Don't wanna be here? Send us removal request.
Text
Bacteriocins and BAM: A Familicidal Love Story
A growing theme in this blog is the stunning ingenuity behind microbial assassinations, and the bacterial struggle for legroom is no exception. Competition for a small pool of available food and space is fierce and has led to the invention of dozens of classes of cell-killing compounds. One of these classes is the bacteriocins which play particularly dirty by singling out for death only close relatives of the perpetrator. A special kind of lectin-like bacteriocin has just been discovered to not enter the cell but target a vital surface protein known as BAM instead. Even family is not safe for these organisms!
You may have heard of one the bacteriocin Ricin. This deadly toxin, produced by the castor oil plant, adheres to a specific kind of sugar on the surface of cells and enters to wreak havoc on ribosomes, the machinery that create proteins. But there are many, many more types such as a pyocins and tailocins.

A rough sketch of LlpAbw, a type of lectin-like bacteriocin in Pseudomonas putida.
Pseudomonads, a famously adaptable bacteria genus found everywhere from hospitals to farms, acquired a special kind of lectin-like bacteriocin (LlpA). Lectins are simply molecules that bind with specific sugars. As pictured above, previous research shows that LlpAs are a two-domain huntress: The lectin-like C-domain attaches to a lipopolysaccharide (LPS) on the surface of the prey followed by a species-specific attack by the N-domain. Fascinatingly, LlpAs do not seem to have any toxic functionality just by looking at their structure. Until now, that was it; we didn’t know much else about how Pseudomonad LlpAs actually worked.
Belgian scientists this week weren’t satisfied and wanted to know how exactly Pseudomonads could distinguish and decimate only similar species using the N-domain. To do this, they took two LlpAs known to kill Pseudomonas fluorescens and P. aeruginosa and looked for mutant colonies that could resist the toxin.
The team found many kinds of mutations. Unsurprisingly, they detected changes to the gene coding for LPS – disguising the sugar that LlpAs need to dock with first would definitely hurt their search-and-destroy abilities. More interesting were several mutations in the gene behind a membrane protein known as BamA.
BamA is the most crucial part of the BAM complex, a five-piece cellular machine that takes newborn pore-shaped proteins known as β-barrels and sticks them in the bacteria’s cell membrane. BAM is essential for life and even in humans is still found in our mitochondria, which descended from ancient bacteria. Suffice to say, the discovery of a link between LlpA resistance and the innocuous BamA raised many questions.
To devise a scheme to prove that LlpAs can’t kill bacteria with changed BamA proteins, the researchers made new hybrid Pseudomonad strains. (Their paper is mischievously titled “Hitting with a BAM”) For example, one P. aeruginosa strain was, through controllable rings of DNA called plasmids, given two BamA genes from itself and another strain. When only its natural BamA was active, the bacteria were vulnerable to PyoL1, their natural LlpA killer. The paper, found, however, that by adding the foreign strain BamA to the cells they suddenly became vulnerable to Pyol2, the LlpA killer of the foreign strain. What’s more, they could then get rid of the natural BamA gene and PyoL1 weakness disappeared, but not PyoL2! Other Pseudomonad species were also tested and the same pattern was found, with few exceptions. It appeared that having a specific BamA protein was the key to being attacked by each unique LlpA bacteriocin N-domain.

A general structure and topology of BamA. Loops 6 is the important source of diversity for LlpA attack.
But what are the actual differences in BamA between strains? Thankfully, that didn’t end up being a hard question. All of the original mutations that granted immunity were found to be in or close to an exposed part of BamA called Loop 6. For even more reinforcement, an analysis of differences between and inside of Pseudomonad species revealed that Loops 4, 6, and 7 are the source of most BamA variety; however, LlpAs only seem to care about the sixth.
Imagine that you are a deadly LlpA compound looking for innocent bacteria to destroy. You are built by your host only able to latch on to specific kinds of BamA proteins using your N-domain, and can’t even detect BamA outside of a small range of Loop 6 codes. Now, you and your cousin coworkers each have different hit lists of different Loop 6 configurations and any Pseudomonad with a Loop 6 close enough to what’s on your list is susceptible to your assault. Therefore it is Loop 6, and not the species, that guides your hunt. More closely related species, however, were found to have matching Loop 6s more often, explaining your familicidal tendencies as an LlpA.
Think of it like a shape/hole matching puzzle. If Loop 6 has holes different from the shapes on your chemical structure, you won’t be able to fit in and bind with it.

A simple diagram of how LlpA chemicals can disrupt BamA, and ultimately murder an unsuspecting family member.
And so, now we know. The many N-terminal domains of LlpAs directly target specific Loop 6 sequences, binding with them as part of the attack. For the record, you needn’t worry about harming your creator; biology is smart and LlpAs created by a cell always target some Loop 6 different from its own. But could LlpAs be attacking BamA itself, not just using it as a stepping-stone?
That answer seems to be a yes. LlpAs, unlike most bacteriocins, don’t have any way to actually get inside the cell. Nor do they have any toxic structures to destroy the cell from the inside even if they somehow got in. What the compounds do have going for them is a very stiff form and strong binding abilities. Loop 6 is important to BAM function and binding to an LlpA could make it stuck and unable to do anything. Also, the vital yet not at all understood C-terminus extension of LlpA is positioned in a way that could block the hole in the BamA pore. Either mechanism would stop BAM from working and make a traffic jam of unfolded β-barrels waiting in line to be added to the cell membrane. Needless to say, the cell is disabled from the outside and won’t last long without them.
LlpAs are just one of many mechanisms used by bacteria to secure a safe space to grow, but as specialized assassins of close relatives they are ruthless. While medicine is interested in their applications as antibiotics, that is an incredibly long way off. Currently, the team is interesting in finding more concrete evidence about how L1pAs bind physically to Loop 6 and how this can lead to the death of the cell.
Statistics say that you’re more likely to be murdered by someone you know. Pseudomonad LlpA bacteriocins are in a deadly embrace with BamA that I think certainly aren’t proving that wisdom wrong.
2 notes
·
View notes
Text
Turning Hostile Marine Fungi Into a Force for Good to Fight Cancer
Just a small sample of the hundreds of potential anti-cancer agents discovered in saltwater fungi.
Fungi are more than just the mushrooms you put on your pizza: Some are giant and colourfully poisonous, while others are so small you wouldn’t even know they were there. What’s more, a great trove of fungi lies in the deep, uncharted regions of the marine ecosystem. Recently these salty sailors exploded in importance in the eyes of scientists trying to create new anti-cancer medicines.
The miraculous chemicals inside of fungi have been long-documented, from ancient homebrew remedies to the world-changing discovery of penicillin in 1928. Many types of fungi are unicellular and must encroach on the territory of hostile bacteria. How do they do this? By excreting specialized chemicals capable of killing all competition. In the case of bacteria, we named these chemicals ‘antibiotics’. Other metabolites (chemicals created by the fungi) have been discovered as potent anti-viral and anti-cancer agents, but until now the majority of research was restricted to land-grown fungi.
Quietly resting with potential is the sea, which holds many fungal treasures yet for medicine. One summary published in January details almost 200 kinds of potent cell-killing metabolites that tease of anti-cancer properties. All kinds of environments were combed across many recent studies, such as deep-ocean, marine sediment, and even mangroves! Any new fungi were tested by observing their ability to kill different human cancer cell lines and some showed deadly efficiency.
Unfortunately, for most metabolites we have no idea how they work. That isn’t a problem for scientists.
New drugs are usually discovered by taking a huge array of chemicals and using computer models and physical tests to look at their ability to do something to a specific process in a cell. In the case of cancer, targeting its ability to infinitely divide and form tumors is the most common tactic. Not only can our marine fungi toxins be used directly in these tests, but we can also use their structural skeletons as blueprints for thousands of new kinds of subtly different chemicals. The more types of chemicals we throw against cancerous enzymes and pathways, the more likely we are to eventually stumble on a winner.
These 200 new metabolites are prime ammunition. Cephalosporins are the only drug made so far from saltwater fungi, in 1964, but that may soon change. In an ironic twist, the deadly chemical killers of the ocean may soon join their land-based brethren in providing us with dozens of powerful new drugs.
1 note
·
View note
Text
Plastic is not good prey - Flipbook!
youtube
https://www.youtube.com/watch?v=WWHInu2A7WM
Today I'm bringing something very different... I tried my hand at a flip book! Garbage polluting the oceans is a Big issue, but less-known to most is the devasting effect microscopic plastic particles can have on zooplankton. Countless studies have shown that some creatures, such as copepods, mistake the plastic for phytoplankton.
This was my first time doing anything like this, and while it was incredibly fun there was definitely a lot of trial and error... and then a bit more error. The binding technique I tried to use ended up not working, meaning I had to salvage the entire thing with cellotape. I also, in hindsight, wish I chose heavier paper. Recording a video of it made flipping it very difficult! Either way, I believe my oscar-worthy plot more than makes up for all that nonsense.






3 notes
·
View notes
Text
Dinoflagellates are Cooking up Their Own Homebrewed Microbial Ballistics
We like to think that weapons are the tools of human warfare – complex machinations invented by our devious minds to hunt and kill. To think that our gadgets and gizmos, like Batman towards Gotham, are what separate us from the simple animals beneath us. New research from last year, however, is adding to a growing library of counterevidence that the natural world is in many ways more fierce and ingenious than humanity. Meet Polykrikos kofoidii and Nematodinium, two microscopic dinoflagellates with their own sophisticated array of ballistic weaponry.
Plankton are aquatic microorganisms mistakenly portrayed as passive due to their inability to fight against a current. In reality, many types of plankton employ agility, toxins, armour, and more for both self-defense and predation. One particularly interesting development is the poorly studied extrusomes, a broad category of projectile-launching organelles that includes toxic syringes and harpoons. Dinoflagellates make up a protist phylum that is often mixotropic; that is, they get energy from both the sun and by ingesting other organisms. Specialized extrusomes called nematocysts are the weapon of choice for these single-celled life forms and the biology of these tiny spear guns is a hot topic for modern research.
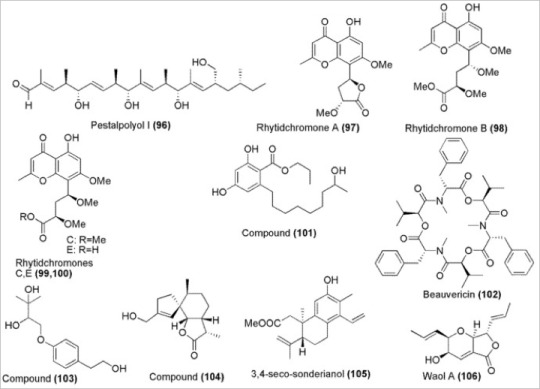
One study, published last year, aimed to find out whether dinoflagellate nematocysts are similar to the stinging mechanisms in jellyfish and anemones of the same name. Introducing the model organism, P. kofoidii. This deceptively explosive species uses a blasting cap–like hatch that hides a pointed stylet attached to a coiled rope called the tubule. When an unfortunate prey comes into contact with the cap, it bursts open and the prey is subsequently impaled and reeled in for consumption. Previously, our knowledge beyond this simple, if deadly, description of P. kofoidii was fairly limited. Using high-resolution video and focused ion beam scanning electron microscopy (FIB-SEM), the researchers set out with the goal of obtaining a detailed protein model of the nematocyst in their sights.
The illustration above shows the fruits of the research team’s labours. Each cell contains four ‘chutes’ which each contain a nematocyst. Plugging the hole, however, is the blasting cap, called a taeniocyst, which is attached via a special linker organelle to the nematocyst. Upon activation, the researchers were surprised to find that the initial projectile is actually the taeniocyst. The long, thin rod sticks onto the prey and possibly could guide the launch of the nematocyst to then spear it, though its exact function is currently unknown.
The mechanics of the nematocyst itself are more complicated. Following the ejection of the taeniocyst, the outside capsule of the nematocyst contracts and forces the stylet up into a sealed nozzle-like cap that is immediately pierced. Next, the ruptured nozzle expands the gasket and allows the tubule to uncoil and launch out and penetrate the prey. In yet another surprise the tubule is actually not the towline used to drag in prey; it quickly dissolves and is replaced by another filament originating from the opposite end of the nematocyst at the attached vesicle. Once firmly anchored, the filament is reeled back in along with P. kofoidii’s next meal!
So the question remains: Is this the ancestor of the jellyfish family of nematocysts? According to the researchers, the answer is no. Jellyfish use a simple corkscrew opening to fire a stinger with a propellant and due to the massive disparity in biology and complexity the chance of one having evolved from the other is slim to none. In fact, the enzyme responsible for the propellant in jellyfish does not even exist in P. kofoidii! Other genetics tests tell a similar tale: Nematocysts found in dinoflagellates are of their own invention.
What about other dinoflagellates? Good second question. Next, the researchers looked for similarities between P. kofoidii and other related species. Again using electron microscopy, they mapped the nematocysts of the elusive Nematodinium. The organelle they found was definitely related to P. kofoidii, using a compartmentalized stylet-nozzle-gasket firing mechanism, but it was also different in a terrifying new way.

Put simply, the nematocyst of Nematodinium is arranged like the famous gatling gun. Beneath a beautiful rose-shaped cap in a single capsule lies no taeniocyst but instead a ring of eleven to fifteen barrels full of painful untethered protist projectile. Historically, details of Nematodinium’s firing mechanism have been murky because the species does not have a blasting cap making it difficult to coax into shooting. The researchers circumvented this problem by discovering that once killed, the degrading weaponry of the cell will spontaneously misfire. What they saw was that the subcapsules expand like accordions, hinting that the propulsion mechanism could be based on structural pressure. It also appears that a single stylet initiates the launch of all barrels, making our gatling gun lookalike act, really, like a microscopic shotgun.
More specific details of the Nematodinium nematocyst are left for the future due to being too small to picture with FIB-SEM, but what we can see points towards strong common ancestry with P. kofoidii. It appears that dinoflagellates are in the middle of an arms race to develop new intimidating tools to attack other cells!
And believe me, complex and powerful means are needed to hunt other dinoflagellates. Many prey species have evolved near-impenetrable armor and their own defensive ballistic extrusomes such as toxicysts, poisonous spears able to kill or disable an overconfident predator. While the researchers admit that the idea that an arms race against these defensive adaptations is driving the creation of imposing ordnance is still a just a theory, you can’t lie that it is a tempting assumption. For example, rocket-stylets are able to puncture thick armor like in P. kofoidii, which is also able to ignore the disabling counter-projectiles launched by its immobilized prey as they are pulled to their inevitable demise. Plus, a system as seemingly overkill as Nematodinium’s multi-barreled shotgun is surely an indicator that these overengineered weapons evolved for a reason in the face of increasingly cunning prey.
Much remains to be learned about predatory dinoflagellates like P. kofoidii and Nematodinium, but this imaging study was a breakthrough into discovering a new world full of makeshift armaments more complicated than those found in any animal. It’s a global missile race headed towards annihilation where the stage is the invisible battlefield floating on the tides.
Further Reading – Detailed reconstructions, models, and video! https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5375639/
4 notes
·
View notes
Text
Quorum Quenching, or how to Blind a Bacteria
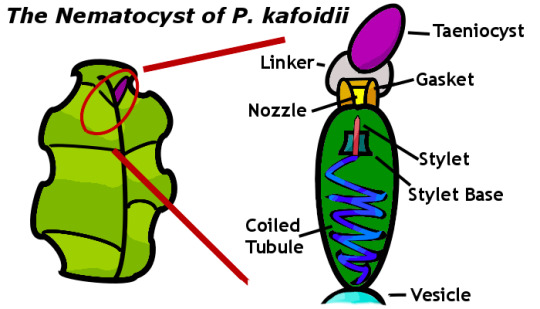
Possibly the simplest statement you could make to describe a bacterial cell is that it doesn’t have any eyes. Can it see anything? Are these cells aware of the world around them, or do they just lifelessly react to simple stimuli? To answer these questions there has been a burst of study on what is known as quorum sensing and its counterpart, quorum quenching.
Quorum sensing (QS) is a chemical technique that individual cells can use to judge the density of other cells around them or, put more simply, how busy their environment is. Gram-positive bacteria passively secrete signaling molecules called autoinducing peptides (AIPs) into the empty space around them. In normal circumstances the concentration of this chemical is too low to be detected by receptors on the surface of the cells. As shown in the figure above, however, when there are a large number of cells close together the concentration of AIPs becomes high enough to beat a ‘threshold’ level of required receptor activation to modify DNA expression! Gram-negative bacteria use N-Acyl homoserine lactones (AHL) in addition to AIP, which can skip the need for receptors entirely by binding directly to internal proteins. By capitalizing on this concentration-dependent gene expression, QS, bacteria can coordinate expensive activities like virulence and phosphorescence!
If QS is how cells can see the world, then quorum quenching (QQ) is the inevitable tool that other cells developed to disrupt it and dominate the environment. QQ works by blocking cell receptors, destroying QS messengers directly, or restricting the creation of messengers by enzymes. Target cells relying off of QS will be fooled and believe that their environment is different than it really is and will express the wrong genes at the wrong times, giving a leg up to the quenching source.
For instance, AHLs are degraded by proteins called amidases and lactonases. The gram-positive Bacillus bacteria are vulnerable to AHLs produced by foreign bacteria and do not use them for their own QS. To defend themselves from these toxic compounds Bacillus produce AiiA, a lactonase capable of breaking down AHL. In this scenario AHL is like a hostile antibiotic that Bacillus has gained immunity to through AiiA, but nearby gram-negative bacteria are left lost and confused without their AHL in the process!
Interestingly, sometimes AHLs are inhibited by the same organism that created them, like in P. aeruginosa. A pathogenic bacteria, P. aeruginosa colonizes host tissues and uses QS to swap energy from mobility-granting pili and flagella to toxins and other biofilm-related activities. When the time comes to swarm to other tissues, the bacteria emit lactonases to disrupt their own QS and revert back to their highly mobile and infectious form! This adaptive ability sheds light on the incredible versatility of QS regulation.
On the flip side, QQ really is rarely benign, even in P. aeruginosa. Past studies demonstrated that P. aeruginosa QS and QQ chemicals slow the growth of common yeast and help to outcompete it. More recent exploration, however, shows that yeast can mount its own defense and release specialized alcohols that muddle with P. aeruginosa QS. This two-sided battle has been frequently described as chemical warfare through QS.
QS is an exploding 21st-century field that is not yet even close to understood. The ability of bacteria to sense their surroundings, and in turn be trapped in an illusory world through QQ, is both amazing as biology and eyebrow-raising for its potential for new medicines. Either way, bacteria are clearly not blobs and much more active than we usually think!
Further Reading
https://academic.oup.com/femsre/article/40/1/86/2467695#61542538
1 note
·
View note
Link
Varroa Destructor - The Destroyer of Bees
It’s a tragedy we’ve yet to solve: Bee colonies across Europe and North America are dying off alarmingly fast in what is known as colony collapse disorder (CCD). In certain areas, like the USA, over 30% of colonies will disappear every single winter. Under great colony stress workers bees in a hive simply fly off and abandon their queen with nothing but a handful of nurses, larvae, and food, dooming her and her hive to extinction. Policy-makers and environmentalists are on the case to save the bees which as pollinators hold huge importance to both nature and the economy, but unfortunately the exact causes of CCD are not so easily broken down and solved.
Enter one chief perpetrator: Varroa destructor, a tiny 1mm UFO-shaped mite with a name suitably dramatic for the damage it inflicts on the hives of the European honey bee. The adult parasite hides beneath the abdominal plates on grown bees where it sucks their haemolymph, their blood, and spreads between colonies as worker bees swarm to establish new colonies or drift away from a collapsed hive. Inside the colony the mites will jump off and lay eggs inside of the combs of developing eggs and larva. These baby mites will feed on their counterpart baby bees and horrifically deform and stunt their growth, simultaneously spreading deadly pathogens such as the deformed wing virus. Ironically, Varroa mites are relatively harmless in their native land of Asia – Asiatic honey bees have biological and behavioural defenses such as grooming and entombing infected larva that neuter the ability of the mite to spread. In European honey bees with no such natural resistance, however, Varroa can spread like wildfire and will quickly stress, compromise, and eventually collapse myriads of hives.
There are many modern perils that threaten the humble honey bee yet they must be protected at all costs. Although more of a microparasite than a micropredator it is a microscopic mass-murderer and I highly encourage you to read more about Varroa destructor in this informational article. While your actions may be limited if you are not a beekeeper the damage this near-invisible bug causes demands as much awareness as possible!
1 note
·
View note
Text
About Me

Hello and welcome to my casual science blog! I’m Andrew Mulholland, a young lad from Fredericton, Canada currently at the tail end of my undergrad in Biochemistry. I’ve spent my entire life living here in a city anyone from a metropolis like Toronto would call just a town and grew up as a weird mixture of Boy Scout and closet nerd. Whenever I go travelling I am proud to represent the Maritimes. I have always been fascinated with science and in school it was never even a question that I would take all the science classes I could, so the natural follow-up was to attend University in that exact subject!
I had many different, changing, passions as a child. At different times I was massively into books, art, sci-fi, fantasy, certain sports like badminton (Though that petered out as I was never good enough to make a team past age 10), games, the outdoors, and more. Many of these things have stuck with me to some degree into adulthood, but most importantly of all, a vivid interest in space as a pre-teen quickly transformed into an interest in almost all natural sciences. These days I have a profound respect for the universe and the physics, the basic essence of everything, that somehow makes everything tick and miraculously allows life to exist. After all, biology and chemistry are the same thing as physics if you keep asking ‘Why?’ often enough. I show my respect for life and the world through enthusiasm with both nature and myself with hobbies and activities like nature walks and weightlifting.
Here in this blog we look at some of the coolest secret displays of nature. Earth can be intense: Everyone can picture a giant squid tearing at a sperm whale trying to eat it, or a pack of wolves stalking a moose. But what about the things we can’t see so easily? For example, the dramatic lives of bacteria, fungi, microscopic crustaceans, and worms? Thin films of deadly hunters, tentacles sucking out innards, and single cells armed with miniature harpoons? If for some reason these ideas don’t immediately pick up your interest then just wait and I will show you the amazing battles for survival that happen at tiniest of scales! Welcome, I repeat, to Battles Beneath the Microscope.
3 notes
·
View notes