Don't wanna be here? Send us removal request.
Text
design snap-fit joints for 3D printing
Snap-fit joints are a fast and also easy means to attach 2 3D-printed components by utilizing interlacing attributes. Not only are they a affordable as well as time-saving link method, but they can additionally decrease the number of parts required in an assembly. Plus, they use the possibility of quick assembly as well as disassembly. This article covers the basics of snap-fit joints (additionally called clips as well as ports) for 3D printing, what to consider when developing snap-fits with plastics and thermoplastics and also which 3D printing process is optimal for producing the very best snap-fits.

Does have a snap-fit tutorial? Prior to (or after) diving into this comprehensive guide, take a look at this cool tutorial on developing snap-fit joints for 3D printing.

What are snap-fit joints (or connectors/clips)? A snap-fit joint is a fairly easy and affordable approach of attaching 2 3d-printed plastic elements. Known as an adapter or clip, a snap-fit normally is composed of a flexible and small projection, like a stud, grain, or hook , as well as a mating clinical depression that disperses and catches the sticking out function. Both functions clicking into place create a durable interlocking link. As soon as the attributes have actually clicked right into area, an undercut holds the two parts of the snap-fit with each other. Depending on the shape of this indispensable undercut, snap-fit assemblies can also be designed to make the interlocking link irreversible. A well-designed snap-fit joint with the right material can be made use of quite a variety of times with no recognizable mechanical exhaustion. There are a lot of sorts of snap-fit types and geometries that comply with the fundamental principles of two interlacing parts. Common kinds of snap-fits: cantilever as well as annular joints Both most commonly utilized as well as commonly reliable types of snap-fit adapters are cantilever and also annular joints Allow's damage these down. Cantilever snap-fit joints. One of the most common snap-fit joint is the cantilever, containing an outcropping (some type of grain or hook) on one end of the component and also an architectural assistance function at the other end. This projection is placed into an opening and also bends back to lock the link into location. Cantilever snap-fits are very easy to design as well as intuitive when it involves setting up as well as disassembly. For lots of applications and cases, cantilevers are one of the most cost-efficient way to link to components. Annular snap-fit joints Annular snap-fit joints use a hoop strain to hold a pushed component in position. Common examples of annular snap-fits are bottle and pen caps. With annular snap-fits, it's possible to create a water resistant seal around the joint. What are the advantages of snap-fitting with 3D printing? While injection molding is usually seen as the more robust way to generate snap-fit joints, 3D printing is a practical alternative (and even a go-to) with the right style as well as materials. 3D-printed snap-fits do not have any of the style limitations related to shot molding-- as an example, draft angles, separation lines, wall thickness and damages-- as well as can be designed and altered effortlessly. This makes them excellent for fast prototyping, where clearance and also fit are critical. This is why designers normally utilize 3D printing for snap-fits in enclosures. What materials are made use of for 3D printing snap-fit joints? Every 3D printing process has its pluses as well as minuses when it pertains to producing get rid of snap-fit connectors. This is due partially to the products used with each of the major additive manufacturing innovations. FDM is one of the most inexpensive way to manufacture snap-fit connectors. While it's absolutely effective, the process has lower accuracy than various other printing methods. If you pick FDM, we recommend using strain-resistant products, such as TPU, nylon as well as aBDOMINAL. SLA resins are likewise a practical alternative for making snap-fit joints, yet they are fairly brittle. Utilizing resins might enhance the possibilities of the snap-fit breaking after duplicated usage. If you print snap-fits with this technology, we suggest long lasting SLA resin. SLS is better than FDM and also shanty town for printing useful snap-fit models and end-use components that will be opened as well as shut often times. The very best material for maximum tear resistance is SLS Nylon. Comparable to SLS, MJF is optimal for making snap-fit adapters. We advise a minimum thickness of 1 mm at the base of the cantilever and a minimum typical overhang deepness of at the very least 1 mm. Snap-fit joints are a quick and also simple way to attach two 3D-printed components by making use of interlacing attributes. A snap-fit joint is a relatively simple and cost-effective technique of affixing two 3d-printed plastic components. A properly designed snap-fit joint with the appropriate material can be used quite a number of times without any type of obvious mechanical exhaustion. Annular snap-fit joints utilize a hoop stress to hold a pushed part in location. With annular snap-fits, it's possible to produce a water resistant seal around the joint.
0 notes
Text
YB1 layout multi-pipeline research and development of innovative antithrombotic drugs
Cardiovascular and cerebrovascular diseases are becoming the biggest disease burden in China. In recent years, sudden death events caused by acute myocardial infarction and cerebral infarction occur frequently, which makes the treatment status of these diseases become the focus of people's attention.
The emergency department of the hospital is almost daily witness the most "life and death speed" place, because of the emergency attack was sent to the hospital in an emergency at the same time, medical experts are also going all out through a variety of advanced treatment means and race against time, hoping to save the lives of more emergency patients. Thrombolysis is an effective treatment method for acute myocardial infarction, cerebral infarction and other cardiovascular diseases based on thrombosis, which is increasingly being used in clinical treatment.
How does thrombolytic therapy race against time and death?
A few days ago, the media reported a news about the ruichang Hospital of Traditional Chinese Medicine using intravenous thrombolytic therapy to successfully treat a patient with acute cerebral infarction: at 14:30 on August 26, this year, a 77-year-old patient Surnamed Shi was sent to the hospital by 120 emergency vehicles because of language barrier, paralysis of the right lower limb for 1 hour. On the way to the hospital, the ambulance staff informed the hospital stroke team of the patient's condition. When the patient arrived at the emergency department, the stroke team had been ready to open a green channel for the patient, through blood routine, coagulation function and head CT examination, diagnosis of acute cerebral infarction.
Due to the patient's critical condition and no contraindications of thrombolytic therapy within the intravenous thrombolytic time window, the stroke team performed intravenous thrombolytic therapy for the patient at 14:55, and the patient turned out to be safe after one-hour treatment.
The news gives us a sense of how thrombolytic therapy is a race against time and death.
A few days later, a similar tense situation played out in Chenzhou No. 1 People's Hospital: At 18:30 on September 5, 66-year-old Lin suddenly suffered from stroke symptoms such as slurred speech and weakness of the right upper limb. His family immediately sent him to the Hospital.
NIHSS score of old forest body 3 points -- NIHSS score is the Abbreviation of neurological function deficit score, which is mainly used to evaluate the neurological function of patients with cerebral infarction. Individualized treatment can be selected, prognosis can be predicted and health education can be carried out by scoring. The green channel for stroke was immediately activated in the emergency department of the hospital, and thrombolytic therapy was started in only 10 minutes under the close cooperation of the stroke emergency team. During the thrombolytic process, Lao Lin's symptoms gradually improved. 24 hours after hospitalization, when the neurologist made ward rounds, his NIHSS score had dropped to 0, that is, he had no symptoms.
As we all know, every second counts in stroke rescue. The earlier intravenous thrombolysis or mechanical thrombolysis is implemented in patients with cerebral infarction, the better the efficacy. DNT time refers to the time from admission to intravenous thrombolytic therapy. Lao Lin's DNT was only 10 minutes, refreshing the record of successful treatment of acute stroke patients in the hospital.
On the same day, the People's Hospital of Mabian Yi Autonomous County in Leshan city, Sichuan Province also successfully implemented its first thrombolytic therapy for acute myocardial infarction, successfully saving the life of a patient with acute myocardial infarction. Acute myocardial infarction, as a representative cardiovascular disease, is also known as the "number one killer" of human health. It has the characteristics of acute onset, rapid change of condition and high mortality. Every treatment is a tug of war with death.
Learned, intravenous thrombolysis treatment in patients with acute myocardial infarction can quickly will have blocked coronary arteries to dredge, for myocardial perfusion again, can make the heart back to the state of survival, and won't cause heart lost vitality, and can reduce the necrosis area, thereby reducing the mortality rate, and within 3 h of stroke onset thrombolysis therapy and PCI therapy effect is quite good.
Thrombosis is the pathological basis of major cardiovascular diseases
In these cases, thrombolytic therapy for acute myocardial infarction, cerebral infarction patients can be called "life-saving symbol" existence. "Thrombolytic therapy" is the use of thrombolytic drugs to dissolve the formation of thrombosis treatment. Thrombosis is a small piece formed by blood flow on the surface of peeling off or repairing the inner surface of blood vessels in the cardiovascular system. Thrombosis and diseases caused by thromboembolism are called thrombotic diseases. Thrombosis is the common pathological basis of the three major cardiovascular diseases (myocardial infarction, stroke and venous thromboembolism) in the world.
Cardiovascular disease has always been one of the most common diseases in China. Currently, cardiovascular disease is the third largest treatment area in China, with huge unmet medical needs and the highest disease burden, with a market size of 212 billion yuan in 2019.
Public information shows that in 2019, there were more than 290 million patients with cardiovascular diseases in China, including 13 million patients with stroke and more than 11 million patients with coronary heart disease. Cardiovascular death, stroke and heart attack are the leading causes of death and disability in China. Statistics show that in 2019, more than 4.5 million patients died of cardiovascular disease in China, with a mortality rate of 2.87%, compared with 1.6% for cancer in China and 2% for cardiovascular disease in the United States.
In 2019, the total hospitalization expenditure for cardiovascular diseases in China was about 70.5 billion yuan. In the same year, the disability-adjusted life-years (DYS) of stroke and ischemic heart disease, two major causes of cardiovascular diseases, reached 44.2 million yuan and 30.1 million yuan, representing a major disease burden in China.
In this context, the prevention and treatment of cardiovascular diseases has also become one of the major initiatives advocated by Healthy China 2030, which sets ambitious goals to significantly optimize the diagnosis, treatment and mortality rate of cardiovascular patients by 2030, including the plan to reduce the mortality rate of cardiovascular diseases to 1.9% by 2030.
Thrombolytic therapy becomes a "life-saving symbol" in the field of cardiovascular disease anti-thrombosis
Thrombosis, the formation of a local blood clot in which arterial thrombosis can lead to myocardial infarction, stroke, acute coronary syndrome and peripheral artery disease; Vein thrombosis can cause pulmonary embolism. Arteriovenous thrombosis is the leading cause of cardiovascular disease and death, and it is also one of the leading causes of death in cancer patients.
Medical studies show that the formation of vascular embolism is a changing process under the interaction of genetic factors and environmental factors. Vascular embolism disease has the complex characteristics of familial, recurrent and different recovery, so active prevention, reasonable intervention and treatment are of positive significance.
Antithrombotic treatment means are mainly based on the degree and location of venous, arterial, distal circulatory system and blood vessels involved, weighing the advantages and disadvantages, using antiplatelet, thrombolytic, anticoagulant treatment. Among the current antithrombotic drugs, antiplatelet aggregation drugs play an important role. After entering the 21st century, antithrombotic drugs at home and abroad have developed into a new era from r&d pipeline to market platform.
At present, a group of emerging participants in the research and development of new antithrombotic drugs have emerged in the market. As one of them, Hong Kong Pharmaceutical Oncolytic has stepped up the layout of multiple thrombolytic drug product pipelines, and the combination of the company's core technology product YB1 and a variety of thrombolytic drugs can treat a variety of thrombotic diseases.

Yb1-rt-pa is the company's first generation of site-directed thrombus ablation product, which is characterized by the rapid site-directed release of thrombolytic drugs through the delivery of YB1 and the release of Urokinase at the thrombus site. Urokinase is produced in the human body by the kidney and directly activates the conversion of fibrinolytic enzyme into fibrinolytic enzyme. Urokinase is the first natural thrombolytic drug extracted from urine in medicine.
In addition, we have also set up the product pipeline of YB1 combined with defibrase and plasminase respectively, which is currently in the development stage. Yb1-rt-pa has been advanced to the pre-clinical stage. The next step is expected to accelerate the completion of the pre-clinical study of this product and apply for IND, expecting to make more new progress and benefit patients around the world.

0 notes
Text
Innovative subdivision therapies emerged, including YB1 immunotherapy
Human anti-cancer has a long history. Global cancer treatment has experienced a long development process. In recent decades, the field of cancer treatment has developed rapidly, from classical surgery, radiotherapy and chemotherapy as the main treatment plan to advanced treatment methods represented by targeted therapy and more recently immunotherapy. Compared with traditional therapies, targeted therapies and immunotherapies benefit cancer patients worldwide in terms of improving efficacy, reducing symptoms or improving quality of life by targeting specific oncogenic pathways and tapping into the patient's immune system, respectively.
Tumor immunotherapy is the latest in a broad line of therapies that control or eliminate tumor cells by stimulating a patient's own immune system to produce or enhance an anti-tumor immune response. Over the past few years, cancer immunotherapy has arguably revolutionized the evolution of cancer treatment. In recent years, the discovery and development of cancer immunotherapy has become a milestone in the field of cancer treatment because of its ability to continuously alleviate the progression of cancer and its generally good tolerance in some patients with advanced cancer.
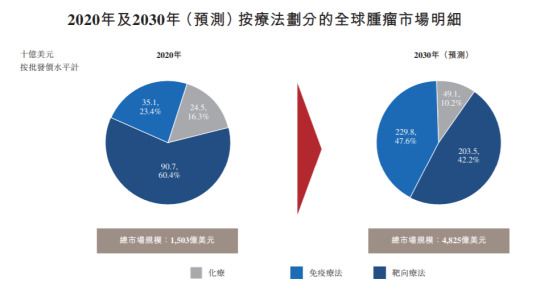
(Credit: Frost Sullivan)
As shown in the chart above, oncology targeted therapies have taken the largest share of the global oncology drug market in 2020, accounting for 60.4% of the total global market share by revenue, according to the report. In the next 10 years, the tumor immunotherapy market is expected to continue to explode, with its market share increasing from 23.4% in 2020 to 47.6%, surpassing targeted therapy to become the largest global market holder among all major therapies. Oncology targeted therapies and immunotherapies are expected to account for approximately 90% of the global oncology market by 2030.
Innovative subdivision therapies are emerging, and tumor immunotherapy is blossoming
While immunotherapy is becoming the mainstay of cancer treatment, new subsets of therapies are emerging that target different research directions, The main types include cellular immunotherapy, immune checkpoint inhibitors (such as PD-1 and PD-L1 inhibitors), cancer vaccines, cytokines and other cutting-edge and emerging immunotherapy products such as oncolytic virus therapy and oncolytic bacterial therapy.
With the emergence and rising trend of innovative therapies mentioned above, the global tumor immunotherapy market is gradually showing a trend of flowers blooming. At the same time, driven by the increasing demand, the market size of global cancer immunotherapy is also expanding and growing, and the industry prospects are attracting the attention of global capital.

(Credit: Frost Sullivan)
According to public information, the market size of cancer immunotherapy in China reached us $1.1 billion in 2019 and is expected to reach US $14.2 billion and US $32.9 billion in 2024 and 2030, respectively, with a CAGR of 67.9% from 2019 to 2024 and 15.1% from 2024 to 2030. The cancer immunotherapy market in the United States was $16.6 billion in 2019 and is expected to reach $49.5 billion and $82.8 billion in 2024 and 2030, respectively, with a CAGR of 24.4% from 2019 to 2024 and 9.0% from 2024 to 2030.
Globally, the market size of cancer immunotherapy was us $29 billion in 2019, and is expected to reach US $95.7 billion in 2024 and US $183.2 billion in 2030, with a cagR of 27.0% from 2019 to 2024. The compound annual growth rate from 2024 to 2030 is 11.4%.
Beware of "high-level duplication" in investment and R&D. Differentiation is the real way to survive
Along with the emergence of a number of innovative therapies, new drug research and development activities for cancer treatment are more intensive and frequent in various segments of the track -- policy guidance and capital enabling also pushed China's medical innovation to a climax, enabling the industry to rapidly enter the fast track of development.
According to official statistics of Drug Evaluation Center (CDE) of STATE Food and Drug Administration, a total of 9,768 applications were accepted in CDE in 2020, 60% of which were for anti-tumor drugs. The 777 clinical trials approved in 2020 covered 150 targets, including 133, 68, 46, 34 and 33 clinical trials on PD-1/PD-L1, VEGF, EGFR, FGFR and HER-2 targets, respectively. With the increasing number of clinical trials with the same target and indication, the speed of clinical trials has gradually slowed down, and the lack of subjects has become one of the factors hindering the innovation and development of new drugs.
In addition, from the point of view of the market, there have been relevant research reports in the industry, worldwide for the same target drug, the first product on the market can obtain 45% of the market, naturally hitting the largest piece of cake; The second to fourth products on the market can obtain 27.9%, 14% and 11.3% market share respectively, and the "gradient" of the share is also obvious. Other products launched later will collectively capture only 1.7 percent of the remaining market share, intensifying competition.
Due to the enthusiastic influx of global capital, more and more biotechnology companies seize the opportunity to continue to emerge, the field of tumor immunotherapy ushered in a large number of emerging participants, the industry track gradually began to show a "congestion" situation, in the investment and research and development of a careless will fall into a "high level of repetition" phenomenon. Under such a situation, xinxing biotechnology companies want to beat many competitors in the popular track. In addition to ensuring the safety and effectiveness of new technology research and development products, it is the way to survive in the fierce competition in the future to maintain differentiation advantages.
In the field of biotechnology innovation and research, it is easier said than done to win by "differentiation". In general, it is even more difficult to differentiate a subdivision of drugs with the same target. Most of them need to expand their competitiveness from the perspectives of technology compatibility and indication range.
On the other hand, it also reflects the relative difference between a new therapy that can really open up a whole new subdivision of research track. Among the existing tumor immunotherapies mentioned above, such as cellular immunotherapy, antibody therapies with immune checkpoint inhibitors such as PD-1 and PD-L1 inhibitors, cancer vaccines, cytokines and oncolytic virus therapy, and oncolytic bacterial therapy, oncolytic bacterial immunotherapy technology is the most emerging research direction. In the pattern of competition in the whole industry its prospects particularly eye-catching.
At present, there are no mature products on the market in China. As the main competitor focusing on the layout of this track, Hong Kong pharmaceutical Oncolytic has invented the world's first oncolytic bacterial carrier YB1, which is also a key technological breakthrough in the development process of oncolytic bacterial therapy.
Soluble tumor YB1 is a genetically programmed engineered bacteria strains of salmonella typhimurium, as carrier can efficient rendering including antibodies, mRNA and protein drugs, such as precision within the targeted tumor hypoxia area, and the tumor inside a large number of replication, can greatly improve the YB1 carrier in the concentration of the solid tumor target location, and release a variety of therapeutic drugs "warhead, Inhibit tumor growth and cause tumor dissolution, and eliminate tumor metastasis, which has great clinical application potential.
As the core technology product of the company, YB1 uses the world's first tumor hypoxic specific targeting technology to achieve tumor targeting, and has obtained the only patent of oxygen-regulated oncolytic bacteria in the world. The YB1 technology is also highly compatible with chemotherapeutic agents, immune checkpoint antibodies and CAR-T cell technologies.
It should be said that the master these technical therapy "differentiated" advantage, whether in Hong Kong RongRong tumor, or other biological industry the road of scientific and technological innovation in enterprise's development is crucial, the future we want to keep the development route of "differentiation" in tumor immunotherapy niche business track in the arena of the bacteria of soluble tumor can make a difference.
0 notes
Text
Preliminary study on YB1 oncolytic bacterial therapy
New cases of several years of growth, continued high fatality rate, under the increasingly severe global cancer development situation, countless top medical researchers, biological science and technology enterprise and the collective power of global capital within the field of cancer therapy, especially has gradually carry the banner of immunotherapy in the industry market ushered in the outbreak.
According to Transparency Market Research (TMR), the Market size of cancer immunotherapy is expected to grow from us $37.5 billion in 2015 to 124.88 billion in 2024, with a compound annual growth rate (CAGR) of 14.5%.
The TMR market is expected to reach $128.3 billion by 2024, according to a report from KBV, a global research firm. Meticulous Research predicted $152.89 billion; Market Research Engine had the highest estimate at $173 billion.
Although precise data are not available on the future growth of the market, it is certain that the global tumor immunotherapy market is growing, and the expansion of the future cancer population and the development of new oncolytic vector technologies are the main reasons for the expansion of the market.
With the rapid development of new oncolytic vector technology, bacterial therapy has attracted much attention
The cancer immunotherapy market is clearly booming, and there are several emerging therapies in different directions in the segmented areas, including antibodies that block inhibitory immune checkpoint pathways, dendritic cell and engineered T cell cell therapies, and vaccines that trigger antigen-specific immune responses in tumors. And oncolytic vector platforms for presenting tumor-specific cytokines and antibody genes.
Among them, oncolytic virus and oncolytic bacteria as the main new immunotherapy vector technology has attracted more and more attention in the industry. In recent years, the innovation of oncolytic vector technology is developing rapidly. Currently, 4 products of oncolytic virus have been approved and launched in the world, and the competition is fierce, while oncolytic bacteria, as a more cutting-edge breakthrough technology therapy, is also attracting attention in the industry market.
Using microorganisms as a carrier, once the tumor gene therapy is an important field of study, because most of the human body tumor (over 90%) will form a solid tumor, and solid tumor microenvironment has a notable characteristic, that is in the center section of the tumor can form a very special low oxygen zone, the hypoxia zone significantly different from normal group, Therefore, the hypoxic microenvironment of solid tumors becomes an ideal target for tumor targeted therapy.
Soluble tumor bacteria as anaerobic or facultative anaerobic microbe, the tumor area for its lack of oxygen provides an ideal place to live - soluble tumor invasive bacteria are a categories of intracellular bacteria, this kind of reaction between microbes and hosts are are mediated through the type III secretion mechanism, this mechanism can be passed to express effect genes at the same time, the nature of the protein expression of a variety of treatment, therefore, Attenuated tumolytic bacteria is an ideal carrier of therapeutic drugs.
BCG · TUBERCULOSIS
BCG Vaccine is a live Vaccine made from the suspension of attenuated Bovine mycobacterium tuberculosis, which can enhance the activity of macrophages, enhance the ability of macrophages to kill tumor cells, activate T lymphocytes and enhance cellular immunity of the body.
The therapeutic BCG vaccine is the only mature and commercially available bacterial drug product, mainly used for postoperative perfusion of bladder cancer. As an oncolytic bacterium, BCG tuberculosis has been used in the treatment of superficial bladder cancer for more than 30 years, and its clinical effect is remarkable. However, its disadvantages are also obvious, that is, its application scope is relatively limited, it can only be used to treat superficial bladder cancer, and it lacks the means of genetic engineering modification.
(The figure shows the clinical studies of BCG combined with various immune checkpoint antibodies in major pharmaceutical companies around the world)
Bifidobacterium - Clostridium spore
Bifidobacterium is a gram-positive, non-motility, rod-shaped, sometimes bifurcated, strictly anaerobic bacteria genus. Bifidobacterium is one of the important members of the intestinal flora of humans and animals. As an important beneficial microorganism in intestinal tract, bifidobacterium has many important physiological functions such as biological barrier, nutrition, anti-tumor, immune enhancement, gastrointestinal function improvement, anti-aging and so on.
It was found that bifidobacterium has anti-colon cancer effect, which may be through affecting intestinal flora metabolism and enhancing host immune response. Adhesion and degradation of potential carcinogens to prevent intestinal cancer; Change intestinal flora; Produce anti-cancer mutagenic substances; Enhance the immune response of the host; To affect the physiological activity of the host.
In 2020, a university of Chicago study found that adding certain bacteria to the digestive tracts of mice enhanced their immune system's ability to attack tumor cells, and further research found that bifidobacteria was the particular strain that modulated the immune response the most. Dr. Thomas Gajewski, project leader of the study, explained that bifidobacteria may trigger an immune response in humans by interacting with free dendritic cells.
Clostridium is a sporozoa, which is a very unique species in nature. When it enters human or animal body, it often causes muscle and soft tissue infection, intestinal diseases and neurotoxic diseases. A c. POROgenes (ATCC13732) sporosporum powder was also used in clinical studies in the 1970s. This may have been a non-standard clinical trial, which was conducted in humans on five tumor patients after the non-pathogenic clostridium caseinate M55 strain was shown. After intravenous administration of spores of Clostridium butyricum M55, the 3 patients with the largest tumors developed oncolytic but no surrounding tissues or smaller metastases. In one case, a temporary clinical benefit was attributed to oncolytic effects caused by clostridium difficile.
In 1978, another trial was carried out for patients with angioblastoma by intra-carotid injection. One week after the injection, most patients' tumors were completely dissolved, but the glioblastoma turned into a brain abscess, and surgery was performed to prevent rupture and death. The results of these studies indicate that despite the established evidence of safety and colonization following intravenous administration, there is an overall lack of clinical benefit due to tumor regeneration from the outer edges of living cells with good blood vessels.
The advantage of bifidobacteria-Clostridium spore oncolytic bacteria is that they are obligate anaerobe and will not be distributed in normal organs. The disadvantage is that it can only be distributed in very large tumors, and can only target the necrotic area of the tumor, oxygen concentration is less than 0.01%, there are no living tumor cells in this area, so the therapeutic effect is limited.
listeria
Listeria is a Gram-positive bacterium, belonging to firmicutes. It is mainly food-borne and is one of the most deadly food-borne pathogens. Listeria has also been widely used in the research of bacterial vectors in the field of cancer treatment. Johnson & Johnson and its subsidiary Aduro Biotech used Listeria as an antigen vector to advance clinical trials. In 2014, Johnson & Johnson and Aduro's listeria project CRS-207 combined with GVAX in the treatment of pancreatic cancer was recognized as a breakthrough therapy by the FDA of the United States, and the trial entered the clinical phase II. However, the follow-up results were not satisfactory, and Aduro finally gave up the promotion plan of THE CRS-207 project.
In January 2019, the FDA halted the enrollment of patients in a phase 3 clinical trial of Advaxis inc. 's AXAL program in Princeton, New Jersey. Advaxis is a late-stage biotech company focused on the discovery, development and commercialization of immunotherapy products, including AXAL, which effectively eliminates human cells infected with HPV. The drug is a bacterial therapy developed by Advaxis' exclusive Listeria immunotherapy technology platform.
In this technique, listeria is introduced into a foreign gene via a plasmid vector to express a fusion protein consisting of an active fragment of the toxin protein LLO and the target antigen E7 protein. The LLO protein derived from Listeria ensures the survival of the bacteria after phagocytosis by antigen-presenting cells (APC) in the human body. AXAL, which has been shown in early trials and preclinical studies, stimulates the body's immune system to recognize the presence of tumor cells and reduce their natural defenses, which in turn encourages the body's own killer T cells to attack the tumor cells.
The FDA's decision to halt Advaxis's bacterial treatment for cervical cancer is the latest setback in the development of a bacterial treatment for listeria. In terms of the strain itself, the advantage of Listeria is that it can carry tumor antigens, but there are two disadvantages: first, many tumor antigens are not obvious, so specific antibodies for tumor treatment is limited; Second, the strain will be distributed in normal organs, affecting human health.
salmonella
Among various kinds of bacteria types, most of the research on salmonella typhimurium, at least more than 10 of genetically engineered based on salmonella typhimurium attenuated strain in preclinical studies, port of medicine soluble tumor biological pharmaceutical co., LTD. The core technology of product YB1 was the first to use synthetic biology technology designed specifically for cancer treatment of salmonella strains.
Though of facultative anaerobic bacteria such as salmonella attenuated abound, but the starting point of past a lot of research is induced by chemical drugs, such as making attenuated bacteria or do some nutrition metabolic gene mutations, while it is possible to realize the characteristics of the tumor target, but can only form a certain scale, such as tumor, liver = 1000: Although the ratio of 1 can reach 10^9CFU/g tissue in the tumor, it can also reach 10^6CFU/g in the liver, which will cause safety risks, and also cause the dose of bacteria can not be injected too much.
From the experimental data, the oncolytic salmonella vector YB1 of Hong Kong medicine has obvious advantages after modification: in vivo, if YB1 is in the tumor region, due to the oxygen content of YB1 is less than 0.5%, its tumor aggregation ability will not be affected, can reach 10^9CFU/g tissue; However, if YB1 is in an environment where oxygen concentration is higher than 0.5%, such as liver and other tissues, YB1 will be cleared quickly, thus achieving great tumor targeting and safety.
As Hong Kong medicine soluble tumor core technology products, with genetic programming modification of salmonella YB1 is currently the world's first successful development of soluble tumor bacteria carrier, and has been applied to pet primary cancer treatment, clinical effect is very outstanding, there is risk of malignant tumor after cured by YB1 pet dog has achieved more than 4 years cancer-free survival.
summary
Throughout the development of bacteria in the field of global cancer immunotherapy therapy, with different types of bacteria as the carrier to develop bacteria soluble tumor therapy, the curative effect in the experiment is endless also and same, some hit stagnate, some effect is prominent, full of unknown laboratory platform in the world, the development process of soluble tumor therapy bacteria to humans bring a surprise, There were regrets, but more than that, there was hope.
0 notes
Text
Oncolytic bacterial immunotherapy has been added to cancer treatment
Cancer treatment is a long-term cause for human beings all over the world. In the face of rising morbidity and mortality, traditional surgery, chemotherapy or radiotherapy can no longer meet the rising demand for anti-cancer.
In recent years, tumor targeted therapy technology, which can achieve the purpose of local extermination by precisely guiding the tumor area and minimizing the damage of surrounding normal tissues, plays an increasingly important role in tumor therapy due to its specificity and targeting, and is becoming the main direction of tumor therapy.
In the field of targeted therapy, the discovery of ideal targets is the root of the development possibility of all new technology therapies. In the study of human solid tumors, scientists have found an excellent target for cancer treatment -- the hypoxic solid tumor microenvironment! This has laid a key foundation for the development and application of many new cancer treatment technologies, including the cutting-edge oncolytic bacterial immunotherapy technology.
Characteristics of solid tumor microenvironment
More than 90% of human tumors are solid tumors, including lung cancer, breast cancer, colon cancer, soft tissue sarcoma, etc. Scientific studies have found that the microenvironment of solid tumors is unique and abnormal. The basic structure of the tumor microenvironment can be roughly divided into three layers: in the circumference of blood vessels, the central layer is fully oxygenated and provides nutrients, enabling cancer cells to accumulate around abnormal blood vessels of tumors; Outside are hypoxic regions containing hypoxic cancer cells; Further out is the necrotic area of the tumor, which has no living cells.
Thus, solid tumors can be distinguished from surrounding normal tissue. The main differences between the two are the characteristics of the cancer cells and the formation of the tumor vasculature, which leads to the formation of areas of hypoxia and necrosis. Due to the inefficiency of vascular network replenishment, solid tumors will have areas of hypoxia and necrosis, where nutrient and oxygen levels are low.
Oxygen levels can vary in different environments. In air, about 21% (150 mm hg) and in mammalian tissues and organs, between 2% and 9% (15-70 mm hg). In the hypoxic regions of most tumors, oxygen levels are approximately below 0.7% (5 mm hg) and below 0.02% under certain severe hypoxic conditions.
Therefore, hypoxia is an ideal target for cancer targeted therapy.
How do bacteria target tumors?
Studies have found that some obligate anaerobe and facultative anaerobe can infiltrate through peripheral blood circulation and preferentially accumulate and proliferate in tumor, that is, have tumor targeting.
The reason may be related to the special microenvironment of tumor: Firstly, the rapid growth of tumor tissue will lead to insufficient blood supply in some areas of tumor, thus forming anaerobic and necrotic areas. It is these anoxic environments that provide a place for bacteria to gather and grow. Secondly, in order to satisfy the growth of tumor tissue, cells in tumor microenvironment will secrete angiogenic factors and reduce the release of angiogenic inhibitors, thus promoting the generation of blood vessels in tumor. However, the blood vessels in tumors are pathologic neovascularization, and their vascular network is deficient in structure and function. They may break, block and branch excessively. Abnormal vascular network is also an important reason for the occurrence of anaerobic zones in tumors. It is for this reason that the researchers speculate that the bacteria target the tumor tissue through gaps in the network of abnormal blood vessels.
In addition, the hypoxic microenvironment in the tumor can cause local deficiency of the immune system, resulting in a greatly reduced ability of the immune system to remove bacteria from the tumor. The tumor tissue with high metabolism and apoptotic cell fragments in necrotic area provide abundant raw materials for bacterial reproduction. These factors allow certain bacteria to exhibit specific tumor-targeting properties.
The role of the immune system in bacteria targeting tumors
In the tumor microenvironment, immune disorder is an important reason for tumor to escape immune monitoring. Recent studies have shown that tumor-infiltrating immune cells (such as macrophages) do not play an anti-tumor role, but promote tumor growth and increase its ability to invade and metastasize. The tumor microenvironment belongs to a chronic inflammatory environment. It has been said that the tumor itself is a wound that will never heal, because there are always a large number of immunosuppressive factors and growth factors such as GM-CSF, IL-6,10,13, VEGF, TGF-β and so on in this environment. These environments eventually lead to dysfunction of immune surveillance.
However, when the human body is infected by bacteria, the bacteria themselves will strongly stimulate the host's immune response. When the bacteria target the tumor, they tip the balance of immunosuppression in the tumor microenvironment. Studies have shown that when bacteria infect tumors, not only do the bacteria activate the neutrophils intensely, but they also introduce this antagonism into the anaerobic areas of the tumor, creating a long-term confrontation.
Saccheri et al. found that bacterial invasion of tumor cells results in a large presentation of tumor-specific antigens. This antigen is delivered directly to dendritic cells (DCs), causing a tumor-specific immune response. Therefore, it is of great significance to study the interaction between bacteria and immune system in the process of tumor infection, which will provide evidence and basis for tumor immunotherapy in the future.
The discovery of salmonella, which targets tumors, and the discovery of oncolytic bacteria have added to the list of cancer treatments
At present, with the rapid development of molecular biology and genetic engineering technology, more and more bacteria have been applied in the field of cancer treatment, including obligate anaerobe Clostridium, probiotic Bifidobacterium, listeria and so on. Among them, gram-negative facultative anaerobe Salmonella typhimurium has the most extensive application prospect.
Previous studies have shown that attenuated salmonella typhimurium can colonize tumor tissue 1,000 to 10,000 times more effectively than normal tissue, but subsequent investigations have shown that any modification of salmonella weakens not only its virulence, It also makes it less effective against tumors.
And because salmonella tends to accumulate in the liver and spleen, residual salmonella can cause a lot of damage if not removed quickly. In addition, because any single tumor treatment has its drawbacks, combination therapy is especially important, but previous studies have failed to highlight the advantages of Salmonella as a delivery warhead.
In 2011, the R&D team of Hong Kong Pharmaceutical Oncology Biopharmaceutical Co., Ltd. realized the efficient programming technology of Salmonella lambda-RED for the first time in the research, establishing the transformation foundation of salmonella synthetic biology. After more than a decade of development, the company created a genetically engineered strain of Salmonella Typhimurium, which it named YB1. The modified version of salmonella, known as YB1, is the world's first carrier of an oncolytic bacterium, marking a crucial technological breakthrough for the idea of using bacteria to treat cancer.
It is reported that Hong Kong Pharmaceutical Oncolytic Biopharmaceutical Co., Ltd. has been focusing on innovative drug research and development in the field of tumor targeted therapy, oncolytic bacteria YB1 is its current primary core technology products, the company is currently promoting the application of YB1 oncolytic bacteria immunotherapy technology in human clinical trials, and has laid out a number of product pipelines. But it should still be some time before its first anticancer drug hits the market.
Although no oncolytic bacteria products in the real sense have been approved for marketing in the world at present, major pharmaceutical companies around the world have begun to enter the layout of oncolytic bacteria track, and have achieved good response in the industry market recently. The field of tumor immunotherapy may soon usher in the spring of oncolytic bacteria therapy.
0 notes
Text
Overview of new technologies in tumor immunotherapy: cell therapy continues to heat up, and oncolytic bacteria reappear
According to official data from the Who's International Agency for Research on Cancer (IARC), there were 19.29 million new cancer cases and 9.96 million deaths worldwide in 2020, both figures representing further increases from 2019. With the increasing incidence and mortality of cancer worldwide, it is becoming more and more urgent to find new technologies and methods for effective treatment of cancer.
In addition to traditional treatment methods such as surgery, radiotherapy and chemotherapy, continuously innovative immunotherapy has gradually become an important development direction in the field of global cancer treatment. In recent years, more and more new therapies have emerged in subdivided fields, promoting the development and expansion of tumor immunotherapy industry.
Today, we take a look at some of the world's most groundbreaking cancer immunotherapies and see which new life science technologies are bringing hope and hope to millions of cancer patients around the world.
CAR T/TCR-T cell therapy
ChimericAntigen Receptor t-cell (ChimericAntigen Receptor t-cell) is the full name of CAR T Cell. CAR T cell immunotherapy (CAR T cell immunotherapy) is a new treatment method for tumor and cancer. It extracted immune T cells from the patients first, and then using genetic engineering technology, writing can make T cells in vitro, identify specific gene fragment of tumor cells and kill tumor cells, and this kind of CAR - T cells in the lab, a lot of training, and then after the expansion of the "enhanced" immune T cells back to patient treatment.
Since Rosenberg first proposed genetically engineered T cell technology in 1986, CAR-T therapy has gone through 35 years. Gene therapy represented by CAR-T cell therapy was rated as "breakthrough therapy" by FDA and accelerated to become a clinical reality. In 2017, the U.S. Food and Drug Administration (FDA) approved ctL-019 (Kymriah) for the treatment of b-cell acute lymphoblastic leukemia (AML) that is refractory or relapsed after at least second-line therapy.
Many domestic biotechnology companies, such as Fuxing Kitt, Yao Ming Junol, Keji Pharmaceutical, Chuanqi Bio and Yongtai Bio, have also entered the competition track of CAR T cell therapy. In June this year, Fosun Kitt's CAR-T product Aquilenxel was officially launched in China. On August 9, the latest public information of Drug Evaluation Center (CDE) of China's State Food and Drug Administration showed that Aquilenxel injection declared by Fosun Kitt was included in the breakthrough treatment drug program. The proposed application for the treatment of relapsed or refractory indolent non-Hodgkin's lymphoma after second-line or higher systemic therapy represents an important step forward in the development of the domestic CAR T cell industry.
Both TCR-T cells and CAR T cells are genetically engineered T cells. Tcr-t cells can recognize tumor-specific antigens from cell membrane surface or intracellular sources, and TCR-t cells targeting NY-ESO-1 have demonstrated good safety and efficacy in clinical trials of refractory and recurrent melanoma, synoviosarcoma, multiple myeloma and lung cancer at home and abroad. T cell immunotherapy is the most likely to achieve a breakthrough in the treatment of solid tumors.
Recently, TCR-T cell therapy continues to heat up, and the market is promising. T-knife Therapeutics, Inc., today announced the completion of a $110 million Series B funding round to bring its TCR-T pipeline into the clinic, expand its scientific team and increase production capacity, according to public information.
Oncolytic virus therapy
Oncolytic virus is a natural or genetically engineered virus that can selectively replicate in tumor tissue and then infect and kill tumor cells or cause lysis of tumor cells, but has no killing effect on normal tissues. Oncolytic therapy is gaining increasing attention from cancer patients and industry markets due to its ability to specifically replicate within tumor cells and cause lysis of tumor cells without affecting normal cells.
In 2004, the first oncolytic virus product Rigvir (ECHO-7 virus) was approved in Latvia for the treatment of melanoma and other cancers; In the following year, China's first oncolytic drug Ankerin (recombinant human adenovirus type 5) was also approved for marketing, which was used for the treatment of primary, clinically advanced and recurrent head and neck tumors and showed very good efficacy in the treatment of advanced nasopharyngeal cancer and bladder cancer. In 2015, THE US FDA approved Amgen's oncolytic drug T-VEC (herpes simplex virus) for marketing for the first time, for the treatment of advanced melanoma, so far the development of oncolytic therapy in the world gradually mature.
In June this year, Daiichi Sankyo announced that its oncolytic virus product Delytact (Teserpaturev /G47∆) was approved in Japan by the Ministry of Health, Labour and Welfare (MHLW) for the treatment of malignant glioma, the fourth oncolytic virus product approved worldwide. At the same time, astrazeneca, Johnson & Johnson, Arna& Pharma and other major pharmaceutical companies began to cluster oncolytic virus distribution, and have entered the oncolytic virus track by adding corresponding departments or acquiring other small and medium-sized pharmaceutical companies.
In terms of domestic enterprises, Hengrui Pharmaceutical, Kanghong Biological, Lepu Medical and so on have a layout in this field, and the development progress of some products has been very advanced. And the current focus on soluble tumor virus therapy research also micro drug, is dedicated to the research and development of a new generation of genetically modified herpes soluble tumor virus with used in the treatment of cancer, and in succession recently announced that its clinical trials of two soluble tumor virus product application were approved, soluble tumor virus therapy at home and abroad to track competition was in full swing, a lively group.
Oncolytic bacterial therapy
Soluble tumor viruses, bacteria and soluble tumor is a tumor immunotherapy soluble tumor carrier category is emerging in the field of therapy, its principle is to bacteria through genetic programming technique for attenuated "good bacteria", and can be used as a carrier to carry drugs targeting tumors and the tumor internal release drugs kill cancer, exert its "soluble tumor" effect.
The idea of using bacteria to fight cancer and treat cancer has a long history of experimental research, but there are very few drugs that have really entered the stage of clinical research and have been on the market. At present, the only drug on the market is therapeutic BCG, which is mainly used for postoperative perfusion of bladder cancer. In 2014, The listeria vector project -- CRS-207 combined with GVAX in the treatment of pancreatic cancer, a collaboration between Johnson & Johnson and Aduro, a biotechnology company, was recognized as a breakthrough therapy by FDA in the United States. However, due to unsatisfactory experimental results, Aduro finally gave up the promotion plan of CRS-207 project.
Until The successful development of the world's first oncolytic bacterial vector by Hong Kong Pharmaceutical Oncolytic Biotechnology Co., LTD. (a domestic biotechnology company focusing on the development of innovative drugs in the field of tumor immunotherapy), it marked another key progress of oncolytic bacterial therapy.
Data show that the RESEARCH and development team of Hong Kong Pharmaceutical Oncolytic Biotechnology Co., Ltd. experienced more than 10 years of research and development cycle, based on synthetic biology, invented a genetically programmed salmonella, and named it YB1; This oncolytic bacterium YB1 can be used as a carrier to deliver therapeutic drugs including antibodies, mRNA, protein drugs, etc., targeting the tumor and releasing drugs internally to attack the tumor and cause tumor dissolution.
The port drug dissolved tumor biological pharmaceutical products currently has completed its soluble tumor bacteria YB1 pet primary cancer therapy in clinical trials, bacteria and has begun to push its soluble tumor immunotherapy of human clinical trials, and the layout of the application of YB1 multiple product line, as to when to have a more mature listed products can actually benefit cancer patients is still unknown.
From the perspective of the world, many international pharmaceutical giants have also begun to flood into the super emerging race track of oncolytic bacteria, including Roche, Merck, Pfizer, Bristol-Myers Squibb and other big pharmaceutical companies have laid out the pipeline of oncolytic bacteria related products. In the future, this subdivision field is bound to be more exciting competition.
summary
Although human has not been completely conquer cancer, but with the development of life science and the progress of new medical technology, the emergence of more and more new cancer treatment technology has let the cancer patients see the hope and light, we are looking forward to the innovative therapy to achieve more breakthroughs, make more patients benefit.
CAR /TCR-T cell therapy, oncolytic virus, oncolytic bacteria, etc. have been running in their respective fields. Tumor immunotherapy is in the ascendant. When will the next "promising star" rise? We'll see!
0 notes
Text
Amazing bacterial therapy. -YB1
Some say born than Eva, Eva, the contemporary young people seemed more interested in having a cat a dog - no according to this, of course, not all, indeed choose pet owners more and more, now the various categories of cats and dogs become more and more important family members, are masters of as close as the family "lexy", for many families bring infinite warmth accompany and spiritual comfort.
However, many people may not know that, just as humans can get cancer, animals can also get cancer, and many pet dogs and cats die of cancer after aging, which is a heavy topic that pet owners have to face.
In October 2015, Noopy was diagnosed with a malignant sarcoma. Within a few months of his diagnosis, Noopy's nasal sarcoma had grown to almost the size of his nose.
Pet cancer affects owners' hearts, YB1 tumolytic bacteria cure dogs with cancer miracle
Cancer pets like Noopy, in fact, there are a lot of, so in pet food, pet grooming and pet training such as "it" of the economy segment consumer products and services more and more popular at the same time, the pet market of medical demand in escalating, also for pet cancer treatment is to make the heart of "lexy" pet parents.
Common pets have sarcoma cancer, melanoma, Noopy is suffered pet sarcoma, traditional treatment is through surgery to remove lump, the tumor is too large or too many and cannot be removed or location cannot reach, can consider to other treatments, including drugs, chemotherapy, immunotherapy (including specific or non-specific stimulate the immune system) and radiation therapy, etc.
However, as with human cancer treatments, chemotherapy or radiation is not suitable for all types of pet cancer and may have side effects. For example, the target of chemotherapy drugs differs between cancer cells and normal cells, but the margins are so small that some normal cells are inevitably destroyed.
Meanwhile, more new gene-based pet cancer treatments are being developed, and Noopy is waiting for a miracle to rewrite his fate. And the miracle did come.
In 2016, Hong Kong Pharmaceutical Oncolytic Biopharmaceutics Co., Ltd. applied its self-developed oncolytic bacterial product YB1 to the treatment of pet primary cancer. Noopy received the treatment with YB1, and the experiment was a great success. After six months of treatment with oncolytic bacterial YB1, Noopy's tumor completely disappeared in October 2016. This 7-year-old poodle is back to health after beating cancer.
Noopy is still healthy and has been cancer-free for more than 4 years after being treated with YB1, making him a miracle in pet primary cancer treatment.
YB1 oncolytic bacteria: Initiating a new era of tumor immunotherapy
The success of YB1 in helping Noopy fight cancer brings hope to many pet families, and the experimental results are also exciting for the development of YB1 oncolytic biopharma in Hong Kong. Although YB1 has not yet been tested in human clinical trials, the toxin-carrying strain yB1-tox has been tested in pet cancer trials, with more than 100 dogs diagnosed with cancer by 2020.
The life of the general situation of pet dog is only more than 10 years old, 10 years of age or older is the dog dog, all sorts of cancer high incidence ages and pet dogs in the cancer and human cancer incidence mode or gene mutations, are very similar, so the dog cancer treatment clinical trial is the most close to the model of human clinical research. At present, the company has screened out very effective tumor types and treatment plans for primary cancer in pet dogs, and the efficiency and safety have been fully demonstrated.
Cancer treatment with bacteria is the application direction of Hong Kong Pharmaceutical oncolytic biopharmaceutical has always been devoted to research, since the successful invention of the world's first oncolytic bacterial carrier YB1, the company is committed to using YB1 to develop mature oncolytic bacterial immunotherapy technology for the majority of cancer patients to bring good news. With YB1 technology being the first to achieve great success in the field of pet cancer treatment, this provides strong evidence for its next application in the clinical study of human cancer treatment.
All along, the port drug dissolved tumor biological pharmaceutical innovation focus on tumor targeting therapeutic areas of drug research and development, relying on the world's leading soluble tumor drug oral bacteria based on synthetic biology platform, the company's goal has always been to provide global revolutionary cancer immunotherapy solution, and at this stage, the port drug dissolved tumor biological pharmaceutical soluble tumor bacteria YB1 is expected with the core technology products, Open a new era in the field of tumor immunotherapy -- oncolytic bacteria will shine in the future.
YB1 technology has clear principle and broad application prospect
At present, more than 90% of human tumors belong to solid tumors, and a very significant characteristic of solid tumors is that due to their vigorous growth, insufficient vascular supply, and vascular malformations, they will form a hypoxic zone (oxygen content less than 0.5%) in the center of the tumor. This is a very significant physical target. In normal tissue, lung tissue has an oxygen content of about 5%, other organs such as liver have an oxygen concentration of 2-3%, and the lowest tissue, bone marrow, has an oxygen content of about 1%. Therefore, tumor oxygen content of 0.5% is a very important solid tumor target, and has universal applicability.
YB1 is a type of salmonella genetically programmed by the r&d team of oncolytic bio-pharmaceutical of Hong Kong medicine. Its advantage after transformation is that, in the human body, if YB1 is in the tumor, due to its oxygen content is less than 0.5%, its aggregation ability in the tumor will not be affected, which can reach 10^9CFU/g tissue; However, if YB1 is in an environment higher than 0.5%, such as liver and other tissues, YB1 will quickly achieve zero clearance, thus achieving great tumor targeting and safety.
The company's research shows that oncolytic bacteria YB1 also has a strong ability to carry drugs. As a carrier, it can efficiently deliver and carry antibodies, mRNA, protein drugs, oncolytic viruses and other "weapons", and can become a "biological missile" to accurately target the hypoxic region of the tumor center, and achieve a large number of replication and amplification within the tumor. The concentration of YB1 vector at the target site of solid tumor was greatly increased. After reaching the tumor, YB1 releases a variety of therapeutic "warhead" drugs, which can inhibit tumor growth and cause tumor dissolution, and eliminate tumor metastasis, which has great potential for clinical application.
At present, Hong Kong Oncolytic biopharma has laid out more than ten product pipelines for the application and expansion of YB1, and will start the human clinical trial study of YB1, looking forward to more research progress in the future, and bring more hope and good news to global cancer patients and cancer treatment field as soon as possible.
0 notes
Text
The soluble tumors bacteria YB1 has pioneered new methods of cancer immunotherapy, and can it pry the market by hundreds of billions of dollars in the future?
The development of life science and technology has completely subverted the social life and medical and health system of human beings in the past hundred years, and the quality of human life has been greatly improved, while completely tackling cancer, a disease that has long plagued human health, has become the common goal of the global medical and scientific community.
In recent decades, the global field of cancer treatment has developed rapidly, and mainstream treatments have evolved from traditional methods such as surgery, radiotherapy and chemotherapy to tumor-targeted therapy and, more recently, tumor immunotherapy, which are better at improving patient outcomes while reducing systemic adverse reactions in patients in treatment. Compared with traditional tumor treatments, targeted therapy and immunotherapy benefit patients in improving efficacy, reducing symptoms or improving quality of life by targeting specific cancer-causing pathways and utilizing the patient's immune system.
Overview of the global and Chinese cancer treatment industry
Currently, the global and Chinese cancer drug market consists of chemotherapy, targeted therapy and immunotherapy. Globally, targeted therapies and immunotherapy account for 83.8% of the global cancer treatment market and are expected to grow to 89.8% by 2030, according to public data reported?
In the Chinese market, targeted therapies and immunotherapy in the field of cancer treatment have greater potential for growth, driven by favorable policies, increasing patient spending power and the introduction of more innovative therapies in China, with the total contribution expected to increase from 36.6% in 2020 to 85.8% in 2030.
Although targeted therapies and immunotherapy approaches are inherently different, in many cases it has been shown that a combination of the two therapies creates synergies, usually one that complements the other, releasing the patient's anti-tumor immunity and thus improving efficacy. This combination has been improved through professional exploration to produce better results in clinical practice and has been shown to be a promising new treatment strategy in the field of cancer treatment.
Oncology immunotherapy will hit the $100 billion Blue Sea market
Tumor immunotherapy has gradually established itself as a pillar in the global field of cancer treatment in recent years, designed to stimulate the patient's own immune system to produce or enhance an anti-tumor immune response to control or eradicate cancer cells.
The discovery and development of cancer immunotherapy in recent years has marked a milestone in cancer treatment and will be an important boost to the growth of the global cancer drug market, as it provides lasting relief and is often well tolerated in several patients with advanced cancer. It is worth mentioning that because tumor immunotherapy works through the patient's own immune system, immunotherapy has fewer side effects than traditional cancer treatments such as chemotherapy and radiotherapy.
The global market for oncology immunotherapy has increased from $9.6 billion in 2016 to $35.1 billion in 2020 and is expected to reach $108.2 billion by 2025, according to the institute's professional report The global cancer immunotherapy market will reach US$229.8 billion over 30 years, with CAGR of 25.3% and 16.3% from 2020 to 2025 and 2025 to 2030, respectively. For the foreseeable future, cancer immunotherapy will usher in hundreds of billions of dollars of blue sea market.
In 2020, oncology immunotherapy accounts for 23.4% of the global cancer drug market, and by 2030 it is expected to account for approximately 47.6% of the global oncology market.
On the domestic market, China's oncology immunotherapy market has reached RMB14.8 billion in 2020 and is expected to grow to RMB88.9 billion by 2025 and further to RMB272.8 billion by 2030, with CAGR of 66.8% and 16.4% from 2019 to 2024 and 2024 to 2030, respectively.
According to the data, oncology immunotherapy will account for 7.5% of China's total cancer drug market revenue in 2020 and is expected to continue growing in the future, accounting for about 39.9% of China's cancer market by 2030.
Will the innovative therapies take advantage of corner overtaking? Tumor immunotherapy shapes the development of new logic
In the face of the growing $100 billion Blue Sea market in the next five years, it is even more important to clarify the path of development in the field of oncology immunotherapy at this stage and in the future, and the choice and layout of the segmented track is about how much market share industry participants can ultimately capture.
After years of evolving cancer treatment paradigms, the development of oncology immunotherapy is now playing an increasingly essential role, subdivision circuits continue to spawn new therapies, by improving the effectiveness of treatment of various types of cancer, to begin to provide cancer patients around the world with more valuable treatment options.
The early stages of the development of global tumor immunotherapy, mainly characterized by immuno-checkpoint inhibitors single therapy, including PD-1/L1 inhibitors and CTLA-4 inhibitors, in a variety of tumors to achieve about 20% ORR, replacing chemotherapy as the treatment standard for a variety of cancer indications. Current mainstream immuno-tumor therapies are characterized by the combination of immuno-checkpoint inhibitors (as cornerstone drugs) with a second immuno-checkpoint inhibitor or different types of cancer therapies (e.g. chemotherapy or angiogenesis inhibitors) with an ORR (objective remission rate) of approximately 40 percent.
Hematoma viral therapy is a new tumor immunotherapy, which, in addition to checkpoint suppression, uses several viruses to selectively replicate and kill tumors directly in tumors, as well as the ability to induce effective, patient-specific anti-tumor immune response. This soluble virus has the potential to produce an immune response to specific tumor antigen groups in individual patients, including new antigens that are uniquely present in tumors.
In recent years, hemovores therapy has become a promising anti-cancer therapy. The soluble virus, which destroys cancer cells without damaging normal tissue through tumor-specific replication, then promotes congenital and adaptive immune responses, demonstrating great potential for cancer treatment. The emergence of germ therapy for Soloma may pry into the larger market of the future.
Hemolytic bacterial therapy is currently more cutting-edge than the tumor virus of the emerging cancer immunotherapy program, because most of the tumors in the human body (more than 90%) will form solid tumors, and solid tumor microenvironment has a significant characteristic, that is, in the center of the tumor will form a very special low oxygen region, this low oxygen region is significantly different from normal human tissue, so the physical tumor oxygen-deficient microenvironment has become an ideal target for tumor target therapy.
The emergence of germ therapy for Hemolytic may pry into the larger market of the future
Hemolytic bacterial therapy is currently more cutting-edge than the tumor virus of the emerging cancer immunotherapy program, because most of the tumors in the human body (more than 90%) will form solid tumors, and solid tumor microenvironment has a significant characteristic, that is, in the center of the tumor will form a very special low oxygen region, this low oxygen region is significantly different from normal human tissue, so the physical tumor oxygen-deficient microenvironment has become an ideal target for tumor target therapy.
As an anaerobic or axial anaerobic microorganism, the anaerobic region of the tumor provides an ideal place for survival - the tumor bacteria is a large class of invasive intracellular bacteria, the reaction between this type of microorganism and the host is mediated by the type III secretion mechanism, which can transmit the expression of the effect gene at the same time express a variety of therapeutic properties of proteins, therefore, detoxifying tumor bacteria is a more ideal therapeutic drug carrier.
In 2011, the core research and development team of HKND YB1 PHARMACEUTUCAL LIMITED. realized the efficient programming technology of salmonella Lambda-RED for the first time in the experiment, established the basis of salmonella synthetic biology transformation, and solved the biosecurity risk in the research and development of bacterial therapy. After establishing the basis for the synthetic biology transformation of salmonella, the company successfully invented a genetically engineered strain of salmonella YB1, a genetically engineered strain of salmonella typhoid, which is also the world's first synthetic biology technology to transform the invention of the tumor bacterial carrier products.
In 2021’s May, YB1 technology-related machine theory was published in Nature Communications, a leading international journal, and studies showed that the safety and efficacy of the synthesis bacterial product had been verified. Thus, with the successful launch of the oncolytic bacterial carriers YB1, bacterial therapy ushered in a new era of development, the clinical application of the hemolytic bacteria is within reach, in the future, can it pry a significant share of the industry market? Believe that time will tell.
0 notes
Text
YB1 -- the world's first oncolytic bacterial carrier
For a long time in the past, cancer was generally regarded as an incurable disease, various malignant tumors were labeled as "terminal disease" and claimed countless lives. Cancer treatment has undergone a long period of exploration and continuous improvement around the world.
In the past century, great progress and breakthroughs have been made in the field of cancer treatment worldwide in terms of technology and methods. With the development of medicine, a variety of cancer treatment methods have been developed at home and abroad, among which surgery, chemotherapy, radiotherapy and immunotherapy are the main pillars. On the other hand, cancer incidence and death rates continue to rise around the world, and people still expect and hope that new anti-tumor drugs or therapies will emerge to benefit patients around the world.
Immunotherapy as' most promising way to beat Cancer '
Cancer immunotherapy has been hailed as the most promising approach to tackling cancer in the past decade. In 2013, Science magazine ranked cancer immunotherapy at the top of the top 10 scientific breakthroughs, igniting new hope for cancer patients.
Since then, from the PD - (L) 1 antibodies to CAR - T therapy, tumor immunology progress is changing many types of cancer treatment criteria, even the development of antineoplastic drugs and cancer treatment paradigm are often rewrite, a large number of new varieties in development and biotechnology companies are at an unprecedented number and scale into the field of tumor immunotherapy.
Tumor immunotherapy plays a role by enhancing the ability of the immune system to target and kill tumor cells. At present, studies at home and abroad mainly involve the following directions: antibodies that block inhibitory immune checkpoint pathways, such as PD1, PDL1, CTLA4 antibodies, etc. Dendritic cell and engineered T cell based cell therapy, such as CAR-T therapy; Vaccines that trigger antigen-specific immune responses in tumors; And oncolytic vector platforms, mainly oncolytic viruses and bacteria, can be used to present tumor-specific cytokines and antibody genes.
In recent years, the emerging oncolytic therapy has attracted extensive attention in patients and the medical market, has attracted many domestic and foreign large manufacturers layout of oncolytic virus pipeline track, and gradually formed a fierce competition pattern. At the same time, a new approach to tumor immunotherapy that is even more cutting-edge than oncolytic viruses, oncolytic bacterial therapy, is beginning to gain traction in the mainstream market.
YB1 -- the world's first oncolytic bacterial carrier
Oncolytic bacteria YB1 is a modified salmonella first developed by Hong Kong Pharmaceutical Oncolytic Biopharmaceutical Co., LTD. As a carrier of oncolytic bacteria, it can be used to develop innovative tumor-targeting drugs. YB1 is also the world's first oncolytic bacterial vector. In the promising field of tumor immunotherapy, this cutting-edge research achievement of Hong Kong oncolytic biopharmaceutical is of great significance in promoting the innovation and development of cancer immunotherapy technology around the world. It is really exciting news.
It is understood that the port drug dissolved tumor biological pharmaceutical co., LTD. Is a focus on tumor targeting therapy innovative drug research and development in the field of biotechnology company, has the world's leading and possess independent intellectual property rights, soluble tumor bacteria based on synthetic biology technology platform, and is committed to offering global revolutionary solution for cancer immunotherapy.
Although the oncolytic bacterium YB1 appears to be "out of the blue" in the industry market, its development has actually experienced years of technological precipitation. In 2011, the company's RESEARCH and development team realized lambda-RED efficient programming technology for Salmonella for the first time in the research, and established the transformation foundation of salmonella synthetic biology. After more than a decade of development, the core research and development team of Hong Kong Pharma Oncolytic Biopharmaceutical has successfully created a strain of Salmonella typhimurium that has been genetically programmed to handle the disease and named YB1.
Data show that the oncolytic bacteria YB1 is also the first strain specially designed for the treatment of tumors by using synthetic biology technology, which has strong drug carrying capacity in addition to its oncolytic properties.
Company research shows that the soluble tumor YB1 bacteria as the carrier, can be efficient presented including antibodies, mRNA and protein drugs, soluble tumor viruses and other "weapons", to incarnate "bio-missile" precise targeting tumor hypoxia in the center of the area, and implement a large number of replication in tumor amplification, greatly improve the YB1 carrier in the concentration of solid tumor target position. After reaching the tumor, YB1 releases a variety of therapeutic "warhead" drugs, which can inhibit tumor growth and cause tumor dissolution, and eliminate tumor metastasis, which has great potential for clinical application.
0 notes
Text
New hope for cancer immunotherapy! Yu Bin's team developed the world's first YB1 oncolytic bacterial carrier
April 15-21 this year marks the 27th National Cancer Prevention and Treatment Publicity Week. The theme of this year is "Healthy Chinese Health Professionals -- Care for Life Science and Cancer Prevention", which aims to promote the important role of family in cancer prevention and cancer prevention and cancer prevention, and at the same time let people have a more scientific and comprehensive understanding of cancer and cancer treatment.
For a long time, cancer has been almost synonymous with "incurable disease" in people's minds. The World Cancer Research Center (WCRC) updated the global cancer case data in 2020, and estimated that there were 19.29 million new cancer cases and 9.96 million deaths worldwide each year. Compared with the previous two years, the number of cancer patients and deaths worldwide increased again. According to statistics, the annual incidence and death rate of cancer in China are 4.569 million and 3.003 million respectively, which means that every minute, 9 people are newly diagnosed with cancer and 6 people die of cancer. These figures are truly shocking.
Immunotherapy has become the main development direction of cancer treatment
With the increasing incidence and mortality of cancer worldwide, the search for more effective cancer treatment technologies and methods has become more urgent. In addition to traditional surgery, radiotherapy and chemotherapy, the development of innovative immunotherapy has gradually become the main direction of cancer treatment, and in recent years, it has developed into a pillar therapy.
The most widely understood immunotherapy for cancer is probably the PD-(L)1/CTLA4 mab, or CAR T cell therapy. But in fact, according to different mechanisms of action, the current immunotherapy drugs can be roughly divided into six categories: one is the immunoregulatory drugs targeting T cells, such as PD-(L)1/CTLA4 mab; Other immunomodulatory drugs, such as TLR/IFNAR1 agonists; Cancer vaccines, such as the BCG vaccine (BCG); Cell therapy, such as CAR-T/TCR-T; Five is the recent very popular oncolytic virus, such as T-VEC; And bi-specific antibodies that target CD3, such as Blinatumomab.
From the perspective of principle, cancer immunotherapy is to fight cancer through the immune system of the human body. In recent years, the innovative development of cancer immunotherapy has indeed fundamentally shaken the inherent pattern of cancer treatment. However, immunotherapy does not work for all patients with solid tumors, and the treatment can sometimes be accompanied by serious side effects. Therefore, how to generate a strong anti-tumor immune response in solid tumors without causing systemic toxicity has become a major challenge for cancer immunotherapy.
While CAR T cell therapy and oncolytic virus immunotherapy are in the ascendant, more cutting-edge oncolytic bacterial immunotherapy has emerged in the field of cancer immunotherapy worldwide, bringing a new hope for cancer treatment!
Yu bin's team successfully developed the world's first oncolytic bacterial vector -- YB1
The research on the use of bacteria as tumor treatment drugs has a history of nearly a hundred years, but few drugs have entered clinical research and been on the market. At present, more than 90% of human tumors are solid tumors, and the vascular system of tumors is obviously abnormal compared with normal cells. Due to excessive bending of blood vessels, slow blood flow, lack of nutrition and oxygen in tumor area, effective diffusion of anticancer drugs will be greatly limited. Therefore, in the hypoxic region of the tumor, the therapeutic effect of anti-tumor drugs will be significantly affected.
Soluble tumor bacteria as anaerobic or facultative anaerobic microbes, tumor area instead of lack of oxygen to offer an ideal place to live - soluble tumor bacteria are a large family of invasive intracellular bacteria, this kind of reaction between microbes and hosts are are mediated through the type III secretion mechanism, this mechanism can be passed to express effect of gene and express many kinds of treatment properties of protein, Therefore, attenuated oncolytic bacteria is an ideal carrier of therapeutic drugs.
At present, the world's first oncolytic bacterial carrier is successfully developed by Hong Kong Pharmaceutical Oncolytic Bio-pharmaceutical Co., LTD. In 2011, Dr. Bin Yu, founder of the company from the University of Hong Kong, and his team realized the efficient programming technology of Salmonella lambda-RED for the first time in their research, establishing the transformation foundation of salmonella synthetic biology. After more than a decade of development, Dr Yu and his team have created a strain of Salmonella typhimurium that has been genetically programmed to handle the disease and named YB1.
YB1 is currently the first strain specially designed for the treatment of tumors by using synthetic biology technology, which not only has oncolytic properties but also has strong drug carrying capacity.
Dr Bin team's research shows that the soluble tumor YB1 bacteria as the carrier, can be efficient presented including antibodies, mRNA and protein drugs, soluble tumor viruses and other "weapons", to incarnate "bio-missile" precise targeting tumor hypoxia in the center of the area, and implement a large number of replication in tumor amplification, greatly improve the YB1 carrier in the concentration of solid tumor target position. After reaching the tumor, YB1 releases a variety of therapeutic "warhead" drugs, which can inhibit tumor growth and cause tumor dissolution, and eliminate tumor metastasis, which has great potential for clinical application.
Dr. Yu Bin: He has been engaged in the tumor immunotherapy industry for more than ten years, contributing to the global cancer cause
Hong Kong Pharmaceutical Oncolytic Biopharmaceutical focuses on the research and development of innovative drugs in the field of targeted cancer therapy, owns the world's leading oncolytic bacteria technology platform based on synthetic biology, and is committed to providing revolutionary cancer immunotherapy solutions to the world.
The founder of the company, Dr. Yu Bin, is also the chief scientist of the company. He is studying under the leadership of Academician Yuen Kwok-yung, a SARS hero in Hong Kong, and Professor Wong Jiandong, a synthetic biology pioneer in China. He has been involved in a variety of research projects, including "Salmonella Salmonella through high-throughput NGS sequencing technology, "Various pathogenic microorganisms such as avian influenza", "application of Lambda-Red homologous recombination system to Salmonella Typhimurium and other pathogenic microorganisms", "development of H5N1 and H7N9 avian influenza genetic engineering vaccine", "IgY with high expression of anti-avian influenza HA The development of transgenic chicken with antibody ", "the development of haemolytic vector Salmonella B1", "the study of haemolytic vector presenting single-chain fragment antibody ScFv", etc.
At present, Dr. Yu bin has been engaged in the field of tumor immunotherapy for many years. He has been practicing for more than 10 years, and has the naming qualification of the only oncolytic bacterial therapy (YB1). His invention of YB1 bacterial immunotherapy has received extensive attention and coverage from major media in the Mainland and Hong Kong. During his doctoral studies, Dr. Yu bin has obtained 2 patents in the United States, 1 patent in 30 Eu countries, and 1 patent in China for his research and invention. He has published more than 10 high-quality English SCI academic papers and achieved fruitful results.
In January 2021, Dr. Yu Bin established Hong Kong Oncolytic Biopharmaceutical Co., LTD as the founder, and obtained the exclusive authorization and commercial development rights of all YB1 patents, and established a long-term strategic partnership with the University of Hong Kong HKU. Under the promotion of Dr. Yu Bin, the product pipeline of Hong Kong oncolytic biopharmaceutical has been expanded to more than ten lines, and YB1 related clinical studies will be fully carried out by the end of 2021, which is expected to contribute to the global cancer cause through more research results in the future.
2 notes
·
View notes