One day, I will go into the wood and never come back. Environnemental chemistry student :)
Don't wanna be here? Send us removal request.
Text
i am supposed to have the energy… to do stuff...?
37K notes
·
View notes
Text
they finally invented an assignment thats not due tomorrow. but the problem is that my body is a machine that turns assignments into assignments due tomorrow
53K notes
·
View notes
Text
Public speaking is actually really easy if you don't respect a single soul in that room. I've had an incredibly easy time delivering speeches when I hated everybody I saw and they all thought I did amazing because my disdain was read as confidence. I don't have any tips for you I'm just telling you a fact
101K notes
·
View notes
Text
Sometimes peace is drinking coffee while looking at November’s gray sky and listening to the entire soundtrack of Interstellar
0 notes
Text
Concept: TMA quotes but it's Barbie
Proof of concept:





12K notes
·
View notes
Text
Happy Birthday, Mr. Jacobi
It’s so weird just thinking that Jacobi is now technically two years older than Kepler was when he passed. Hope that Jacobi celebrates somewhere, probably lighting up fireworks 🎇
11 notes
·
View notes
Text
hey if you're trans in the us i love you. hey if you're queer in the us i love you. hey if you're a person of color in the us i love you. hey if you're a woman in the us i love you. hey if you're disabled in the us i love you. i love you i love you i love you
48K notes
·
View notes
Photo

The first simulated image of a black hole was calculated with an IBM 7040 computer using 1960 punch cards and hand-plotted by French astrophysicist Jean-Pierre Luminet in 1978.
77K notes
·
View notes
Text
The atomic radius, shielding, and the lanthanide contraction
As you hopefully already know, there are several trends of the elements' properties that you can squeeze out of the periodic table. The one I want to focus on in this post is the atomic radius.
The shorter and simpler explanation is as follows: if you move from the top to the bottom, the radius increases, which is simply the result of more electron shells (“layers”) being added to the atom. Moving left to right, the radius decreases and that is caused by the growing positive charge of the nucleus attracting the valence electrons more strongly like the swole doge and therefore shrinking the atom.
But chemistry is a science, and the sciences are beautifully complex, so let me tell you about this one intricacy affecting the radius. We’re going to need some knowledge of the electronic structure of an atom, but I'll try to fit all that you really need to know inside a single paragraph.
The compact version is this: inside the atom, there are little pockets of space where electrons can be, so to say. Those pockets are called orbitals and they have all sorts of funky shapes. The s orbitals, for example, are ball-shaped, while the p orbitals look roughly like balloons attached to the nucleus. (They're sometimes described as dumbbell-shaped. What sort of dumbbell looks like that. They're balloons.)

[source]
Back to the radii! I'm going to use the second period as an example here, but there's nothing special about it. It just happens to be quite a simple case. Lithium and beryllium only have s orbitals, so that's not very interesting, but the rest of the period has both s and p orbitals.
As we move from lithium, across the whole period, and to fluorine (there's no need to engage the noble freaks), three important things happen: the radius decreases, each next element has one more proton in the nucleus than the previous one, and also each next element has one extra valence electron. Duh. The number of electrons "inside" doesn't change, it's only the outermost shell that gains electrons.
But remember, an electron is not a marble sitting in one point at all times. When it comes to electrons in atoms, it's better to imagine them as lil clouds of negative charge in the shape of their respective orbitals. As a result, the core electrons obscure the nucleus from the valence electrons. Not entirely! But the attractive force felt by the valence electrons isn't just equal to whatever Coulomb's law would give us - it's a bit smaller. This is called the shielding effect and the charge that valence electrons actually "feel" from the protons in the nucleus is called effective nuclear charge. The atomic radius is therefore a tad bigger than we could assume without acknowledging shielding (but the trend of a decreasing atomic radius across the periodic table still stands, of course).
So, what about the lanthanides, those poor, misunderstood outcasts thrown under the periodic table?

God I love pubchem's periodic table it's so pretty!
There's no plot twist here, they don't break the trend. Cerium is the biggest one, lutetium the smallest. So what gives? See, lanthanides begin filling up their f orbitals, whose shapes are so absurd even I am afraid of them.
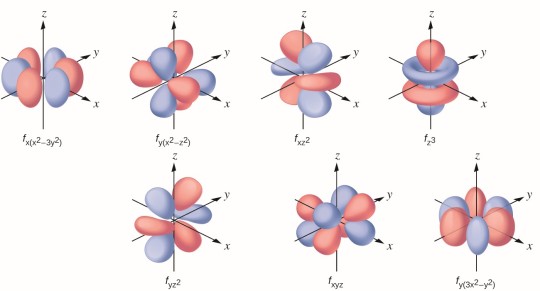
[source]
Because they're so ridiculous, they are supremely bad at shielding the positive charge of the nucleus, which in turn makes the lanthanides significantly smaller than they would be if this effect didn't take place - so much so that we call this phenomenon the lanthanide contraction. Actually, the f orbitals suck at shielding so bad that the radii of the (d-block) metals of the sixth period are very similar to the radii of the elements in the period above them - even though they should be much larger!

[source]
Really. Chemistry is like psychology for electrons. Everything that happens here is caused by these guys' weird behavior.
78 notes
·
View notes
Text
i’m not lying on the floor physically but i am lying on the floor spiritually
195K notes
·
View notes
Text
fellas
fellas is it gay to buy 50 pounds of fireworks for your employee & throw them a suprise employment anniversary party with just the two of you
91 notes
·
View notes
Text
the right hairstyle for your face is the one that makes you smile when you see yourself in the mirror btw.
66K notes
·
View notes
Text
The last season is absolutely horrendous rip

849 notes
·
View notes
Text
Being a demi aroace is interesting. I used to feel absolutely nothing about anyone and now I am filled with lust and love for some fucking guy who's very special to me... It's funny and sort of embarassing for me ngl
716 notes
·
View notes

