with severe hepatic impairment.
(2) The dose for patients with renal impairment does not need to be adjusted.
5. Blood drug concentration monitoring:
most patients do not need routine monitoring of therapeutic drug levels. Sirolimus plasma levels should be monitored in the following patients: children, those with impaired hepatic function, those taking concurrent potent CYP3A4 inducers and inhibitors, and/or those with significantly reduced or discontinued cyclosporine doses. In a controlled clinical trial of concomitant cyclosporine administration, the mean trough whole blood sirolimus concentration measured by immunoassay was 9 ng/ml in the 2 mg/day group and 17 ng/ml in the 5 mg/day group. Results obtained with other assays may differ from those obtained with immunoassays.
6. Instructions for dilution and administration:
the prescribed amount of this drug oral solution should be drawn from the bottle using an oral applicator. Pour the exact amount of this medicine from the dispenser into a glass or plastic container filled with at least a quarter cup (about 60 mL) of water or orange juice (do not dilute with other liquids, especially grapefruit juice). Stir well and drink immediately. Take at least 1/2 cup of water or orange juice (about 120ml), add it to the same container to rinse, and drink all of it immediately.
7. Disposal and disposal:
Since this medicine is not absorbed through the skin, there are no special precautions. However, in case of accidental direct contact with skin or mucous membranes, wash thoroughly with soap and water; rinse eyes with clean water.
Common Specifications
(1) Sirolimus tablet: 1mg.
(2) Sirolimus capsule: 0.5mg.
(3) Sirolimus oral solution: 50ml: 50mg.
Storage method
Sirolimus Tablets, Sirolimus Capsules:
Shield from light, airtight, and store below 25°C.
Sirolimus oral solution:
1. The bottled oral solution should be stored in a refrigerator at 2-8°C away from light. Once the vial is opened, the medicine should be used up within 1 month. If necessary, the patient can store the vial at room temperature (up to 25°C) for short-term storage (eg several days, but not more than 30 days). The drug should be taken immediately after dilution.
2. The bottled sirolimus oral solution may be slightly cloudy when refrigerated. If turbidity appears, place this product at room temperature and shake gently until the turbidity disappears. The appearance of turbidity does not affect the quality of the product.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Advantages of Anti-HBV
Dalbavancin manufacturer-Minbiotch
0 notes
Text
Specific contraindications for the use of Simorox tablets
1. Sirolimus is only for use by physicians experienced in immunosuppressive therapy and the management of kidney transplant patients. Patients receiving this drug should be treated in an institution equipped with appropriate laboratory and auxiliary medical facilities and personnel. Physicians responsible for maintenance therapy should have complete information necessary for patient follow-up.
2. It is recommended to use sirolimus in combination with cyclosporine and corticosteroids.
3. Sirolimus is taken orally, once a day, with or without food.
4. The bioavailability of crushed, chewed, or cut tablets has not been established and such use is not recommended. Oral solutions should be prescribed and instructed to use in patients who cannot take tablets.
5. Sirolimus should be started as soon as possible after transplantation. It is recommended that sirolimus should be taken 4 hours after cyclosporine oral solution (modified) and/or cyclosporine capsules (modified) [cyclosporine microemulsion (modified)] (see [Drug Interactions]). ]).
6. Frequent adjustment of the sirolimus dose based on unstable sirolimus plasma concentrations may lead to overdose or underdose because of the longer half-life of sirolimus. Once the maintenance dose of sirolimus is adjusted, patients should continue to take the new maintenance dose for at least 7-14 days before further dose adjustments are made under blood level monitoring.

In most patients, dose adjustments can be calculated based on a simple ratio: new sirolimus dose = current dose × (target plasma concentration/current plasma concentration).
When the trough concentration of sirolimus needs to be greatly increased, a loading dose can be considered on the basis of the new maintenance dose: sirolimus loading dose = 3 × (new maintenance dose - current maintenance dose).
The maximum administered dose of sirolimus should not exceed 40 mg/day. If the estimated daily dose of sirolimus exceeds 40 mg due to an additional loading dose, the loading dose may be administered over two days. Sirolimus trough levels should be monitored for at least 3-4 days after taking a loading dose.
7. The 2 mg sirolimus oral solution has been proven to be clinically equivalent to 2 mg sirolimus tablets, and therefore, can be interchanged in equal amounts. However, the clinical equivalence of larger doses of sirolimus oral solution to larger doses of tablets is unknown (see Pharmacokinetics).
8. To minimize differences in the absorption of sirolimus, this drug should be taken consistently with or without food. Grapefruit juice slows CYP3A4-mediated metabolism of sirolimus and potentially enhances P-glycoprotein (P-gp)-mediated reverse translocation of sirolimus from intestinal epithelial cells to the lumen and is therefore not available In the delivery of sirolimus.
9. Combination of sirolimus and cyclosporine in patients with low to moderate immune risk: For new kidney transplant recipients, the combination of sirolimus with cyclosporine and corticosteroids is recommended. The loading dose of sirolimus should be taken for the first time, that is, 3 times the maintenance dose. The recommended loading dose for kidney transplant patients is 6 mg and the maintenance dose is 2 mg/day. To maintain sirolimus plasma levels within the target range, sirolimus plasma levels should be monitored. Although a loading dose of 15 mg and a maintenance dose of 5 mg/day have been used in clinical trials to be safe and effective, the efficacy of doses above 2 mg in renal transplant patients is unclear. The overall safety profile of patients taking sirolimus oral solution 2 mg daily was better than that of patients taking sirolimus oral solution 5 mg daily.
10. Dosage adjustment
(1) Low body weight patients:
The starting dose for patients aged 13 years and above but weighing less than 40kg should be adjusted to 1 mg/m2/day based on body surface area. The loading dose should be adjusted to 3 mg/m2.
(2) Patients with hepatic impairment:
It is suggested that the maintenance dose of sirolimus in patients with hepatic impairment can be reduced by about 1/3 to 1/2. The loading dose of sirolimus does not require adjustment: (see Pharmacokinetics). In patients with hepatic impairment, monitoring of trough sirolimus concentrations is recommended.
(3) Patients with impaired renal function:
No need to adjust the loading dose of sirolimus. No dose adjustment is required for renal impairment.
(4) Pediatric use:
The safety and efficacy of sirolimus in children under 13 years of age have not been established. The safety and efficacy of sirolimus have been studied in children 13 years of age and older with low to moderate immune risk. The use of sirolimus in this population of children 13 years of age and older is supported by adequate, well-controlled clinical trials of adult sirolimus oral solution. In particular, pharmacokinetic data in pediatric kidney transplant patients were analyzed in these trials (see Pharmacokinetics).
(5) Medication in elderly patients:
No dose adjustment is required.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier, Welcome to visit our website.
0 notes
Text
The development process, efficacy, usage, and dosage of sirolimus

Sirolimus, also known as rapamycin, is a macrolide antibiotic immunosuppressant for medical use.
R & D process
In 1975, Vezina et al. of Ayerst Laboratory in Canada isolated Streptomyces hygroscopicus, which can produce sirolimus, from soil samples of Easler Island in the Pacific Ocean.
In 1977, sirolimus was found to have immunosuppressive effects, and in 1989, sirolimus was tried as a new drug for the treatment of organ transplant rejection. After phase III clinical trials, the oral solution of sirolimus developed by Wyeth Pharmaceuticals was first marketed in the United States in October 1999, and the FDA allowed it to be used as a relatively safe drug in clinical prevention and treatment of renal transplant rejection. Since then, 1mg tablets have also been marketed in the United States, and have been approved to be used in combination with cyclosporine and steroids for anti-rejection in kidney transplant patients.
Afterward, the US FDA approved the new label of Wyeth's immunosuppressant Rapamune (sirolimus, sirolimus) (I) for maintenance use in the prevention of renal transplant rejection after ciclosporin is withdrawn in patients using cocktail therapy. . This new indication allows cyclosporine to be withdrawn 2 to 4 months after kidney transplantation in patients with low-to-moderate immune risk. calcineurin inhibitor
The mechanism of sirolimus as an immunosuppressant is that it can block the late reaction (proliferation) of T lymphocyte activation, inhibiting the entry of cells from the G1 phase into the S phase, and blocking the interaction between interleukin-2 (IL-2) and its receptors. Combined, Tc and Td cells cannot become sensitized T lymphocytes with immune response, and play their immune role.
A new study found that sirolimus can effectively reduce the incidence of skin cancer after kidney transplantation. Patients who received sirolimus had a higher risk of developing new squamous cell carcinoma than those who received cyclosporine or tacrolimus, according to Sylvie Euvrard, MD, of the Hospices Civils de Lyon in Lyon, France, and colleagues halved (RR 0.56, 95% CI 0.32 to 0.98). "We speculate that there may be a specific antitumor activity of sirolimus that could explain the reduction in new skin cancers, rather than the reduction in immunosuppression," they wrote. Possible role of sirolimus The mechanism is the inhibition of angiogenesis and cell proliferation, which also belongs to the antiviral effect.
Efficacy
This product is used to prevent organ rejection in patients 13 years of age or older who have received kidney transplants. Sirolimus is recommended in combination with cyclosporine and corticosteroids. Therapeutic drug plasma concentration monitoring is recommended for all patients receiving sirolimus.
Dosage
There may be differences in the usage and dosage of this product in different dosage forms and specifications. Please read the specific drug instructions for use, or follow the doctor's advice.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier. Welcome to visit our website.
0 notes




