Don't wanna be here? Send us removal request.
Text
Health Complexities in Pediatrics: The Saban Research Institute’s Annual Symposium

The Saban Research Institute's Annual Symposium will take place on February 19, 2020. This year's theme is Health Complexities in Pediatrics: Investigating Systems in Action and Interaction
The Saban Research Institute Annual Symposium highlights exciting strides that researchers at Children’s Hospital Los Angeles, the University of Southern California and from around the globe are making in understanding the developmental origins of health and disease. Each year, the theme of the symposium reflects a research priority area of The Saban Research Institute and the University of Southern California. This year’s symposium, “Health Complexities in Pediatrics: Investigating Systems in Action and Interaction,” features research on the complex system of interactions and factors—from environmental to the cellular level—affecting children’s health, well-being and long-term outcomes. Join us for this unique opportunity to hear from experts as they discuss a topic of tremendous relevance to our research enterprise. When: We dnesday, Feb. 19, 2020 | 8 a.m. – 5 p.m. Where: The Saban Research Institute of Children’s Hospital Los Angeles 4661 Sunset Blvd., Los Angeles, CA 90027 RSVP: 2020sabansymposium.eventbrite.com For questions, please email [email protected]

7 notes
·
View notes
Text
At the Heart of Research
Scientists at Children’s Hospital Los Angeles discover a mechanism that zebrafish use to regenerate damaged heart tissue. This research could lead to better treatments for babies in need of heart repair.
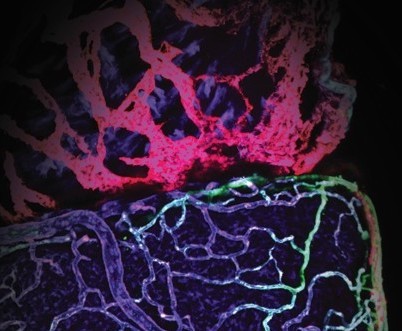
Cardiac lymphatic vessels (seen here in red) in the zebrafish heart help the fish repair damaged tissue after injury.
One of the reasons coronary heart disease is so deadly is that fluid build-up and scarring can develop in the heart tissue. This prevents the heart from contracting properly, impacting its ability to supply the body with fresh blood. If scarring is extensive, heart failure can result.
“We’re interested in zebrafish because of their incredible capacity for regeneration,” says Michael Harrison, PhD, a Research Associate in CHLA’s Saban Research Institute. These inch-long creatures are often studied in the field of cardiac repair because they can recover after extensive heart injury. “Zebrafish can clear the damaged tissue and replace it with fresh, functioning heart muscle,” he says. The question is: How are they able to do this?
Dr. Harrison works in the laboratory of Ching-Ling (Ellen) Lien, PhD, where the overall research goal is to uncover the mechanisms used by zebrafish to recover from injury. The answers gained from this research could help develop new treatments for babies with congenital heart defects or individuals who have suffered heart attack or other cardiac injury.
In addition to arteries and veins, many animals – including humans – have a network of lymphatic vessels. This system clears fluid and debris from tissue. Their malfunction may cause fluid build-up or limited clearing of damaged cells after heart injury in humans. “We don’t see that occurring in the zebrafish,” says Dr. Harrison, “so we began to investigate the cardiac lymphatic system in our laboratory.”
Lymphatic vessels are clear, unlike their larger and more colorful blood vessel counterparts. In addition to being hard to see literally, they have also flown under the radar in cardiac regeneration research. In fact, it was not known whether zebrafish even have cardiac lymphatic vessels. Dr. Harrison and Dr. Lien determined that zebrafish do have these vessels and that they could be a critical factor in heart repair. These findings suggest that the cardiac lymphatic system could be an important research focus in the field of cardiac repair.
In zebrafish with heart damage, the team made a striking observation – the cardiac lymphatic vessels grew and expanded to cover the wound area. When the investigators blocked this process, the fish were unable to repair their heart damage. “These experiments showed that the lymphatic vessels in the zebrafish heart are doing something critical,” says Dr. Harrison, “there’s definitely an important role there.”
Characterizing this role will be the subject of ongoing research. Understanding how the lymphatic vessels are aiding in repair could point scientists toward a treatment target. “We are looking very closely at these vessels, says Dr. Lien. “We think this is perhaps a new frontier in heart regeneration research.”
The study was recently published in the journal eLIFE. Additional authors on the study include Xidi Feng, Guqin Mo, Antonio Aguayo, Jessi Villafuerte, Tyler Yoshida, Caroline Alayne Pearson, and Stefan Schulte-Merker.
4 notes
·
View notes
Text
A Higher Resolution Image of Human Lung Development

The invention of interactive map applications has revolutionized wayfinding, providing an unprecedented level of information far beyond what printed road maps can offer. Researchers at Children’s Hospital Los Angeles are giving us a similar look into the anatomy of the human lung, and their findings could help babies breathe easier.
Infants born prematurely often suffer from poor lung development and can face life-threatening consequences. In order to provide novel treatments for these babies, we must first understand how cells of the lung differentiate and grow. But there are large knowledge gaps that must first be filled. Denise Al Alam, PhD, leads a team that studies development of the lung on a molecular and cellular level.
A new study, published online in European Respiratory Journal, marks an important milestone. “This is one of the very first studies looking at how the human lung develops at the single cell level,” says Dr. Al Alam. The study tracked the fate of these cells over time, showing a trajectory of cell development. Knowing when certain cell types are differentiating gives investigators a very detailed, time-lapse map to lung development.
Dr. Al Alam’s team focused on the development of two types of cells in the lung. Airway smooth muscle cells line structures such as the trachea, bronchial tubes, and smaller branches. Vascular smooth muscle cells are found in the walls of blood vessels. Both types of cells add muscle tone and stability while also mediating necessary movement within these structures. But they are often implicated in different disease pathways.
“The problem is that we can never really study one cell type without also studying the other,” says Dr. Al Alam, investigator in the Developmental Biology and Regenerative Medicine Program at The Saban Research Institute of CHLA. Up until now, investigators have used a marker – known as ACTA2 – to identify these cells. But the marker doesn’t distinguish between the two smooth muscle cell types. Although both types of cells are smooth muscle they are implicated in totally different pulmonary diseases. “We need to study the two types of cells independently to understand what happens in different lung disorders.” Fortunately, her work has solved this problem that has hindered research in the field.
Dr. Al Alam’s team has identified molecular markers that are unique to each subset of cells. This will allow investigators to differentiate between the two groups, which will benefit respiratory research efforts worldwide. Developmental disorders such as COPD, asthma, and bronchopulmonary dysplasia can now be studied separately from related, but distinct, conditions that arise later in life, such as pulmonary hypertension.
“It’s been decades since we’ve introduced anything into the clinic to treat lung diseases associated with prematurity,” says Dr. Al Alam. “We are putting together critical pieces so we can get these babies the treatments they need.”
The first author of the study was Soula Danopoulos, of The Saban Research Institute of CHLA. Other authors on the study were Soumyaroop Ghattacharya and Thomas J Mariani of the University of Rochester. The study was funded by the National Institutes of Health National Heart, Lung, and Blood Institute (R01HL141856, R01 DA037447) and the Hastings Center for Pulminary Research.
4 notes
·
View notes
Text
Well, That’s a First!
California’s first Surgeon General, Dr. Nadine Burke Harris, visits Children’s Hospital Los Angeles

One of the reasons to conduct research at a pediatric institution is that getting evidence-based answers sooner can lead to real changes in health outcomes for children. These benefits persist throughout an individual’s lifetime.
Pat Levitt, PhD, Chief Scientific Officer and investigator at CHLA’s Saban Research Institute runs a laboratory that focuses on early child development. He and others at CHLA study how early life stress adversely affects brain and body development. Their research shows that factors in the social environment can significantly impact brain and metabolic functioning. If we can use these discoveries to shape pediatric care and buffer early life stress, real differences can be achieved in lifelong health.
You know who agrees with this? Nadine Burke Harris, MD, MPH, FAAP, California’s current, and first, Surgeon General. Long before her appointment, Dr. Burke Harris began focusing on increasing health equity among diverse populations in California. She is passionate about using evidence-based practices to better serve children. Dr. Burke Harris was invited by Dr. Levitt to present at Pediatric Grand Rounds at CHLA and meet with leadership and clinical trainees.
Her presentation was stirring - it was based on data and hypotheses, but it was also full of passion and hope. Dr. Burke discussed the impact of adverse childhood experiences (or ACEs) on an individual’s health: ACEs increase risk for cardiovascular disease, cancer, diabetes, and mental illnesses later in life. She also presented plans to have California at the forefront of developing programs to mitigate the impact of ACEs.
Dr. Levitt shares the Surgeon General’s drive for translating research answers into real changes in health policy. He and Dr. Burke-Harris served on a committee that led to the July 2019 report Vibrant and Healthy Kids: Aligning Science, Practice, and Policy to Advance Health Equity. This publication represents an important step in translating scientific discoveries into real changes in health policy and pediatric practice.
Publication citation:
National Academies of Sciences, Engineering, and Medicine 2019. Vibrant and Healthy Kids: Aligning Science, Practice, and Policy to Advance Health Equity. Washington, DC: The National Academies Press. https://doi.org/10.17226/25466
Download for free here:
https://www.nap.edu/download/25466
0 notes
Text
CHLA Scientists Develop Breakthrough In Vitro Model

Scientists at Children’s Hospital Los Angeles develop first physiologically-accurate in vitro model of the human kidney glomerulus, representing a milestone in the field of kidney disease research
Kidneys work to constantly filter blood and remove toxins from the body. Conditions such as chronic kidney disease (CKD) are characterized by a reduced ability to perform this essential function. CKD incidence is growing and more than 1.4 million individuals depend on dialysis or kidney transplant for survival. Development of new treatments requires an understanding of the mechanisms of the disease progression, but scientists have not been able to accurately model kidney filtration in vitro – until now.
In a landmark study published in Nature Communications, scientists at Children’s Hospital Los Angeles demonstrate an in vitro kidney model that could change the course of research for diseases like CKD.
The kidney contains specialized structures called glomeruli. Within each glomerulus is a filtration barrier made up of two thin layers of highly specialized cells and a membrane that acts as a selective filter. As blood moves through each glomerulus, toxins and small molecules can pass through, while proteins and other important components are kept in the bloodstream. “This filtration process breaks down in patients with kidney disorders,” explains Laura Perin, PhD, who is co-senior author on the study along with Stefano Da Sacco, PhD. “But because we haven’t had a good in vitro model, we still don’t know the mechanisms of injury to the glomerulus in CKD.”
Dr. Perin and Dr. Da Sacco conduct research in the GOFARR Laboratory for Organ Regenerative Research and Cell Therapeutics in Urology along with co-director Roger De Filippo, MD, at CHLA’s Saban Research Institute. The lead author on the study was CHLA postdoctoral research fellow Astgik Petrosyan. Together, the team studies the structure of the glomerulus to better understand how and why their ability to filter blood breaks down.
“A big challenge in the kidney research field has been trying to replicate the glomerulus in vitro,” says Dr. Da Sacco. “In particular, the glomerular filtration barrier is very difficult to recreate in a lab using standard techniques.” Because of this, most published studies have used an artificial membrane between the two cell layers. While fluid can be exchanged, the cells cannot communicate across this membrane in the same way they do biologically. “This results in a model that doesn’t really filter properly,” he explains.
The critical component missing from current experiments is a filter that is selective and allows proper cell-to-cell communication. Dr. Da Sacco and Dr. Perin set out to grow healthy kidney cells in a way that allowed for the natural glomerular barrier to form, just as it does in the body. Using specialized, compartmented containers called OrganoplatesTM, the investigators did exactly that.
The result?
A model glomerulus that functions nearly identically to that found in real kidneys. They are calling this model, which is derived entirely from healthy, human kidney tissue, a glomerulus on a chip.
On one side of the cells, investigators add fluid and, on the other side, they collect what the ‘glomerulus’ filters, which is called the filtrate. In their experiment, the scientists added blood serum from healthy individuals. Without the use of a manufactured filter, the team’s in vitro glomerulus behaved as human kidneys are expected to act: proteins remained in the serum while smaller molecules passed into the filtrate. “The barrier that our cells naturally formed is selective, just as it would be in a fully-functioning kidney,” says Dr. Da Sacco. “It is remarkable.”
This model represents a substantial leap forward from the current standard of in vitro kidney research. “Our system behaves like a biologically, physiologically correct glomerulus,” says Dr. Perin. “This opens up the door for us to understand what we still don’t know – the molecular mechanisms of injury in CKD and, more importantly, how to prevent damage.”
While this seemed a distant goal in the past, Dr. Da Sacco and Dr. Perin are already recreating and studying the disease state in their model. When the investigators added serum from patients with CKD, they found that the glomerulus exhibited the same type of damage observed clinically: proteins began to leak through the compromised filter. Protein levels measured in the experimental filtrate matched patient clinical filtrate samples with a correlation of approximately 90%.
This breakthrough paves the way for numerous clinical applications. In the burgeoning era of personalized medicine, a preparation such as this can be used to examine molecular mechanisms of kidney damage in individual patients. Disease progression can also be monitored over time using serial blood sampling. Also, the model could be used for screening new drugs prior to human clinical testing.
Dr. Perin and Dr. Da Sacco, who are also Assistant Professors in the Keck School of Medicine at USC, are co-senior authors on the publication. Other authors are Paolo Cravedi of Icahn School of Medicine at Mount Sinai, NY; Valentina Villani of CHLA; Andrea Angeletti of the University of Bologna, Italy; Joaquin Manrique of Complejo Hospitalario de Navarra, Pamplona, Spain; Alessandra Renieri of Azienda Ospedaliera Universitaria Senese, Siena, Italy; and Roger De Filippo of CHLA.
The study was funded by the GOFARR Foundation and the Schenkman Family, grants from the Alport Syndrome Foundation, a TSRI Research Career Development Award, and a Wright Foundation Pilot Award. OrganoplatesTM were purchased from MIMETAS, who the authors wish to thank for providing invaluable training, technical support, and assistance.

Healthy human kidney cells (visible in red and green in this fluorescent image) grow in layers to form a filtration barrier in vitro. Image courtesy of Dr. Perin and Dr. Da Sacco.
3 notes
·
View notes
Text
Peter Chiarelli, MD, PhD Wins Prestigious Award for Nanoparticle Research

When it comes to pediatric brain cancers, research and treatments have made great strides. But some cancers do not respond to conventional treatments or grow deep within delicate areas of the brain, where surgical removal can be impossible.
Peter Chiarelli, MD, PhD, has received an award to support his research in the field of pediatric brain cancer. The Joint Pediatric Section of the American Association of Neurological Surgeons together with the Congress of Neurological Surgeons (AANS/CNS) awarded the prestigious grant to Dr. Chiarelli to fund his ongoing research in advancing radiation therapy. With a background in physics, chemistry, and medicine, Dr. Chiarelli is converging multiple fields of study to advance treatment paradigms of pediatric cancers.
Nanoparticles – ultra small molecules with a long list of potential functions – have revolutionized medicine. Nanoparticles are only about one millionth of a millimeter in diameter, making them 1,000 times smaller than the average human cell. They can be coated in medicine to deliver treatment or filled with dye for imaging purposes.
Dr. Chiarelli’s research explores an exciting new use for nanoparticles. He hopes to use them to increase specificity and effectiveness of radiation therapy for pediatric cancers. Dr. Chiarelli is working on a novel nanoparticle that can find and target tumors based on a specific marker the cancer cells have on their surface. When the patient receives radiation therapy, the nanoparticles within the cancer cells concentrate the radiation treatment, making it much more toxic to the cancer cells. The surrounding, healthy tissue will simply receive the same radiation beam because nanoparticles won’t invade normal cells. Preclinical studies are yielding promising results.
This type of innovation could revolutionize the field of radiation therapy. If cancer cells are killed more efficiently, clinicians may be able to reduce the dosage of radiation or the number of needed treatment sessions. While these nanoparticles are not yet in use clinically, Dr. Chiarelli’s work is certain to make an impressive impact on our ability to build hope and create healthier futures here at CHLA.
4 notes
·
View notes
Text
Precision Medicine for Pediatric Cancer

Research performed over the last several decades has led to an increased understanding of the genetics of cancer. The clinical application of this knowledge for pediatric cancer has lagged behind studies performed for adults. In a perspectives article published in the prestigious journal Science, Dr. Jaclyn Biegel, from Children’s Hospital Los Angeles, and Dr. Alejandro Sweet-Cordero, of the University of California, San Francisco, survey the landscape of this young field and present opportunities for using genomic information to advance a new era of care for children with cancer.
Cancer arises from genetic changes, including DNA mutations, that are either present at birth, or are acquired over time. But pediatric malignancies often develop from a very small number of mutations, only some of which overlap with the types of mutations seen in adult cancers. This makes diagnoses more challenging because tests developed for adult cancers are not necessarily effective for pediatric patients.
OncoKids® was one of the first next-generation sequencing panels to detect DNA and RNA changes that characterize pediatric cancers. The panel was developed at Children’s Hospital Los Angeles under the guidance of author Jaclyn Biegel, PhD, FACMG, Director of CHLA’s Center for Personalized Medicine. The OncoKids® panel provides a molecular diagnosis, informs prognosis, and highlights novel therapeutic targets across the broad spectrum of cancers in children, including leukemias, brain tumors and other solid tumors.
Dr. Biegel also explains that germline testing – which uses a sample of a patient’s blood to identify future cancer risk – is a critical component. “To truly achieve personalized medicine in pediatric oncology, we need to be able to determine if a child is genetically predisposed to develop cancer,” she says.
5 notes
·
View notes
Text
Children’s Hospital Los Angeles Hosts 10th Annual St. Baldrick’s Head Shave Event

For the tenth year in a row, doctors, family members, and the Los Angeles community came together to support cancer research at the St. Baldrick’s Annual Head Shave Event. This year’s event held particular significance for one of CHLA’s doctors, Robert Seeger, MD, MS (pictured below), who shaved his head for the 10th and final time.

As former Division Head for Basic and Translational Research of the Center for Cancer and Blood Diseases and longtime Director of Cancer Research at CHLA, Dr. Seeger has devoted his career to leadership and furthering cancer research. He is a highly-respected member of the CHLA community and we were proud to celebrate his final head shave! To top it off, Dr. Seeger’s son had his head shaved alongside his father. The successful event raised more than $10K for pediatric cancer.
3 notes
·
View notes
Text
Mental Healthcare ... in the NICCU?

Bringing a baby into the world involves many firsts – mothers and fathers are discovering their new roles, babies are learning what it means to live outside the womb, and the family is forging a relationship and bonding. But what happens when this time of uncertainty is complicated by medical issues?
Many infants born premature or with other complications often forego their first weeks or months at home for a stay in the neonatal intensive care unit. The NICCU is designed to deliver critical medical care to babies in need but can be traumatic for infants and their families, alike. In the Early Childhood Mental Health Program at Children’s Hospital Los Angeles, clinical psychologists Marian Williams, PhD, Patricia Lakatos, PhD, and a team of infant-family mental health specialists work towards greater mental health awareness in the NICCU.
Infants may not be the first age group called to mind in discussions of mental health. Yet, for babies in critical medical condition, Dr. Lakatos says an “infant mental health-informed perspective” could reduce stress and improve bonding with parents. This means not only focusing on the physical needs of the child but also the emotional and mental needs, not an easy task for newborn infants who cannot make their voices heard.
In an article published in Journal of Clinical Psychology in Medical Settings, Dr. Lakatos, Dr. Williams, and co-authors Tamara Matic, MD, and Melissa Carson, MD, advocate for a third component of the NICCU family – the relationship between baby and parents. “A lot of mental health work in NICCUs currently focus on either the mental health of parents or on the baby’s development,” says Dr. Williams, who is also the Director of the Stein Tikun Olam Infant-Family Mental Health Initiative at CHLA. “We also want to focus on the relationship between babies and their parents.”
Many parents of children in intensive care units experience symptoms of post-traumatic stress, which can threaten bonding with a newborn baby. In order to support the developing relationship between parents and their new baby, the CHLA infant mental health team turned to a model of intervention that has demonstrated success in families who have undergone trauma. Child-Parent Psychotherapy – or CPP – addresses the parent-child relationship directly, nurturing and advocating for it in its own right.
With funding from the Stein Tikun Olam Infant-Family Health Initiative, Drs. Williams and Lakatos, and the team were able to adapt CPP to the NICCU setting at Children’s Hospital Los Angeles. Their publication describes how the established, evidence-based CPP model can be used to nurture developing infant-parent relationships in the NICCU. While it has been implemented in other settings, CPP is not commonly integrated into NICCU patient care.
CPP is a flexible model that has multiple levels of intervention, depending upon individual family needs. Sessions with trained CPP providers can vary in number or duration, with the aim of restoring a developmental trajectory for parent and child. CPP providers advocate for mental health needs of parents and babies, working alongside their medical and social work colleagues. “When babies are in the hospital, we need to think about them, their parents, and their relationships,” says Dr. Lakatos.
Appropriately, NICCU medical staff focus on the acute physical needs of the child. Dr. Williams sees clinical psychologists in a necessary, complementary role. “These babies are eventually going home,” she says. “They are missing out on their bonding time, but there is great potential for resilience. Being mindful of the stressors these families are facing helps them feel understood and can set them on a positive trajectory.”
3 notes
·
View notes
Text
What Makes a Cell Turn Cancerous?
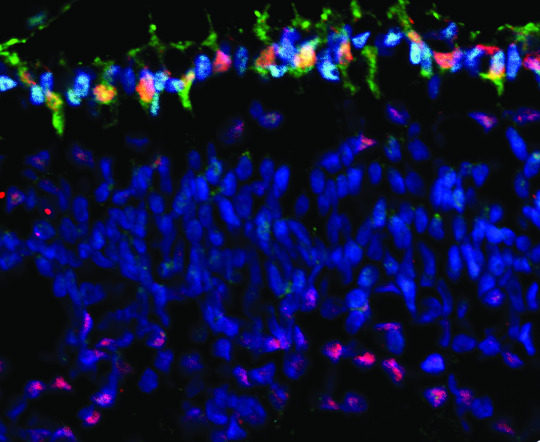
A cancer gene called MYCN (red) appears in high levels in cone cells (green). These cone cells can go on to form retinoblastoma tumors. Image provided by Dr. Hardeep Singh, Children's Hospital Los Angeles.
Retinoblastoma is a tumor of the retina that generally affects children under 5 years of age and accounts for approximately 4% of childhood cancers. If not diagnosed early, retinoblastoma may result in loss of one or both eyes and can be fatal. David Cobrinik, MD, PhD, of The Saban Research Institute and The Vision Center at Children’s Hospital Los Angeles, studies the development of this cancer. His team showed that retinoblastoma arises from abnormal proliferation of a cell type called cones in the retina, the light-sensing layer behind the eye. A mutation in a tumor-suppressing gene called RB effectively releases a brake on cell growth, causing cones to grow out of control and form a tumor. Children who inherit a mutated form of RB have more than a 95% chance of getting retinoblastoma. Given this strong correlation, an understanding of how RB mutations affect cone cells could lead scientists towards an intervention in the disease.
Dr. Cobrinik received a $1.6M grant from the National Cancer Institute of the NIH to study what causes cone cells to proliferate and form tumors. Though the genetic mutation is identified, we still must understand how RB mutation affects cone cells. What makes a cell turn cancerous? Why are cone cells vulnerable to this mutation? To answer these questions, Dr. Cobrinik explains, we must understand the normal function of RB and what it is doing to block tumor formation in healthy individuals. “If we can understand this,” Dr. Cobrinik says, “we might have the opportunity to prevent the tumor process.” While his research is focused on retinoblastoma, understanding how tumor-suppressing genes affect healthy cells could open up avenues for treatment in many cancer types.
19 notes
·
View notes
Text
Our #WomenInScience Rock!

They wear lab coats in place of capes, safety goggles instead of masks, and wield pipets - not swords. But they are still our heroes.
At CHLA, we are proud to honor women in science for International Women’s Day!
Please visit www.chla.org/womeninscience to learn about 6 talented women who are saving lives from the laboratory.
4 notes
·
View notes
Text
Solving the Mystery of the Silent Stroke
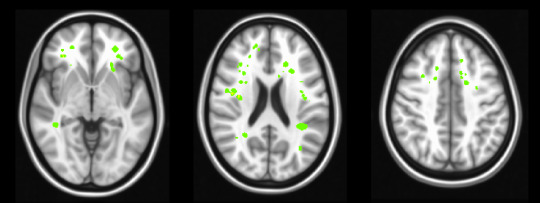
When blood flow is blocked in the brain, a small portion of brain tissue starves and dies – this is known as a stroke. By the time a patient with Sickle Cell Disease (SCD) is 30 years old, he or she has a 50% chance of having a stroke. Children’s Hospital Los Angeles physician and investigator John Wood, Ph.D. is out to discover why. He has dedicated his career to better understanding SCD and improving care for patients with this disease.
SCD occurs when a genetic mutation affects hemoglobin, the component of red blood cells that carries oxygen. This decreases the ability of blood cells to deliver oxygen to tissues throughout the body. It also causes blood cells to deform into a characteristic crescent shape and lodge into the smallest blood vessels, blocking blood flow. This causes excruciating bouts of pain called ‘crises,’ which can severely reduce quality of life. But lurking under the radar is a hidden complication in SCD: the silent stroke.
“We have learned a lot about how to prevent the large vessel strokes,” says Dr. Wood. He is referring to strokes that have immediate, catastrophic effects such as blindness or paralysis. “But patients with SCD still suffer from silent strokes.” Though they are called silent, these strokes can have debilitating effects on the brain’s executive function, which is the ability to execute complex tasks needed for things like maintaining a job or doing well in school. On the surface, the reason for these strokes seems clear: damaged blood cells carry less oxygen, which causes oxygen deprivation in the brain. Yet, Dr. Wood measured total oxygen delivery to the brain and found no decrease in SCD patients. This is because the body compensates for decreased blood oxygen by increasing total blood flow to the brain.
Dr. Wood was left with the question of why patients with SCD continued to suffer strokes. “I began to think, ‘maybe there’s a distribution problem,’” Dr. Wood says. “Total blood flow is fine, but is it going where it needs to go?” Using an advanced imaging technique called arterial spin labeling to measure blood flow, Dr. Wood’s team found a disparity among brain areas. While total oxygen delivery to the brain was unchanged, oxygen delivery to the white matter was reduced by more than a third – 35% – in patients with SCD. Critically, this part of the brain – the white matter – is where the majority of silent strokes occur in these patients.
Grey matter is composed of neurons, the brain cells that store information. White matter is the network of highways in the brain that neurons use to transmit this information. Dr. Wood’s finding demonstrates that the body differentiates between grey and white matter, clearly prioritizing neurons. This makes sense because keeping neurons alive is critical for survival. Strokes in grey matter are immediately debilitating, while strokes in white matter appear silent because they simply cause informational processing to slow. Though they may not cause paralysis or major motor deficits, effects of white matter silent strokes can greatly impede important aspects of a person’s day-to-day life. Dr. Wood’s study gives us a much better understanding of why these strokes are occurring, knowledge that is necessary before medicine can effectively treat this aspect of the disease.
Results of this study were published recently in the American Journal of Hematology by lead author Yaqiong Chai, a senior PhD candidate. The study was funded by the National Heart Lung and Blood Institute of the National Institutes of Health.
Other authors on the study include Adam M Bush, PhD; Julie Coloigner, PhD; Aart J Nederveen, PhD; Benita Tamrazi, MD; Chau Vu, MS; Soyoung Choi; Thomas D Coates, MD; and Natasha Lepore, PhD.
Image caption: silent cerebral infarcts (strokes) are shown in green, overlayed on a structural MRI image. Note that the strokes are found predominantly in the white matter of the brain.
3 notes
·
View notes
Text
I Heart Zebrafish
How a fish smaller than a pinky finger holds big clues to saving hearts
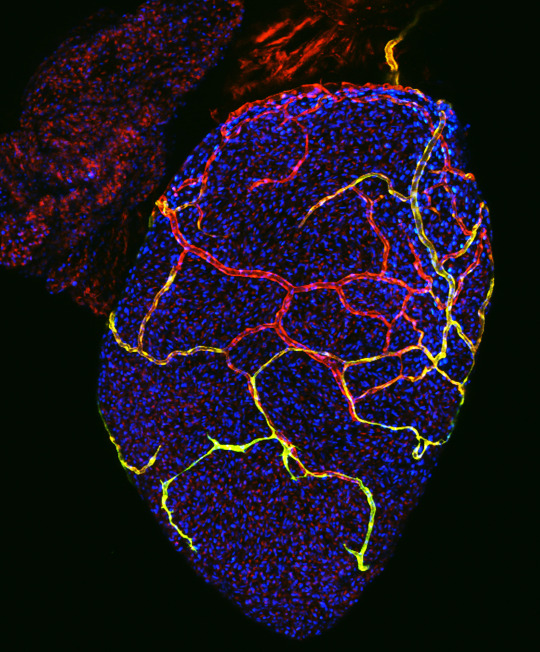
What do zebrafish and newborn babies have in common? Both are small, yet hold vast potential for the future. But these two organisms are more intertwined than you might think.
Ching-Ling (Ellen) Lien, PhD, of Children’s Hospital Los Angeles studies zebrafish because of their incredible regenerative capacity. The heart of this inch-long creature is no wider than the tip of a pen, yet its cells are mighty, allowing it to regenerate after extensive damage. Dr. Lien is looking to uncover the molecular pathways zebrafish use to regenerate so that medicine can one day apply this knowledge to regrow human hearts.
Heart failure is the leading cause of death worldwide, and not just for adults. Babies born with congenital cardiac defects can also experience heart failure because the physical structure of this vital organ is simply not strong enough. If new heart tissue can be regenerated by a patient’s own body, babies with severe heart defects wouldn’t have to depend on transplants.
While we are not there quite yet, Dr. Lien has made key discoveries that are bringing us closer to this goal. For example, she studied genetic zebrafish mutants to pinpoint key genes in the regeneration pathway. “Our lab is the first to discover that a signaling pathway called Cxcl12-Cxcr4 is critical for the development of coronary blood vessels,” she says. “These vessels support the growth and regeneration of heart tissue in the zebrafish. This opens the door for our understanding of the early signaling events in tissue regeneration.”
Findings like these show us that zebrafish, for their miniscule size, provide important information we can someday use to help babies thrive.
Image caption: coronary blood vessels surround and nourish a zebrafish heart
Image courtesy of Ellen Lien, PhD, Children’s Hospital Los Angeles and the Cellular Imaging Core at Children’s Hospital Los Angeles
8 notes
·
View notes
Text
Wired for Obesity
Scientists pinpoint a set of genes that may wire the body weight center of the brain
Eating and exercise have the power to shape our bodies but those activities are controlled by hard-wired circuits in the brain. This means that when these neural circuits are developing is a critical time for determining how our brains will regulate our body weight, even in adulthood. What remains a mystery is just how these circuits achieve the proper wiring. Why do certain brain cells connect to one area while specifically avoiding other, nearby cells? Understanding how brain cells in the hypothalamus form these specific, complex connections – and how this process can be adversely affected – could provide insight into the development of childhood obesity.
In a study published recently in the journal Cell, investigators at Children’s Hospital Los Angeles and the University of Cambridge uncover key genes that guide the process of brain development. These genes instruct the body to build a set of molecules that act as a road map, guiding developing neurons to form circuits in the body weight center of the brain.
Sebastien Bouret, PhD of the Saban Research Institute at CHLA leads a team of researchers that study the formation of these circuits. “We know that the brain, in particular an area called the hypothalamus, has a very important role in the regulation of food intake and blood sugar,” explains Dr. Bouret, who is also an associate professor of pediatrics at the Keck School of Medicine of USC. Researchers have focused on the hypothalamus for years in an effort to study the epidemic of obesity, which affects nearly 14 million children and adolescents in the United States. “What we don’t yet understand,” he says, “is how these circuits in the hypothalamus are being organized. We want to know how the brain puts itself together and what exactly governs that process.” Understanding this is key because circuits must be established properly in order for the brain to ultimately perform complex functions like maintaining proper weight.
Enter the semaphorins – small signaling molecules found in abundance in the developing hypothalamus. Bouret wondered whether these semaphorins could help form body weight circuits. Dr. Sophie Croizier, who led the study in Dr. Bouret’s lab, blocked semaphorin signaling in cells of the hypothalamus. She discovered that brain cells no longer grew the way they were supposed to, showing that semaphorin provides an essential map for them to follow. In addition to connections failing to establish, loss of semaphorin action in a preclinical model also caused elevated body weight. “What we are seeing is that semaphorins are guiding and shaping development of hypothalamic circuits that ultimately regulate calorie intake,” explains Dr. Bouret.
But the story does not stop here.
Professor Sadaf Farooqi, PhD, FRCP, FMedSci from the University of Cambridge was also analyzing genetic information from individuals with obesity. Dr. Farooqi’s team tested 1,000 DNA samples and found that individuals with early-onset obesity harbored more rare mutations in genes involved in semaphorin signaling than healthy individuals. The finding that people with obesity have these mutations shows that semaphorins are important in maintaining healthy body weight. “We have now discovered the genes that establish the precise neural connections that form these circuits,” says Dr. Agatha van der Klaauw, who led the study in Dr. Farooqi’s lab and is co-first author on the paper.
This study reveals a much clearer picture of what occurs in the developing brain. Semaphorin signaling appears to shape the physical architecture of the brain and influence circuitry governing body weight.
9 notes
·
View notes
Text
What Can Blood Flow Tell Us About Autism?
With autism diagnoses on the rise, science is on the hunt for answers. New research at Children’s Hospital Los Angeles uses advanced imaging techniques to uncover some clues.
Each year, one in 59 children are diagnosed with Autism Spectrum Disorder (ASD), a condition that impacts a child’s social, emotional, and behavioral development. The rate of diagnosis has grown, tripling in the last 15 years. While genetic and environmental influences have been implicated as potential causes of ASD, little is known about its neurobiology.
Now, researchers at Children’s Hospital Los Angeles have brought us one step closer.
In a recent study, CHLA’s Bradley Peterson, MD, uncovers a direct link between altered brain activity and social deficits in ASD. Peterson’s group studied 44 individuals with ASD and compared them with 66 typically-developing participants. Groups were matched for age, sex, and IQ.
Peterson’s team measured two types of information. First, the group used a method called arterial spin labeling, which measures blood flow through the vessels of the brain. Because active parts of the brain need the most oxygen and nutrients, more blood flow to an area signals increased brain activity. Second, the team measured levels of NAA, an amino acid byproduct commonly used as a marker of healthy neurons.
“This is a multimodal imaging data set,” explains Peterson, Director of the Institute for the Developing Mind at CHLA and Professor of Pediatrics at the Keck School of Medicine of USC. “Each modality gives us a different window into the brain. We are able to look through both windows at once to tell us much more about what’s going on in the brains of these individuals.”
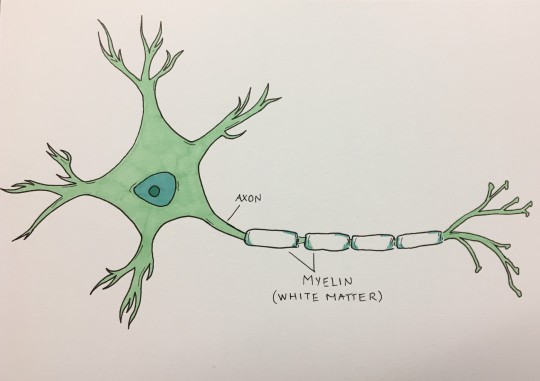
Scans revealed a striking pattern in the part of the brain called the white matter.
Our brains have about 100 billion cells, which communicate with each other through long, wire-like branches called axons. These axons are coated with myelin, a specialized wrapping – like wire insulation – that helps the messages flow faster from one cell to another. Because myelin appears white, communication pathways between cells are collectively called white matter. Cell bodies, or gray matter, are not coated as extensively in myelin and therefore do not appear white.
Studies show that communication between distant brain cells is disrupted in ASD due to fewer long-range connections between cells and thinner myelin. Given these differences in white matter, decreased blood flow and activity in this region would make sense.
Yet, Peterson and his team found exactly the opposite.
In the study, the researchers found what is called hyperperfusion – increased blood flow, indicating more brain activity – throughout large portions of white matter in participants with ASD. Perhaps even more striking, these activity rates were correlated with ADOS scores; ADOS is a tool used by doctors to help diagnose ASD.
If white matter is compromised in ASD, one might expect decreased activity in this region, not increased. Peterson explains that this finding likely reveals an attempt to compensate for underlying white matter problems. “If the drive train in your car is compromised, you have to hit the gas harder to achieve the same speed,” he explains. Similarly, supporting cells in the brain that create and maintain the myelin wrapping seem to be working overtime to counteract deficits in the underlying axon. This compensatory mechanism may partially explain why many individuals with ASD are high functioning. “This correlation of perfusion and ADOS is absolutely key,” emphasizes Peterson, “because it shows that the higher the blood flow, the more ASD symptoms the participants have. This very solidly supports the idea of compensation.”
Looking in the second window – measuring NAA concentrations as a marker for healthy neurons – revealed support for this idea of compensation. “We found that in participants with autism, the lower the NAA concentrations were, the higher their perfusion was at those points in the brain,” Peterson says. In other words, areas with the lowest levels of healthy neurons were also the ones with the highest activity and blood flow.
The majority of autism research has focused on other parts of neurons, as opposed to axons. Peterson’s study marks the first to correlate widespread white matter hyperperfusion, healthy neuron biomarkers, and symptom scores. The study paves the way for understanding more about how the brain may compensate for compromised white matter signaling. “Axons and their supporting cells have not been a major focus in autism research,” Peterson says. “My hope is that this study will help to refocus attention on the cell types and brain areas that are critical in understanding the neurological basis for ASD.”
Additional authors on the study include Ariana Zargarian, Jarod B. Peterson, Suzanne Goh, Siddhant Sawardekar, Steven C. R. Williams, David J. Lythgoe, Fernando O. Zelaya, and Ravi Bansal.
Image caption:
A neuron sends messages through its axon, which appears segmented in the image due to the myelin wrapping. Myelin speeds up messages sent from one neuron to another.
3 notes
·
View notes
Text
Bayanihan (buy-uh-nee-hun): Working Together to Effect Change
Bayanihan is a Filipino term that means working together in the community to accomplish a difficult task.

It is a generation ago. A young girl named Joyce was born in Historic Filipinotown, Los Angeles – where many immigrants from the Philippines often first settle. Her parents and grandparents came to the United States, like so many others from the Philippines, a journey born of the promise for a better life for generations to come. This journey is not easy, nor does it stop once new roots are carefully planted and tended in their new community. Immigrants are burdened with desires to maintain their strong cultural traditions while learning the customs of their new country. External factors like bullying and discrimination further complicate the struggle. Joyce sees firsthand how these difficulties can translate into poor health outcomes in her community.
No longer a little girl, Joyce Javier, MD, MPH, MS, has earned degrees in medicine and public health to arm herself to make a change. She seeks to continue the journey whose first steps were taken not long ago by her family. Dr. Javier makes this voyage for her own daughters and for the rest of her Filipino community. But hers is not a geographical journey. She will not have to brave leaving her own country in search of opportunities like her grandparents and parents did. Instead, she seeks to better the lives of generations to come right here in Los Angeles. Dr. Javier partners with the community to address and tackle the issues they face. As it turns out, these issues are life and death.
Asian American and Pacific Islanders age 15-24 are the only racial/ethnic group for whom suicide is the number one cause. Suicide. It bears repeating.
This staggering fact demonstrates a severe necessity to better serve the mental health needs of minority populations. “As a pediatrician and a mother of two daughters in their pre-teens, these are scary statistics,” says Dr. Javier. External pressures such as bullying or racism can affect an individual’s mental health. Ideally, mental health should be addressed early on. Dr. Javier says it’s “not just mental health, but building resilience so that kids have the tools they’ll need to overcome life’s hardest challenges.”
Dr. Javier works closely with other clinicians and leaders of her community to recruit families into a parenting program called The Incredible Years®, which has demonstrated success in improving family life. While this may seem simple, it is not. Parents are hesitant to enroll, likely because they feel like they are asking for help. Yet, once families complete the program, parents report reduced stress and improved family dynamics. These are protective factors against youth conduct problems and devastating outcomes such as suicide.
In a recent study published in Pediatrics, the official journal of the American Academy of Pediatrics, Dr. Javier and co-authors, Dean Coffey, Lawrence Palinkas, Michele Kipke, Jeanne Miranda, and Sheree Schrager demonstrate a successful method for recruiting families to participate in the parenting program. Along with parents in the community, Dr. Javier made a video (available to watch here) featuring parents and grandparents of the community who had participated in – and benefitted from – The Incredible Years® program. This culturally-tailored video was a success in Dr. Javier’s randomized controlled trial. From here, there is nowhere to go but up for Dr. Javier’s undertaking.
It is clear that she is on a path to drastically improve the lives of those in her community. By partnering with parents like herself, she is making a change. For more information on her study, please visit https://filipinofamilyhealth.com/.
Image caption: Dr. Javier as a young girl
3 notes
·
View notes
Text
Nature, Nurture, and Neurodevelopment
CHLA’s Dr. Bradley Peterson weighs in on what it takes to develop a human brain

In a public town-hall style event, CHLA’s Dr. Bradley Peterson spoke amongst a panel of early childhood specialists. The event was organized by KPCC In Person and hosted by Priska Neely, who covers early childhood and the issues young children face.
In addition to Peterson, the well-rounded panel of four included author Roma Khetarpal, UCLA pediatrician Adam Schickedanz, and early childhood education expert Schellee Rocher. Each panelist spoke one-on-one with Neely before fielding a wide range of parenting questions from the audience.
Designated by Neely as the “brain guy,” Peterson painted an elaborate picture for the event’s host and the audience about neurodevelopment. “You can think of the developing brain as a sphere,” he began. “A cross-section of this sphere through its middle might resemble a bicycle wheel. It’s organized with spokes radiating outward towards the tire, or surface of the brain.” Peterson likened these spokes to neurons. “Neurons are the brain cells that pass information and allow us to think, behave, and have emotions,” he explained.
Peterson went on to say that, at birth, the architecture of the brain is very sparse compared to what it will soon become. Soon after birth, he said, the brain cells begin to sprout and grow dendrites – the branching, bushy structures that receive information from other brain cells. Cells begin to connect to one another, forming dense, complex circuitry. “Unbelievably quickly,” Peterson continued, “in the first months of life, the spokes begin to fill in.” The architecture of the brain is being built at lightning speed in this first year of life. It is this growing complexity, he said, that allows new information to be passed from one part of the brain to another. This dense web of interconnected brain cells sets the very foundation of all emerging capacities in early childhood – things like walking, talking, thinking, and relating socially to others.
Sometimes, the brain is compared to a computer. This analogy speaks to the brain’s incredible computational capacity, though it does not acknowledge some of its more mysterious aspects– things like personality or a person’s ability to love. Peterson expanded upon the intrigue of the brain’s capabilities, explaining that the ability to learn and physically reshape itself sets it apart from its machine counterpart. “A one-year-old is just beginning to walk and say a few words. In the next year or so, that child will be capable of hundreds of words. No computer can teach itself to do that. This ability is all due to the developing architecture of the human brain.”
This ability to learn can be found in our DNA long before a brain even begins to develop. Written into the very code from which all our proteins, cells, and tissues are born is the ability of the brain to be molded by our surroundings, a stunning feat of adaptability. Unlike a computer, the human brain grows and learns in a way that is largely dependent upon experience, which actually shapes the physical architecture of the brain. Peterson explained that the dense, bushy web of interconnected cells in a two-year-old’s brain is actually richer in connections than in an adult’s brain – 50% more dense. The environment, he said, guides the brain in which connections will survive and become permanent, while pruning the irrelevant circuits. “It’s a dance between the developing organism, with all its hard-wired programming, and the environment – each interacts with, and affects, the other.” What we are left with is imagery of a blossoming array of neural connections that begins to explode within the first two years of life, and is then masterfully whittled away as we are shaped by our experience. The mature brain is perhaps less a computer than it is a sculpted work of art made by our genes and the world that surrounds us.
Click here to visit the KPCC In Person event page with full video of event.

7 notes
·
View notes