Text
Editing gene editing out of the definition of genetic engineering
The Royal Society kickstarted the national conversation on the use of gene editing. It says that it is “important that New Zealand develop its own view”, but it is using language that better constructs its desired outcome: that “it’s time for an overhaul of the regulations”.
In New Zealand, the European Union and Australia, gene/genome editing techniques are processes that trigger regulations of the products. In all these jurisdictions industry and some public sector scientists are campaigning to either remove this trigger or exempt some processes. The campaign has worked in Australia to deregulate some, but far from all, gene editing processes.
I’ve written before about the different forms of regulation. In this article, I’m going to focus on the push to define genome editing as out of scope of our Hazardous Substances and New Organisms (HSNO) Act, the legislation that is specific to evaluating the living things we make using gene technology. The arguments being used in New Zealand are common everywhere. One whopper I’ll address is that genome editing techniques are not in vitro techniques, those that define genetic engineering.
Manipulation of the in vivo/in vitro distinction that qualifies some techniques for regulation is catching out vulnerable science journalists, leading to little critical coverage of the argument on regulation. Here is an example from New Zealand media:
As part of a series on gene editing, The Royal Society Te Apārangi’s Gene Editing Panel has produced a paper looking at whether New Zealand’s regulatory framework is still fit for purpose…To be genetically modified the genes need to have been modified or derived from something made “in vitro” – a laboratory vessel. Science has advanced to the point where CRISPR can be applied in living cells, not in a laboratory vessel. The current definition in the main piece of legislation created to regulate gene technology doesn’t reflect where science is at. Other legislation could also come into play when making decisions around the release of a genetically-edited organism, but because much of the legislation was written before the science, it’s a bit like trying to jam a square peg into a round hole.
CRISPR in the above quote is shorthand for one site-directed gene/genome editing technique using a guide RNA (CRISPR) and a nuclease (Cas9). It doesn’t matter that the reporter only mentions this special case because her argument applies to all site-directed techniques including ZFN, TALENs and oligonucleotide-directed mutagenesis.
There are so many things wrong in this short text that it is hard to know where to start to unravel it. I’ll start with the biggest and most misleading statement: “Science has advanced to the point where CRISPR can be applied in living cells, not in a laboratory vessel.” This little gem is also tripping up many of my colleagues and regulators.
In vivo The reason that it is important is that the use of ‘in vitro techniques’ is a key trigger for regulation in countries with “process-based” regulations. The term is not identical in all jurisdictions but it is the touchstone in New Zealand’s legislation. A High Court decision in 2014 confirmed that it was. It may seem surprising at first, but the same Court rejected the notion that modifying genes or other genetic material in vivo - within the cell – did not make them in vitro techniques.
That might sound strange at first, but the Court had solid grounds for doing so. If we were to say that gene modifying techniques must only modify genes or other genetic material in vitro, there would be no genetically modified organisms. This is because all the techniques used since the late 1970s have an in vivo stage where the genes or genetic material of a living organism are modified (see Figure). To deny that genetically modified organisms exist would be obvious nonsense and out of step with intellectual property rights law and the global consensus expressed through many international agreements from Codex Alimentarius through TRIPS on to the Convention on Biological Diversity.
The poster-child technique of genetic engineering is to insert a recombinant DNA molecule into a living cell (Figure). Alone in a test tube, a recombinant DNA molecule is neither an organism nor is it alive or able to reproduce. Putting it into a living cell is a different matter. The recombinant DNA molecule modifies the genetic material by integration into or recombination with another DNA molecule (modifying genes) or by self-directed replication (modifying genetic material). This all happens in vivo.
I’ve adapted a nice cartoon made by Rebecca Mackelprang to illustrate the steps that techniques which everyone agrees are genetic engineering have in common with the steps of the techniques called genome editing (Figure). (Note, that was not Dr Mackelprang’s use of the cartoon.) When you align the procedures you see pretty much the same use of in vitro techniques too. The change in the living cell in all cases is due to enzyme-mediated reactions that result in a change to genes or other genetic material. The ingredients are different, as they would be for a chocolate or vanilla cake, but both are cakes after baking. The exception may be chemical and radiation mutagenesis which are defined as creating genetically modified organisms (but usually exempted from regulations).
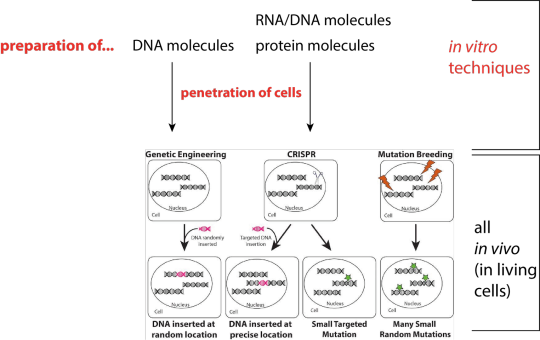
Figure of a common depiction of genetic engineering and genome editing techniques, adapted to show the respective in vitro (highlighted in red) and in vivo stages resulting in modified genes or other genetic material. Modified from https://theconversation.com/organic-farming-with-gene-editing-an-oxymoron-or-a-tool-for-sustainable-agriculture-101585. Rebecca Mackelprang, CC BY-SA
Genetic material The term ‘genetic material’ used in legislation may be contributing to the misunderstandings of place – either in vivo or vitro - where genes or other genetic material are modified. For a large proportion of my molecular biology trained colleagues, genetic material means essentially the same thing as genes, and genes for them mean DNA. In the phrase ‘genes or other genetic material’ used in the HSNO Act, I imagine that they think that the genetic material is both DNA and RNA. However, the legislation written as ‘genes or other genetic material’ was not as trivial in meaning as “DNA or RNA”.
Beginning with the 22nd Session of the Conference of FAO (held in 1983) it was decided that the “‘base collection of plant genetic resources’ [genetic resources are defined as genetic material with value] means a collection of seed stock or vegetative propagating material (ranging from tissue cultures to whole plants)…” (emphasis added to UNFAO 1983). Therefore, and for some considerable time1, genetic material has been a term to mean organisms, seeds, zygotes and cuttings, not just DNA or chromosomes. Treatments that modify organisms, seeds, zygotes and cuttings using in vitro techniques create genetically modified organisms. All of these treatments have at least a final in vivo step.
The reactions that modify genes or other genetic material using older techniques also happen in vivo but rely on critical in vitro steps including making the mutagen (e.g. a recombinant DNA molecule) and penetrating a living cell to cause the mutagen to change genes or other genetic material. The EU directives describe it similarly as “the formation of new combinations of genetic material by the insertion of nucleic acid molecules produced by whatever means outside an organism, into any virus, bacterial plasmid or other vector system and their incorporation into a host organism in which they do not naturally occur but in which they are capable of continued propagation.” You see they carefully separate the “nucleic acid molecules” from the “genetic material”; only the latter is modified.
This is the same for all the newer editing methods too. They require penetrating a living cell with the mutagen (e.g. an editing protein or its mRNA). Recombinant DNA molecules (“nucleic acid molecules”) are made using in vitro techniques, but so are the editing proteins and in the case of CRISPR/Cas9, also the guide RNA (a nucleic acid molecule).
Journalist beware! Beware science journalists when you are told that genome editing is “new” and “different” from genetic engineering. Editing is not new because the ability to use site-directed techniques in vivo predates the discovery of the current editing proteins such as ZFNs, TALENs and Cas9 (of CRISPR/Cas9). Site-directed techniques such as oligo-nucleotide mutagenesis were being used as early as the 1980s in some organisms. What is different now is that the editing proteins work in just about all organisms and they are in some cases even more efficient and flexible than the older techniques. However, it is misleading to say that the laws in Australia, the European Union and New Zealand, among many other countries, were written when such techniques were not even imagined. They were written well after such techniques were in use (for review see Heinemann 2015).
The conversations happening in many countries simultaneously around the world on regulating genome editing has disproportionately privileged certain voices that have forgotten the roots of their science. I would also fail the test as a bona fide science historian! Therefore, my purpose here is not a history lesson but to add another dimension to the conversation that I don’t see from our scientific organisations such as the Royal Society. Sadly, I’ve also not seen much effort from our New Zealand science media to challenge the scientific institutions’ roles in this conversation.
1(A) “Referring to genetic material as “any material of plant origin, including reproductive and vegetative propagating material, containing functional units of heredity” has a long tradition in management of GRFA [genetic resources for food and agriculture], and is consistent with classical applied genetics because the predominant tool is breeding. The agricultural genetics literature from the 1940s explicitly equated ‘genetic material’ with that which could recreate the plant (seeds or propagules) (Weiss, 1943)” (Heinemann et al. 2018). (B) “(a) ‘plant genetic resources’ means the reproductive or vegetative propagating material of the following categories of plants: • (i) cultivated varieties (cultivars) in current use and newly developed varieties; (ii) obsolete cultivars; (iii) primitive cultivars (land races); (iv) wild and weed species, near relatives of cultivated varieties; (v) special genetic stocks (including elite and current breeders' lines and mutants);” (UNFAO 1983). (C) “Genetic resources (GRs) refer to genetic material of actual or potential value. Genetic material is any material of plant, animal, microbial or other origin containing functional units of heredity. Examples include material of plant, animal, or microbial origin, such as medicinal plants, agricultural crops and animal breeds” (emphasis added). Source: World Intellectual Property Organization (WIPO) https://www.wipo.int/tk/en/genetic/
References
Heinemann, J.A. 2015. Expert scientific opinion on the status of certain new techniques of genetic modification under Directive 2001/18/EC. Commissioned by Greenpeace International.
Heinemann, J.A.; Coray, D.S.; Thaler, D.S. Exploratory fact-finding scoping study on “Digital Sequence Information” on genetic resources for food and agriculture. Background Study Paper NO. 68. Background Study Papers: United Nations Food and Agriculture Organisation; 2018.
UNFAO. Resolution 8/83 International Undertaking on Plant Genetic Resources. The Conference; 1983.
0 notes
Text
Is product-based regulation of biotechnology code for no regulation?
The Royal Society released a discussion paper to serve the national conversation on the use of gene editing in primary industry. They say that they want our views, but Royal Society representatives in the media seem to know what the answer should be already: deregulate. This seems all too familiar.
Father: Son, we need to have a new conversation about mowing the lawn.
Son: Why?
Father: The old conversation hasn’t worked.
Son: OK. Well, let me tell you about the mower's bad wh…
Father: Just do it.
Son: Wait, what? I was just telling you that…
Father: This lawn frankly is a mess and is indefensible. You need to fix it up.
Son: Thanks for the conversation, dad.
Sometimes a conversation is a willing exchange of views between different people. But conversation can also mean that someone just has something to tell you. I’m getting the impression that this is what the Royal Society means when it asks us to discuss gene editing.
The Society’s discussion paper is described as aiming "to continue our national conversation about the use of gene editing in primary industry.” I can’t find anyone other than the research/entrepreneurial research community, including Society leaders, and some industry groups that have asked for the conversation on genetic engineering to start again. The Society has not said why it has prioritised this national conversation and not, say, sustainable ecological agriculture and forestry - or reducing food waste.
From what I can see of the conversation, it paraphrases something like this: “New Zealand, you need to rethink your position on genetic engineering technologies, such as gene editing, because the old conversation hasn’t worked and its your fault. Moreover, that is frustrating to some of our members.” That at least is what comes through in media coverage around the releases of the reports.
Narrow problem formulation
By instigating the discussion the Society gets to define the problem and chose a formulation suited to a solution using gene editing. For example, the problem with Douglas fir was defined as wilding conifers (and secondarily allergic reactions to them), not say, forest management, production models or producer liability.
It only considers one solution, making new fir trees sterile using gene editing. This is to stop further escapes and reduce the impact of allergens from the trees. Would that work? I don’t know because the report didn’t say how long it would take, or if it was even possible, to eliminate fertile weed populations that have already established. It asserts that more sterile trees might reduce the number of people who develop allergies, but doesn’t provide evidence that existing wilding pines don’t already saturate exposures or that most people suffering from pine pollen don’t live near forests that will continue to produce pollen.
Gene editing not banned
Neither New Zealand nor Europe has shut the door on products made using gene editing despite claims to the contrary. Both jurisdictions have defined them true to their respective legislation which provides mechanisms to adopt these products for use in food and the environment. These laws were the product of normal democratic processes and are harmonised to an even larger global consensus on the right of countries to regulate genetic engineering. Such international instruments are, among others, the Codex Alimentarius and the Convention on Biological Diversity (and its sub-treaties). Most of the world subscribes to these, including New Zealand.
Regulation of technology is normal
Biotechnology is not unique in being regulated, and products from regulated technologies are both commercially successful and available. They include airplanes, cars, radioisotopes used in medicine and construction, drugs, vaccines, and guns. Indeed, not only are airplane manufacturers and airlines highly regulated, but they have enjoyed a high degree of public trust despite the occasional disaster. They don’t blame regulation for them not bringing their products to market.
Royal Society leaders and others are promoting what they call product-based regulation to replace what they characterise as existing process-based regulation for biotechnology.
Those promoting product-based regulations also say that existing products of genetic engineering are safe. And well they should be. They came through process-based regulatory methods designed to ensure their safety. Even in countries that only require product-based evidence, the testing was informed by process-based evidence in preparation for regulators in countries such as ours.
Product & process regulation useful
There are virtues to both kinds of regulation, and some optimal mix of the two might secure for the public, and the farmer, the best of both worlds. Although this remains only speculation until they say exactly what they mean by product-based regulation. Nevertheless, here are a couple of examples.
Both airplanes and drugs are regulated on process and product. Airplane manufacturers not only have to prove that their final product is safe, they also have to comply with process-based regulations throughout the design and ongoing manufacturing stages. This is also true of the pharmaceutical industry’s drugs and vaccines.
A recent example of the value of process-based regulation was the intervention of a federal court in the US to halt the distribution of 3D printer plans to make undetectable handguns. Purely product-based legislation might have prevented the regulator from interfering until the guns were being distributed. Way too late for undetectable firearms. Unfortunately, it might be too late in that case anyway.
Product-based advocates frequently complain about the arbitrary approach to testing crops made through gene transfer genetic engineering or gene editing but not old-style chemical and radiation mutagenesis. All are officially forms of genetic engineering in New Zealand so if you are concerned about the safety of the latter, then yes we should alter legislation to test crops made this way. Oddly, I never hear advocacy for this, just advocacy for reducing safety checks on other things.
There are a couple of important differences between chemical/radiation mutagenesis and gene editing. One difference is how they are regulated. Have you ever tried to buy a cesium source for use in your garage, or order a potent chemical mutagen online? If you have, you will know that it won’t end well for you because these tools are regulated. Access to radioisotopes and harmful chemicals is restricted to particular applications and those who may use them. In contrast, access to the tools for gene editing can go unnoticed in countries with product-based legislation. Researchers at a Canadian university made a point of this, assembling a horsepox virus (a relative of smallpox) using similar but even more expensive tools that they ordered “through the mail”.
Another difference is that it can take much more time to change the genes you want to change using these tools. That also limits the kinds of changes that you can make. So when we talk about the precise and small changes by gene editing, we are telling you only part of the story. Some desirable traits would only require this, but others won’t. You can serially apply gene editing techniques to make dramatic changes in one place in the genome, and you can apply the tool once to many places in a genome. Because it is fast, you can make huge changes fast. To know that these changes exist depends on using risk assessment techniques that are informed by how the changes were made. Of course, it also depends on the tools being used by those who are willing to tell you what they did.
If not process, then what?
In one of its scenarios, the Society discussion paper recommends genome sequencing as a risk assessment technique that could be used to find either intended or unintended changes in gene edited organisms. But it doesn’t tell you who will require such an investigation if only the product were regulated. If whole genome sequences of gene edited crops are to be routine parts of assessment of agricultural products, then the Royal Society is still promoting what we have now: regulation triggered by process applied to a product.
If whole genome sequences and other techniques used to assure the safety of gene editing products aren’t required, then the Society isn’t promoting a form of product-based regulation, it is promoting no regulation because there is nothing to trigger legislation leading to a risk assessment of the product. That is really a very different conversation.
0 notes
Text
Are government regulators communicating with citizens or using language to avoid responsibility?
The modern framework of public sector oversight of private sector safety evaluations is designed to reduce harm to trade. The cost to the private sector of requiring additional safety information (both qualitatively and quantitatively different) is immediately weighed against the social cost of incorrect assessments. A regulator faces real pressure from the concentration of private wealth and power through both political manipulation of the agency and legal action. In contrast, the citizenry rarely create an equivalent threat in the short term.
Have you ever participated in consultation with a government safety regulator when they were evaluating a product that cannot be fully described for release into a complex environment that has not been fully described? If you have, then you will be familiar with the language barrier between them and the citizen, or them and the independent subject expert. It takes a considerable investment of time and study to become familiar with the quasi legalise of the process and to decode the complex linguistics used by regulators. If you have not, then the story below will probably appear farcical. But that may be justification enough to read it.
Galactic Standards Agency NZ vs. Nicolaus Copernicus, or how modern risk assessment might appear to a Renaissance astronomer*
*As far as possible, the text is an accurate reconstruction of the reasoning and level of communication of regulators in this universe. Where necessary, names and details were changed to protect the innocent.
In a parallel universe near here, rocket science developed on Earth in the equivalent of our 16th Century. As the rockets achieved the ability to penetrate Space, private enterprise could consider commercial opportunities there. This new ability was hoped to be a source of great benefit. But it also might be dangerous. To ensure that human health and environment were adequately protected against the latter, governments wrote laws that created safety regulators. One of these was the Galactics Standards Act 1535 which created Galactics Standards Agency of Noty Zunis (GSANZ).
GSANZ oversees safe use of rocket science to ensure maximum benefit of the new technology and thereby also limit the liability of applicants seeking release for commercial purposes. If GSANZ determined that a use was safe, then the applicant was shielded indefinitely from liability for any unanticipated or unintended adverse effect. Nevertheless, the companies did have an “ongoing duty of care” where the “developer is expected to monitor for existing and emerging risks that may be associated with its product and notify regulatory authorities whenever new information is uncovered". However, there was no provision for requiring post-release monitoring plans from rocket builders, or mechanisms for assessing the success of these plans even if they did exist.
By unknown means, the diary of one N. Copernicus was recovered in a long-closed section of the library of the Frombork Cathedral Chapter. His diary apparently records excerpts of the regulator justification for a decision in his Universe. What follows is the voice of the regulator as recorded by Copernicus.
********************************************************
1.4 Reasons for accepting the Application
The Application was accepted for assessment because: it complied with the procedural requirements under subsection 22(2) of the GSANZ Act; it related to a matter that warranted the variation of a galactic regulatory measure; it was not so similar to a previous application for the variation of a regulatory measure that it ought to be rejected.
1.5 Procedure for assessment
The Application was assessed under the General Procedure.
2. Summary of the findings
On 9 May 1551 Ptolemaics Inc. (the Applicant) applied to Galactic Standards (the Authority) for approval to release an asteroid into the Earth system of planets in order to accelerate the special rock into an independent and distant orbit around the Earth. The purpose is to use the rock as a platform on which Earth tourists can one day land and enjoy an espresso.
The nature of the modifications to the rock for the purposes of using it as a cafe are not relevant to the legislation of GSANZ. Any issues relevant to that use are considered by the Food Standards Agency and the Office of Galactic Traffic Regulator.
The Authority has considered the risks to Earth and other Heavenly Bodies should release of the asteroid be approved. While this would be the first purposeful release of an asteroid into the system, and the rock is intended to be closer to the Earth and her Moon than other asteroids, the Authority considers that the risk of this asteroid smashing into the Earth and her Moon, or any other Heavenly Body, to be no greater than any current risk posed by asteroids. This is because the asteroid is not the only large rock in orbit in the Earth system and all orbiting rocks pose some risk of colliding with the Earth, her Moon or one of her planetary neighbours and their moons.
Ptolemaics has submitted a detailed dossier of scientific studies showing that the rock could safely be launched into orbit and once there would safety inhabit that orbit. They have conducted a series of launch tests with stones and some gravel that were boosted into space. Some statistically significant differences in meteor activity were detected, but no biologically relevant effects were found.
The rock is expected to accelerate toward Jupiter where the planet’s gravity would boost the velocity of the rock to that necessary to establish its intended orbit.
The Authority has determined that the Ptolemaics asteroid is as safe as naturally-occurring asteroids and therefore recommends to the Ministerial Council that the asteroid be given de-regulated status under the provisions of the Galactics Standards Act.
2.1 GSANZ Act assessment requirements
2.1.1 Cost/benefit analysis
The Authority has also conducted an extensive cost/benefit analysis on the granting of de-regulated status to the safe release of an asteroid.
The Office of Best Practice Regulation (OBPR), in a letter to GSANZ dated 24 November 1541, granted a standing exemption from the need of the OBPR to assess if a Regulatory Impact Statement is required for the approval of additional asteroids. The exemption was provided as applications relating to asteroids are considered as minor, machinery and deregulatory in nature.
GSANZ undertook a cost benefit analysis (see below). The analysis concluded that costs that would arise from a galactic regulatory measure developed or varied as a result of the Application/Proposal do not outweigh the direct and indirect benefits to the community, Government or industry that would arise from the development or variation of the galactic regulatory measure.
A consideration of the cost/benefit of approving the draft variation is not intended to be an exhaustive, quantitative dollar analysis of the options and, in fact, most of the impacts that are considered cannot be assigned a dollar value. Rather, the analysis seeks to highlight the qualitative impacts of criteria that are relevant to each option. These criteria are deliberately limited to those involving broad areas such as trade, consumer information and compliance.
1. Every once in a while the asteroid will come between the Sun and Earth and cast a shadow.
Cost: Less direct solar radiation for crop growth and sun tanning. This is considered to be negligible because the rock is pretty small.
Benefit: Will contribute to efforts to reduce global warming and reduce skin cancer. These benefits were considered nearly negligible but a net positive.
2. For those who believe that the Earth system was created by God and is immutable-
Cost: This might shake their belief system. Unlikely to really change their beliefs because, if they really do believe this then they’ll find some way to reconstruct their beliefs.
Benefit: This might shake their belief system. Other religions may get new members.
3. For those who may one day ride in spaceships.
Cost of not granting de-regulated status: No place to land for an espresso. Loss of commercial activity derived from taking people to land on an asteroid where espressos are made.
We consider this to be a significant loss of options to future generations. The loss of a reason to build spaceships and espresso machines could have far reaching economic repercussions.
Benefit: There is a hypothetical risk that when the espresso machine and spaceship are invented, the country(ies) in which they are manufactured might challenge all other countries before the WTO, an international trade regulation body, arguing that a historical failure to approve a safe asteroid could be construed as a historic non-tariff trade barrier. Therefore, approval now will mitigate that risk and thereby provide a net benefit.
GSANZ therefore concludes that there are only benefits and no risks to approving the release of Ptolemaics' new asteroid.
2.2 Summary of issues raised in the submissions
GSANZ has held two rounds of public consultation on the application to release an asteroid into the Earth system. The process by which GSANZ considers standards matters is open, accountable, consultative and transparent. Public submissions are called to obtain the views of interested parties on issues raised by the application and the impacts of regulatory options.
Public submissions were invited on a draft variation which was released for public comment between 16 December 1551 and 10 February 1552. The call for submissions was notified via the Notification Circular, media release and through GSANZ’s social media tools and the publication, Galactic Standards News. Subscribers and interested parties were also notified.
At the Initial Assessment stage, 2003 submissions were received. Of these, 1998 were from groups opposed to the release of the asteroid. Most of these objected to the release of any large rock at speed into the Earth system fearing some cataclysmic event that is hard to predict or exclude. GSANZ has assessed the release and found that the asteroid is as safe as any other large rock traveling at speed in the Earth system, is satisfied that the release of the rock is safe and thus has answered these submissions.
Four submissions were received that supported the release of the rock.
One submission was received from Nicolaus Copernicus, an astronomer, of Frambark.
At the Draft Assessment stage, a further 57 submissions were received. Fifty-five were in favor of the Authority’s draft recommendation to de-regulate the Ptolemaic asteroid. One submitter was on principle against the release of large rocks traveling at speed. The other submission was again from Nicolaus Copernicus. His two submissions identified specific and detailed concerns that we address below.
2.2.1 Issues raised specific to the Application
GSANZ requires that the release of large rocks into the Earth system be shown to be as safe as naturally-occurring rocks. In this case, the rock is not intended to impact with Earth, her Moon or any other rock, planet or moon in the Earth system, so the chances of it doing that are low. Thus, even in the unlikely event that the asteroid would collide with another Heavenly Body, it is an effective impossibility that it or any debris from the collision would impact life on Earth.
N. Copernicus argues that the Ptolemaics asteroid trajectory and final predicted orbit were calculated using a flawed model of the Earth system. He has provided references from scientific and philosophical publications wherein authors speculate about the geocentric system as a heliocentric, or ‘S-O-L-A-R’, system. This highly controversial hypothesis posits that the Sun is the centre of the Universe and that all bodies, including Earth, orbit the Sun, rather than the well-established understanding that all bodies orbit the Earth.
A few other astronomers have speculated on a Sun-centric Universe. Aristarchus of Samos’ theories had noticeable similarities to those of N. Copernicus. Although Galileo and Kepler have published data that can be interpreted as consistent with a heliocentric system such as proposed by Copernicus, there is no scientific consensus on the organisation of the Universe around the Sun. The totality of evidence, including that given to us by God, is consistent with a geocentric system where all Heavenly Bodies circle the Earth. Moreover, the orbit of every observable Heavenly Body can be described using retrograde motion mathematics. The accuracy of description is good. Even if new Heavenly Bodies are entering the Earth system, GSANZ is satisfied that no new scientific information of significance to this safety assessment would come from testing the prediction of retrograde mathematics. Moreover, neither Aristarchus’ nor Copernicus’ theories have received much scientific attention and neither of their works has benefited from replication. Indeed, N. Copernicus’ work has been the subject of significant criticism because he has assumed planetary objects to move in large circles around the Sun, rather than in elliptical orbits. The proposal that new asteroids can exert biological effects upon humans is both controversial and highly speculative as has been previously discussed by GSANZ.
Accepting for a moment that the unlikely heliocentric model of the Universe were valid, N. Copernicus calculates that the asteroid would exit Jupiter orbit with enough momentum to potentially fragment Earth’s Moon on impact. According to his calculations, the chance of a lunar collision is 1% in the next 100 years (or 0.01%/year). There is a similar chance that the asteroid would be captured by Mars, or collide with Mars. There is also a 10% chance that in 50 years the asteroid would impact the Sun. Finally, the chance of a direct impact with Earth is 0.25% over 30 years, rising to 10% cumulative probability over the next 200 years.
Based on this conjecture, N. Copernicus has made the following recommendations.
1. The GSANZ should confirm through direct telescope observation that other naturally-occurring large rocks that are new to the Earth (Solar) system follow a predicted orbit using the Ptolemaic model of the Universe.
GSANZ response: Currently the largest telescope on the planet is Mr. Galileo’s plano-convex objective with a focal length of about 30-40 inches, and a plano-concave ocular with a focal length of about 2 inches. N. Copernicus says that using this scope, Galileo has told him that he can see moons around Jupiter and suggests that there is a theoretical possibility that the asteroid could collide with one of these moons, or be moved out of its planned trajectory by one or more of the said moons. However, the Authority cannot accept these putative findings, as independent experts chosen by the Authority, who use standard telescopes, have been unable to confirm the existence of any moons around Jupiter. Furthermore, even if moons do orbit Jupiter, they are currently at risk of being hit by rocks and these may also theoretically strike other Heavenly Bodies, including Earth. The Authority is satisfied that the Ptolemaics asteroid is as safe as any other rock in the Earth system.
Therefore, GSANZ considers N. Copernicus’ experiment to be of academic interest, but not important for determining the trajectory and hence the safety of the asteroid.
2. GSANZ should confirm the calculations provided by the Applicant that suggest that the asteroid would have no effect on human health should it collide with Earth. GSANZ should confirm that the calculations were assisted by state-of-the-art idiot savant and slide rule.
GSANZ response: The Applicant has submitted detailed calculations of the orbit of the asteroid. The Authority is satisfied that the Applicant has provided sufficient detail for the Authority to determine the safety of the asteroid as the Applicant has provided details of the orbits that could occur from a launch error range of as much as 2%. In none of the ten possible orbits calculated, is the Earth at risk of a collision. The Authority agrees that a slightly higher launch error rate may significantly increase the number of possible orbits, but based on these previous calculations, the Authority is confident that none of the possible orbits would increase the likelihood of the asteroid colliding with the Earth, its Moon or any other Heavenly Body. As such, the calculations provide qualitative and sufficient evidence of the absence of any risk to Earth and the use of the idiot savant and slide rule would not significantly increase the usefulness of the calculations.
Moreover, the Applicant has provided the Authority with calculations for a worst-case scenario, wherein the asteroid does strike Earth at speed. The maximum speed of the asteroid was found to be only 0.075% of the speed calculated by N. Copernicus (who neglected the frictional co-efficient of the ether and the force of predicted solar winds). Combined with the asteroid’s zero weight in space, the impact would be less than that of a feather falling from the Tower of Pisa.
3. GSANZ should demonstrate that either it or Ptolemaics have a post-release monitoring plan and that the launched rock can be recalled should its trajectory be determined to threaten Earth in the future.
GSANZ response: Post-release monitoring is not a requirement of the Galactics Standards Act 1535. Furthermore, any unanticipated harms that might arise post-release are covered by the Applicant’s ongoing duty of care.
An Expert Advisory Group (EAG), involving observatory personnel and representatives of the Noty Zunis jurisdiction was formed by the Galactic Earth System Regulation Standing Committee’s Implementation Sub-Committee to identify and evaluate appropriate methods of analysis associated with all applications to GSANZ, including asteroid applications. The EAG indicated that for asteroid applications, the description of length, width and girth of the asteroid is sufficient data to be provided for analytical purposes.
Using this information, anyone with a telescope would have the capability to develop a detection method. This information was supplied by the Applicant to satisfy the requirement for detection methodology in the GSANZ Application Handbook.
As GSANZ has determined that there is no risk to human health from the release of the asteroid, there is no need to require post-release monitoring or a recall mechanism. The asteroid is a body of only 500 kilometers long, 500 meters thick and 4 kilometers wide. Illuminated by the Sun, the asteroid would be visible using the most advanced telescope only until it reached a distance of half way to the Moon. Thus, post-release monitoring would not add to the assurance that the asteroid was safe.
3 Decision
The draft variation to Standard 1.5.2 of the current Code, as proposed following assessment, was approved without change. As a consequence, a draft variation to Schedule 26 of the revised Code was also approved.
The approved draft variation to Standard 1.5.2 of the current Code takes effect on gazettal.
1 note
·
View note
Text
Correlation is not causation
27 November 2014
A heavily promoted meta-analysis claiming to demonstrate the benefits of GM crops especially in developing countries does no such thing. It fails to separate from the GM trait the effects of confounding variables that are known to dramatically affect all measured parameters. Commentators are presenting misleading interpretations.
A new meta-analysis of 147 studies is cited as evidence that GM crops have made substantial yield and profit increases and reduced pesticide use (Klumper and Qaim, 2014). However, it fails to differentiate between, on the one hand, differences in farmer income, ability to irrigate, and access to support—and importantly access to elite germplasm—and, on the other hand, the contribution of the GM trait. In other words, the meta-analysis confuses correlation with causation.
Those quick to overstate the power of this study seemingly ignore that it is prone to distortion from environmental and temporal heterogeneity because it is a collection of mostly short-term observations lacking geographic continuity, disconnected in time and without uniform standards of measurement. This it has in common with some other meta-analyses that have been used to make similar claims about the certainty of benefits from GM cropping systems.
Findings at a glance*
The results cannot be generalized to the global experience. Almost all studies come from just three countries: South Africa, the US and India (Figure 1).
Almost all studies were under three years long, with ~60% of studies just one season (Figure 2).
Almost all time periods measured were prior to the rise of resistant weeds and pests, making measured benefits a short-term phenomenon.
Only three crops were examined: cotton, maize and soybean. There was no coverage of canola, sugar beet, alfalfa or papaya.
Nearly 80% of studies are on Bt (insecticidal) crops, despite the majority of GM crops for the majority of time being HT (herbicide tolerant) (Figure 3).
Over 50% of the studies are just Bt cotton. Only 14% of the studies were of HT soybean despite this combination of crop and trait being the longest in commercialization. Moreover, these minority studies are split over multiple countries, making them anecdotal.
Any of six different variables qualified a study for inclusion, reducing the number of comparisons on some variables to well below a sample size of 147. The mix of methodologies in the underlying research papers included is so varied that the collection has almost no power of true replication.

Correlation not causation
Does the meta-analysis demonstrate that GM crops are in general superior to non-GM counterparts for yield, pesticide use and profitability? No. At best, taking the meta-analysis at uncritical face value, it presents a correlation between GM crops and some measured benefits. It does not provide evidence that GM-traited crops are responsible for the benefits. When one looks deeper than face value, the correlation itself is weak.
Many of the underlying studies that I examined* were based on farmer surveys (rather than actual measurements). Not only do farmer surveys weaken the strength of the overall conclusions, but some of the underlying studies used in this meta-analysis have been subject to significant methodological criticism (Glover, 2010c). Studies 57 and 59 (and the research groups responsible for studies 45-46, 55-59 and 100) were described this way by Glover (2010c)—
Work by other researchers has shown that these are not simply mischievous speculations. Fundamental questions have been raised about the Pray–Huang group’s conclusions, with regard to both the finding of reduced pesticide use and the attribution of such an effect to the adoption of Bt-cotton technology per se.
Many of the underlying studies were surveys of outputs from farmers, some of whom grew GM crops and some who did not. For example, at least 14 studies (10-17 and 52-55 on cotton and 41-42 on maize) appear to have assembled data from an area where some farmers were growing GM and other farmers conventional versions of the crop. This is nearly a third of the studies I examined. The meta-analysis should have, but did not, take into account any systematic differences between farmers who adopt or do not adopt a particular variety to ensure that differences are due to the GM trait and not something else.
Studies that compare farmers who adopted GM crops with those who may have been growing saved seed or other commercial varieties are introducing significant variables, e.g., different wealth or other characteristics of the farmer or farm. GM crops are also elite varieties, products of the latest breeding that has nothing to do with the GM trait. Companies selling high-cost GM seeds do not undermine their market by breeding the GM-based trait into poorly performing germplasm. Those farmers adopting GM are also adopting both the latest germplasm and a management program designed by the seed seller. GM seed companies have programs that finance small scale, early adopting farmers (Glover, 2010a). Other farmers are working with germplasm that is not related to the GM versions and they do not have access to the same level of outside support. Studies collected for the meta-analysis are biased toward early adopters.
The problem is key because almost all studies have focused on the years immediately following the introduction of Bt cotton, when yield differences mainly reflect the agricultural prowess of a biased group of early adopters (and also reflect how this group happened to fare their first time trying a new technology).Crost et al. found that in “cross-sectional analysis of the type used in most of the previous studies on Bt cotton, more than half of the observed yield effects would be due to self-selection effects”… A related problem is bias in cultivation practices: prior to the institution of price caps in some [Indian] states in 2006, Bt seeds cost four times as much as conventional seeds, and would have been planted in the fields with best irrigation and then benefited from unusual care and expense. —(Stone, 2011)
In effect, these studies are a measure of the impact of breeding and socio-economic factors, not GM. The GM trait may correlate with greater yield or profitability, but that correlation is not a demonstration that GM is the cause of these benefits.
Study choices
The authors of the meta-analysis were candid in their selection procedures. To be included, a study had to provide some original data (and not be just another meta-analysis). They also included multiple studies with duplicated data—
In some cases, the same results were reported in different publications…several publications involve more than one impact observation, even for a single outcome variable, for instance when reporting results for different geographical regions or derived with different methods (e.g., comparison of mean outcomes of GM and non-GM crops plus regression model estimates). In those cases, all observations were included.
The addition of an entirely new paper to add one small data contribution inflates the power of the meta-analysis. For example, studies 34 and 35 comprise a conference presentation and later publication of the same dataset. Plagiarism software found a 71% identity between the publications, and most of the differences were just cosmetic. There may have been a unique data point between the two, but this small difference added another entire paper to the list.
Meanwhile, while entire studies were included for the sake of a small additional piece of data, one of the largest studies from a developed country was excluded from the meta-analysis. This study, on one of the longest used GM crops, examined both yield and profitability characteristics of GM cotton over 4 years in the same place (the US State Georgia, see Jost et al., 2008). The study is over a long period of time compared to most of those used in the meta-analysis, and was conducted over consecutive years in a country where all farmers are able to access the latest germplasm. That study did not, however, find that GM crops were superior to conventional.
In fact, the Jost et al. study found that GM-traited seeds eroded farmer profits because of their high cost. Worst performing was the HT GM cotton. In this regard, it is noteworthy that HT studies are a minority in the meta-analysis, despite HT being the most common GM trait and the longest in commercial use. Jost et al. concluded—
Collectively, these results indicated that profitability was most closely associated with yield and not with technology.
That is the key. If farmers using obsolete germplasm are compared to those using the latest elite varieties (packaged with a GM trait) and provided with support to optimize management, the latter will be found to have greater yields. Nothing about this proves the benefit of GM. Moreover, those with greatest yield make more money. Again, the outcome is correlated with yield and GM. But only yield is causative and this is determined by factors other than the GM trait.
Contrasts
The meta-analysis seems more suited to providing a snapshot of two agroecosystems, India and South Africa, comparing production of just Bt cotton (and perhaps maize). Drilling down into the studies specific to those agroecosystems could be useful, rather than conflating them with one-off measurements from many other countries and mixing together on farm studies and field tests as was the case with this meta-analysis.
Confusingly for me, the authors say that they excluded studies on sugar beets, papaya and canola (oilseed rape) for precisely the reason that there were, in their view, too few studies outside of a small number of countries. But then to include a few studies on HT soybean mainly in the US (with 3 studies in nearby Canada and only one other study from Romania) seems inconsistent.
The meta-analysis at its most authoritative is not a validation of GM cotton, soybean and maize superiority, much less a general endorsement of all the kinds of crops that have been genetically modified. Realistically, the meta-analysis is built on some shaky underlying information and lack of detail on breakdowns where there is greatest replication power (Glover, 2010c; Stone, 2011).
The authors also ignored our analysis of performance of crops grown at scale in countries that are matched for hemisphere, access to elite germplasm, mechanization and farmer support for the longest ever measured period of over 50 years (Heinemann et al., 2014b). Our study did not compare matched GM/conventional crops grown side-by-side. However, neither did most of the studies in the meta-analysis as I indicated above. Study 30 of the meta-analysis was explicit about that—
It is important to emphasize that this is only a cross-sectional survey. It does not represent a side-by-side comparison of GMO and non-GMO crops. It represents a picture of what Iowa farmers experienced.
Our long-term study was criticized for this very thing, but some of the same voices are silent about the issue when it is used to produce evidence of GM crop benefit.
Moreover, our long-term study assembled measured farm outputs, so it should have met the criteria for inclusion. However, when we examine the use of GM crops in North America and elite equivalents in Western Europe over the short period of GM adoption, we find yield stagnation or decline in North America (Heinemann et al., 2014a).
Importantly, we also found the same yield penalties apply to wheat grown in the US but not Western Europe. Wheat is a major non-GM crop in the US and Western Europe. This is further evidence that one needs to consider the entire biotechnology, management and innovation package under which agriculture is conducted in order to determine which tool in the toolbox will be truly useful.
Lessons
It is very difficult to get solid indicators of the actual contribution GM cropping makes in places in which it is being used. This is why I question the unequivocal statements made by some scientists about benefits. Their conclusions appear to be uncoupled from the strength of the data.
All studies have their weakness. It would have been useful if we could have had massive side-by-side comparisons of GM and non-GM plants and management systems for our 50-year study. That kind of data at that scale does not exist. But we did balance as much as possible the larger variables of wealth, education and biotechnology. Likewise, it would have been useful if the latest meta-analysis would have included all relevant studies, more crops and only side-by-side comparisons, rather than mixing data in all ways possible.
GM plants are not science: they are products made by scientists. They may or may not do what their developers hope for them. Likewise, alternatives to these products may also fail to live up to expectation. Therefore, we need proper holistic evaluations of the tools in order to inform policy on the future of agriculture.
Public policy requires, and small farmers need, a more rigorous and dispassionate evaluation of the pros, cons and opportunity costs of GM technology and especially a more careful analysis of how it can be designed and made available in forms that could enable it to be authentically ‘pro- poor’. —(Glover, 2010b)
Skepticism about the performance and value of one or more GM products is not a rejection of science or scientists, any more than switching power companies is a rejection of electricity. Those claiming that these products are science are failing to distinguish between science and technology. Perhaps this is not unexpected given that the three words are now spoken as if they were one word. They never have been and are not now, and it would serve us well to remember that.
*How my analysis worked
I looked at ~one-third of the studies, a significant subsample. I applied no rule, simply taking the first 49 of 147 studies (Table S1 of original paper). In the end, I had to consider the first 55 studies because I either could not find or was unable to access relevant details of studies 5, 7, 18, 38, 43 and 48. Only 2 traits are covered: insecticidal Bt and herbicide tolerance (HT).
One study had data from two countries. From this subsample and a scan of the titles of other studies, I estimate that the geographical diversity is largely saturated in the first 55 studies with the total number of countries from which data was obtained for at least 1 study estimated at being ≤15.
References
Glover, D. (2010a). The corporate shaping of GM crops as a technology for the poor. J Peasant Stud 37, 67-90.
Glover, D. (2010b). Exploring the Resilience of Bt Cotton's ‘Pro-Poor Success Story’. Develop Change 41, 955-981.
Glover, D. (2010c). Is Bt cotton a pro-poor technology. J Agrarian Change 10, 482-509.
Heinemann, J.A., Massaro, M., Coray, D.S., and Agapito-Tenfen, S.Z. (2014a). Reply to comment on sustainability and innovation in staple crop production in the US Midwest. Int J Ag Sustain 12, 387-390.
Heinemann, J.A., Massaro, M., Coray, D.S., Agapito-Tenfen, S.Z., and Wen, J.D. (2014b). Sustainability and innovation in staple crop production in the US Midwest. Int J Ag Sustain 12, 71-88.
Jost, P., Shurley, D., Culpepper, S., Roberts, P., Nichols, R., Reeves, J., and Anthony, S. (2008). Economic comparison of transgenic and nontransgenic cotton production systems in Georgia. Agron J 100, 42–51.
Klumper, W., and Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9, e111629.
Stone, G.D. (2011). Field versus farm in Warangal: Bt cotton, higher yields, and larger questions. World Develop 39, 387-398.
0 notes
Text
Science vs assumption
13 November 2014
This new trait is based on the expression of what are called double-stranded (ds)RNA molecules. These molecules influence the expression of genes. The types of dsRNA molecules relevant to this essay are also called, among other things, siRNA, miRNA and microRNA, and cause ‘silencing’ effects such as RNAi (interference), PTGS (post-transcriptional gene silencing), and DNA methylation. While we are most familiar with the ability of dsRNA molecules to either ramp down or prevent translation of a messenger RNA (mRNA) into a protein, dsRNA can also up-regulate some genes and can interfere with RNA that is not mRNA. For a technical review, see 1, 2, or 3.
The use of dsRNA molecules as gene regulators appears to be nearly universal in biology. Nevertheless, our knowledge of the biochemistry is still incomplete. Our ability to predict the cascade of genes affected by any particular dsRNA molecule is poor. Therefore, I hold the view that novel dsRNA molecules created through genetic engineering techniques or applied to the surface of crops in the form of pesticides should be tested for the potential to cause human health effects and unintended environmental effects (1).
However, at least one food safety regulator, Food Standards Australia New Zealand (FSANZ), disagrees that novel dsRNAs in food could have human health effects. I find that regulator’s arguments for not undertaking a risk assessment unconvincing, but I do agree that there has been a lack of proof that ingested dsRNAs from plants have a biological effect on people (or mammals in general).
A key plank in the argument made by the food regulator and some scientists is that ingested dsRNAs are too fragile to survive digestion and therefore we cannot be ‘exposed’ to them in an active form. A second plank in the argument is that the concentration of dsRNAs in food would be too low to have an effect even if they were taken up. These arguments are rapidly losing their power to convince as a growing body of scientific evidence suggests that nature doesn't share their assumptions.
Differences between food and environment regulators
Furthermore, risk assessment by food regulators does not take into account environmental effects. Therefore, their opinions are not relevant to the potential for adverse effects to arise in the environment and are not the final word when it comes to generating the full picture of GMO safety, for people or the environment.
The US Environmental Protection Agency (EPA) recently concluded a large-scale evaluation of dsRNA-based pesticide products and whether or not existing risk assessment frameworks are sufficient to evaluate them for safety. The answer, succinctly, was ‘no’.
Overall, the Panel agreed with the concerns raised by the EPA regarding the inadequacies of the current environmental fate and non-target effects testing frameworks for dsRNA PIPs [plant incorporated protectant] and exogenously applied dsRNA products. Uncertainties in the potential modes of action in non-target species, potential for chronic and sublethal effects, and potential unintended consequences in the various life stages of non-target organisms are sufficient justification to question whether the current Agency framework for ecological effects testing is applicable to dsRNA PIPs or exogenously applied non-PIP end-use products. Due to the modes of action of RNAi, no one set of test species will serve as an adequate representation of non-target species for all pesticidal products using RNAi technology. The classic approach of developing and assembling effects data for a standard set of test species will likely not work well for this technology.
The EPA evaluation involved a standing group of scientists taking both written and oral submissions from scientists and other interested persons from around the world. Despite the lack of definitive proof of biological activity from dsRNA molecules ingested by mammals, considerable uncertainty remains. This is because it is unlikely that dsRNA in actual food will be pure, the form used in laboratory studies, but will instead be protected by other kinds of molecules, like those that form ‘exosomes’. EPA therefore said that degradation of dietary dsRNA cannot be assumed. They “recommended experimental testing of the mammalian blood and exposed tissues be done to ensure that the siRNAs processed from the PIP dsRNAs are not present,” to confirm that they have been degraded “since these could have off-target effects after human consumption” (emphasis added).
Importantly, ingestion is not the only exposure pathway. The EPA also wants other exposure pathways such as via the lungs through inhalation, or through contact with skin or mucosa, to be tested. These pathways could produce very different exposure potentials. The EPA highlighted that other exposure pathways remain unexplored and may at times be more relevant than ingestion.
Moreover, the argument that RNA won’t survive digestion is hypothetical because there are few studies on dsRNA stability through digestion, and none that prove complete removal of dsRNA at the stomach acidity levels typical of different kinds of consumers. Consequently, the EPA Panel “recommended that the stability of dsRNA in individuals that manifest diseases, immune compromised, elderly, or children be investigated.”
The EPA also noted that bioinformatics and use of long dsRNAs does not guarantee absence of risk. “While ‘long’ dsRNA” which may be produced by the genetic engineering of the plant “may have no similarity to mammalian genes, processing of dsRNA into shorter siRNAs may present additional issues if these siRNAs have a high degree of similarity to sequences in non-target species including mammals.” The short active form may have many more targets than predicted from the intended longer form. “Chances of off-target binding increase as the siRNA becomes shorter and if sequences mismatches between target and off-target sites occur,” the EPA Panel said. Off-target effects can result in unintended silencing of other genes in animals or humans, potentially causing unanticipated adverse effects.
Thus EPA has come substantially to the same conclusions we did in our previous two publications, 1 and 2. This will be disappointing news for the likes of Profs. Rick Roush and Peter Langridge, who have publicly stated precisely the opposite without providing any evidence, via the Science Media Centre and other media outlets.
Dietary dsRNA back in the spotlight
Probably more troubling to the scientists and regulators who have attempted to paint a one-dimensional picture of dsRNA risk assessment will be two new publications. The first demonstrates that dsRNAs (miRNAs) found in cow milk are biologically active in humans. The research, published in the Journal of Nutrition, found that the miRNA in the cow milk survived digestion and could alter gene expression. The authors said: “We conclude that miRNAs in milk are bioactive food compounds that regulate human genes” (2).
These are the very things that prominent regulators such as FSANZ, some scientists and the editor of Nature Biotechnology said couldn’t happen. The focus of their previous dismissals was a paper that reported that dsRNA of plant origin could be detected in human blood, alter gene expression in human cells in culture, and alter genes in the liver of live mice fed these plants (3). The editor of Nature Biotechnology said that the detection of plant dsRNAs in human blood and mice organs “went against a large body of research in which the systemic administration of double-stranded RNAs was shown incapable of triggering the RNA interference pathway in humans (and mice).” However, the editor failed to acknowledge the full range of studies that report detections of exogenous dsRNA in humans and animals, e.g., Wang et al. (2012) (4) and Lam (2012) (5). This new study should raise more questions about the editorial objectivity of Nature Biotechnology, and the subjective views of some food safety regulators.
The first study showed that dsRNA in breast milk could be transferred via ingestion, survive digestion and cause changes in gene expression, invalidating claims that it could not. This next study provides the latest evidence that dsRNAs derived from plants are found in human and pig breast milk, packaged in exosomes (6). “Our study shows that plant miRNA molecules are abundant in human and porcine breast milk exosomes,” the authors said. The obvious recipients of these dsRNAs would be babies. This study establishes that novel dsRNAs introduced into plants or animals by genetic engineering, or sprayed onto plants as a pesticide, may very well survive digestion and accumulate in some tissues.
Proper regulatory review needed
Our diets are full of dsRNA. It is a natural component of all life. Our diets are also full of proteins. However, no credible dietitian or toxicologist would suggest that if all proteins in a carrot are safe, then all proteins a carrot can be made to make would be safe. That is why risk assessments consider novel proteins in genetically modified food products. Yet this same argument is used to pre-determine the safety of dsRNA. For example, FSANZ says: “There is no scientific basis for suggesting that small dsRNAs present in some GM foods have different properties or pose a greater risk than those already naturally abundant in conventional foods.”
This kind of argument might have had some validity when we thought RNA was biologically inert except when used as a template for the production of proteins. But now we know that RNA has, when in double-stranded form, activities that transcend its informational content as a template. That activity is revealing surprises on a nearly daily basis. It is being pressed into service as a form of biotechnology product (sometimes even unwittingly, see: 7), and those products are meant for our food and environment. As my colleague, toxicology Prof. Ian Shaw, said on the Science Media Centre site: "currently data relating to dsRNAs and their effects are not required as part of the dossier supplied to a regulatory authority (e.g. FSANZ) as part of the regulatory process for assessing GM foods. Therefore, dsRNAs are not considered during the risk assessment. I agree with Heinemann et al; THEY SHOULD BE.”
Denying that dsRNAs deserve to pass a risk assessment based on valid measures of their potential to cause an adverse effect is only creating more unnecessary distrust in a technology that has some promise.
References
1. Heinemann, J.A., Agapito-Tenfen, S.Z. & Carman, J.A. A comparative evaluation of the regulation of GM crops or products containing dsRNA and suggested improvements to risk assessments. Environ Int 55, 43-55 (2013).
2. Baier, S.R., Nguyen, C., Xie, F., Wood, J.R. & Zempleni, J. MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers. J. Nutr. 144, 1495-1500 (2014).
3. Zhang, L. et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 22, 107-126 (2012).
4. Wang, K. et al. The complex exogenous RNA spectra in human plasma: an interface with human gut biota? PLoS ONE 7, e51009 (2012).
5. Lam, E. (2012). WO 2012135820 A2. http://www.google.com/patents/WO2012135820A2
6. Lukaski, A. & Zielenkiewicz, P. In silico identification of plant miRNAs in mammalian breast milk exosomes - a small step forward? PLoS ONE 9, e99963 (2014).
7. Sanders, R.A. & Hiatt, W. Tomato transgene structure and silencing. Nat. Biotechnol. 23, 287-289 (2005).
1 note
·
View note
Text
Ultimate experts
20 October 2014
I addressed this question in a recent blog on The Conversation. The short answer is that they don’t routinely rely upon sources of evidence that have been through a process of blind peer-review at the time that they make their conclusions about the safety of these products. This isn’t to say that their conclusions are necessarily wrong as a result. However, where the ultimate product is trust, it is relevant how society views sources of information.
It was a choice by governments to use industry-sourced - and frequently secret - information for use by regulators, rather than to use data generated by disinterested scientists and that could be contested by free exchange of materials and protocols.
It was a political decision to make the person or organisation wanting to place a product, such as a GMO, on the market legally responsible for demonstrating that it is safe. This has proved to be contentious, because the company or organisation given this responsibility also has an interest in the product being found safe. -COGEM
A new open access article by Zdziarski et al. in the leading risk assessment journal Environment International reports for the first time the breakdown of availability of peer-reviewed articles using rat feeding studies to assess adverse effects of GM products. This research considered both the number of peer-reviewed articles in total, and those that had had at least this level of quality assurance at the time the product was being reviewed by a government regulator.
The authors identified 47 GM crop plants that were approved by at least one food safety regulator somewhere. There are more than this number approved, but the authors limited the search to GM plants of three modifications - a kind of herbicide tolerance (glyphosate tolerance) or a kind of insect resistance (by expression of cry1Ab or cry3Bb1). Herbicide tolerance and insect resistance are overwhelming the two most common traits in commercialised crops, however, making this paper broadly relevant.
For these 47 approved products, only 18 published, peer-reviewed studies could be found. These studies were restricted to only 9 of the 47 approved GM food crops. The lack of studies isn’t the only interesting finding. Critically, many of this small number of studies also failed to adequately describe the methodology, other basic information needed to determine the level of confidence in the results, or even the results!
Study limitations
There are two limitations to the Zdziarski et al. (2014) study. The first is that it only considers one of the kinds of studies that may be used to assess safety, namely histopathological evidence from rats fed the product. There may have been in existence and available to the regulator other kinds of studies (e.g., compositional analyses, use of different animals) that had been published. However, from my experience, that too is unlikely at the time of the assessment. In any case, animal feeding studies are routinely done by the parties (public or private) that produce these products for food safety approval and as a consequence the information is routinely available to regulators. Therefore, the information should be capable of being published. The focus on rat feeding studies and histopathology adopted by Zdziarski et al. was a reasonable endpoint to survey when asking the general question of how much data provided to regulators ultimately is blind peer-reviewed.
Second, not all regulators require data from animal feeding studies much less specifically rat histopathology. Food Safety Australia New Zealand (FSANZ), for example, does not. It believes that such studies do not add to its confidence when determining the safety of food derived from GM plants. At other times, however, FSANZ does recognise the value of histology. For example, FSANZ did view histological evidence as useful for investigating the causes of stomach inflammation observed in an earlier study. It said that: “The presence of “inflammation” was determined by visual appearance (reddening) only, without any microscopic (histological) confirmation. This is not considered a reliable method for establishing the presence of true inflammation.” FSANZ appears to believe that histology should be a part of proving harm, but unnecessary for establishing safety. A mixed message from regulators might reduce the priority manufacturers place on histology as part of a safety assessment.
What the public hears
The chief scientist of FSANZ, Dr. Paul Brent said on Radio New Zealand National that: “It isn’t the case that all of the information we get from industry isn’t published. In fact, much of it is published in peer-reviewed journals” (Nine-to-Noon, 28 March 2013). When statements such as this are confronted with peer-reviewed research suggesting that the very opposite is true, who is the public supposed to believe?
Even if much of the research Dr. Brent refers to were eventually published – and this is a big IF – the defensive stance by the regulator only raises additional concerns. First, as the Zdziarski et al. study shows, even less research has been through a quality assurance process independent of the regulator at the time the regulator recommends that the product be approved for use in food. The often multi-year gap between approval and publication is not reassuring. Moreover, papers published after regulatory approval might have different information in them than was used by the regulator. It would be interesting to compare the propriety studies given to the regulator with those eventually published.
Second, some kinds of studies are more important than others for food safety. Which those are may be debated, or may differ depending on product, use or consumer. But the upshot is then that not all products may receive the same kind of testing. We don’t have a solid idea of how comprehensively these products are tested by the same or similar methodologies and how uniformly these tests are applied to all products. For example, were the other 38 relevant GM products not tested for histopathology, or tested but the data not published? Finally, assuming that food safety studies would be at least as, if not more, rigorous than environmental safety studies, one can only wonder how a survey of this type, with a focus on environmentally relevant endpoints, would turn out.
It has been argued that the regulator is the peer reviewer. Some might say that it would be a nonsense to suggest that a journal’s peer-review system was better at evaluating safety studies than is a regulator. However, when regulators such as FSANZ issue opinions on safety or critiques of actual peer-reviewed science but fail to disclose the names and qualifications of its own authors, it only undermines public confidence. For example, I’ve been repeatedly ignored when I’ve asked FSANZ, consistent with their policy of transparency, to reveal the names and qualifications of those authoring particular opinions in the agency’s name.


A general problem
The issues raised by the new study and my own experience are not unique to safety testing products of biotechnology. A study by Boone et al. (2014) looking at the pesticide industry, found similar problems there:
Pesticide use results in the widespread distribution of chemical contaminants, which necessities [sic] regulatory agencies to assess the risks to environmental and human health. However, risk assessment is compromised when relatively few studies are used to determine impacts, particularly if most of the data used in an assessment are produced by a pesticide's manufacturer, which constitutes a conflict of interest. –Boone et al.
Not only are the problems the same, but so are the solutions.
Although manufacturers who directly profit from chemical sales should continue to bear the costs of testing, this can be accomplished without COIs by an independent party with no potential for financial gain from the outcome and with no direct ties to the manufacturer. –Boone et al.
Despite the constraints on regulators by their legislation, resources and political masters, I think that they routinely do a good job. I certainly would not trade the existing regulators in my country for no regulation. If we want better outcomes of regulation, we need better laws and society needs to be a more active participant.
Still, sometimes the regulator does not serve itself well. Hyping the quantity and quality of evidence it uses and understating conflicts of interest creates cracks that serve as footholds for climbing doubts.
1 note
·
View note
Text
Response to Robert Wager, science journalist
The other blog was titled: “If it weren’t for false balance, there’d be no balance at all.” The blog was about a Reuter’s reporter, Carey Gillam, who was attacked by blog writers over their suggestion that she was (but they weren’t?) manipulating the GM debate. My main point was that New Zealand academics, who are statutorily obligated to serve as critic and conscience of society, rely heavily on the media to make our service relevant. Silencing the journalist is to silence the academic. That is the point that made my blog relevant to the AFA site.
Turning to the GM debate
Wager said that Gillam “has repeatedly published articles that cite junk science that has never been repeated.” Wager is, I think, @RoberWager1 on Twitter. He describes himself as a science journalist, not a scientist. Gillam also does not claim to be a scientist. I couldn’t find any bylines for Wager in major media outlets, only blogs and comment sections below online articles. To the best of my knowledge, then, Wager is also not a scientist* and not clearly better equipped to make these judgments than is Gillam.

The sophism “never repeated” deserves some unpacking. First and importantly, the vast majority of studies on GM products have never been repeated, replicated or even published in a peer-reviewed journal. The studies on these products are seen by government regulators well in advance of any attempt to publish them (COGEM, 2013). A few studies used by regulators may later be published. But at the critical time when a product is being evaluated for safety it does not benefit from an independent, blind peer-review of the type that generally is used by science journals. And peer-reviewed research has found that the inherent conflict of interest in this practice is not properly balanced by regulators (Boone et al., 2014). Yet Wager’s concerns are only about those studies critical of some GM products that have passed through this more rigorous, blind peer-review process.
Secondly, the published research literature rarely if ever provides a comprehensive evaluation of any single product tested in all ways relevant to a risk assessment. For example, try selecting one GM product of interest, say the high oleic acid soybean DP-305423-1, and then attempt to find and list all the published peer-reviewed studies about that product. Now compare that list to the international food safety guidelines produced by the joint body of the UN Food and Agriculture Organisation and World Health Organisation - the Codex Alimentarius Commission (CAC, 2003) - and to the environmental risk assessment guidance produced under the Convention on Biodiversity. As a colleague at my University is fond of saying, 'I’ll eat my hat' if you can find a product that has been tested by every step in these guidelines and if the results appear in a peer-reviewed journal. I’d be especially interested in seeing all the published peer-reviewed safety studies of GM products tested in their cooked or processed forms as appropriate for the society that will use the product, and as recommended by Codex Alimentarius -
47. The potential effects of food processing, including home preparation, on foods derived from recombinant-DNA plants should also be considered. For example, alterations could occur in the heat stability of an endogenous toxicant or the bioavailability of an important nutrient after processing. Information should therefore be provided describing the processing conditions used in the production of a food ingredient from the plant. For example, in the case of vegetable oil, information should be provided on the extraction process and any subsequent refining steps.
This last point is relevant to something else Wager said: “As for you implying companies dictate testing protocols, you are completely wrong. Every GE crop must meet testing protocols determined by internationally determined criteria. The OECD/WHO guidelines are the best place for you to go see for yourself.”
I never implied that companies dictate testing protocols. (Wager’s technique of manufacturing an argument, then crediting it to me, and then telling me I’m wrong is a tactic I have seen before, however.) Moreover, I am indeed familiar with the international guidance on testing. I’ve read the all-important guidelines issued by FAO/WHO and other agencies. In fact, I’ve done more than this. I’m part of the Ad Hoc Technical Expert Group that has been developing environmental risk assessment guidance under the Cartagena Protocol on Biosafety.
It is you, Mr. Wager, who seems unfamiliar with how the process works. Safety regulators are not bound to follow the guidance, or every recommendation in the guidance. For example, Food Standards Australia New Zealand openly says that: “We do not generally require food manufacturers to submit feeding studies in animals as part of an application for a GM food approval.” Nevertheless, Codex Alimentarius guidance allows regulators to ask for animal feeding studies. As I said above, Codex Alimentarius also suggests doing studies with cooked and processed materials relevant to home preparation. Where are all these studies, Mr. Wager? So regulators do not have to strictly follow the guidelines. They can choose to not require some testing that is recommended in the international guidelines.
Finally, Wager says: “If every food safety authority in the world, every health authority in the world and every National Academy of Science in the world all agree on the safety of GE crops and derived food would you not call that a consensus? If not then what do you think they all missed when they came to that conclusion...”.
This sophism unfortunately again requires unpacking.
Government regulators
What Mr. Wager suggests is hypothetical and unrealistic. Every ‘food/health’ authority in the world has not come to the same conclusion on every GMO. Most countries in the world have not conducted their own evaluation even of the industry-supplied data, much less conducted independent scientific testing. These countries, mainly the poorest, usually accept the findings of other regulators or may impose trade barriers while they develop capacity to do their own evaluations. Even among the countries that have done evaluations, there have been differences of opinion. Should I attempt to imagine the nonexistent situation where all countries have come to the same conclusion, that still would be about existing GM products, not all possible GM products.
Government safety regulators also don’t endorse products. When they approve a product it is based on their conclusion that the company-supplied data has not raised safety concerns. The regulator places any long-term liability for unforeseen damage caused by the product on the manufacturer. For example, Food Standards Australia New Zealand says:
In Australia and New Zealand, as in most other countries, the responsibility for post-market monitoring is covered by an ongoing duty of care on the part of the developer. The developer is expected to monitor for existing and emerging risks that may be associated with its product and notify regulatory authorities whenever new information is uncovered [emphasis added].
This is not an endorsement of the product by a food safety authority, but an approval to release the product based on the regulator’s trust in the manufacturer.
Here is an instructive example. The US Environmental Protection Agency recently issued a white paper on the risk assessment of GM crops engineered to produce a form of RNA, called double-stranded (ds) RNA, intended to be toxic to insect pests. With respect to a human health assessment, they say on the one hand that: “There is no convincing evidence that ingested dsRNA is absorbed from the mammalian gut in a form that causes physiologically relevant adverse effects.” Then they say on the other hand that they recommend among other things that the stability of:
different structural forms of dsRNAs be addressed for dermal and inhalation routes of exposure;
dsRNA in individuals that manifest diseases, immune compromised, elderly, or children be investigated.
That EPA does not have evidence of significant adverse effect through oral exposure does not mean that they endorse the safety of crops expressing dsRNA. Quite responsibly, they acknowledge the knowledge gaps and suggest what kind of research is needed for proper risk assessment (Heinemann et al., 2013; Lundgren and Duan, 2013). Superficial generalisations about what these agencies say is misinformation.
National and professional science organisations
Wager also misrepresents what national science bodies conclude. They at best retrospectively issue statements about existing GM products, not those in development or that could come in the future. Furthermore, there is no generic endorsement of GM products. For instance, I have never seen the US National Academies of Science say:
“We recommend that you eat such-and-such a GM corn product grown from seed made by so-and-so and treated with their own proprietary herbicide.”
Based on what some national science bodies actually say, one could at best argue that their statements are evidence of a convergence of opinion on the historic use of the technique for altering food crops. I respect those opinions. But I don’t respect them being turned into propaganda.
Conclusion
Wager’s statements ignore this far more nuanced and responsible position taken by the food and health regulatory agencies, national science bodies, and the broader scientific literature on risk assessment. Moreover, human health is just one part of the risk assessment that matters to people. So even narrowing it to this topic creates a sophism.
The 'scientific consensus' is a decision-making tool. The 'scientific consensus meme' is being used in the social media to substitute for science an allusion to authority. The scientific literature is not black and white. It is messy and equivocal. This doesn’t mean that existing commercial GM crops have definitively been shown to cause significant adverse human health effects. But it does mean that vigilance of them and their associated pesticides is a legitimate, common if not universal, desire.
*Since writing this blog, Mr Wager has contacted me to say that he is a scientist and directed me to his appointment at Vancouver Island University. I thank him for this clarification. Even with this in mind, I stand by my article: it is not clear why he would expect a professional journalist to weigh in on the technical details of a clash between scientists or why he would expect her to value authority-based arguments above fairness and transparency. More on that in the comment section here: http://academicfreedom.kiwi.nz/2014/09/werent-balance-thered/#comment-1221
References
Boone, M.D., Bishop, C.A., Boswell, L.A., Brodman, R.D., Burger, J., Davidson, C., Gochfeld, M., Hoverman, J.T., Neuman-Lee, L.A., Relyea, R.A., et al. (2014). Pesticide regulation amid the influence of industry. Bioscience in press.
CAC (2003). Guideline For The Conduct Of Food Safety Assessment Of Foods Derived From Recombinant-DNA Plants. Codex Alimentarius Commission.
COGEM (2013). Where there is smoke, is there fire? Responding to the results of alarming studies. COGEM Topic Report Dutch Commission on Genetic Modification CGM/131031-01.
Heinemann, J.A., Agapito-Tenfen, S.Z., and Carman, J.A. (2013). A comparative evaluation of the regulation of GM crops or products containing dsRNA and suggested improvements to risk assessments. Environ Int 55, 43-55.
Lundgren, J.G., and Duan, J.J. (2013). RNAi-based insecticidal crops: potential effects on nontarget species. Biosci 63, 657-665.
1 note
·
View note
Text
Vaccine and climate as sophistry on GMOs
A recent post on Twitter caught my eye:

The generic “GMO” is also misleading when used by the people making these sophisms: "...unsound or misleading but clever, plausible, and subtle argument or reasoning." GMO means, in the context of the discussion, commercialised genetically modified crop plants, certainly not all GMOs or research that uses similar techniques. Person is not the only one to make these kinds of links. Keith Kloor, David Tribe and Rick Roush, among others, have attempted to do the same and the practice has been criticised effectively here.
Vaccines
To be clear: people (including me) who accept the use of vaccines do so for many reasons. Among them are:
the considered judgment that vaccines do more good than harm for society and/or the individual;
appreciation of the quality and quantity of both efficacy and safety testing, that, while it has flaws, is still about the best we seem to be able to achieve;
that vaccines are administered by medical practitioners who are among the most highly trained work force on the planet, and they are subject to extensive and ongoing monitoring and legal liability; and
critically, vaccines are legally administered only after receiving informed consent from the recipient who receive clear, research-informed instructions about how to recognise adverse effects.
In other words, the ‘science’ is necessary but not sufficient cause for me to accept this technology in my body.
Moreover, the science has almost nothing to do with the vaccine controversy. The meme is subtly equating the technological products with ‘science’ itself. The science in vaccines was in the discovery that exposure to antigens can create an immune response sufficiently vigorous and long lasting as to provide protection against inoculation by some disease-causing creatures. The science identified those creatures and dissected them so effectively that vaccine candidate strains and parts could be identified. Finally and importantly, the science was behind all the sophisticated delivery techniques we have and the procedures for monitoring adverse effects. None of these science contributions per se are controversial.
The controversy starts and ends with the product. They are the result of a (usually) commercial process. Proprietary secrets surround the products and the decisions made during their development. The history of decisions made cannot be thoroughly interrogated by the public to confirm that manufacturers have always identified the best and safest products or the best possible products among alternatives, when there were choices. For example, when identifying lead compounds for drug development, there may be many different potential drugs. Which one is chosen for commercialisation may have as much to do with what competitors are developing or with considerations of which are more easily defended against patent infringement as with interests in efficacy or safety.
Secrecy drives mistrust. This is not to say that the mistrust is with ‘large corporations’. Public research organisations are also increasingly operating under the same incentive schemes as the private sector and are more frequently demonstrating the same behavior and potential to cop a share of the mistrust thus generated (reviewed in chapter 8 of Heinemann, 2009).
If the controversy on vaccines is truly in the past, it would be because governments and professionals with no vested interest in the products have succeeded in establishing an ongoing and adequate regulation of the pharmaceutical industry (Table). Moreover, that trust is earned product-by-product. The eradication of smallpox does not exempt a future manufacturer from extensive safety and efficacy testing and monitoring of new vaccines. Past success should not make us complacent to the possibility that an unsafe vaccine might be pushed onto a future market.

So if GMOs and vaccines have anything metaphorically in common, perhaps this is what it should be. GMOs used in agriculture should have independently verified benefits demonstrated by more than a few single year field trial comparisons and be transparent to establish that the trials were rigorously conducted and controlled for variables. This would preclude, for example, comparing elite germplasm with the GM trait to older varieties without the GM trait, and comparing a field of GM crops with built-in insecticide to a field with no alternative pest management system.
"A long-contested debate centres on the potential for GMOs to contribute to greater sustainability in agriculture. The issue is multi-layered and depends fundamentally on the comparators and practices that GM technology would replace. For example, a GM technology resulting in reduced use of pesticides could be more sustainable than a conventional system relying on pesticides, but this GM/reduced-use system would score less well if compared with an organic system that used no pesticides" (Pretty, 2001).
Climate Change
The debate on efficacy and net benefit of GM cropping systems also differs from the debate on climate change. Increasingly frequently the term ‘scientific consensus’ is used to disparage any scientific scrutiny of GMOs by metaphorical linkage to climate change denial. However, that metaphor also fails in important ways.
First, scientific studies that find potential adverse effects from some GM products or the combination of the GM crop and its intended co-use with a ‘chemical’ pesticide, are not bucking the scientific establishment. Like all research findings, there will be flaws in these studies and there will be those scientists and commentators who value the findings more than others. This is also true of studies that claim absence of adverse effects. In other words, all these studies are part of the consensus of how science is done rather than a consensus of what results are acceptable.
Science is not ruled by popularity of the findings, and thus consensus conclusions are not themselves science. Real consensus exercises are ways to draw considered and qualified conclusions. They are at best a way to make decisions about products, set research priorities or agree on who to invite to the next conference.
This is also true of climate science. Not everything the International Panel on Climate Change (IPCC) says or predicts is certain. Nevertheless, there is such a quality of accumulated evidence gathering from climate science as to suggest that action is needed to curb probable anthropogenic accelerants. Surprisingly, the so-called consensus of science on GM crops does not come from any actual effort to achieve and report a consensus, but mainly on the assertion of consensus by those who have access to media, social networking or government.
This claimed consensus does not exist. In fact, the closest exercise on agriculture to the IPCC was the International Assessment on Agricultural Knowledge, Science and Technology for Development (IAASTD), and the conclusions were not in line with these other ‘consensus memes’ (Kiers et al., 2008). The IAASTD recommended more research and investment in biotechnology that improved soils, reduced pest pressure and increased farmer incomes rather than agricultural models that extend the expensive high input dependences behind the green revolution, as do commercialised GM crops.
Second, most studies that conclude that a particular GMO does not cause a particular adverse effect in a chosen context nevertheless find evidence of adverse effects. However, many authors conclude that, on the basis of the strength of their study or other considerations, that they do not believe their findings point to a safety concern. To gloss over this nuance is poor science and poor science communication. We must be aware of the uncertainty in the primary data to remain vigilant of unintended and unanticipated potential adverse effects. Noting these is not necessarily problematic in an environment of transparent and trustworthy regulation, as has been proven by the adoption of many medicines that also have adverse effects.
Messages that are compatible with climate change skepticism and hostile to ongoing scientific and public scrutiny of GMOs tend to align with large industries and public institutions with massive financial stakes in the adoption of the message. Conveyors of the message may not themselves represent any of these stakeholders, but their vocal champions on social media and their access to media and other important outlets are both very likely conditioned by these concentrated financial powers.
It would be naïve to think that the implications of having an opinion harmonious with power would attract the same personal or professional risk as having an opinion discordant with the interests of those who hold financial or social power.
Strikingly, this is the pattern I perceive in the celebrity of climate change skeptics and resistance to better regulation of GMOs. Unlike those who see this as a political ‘right’ and ‘left’ issue, I instead see this as an alignment issue. Climate change skeptic celebrities are often hostile to GM regulation, positions reconciled as industry-friendly. A public concerned about the effects of climate change may also want GM products labeled, positions reconciled by wanting to have a say in how the planet is being used.
Sophism not science
The intellectually dubious conflation of the public discourses on vaccine and GM crops, and GM crops and climate change, is in my view a sophism because it attempts to smear, on purpose or unintentionally, desires for proper regulation of products in food and the environment with unrelated controversies.
None of the proven solutions - transparency in product development and testing as well as robust regulation and monitoring - appears to be a high priority of those propagating the memes and thus they miss the key lesson such comparisons could hope to teach. By this I mean that, where ‘science won’ (as qualified as that characterisation may be) in those other controversies, it was due to greater transparency in research and consensus forming activities (eg, IPCC) and better regulation (eg, vaccines), not belittling or patronising the public understanding of science and commerce.
References
Heinemann, J.A. (2009). Hope not Hype. The future of agriculture guided by the International Assessment of Agricultural Knowledge, Science and Technology for Development (Penang: Third World Network). Free access here: Hope Not Hype.pdf
Heinemann, J.A., and El-Kawy, O.A. (2012). Observational science in the environmental risk assessment and management of GMOs. Env Int 45, 68-71.
Kiers, E.T., Leakey, R.R.B., Izac, A.-M., Heinemann, J.A., Rosenthal, E., Nathan, D., and Jiggins, J. (2008). Agriculture at a Crossroads. Science 320, 320-321.
Pretty, J. (2001). The rapid emergence of genetic modification in world agriculture: contested risks and benefits. Environ Conserv 28, 248-262.
2 notes
·
View notes