#API custom synthesis
Explore tagged Tumblr posts
Text
Custom Synthesis Manufacturing Company | Saurav Chemicals
Explore premium custom synthesis services at Saurav Chemicals. Where our team of scientists specializes in crafting intricate molecules customized to meet your specific requirements.

#Custom synthesis#Custom synthesis services#Custom synthesis manufacturing#API custom synthesis#Contract chemical manufacturing
1 note
·
View note
Text

Vidgastech – Ruxolitinib Intermediates Manufacturer
Vidgastech is a leading manufacturer and global supplier of Ruxolitinib Intermediates. We specialize in high-purity pharmaceutical intermediates for API production, supporting the global pharma industry with quality, consistency, and innovation.
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#Ruxolitinib Raw Material#API Supplier#Vidgastech#GMP Manufacturer#Bulk Drug Intermediates#Custom Synthesis
0 notes
Text

In line with our expanding presence, we continue to collaborate with trusted clients and partners who share our long-term vision. Our consistent focus on precision, quality, and performance shapes every aspect of our service. We cater to sectors like Pharmaceuticals, Agrochemicals, and Specialty Chemicals, backed by robust CRO and CDMO support.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
How Salesforce Developers Shape the Future of Project Management Success?
The ever-changing field of project management has made technology developments crucial to the achievement of desired results. With the help of knowledgeable developers and consultants, Salesforce is a platform that can truly alter businesses, even in the face of an extensive number of competing offerings.
A Salesforce consultant will have a huge influence on how project managers succeed in the future. They will use Salesforce's features to improve teamwork, accelerate efficiency, and streamline procedures.
In this blog, we'll reveal the critical role that Salesforce developers play in influencing the success of project management. We'll explore their experience streamlining processes, streamlining work, and customizing solutions to drive productivity and cooperation in the fast-paced project environments of today.
Customized Solutions Crafting
Explore the ways in which developers modify modules, improve user experience, and guarantee scalability to ensure future-proofing of Salesforce systems.
Adapting Salesforce Modules:
The modules in Salesforce's suite are easily navigated by developers, who may easily customize features to fit project workflows. Whether creating complex workflows, setting unique items, or connecting third-party apps, developers take use of Salesforce's adaptability to create solutions that align with project goals.
User Experience Enhancement:
Developers may simply explore the modules in Salesforce's suite and modify functionalities to suit project procedures. Whether establishing custom items, integrating third-party apps, or building intricate workflows, developers leverage Salesforce's flexibility to build solutions that support project objectives
Scalability and Future-Proofing:
Future-focused, scalable, and flexible solutions are designed by developers. They future-proof project management systems by foreseeing possible expansion and changing needs, providing the groundwork for long-term success and adaptability.

Seamless Collaboration Integration
Examine how seamless collaboration integration may strengthen teamwork, bridge systems, and enable data-driven decision-making.
System Integration:
By utilizing middleware and APIs, developers can plan the smooth connection of Salesforce with other vital programs and systems. Integration facilitates data flow and guarantees a cohesive environment through connections with project management software, communication tools, and enterprise resource planning (ERP) systems.
Collaborative Workspace:
Within Salesforce, developers create collaborative workspaces that enable teams to share insights, interact in real time, and centralize communication. Transparent communication and knowledge sharing are facilitated by features like Chatter, Communities, and interfaces with Slack and other collaborative applications.
Data-Driven Decision Synthesis:
Developers facilitate the extraction of meaningful insights from heterogeneous data sources for project stakeholders by providing integrated analytics and reporting functionalities. Through the synthesis of data in Salesforce, ranging from project status to customer feedback, stakeholders can efficiently minimize risks, make well-informed decisions, and drive strategic objectives.
Automation for Enhanced Efficiency
Investigating data synthesis, collaborative workspaces, and efficient procedures for well-informed decision-making.
Workflow Automation:
Developers use Salesforce's automation features, such Flow and Process Builder, to standardize procedures and automate time-consuming tasks. They manage workflows that reduce human error, speed up task completion, and increase overall efficiency by specifying triggers, actions, and approval processes.
AI-Powered Insights:
By using artificial intelligence (AI) tools such as Salesforce Einstein, developers are able to introduce intelligence into project management procedures. AI-driven insights enable project teams to make data-driven decisions quickly, from sentiment analysis that measures stakeholder satisfaction to predictive analytics that predicts project timeframes.

Mobile Optimization:
Salesforce is optimized for mobile devices by developers who understand how important mobility is in today's dynamic work environment. They ensure that project stakeholders can access vital information and complete activities while on the go by utilizing native app development and responsive design, which promotes responsiveness and productivity.
Conclusion
In conclusion, Salesforce developers are the engine of innovation, using the platform's potential to entirely rethink the project management sector in conjunction with Salesforce consulting experience. By means of customization, automation, and integration, they facilitate enterprises in achieving unparalleled levels of efficiency, collaboration, and success. The combined experience of consultants and Salesforce developers will be essential in steering project management's future course toward even higher success and quality as it develops.
FAQs About Salesforce Developers and Project Management
How do Salesforce developers contribute to project management success?
Salesforce developers streamline project workflows, automate tasks, and customize solutions, enhancing efficiency and collaboration for project teams.
What skills do Salesforce developers bring to project management?
Salesforce developers possess expertise in coding, data management, and platform customization, enabling them to tailor solutions that align with project goals and requirements.
Why is Salesforce considered crucial for future project management?
Salesforce's robust platform offers scalable solutions, real-time insights, and seamless integration capabilities, empowering project managers to drive innovation and achieve project success efficient
#remote work#technology#hire salesforce developer#hire salesforce consultant#project manager#tech jobs#Future of businesses
4 notes
·
View notes
Text
High-Quality Atorvastatin Impurity Standards for Accurate Pharmaceutical Research
Atorvastatin is one of the most widely prescribed statins, primarily used to lower cholesterol and prevent cardiovascular diseases. As a crucial component in generic and branded formulations, atorvastatin must be tested with the highest standards of precision and accuracy. At Aquigen Bio, we offer an advanced range of atorvastatin impurity standards to support pharmaceutical development, quality control, and regulatory compliance.
Why Are Atorvastatin Impurity Standards Important?
In drug development and production, the identification and quantification of impurities play a critical role in ensuring safety, efficacy, and compliance with international guidelines such as ICH and FDA. Impurity profiling of atorvastatin helps manufacturers meet stringent quality requirements, providing confidence in the drug’s therapeutic effectiveness.
Aquigen Bio’s impurity standards are synthesized and characterized with high purity and traceability, ensuring accurate results in analytical testing.
Explore Our Range of Atorvastatin Impurity Standards
Our collection of atorvastatin-related impurity standards includes rare and stable isotope-labeled compounds to support bioanalytical and pharmacokinetic studies.
1. (3R,5S)-Atorvastatin Calcium salt
This stereospecific calcium salt form of atorvastatin plays a critical role in chiral impurity testing and stereoisomer profiling. Its precise structure helps ensure the enantiomeric purity of the active pharmaceutical ingredient (API).
2. 2-Hydroxy Atorvastatin D5
This deuterated metabolite is valuable in mass spectrometry applications. The presence of five deuterium atoms enhances sensitivity and precision in LC-MS/MS quantification, making it an ideal internal standard in pharmacokinetic and metabolic stability studies.
3. 2-Hydroxy Atorvastatin D5 Disodium Salt
The disodium salt form improves solubility and stability, offering a reliable reference standard for bioavailability and dissolution testing. It’s especially useful in developing robust analytical methods for complex matrices.
Why Choose Aquigen Bio?
Aquigen Bio stands out as a trusted partner for high-purity impurity standards. Here’s what makes us different:
GMP-compliant synthesis
Certificate of Analysis (CoA) with detailed characterization data
Global shipping with temperature-controlled logistics
Customized solutions for R&D and commercial needs
Whether you’re developing a generic atorvastatin formulation or optimizing quality control processes, our range of impurity standards supports every stage of pharmaceutical analysis.
0 notes
Text
High-Quality Benzylhydrazine Dihydrochloride by Jay Finechem
Benzylhydrazine dihydrochloride (CAS No. 20570-96-1) is a key intermediate in pharmaceutical and organic chemical synthesis. Known for its reactivity and role in preparing active pharmaceutical ingredients, it demands high standards of purity and consistency. Jay Finechem, a reputed Indian chemical company, is a trusted Benzylhydrazine dihydrochloride manufacturer, offering premium-grade material tailored to industrial needs.
Jay Finechem’s expertise in producing niche fine chemicals ensures reliable quality, technical accuracy, and compliance with global regulatory expectations. This makes them one of the most preferred Benzylhydrazine dihydrochloride suppliers for pharmaceutical companies and research institutions worldwide.
CAS No. 20570-96-1 Manufacturer with Global Standards
As a recognized CAS No. 20570-96-1 manufacturer, Jay Finechem delivers Benzylhydrazine dihydrochloride with consistent specifications and complete documentation, including a Certificate of Analysis (COA) and MSDS. The company’s processes are governed by GMP-compliant practices, allowing customers to trust the quality at every stage of procurement and application.
Their R&D-backed approach and stringent quality checks ensure that the chemical meets the exact parameters required for laboratory research and API development. This commitment to excellence positions them among the leading CAS No. 20570-96-1 suppliers in the global fine chemical market.
Leading Indian Supplier of Benzylhydrazine Dihydrochloride
Operating from Vapi, Gujarat, a prominent industrial zone, Jay Finechem stands out as a top CAS No. 20570-96-1 Indian manufacturer. Their location gives them a strategic advantage in terms of raw material sourcing, compliance infrastructure, and timely logistics.
As a Benzylhydrazine dihydrochloride manufacturer in India, Jay Finechem combines modern production capabilities with a strong emphasis on safety, environmental compliance, and scalability. Whether you are looking for lab-scale quantities or bulk production, the company provides reliable solutions tailored to customer requirements.
Trusted Benzylhydrazine Dihydrochloride Supplier in Vapi and Across India
Jay Finechem is not only a major Benzylhydrazine dihydrochloride Vapi-based supplier but also serves clients across the country and abroad. Their dedicated technical team offers full customer support from inquiry to delivery. Clients appreciate their transparent dealings, stable pricing, and consistent product availability.
For industries looking to buy Benzylhydrazine dihydrochloride in India, Jay Finechem is a dependable source for purity, compliance, and timely delivery. As an established Benzylhydrazine dihydrochloride supplier in India, the company supports both domestic industries and global exporters with competitive pricing and top-tier customer service.
Why Choose Jay Finechem?
CAS No. 20570-96-1 supplier with stringent quality control
In-house testing labs and documentation for every batch
Efficient logistics from Vapi, a chemical manufacturing hub
Reliable bulk and custom packaging options
Whether you're a pharma company, research lab, or international distributor, Jay Finechem is your trusted Benzylhydrazine dihydrochloride manufacturer in India, delivering uncompromised quality and professional service.


0 notes
Text
Bio-Synth: Redefining Pharmaceutical Excellence in Antihistamines
In today’s competitive pharmaceutical landscape, innovation and quality assurance have become non-negotiable. Bio-Synth, a prominent name in the life sciences sector, stands at the forefront of delivering high-quality antihistamine solutions. With a strong focus on research, compliance, and customer satisfaction, the company is gaining global recognition for its role in advancing healthcare outcomes—particularly in the allergy treatment domain.
Among Bio-Synth's core specialties is the development and supply of advanced molecules for second-generation antihistamines. These include active substances tailored to meet stringent Bilastine API Specification standards. By prioritizing consistency, purity, and bioavailability, Bio-Synth ensures that its products align with international regulatory benchmarks. Their well-characterized antihistamine compounds offer therapeutic reliability, reinforcing the company's commitment to global health.
Another critical area of focus lies in the preparation of essential chemical compounds used in antihistamine production. Bio-Synth has built a strong infrastructure for producing bilastine intermediates, with modern facilities equipped to handle high-volume and high-purity synthesis. This makes the company a trusted choice among partners looking for scalable and reliable sourcing options.
India has emerged as a pharmaceutical manufacturing hub, and Bio-Synth has harnessed this advantage to establish itself among the leading bilastine intermediates manufacturers in India. Leveraging cutting-edge technology and a highly skilled workforce, the organization ensures cost-effective solutions without compromising on quality or compliance.
To conclude, Bio-Synth exemplifies what it means to blend scientific expertise with industrial excellence. With a steadfast commitment to innovation and quality, the company continues to play a pivotal role in the antihistamine sector—delivering value across global markets and setting new benchmarks in pharmaceutical manufacturing.
0 notes
Text
Is Retatrutide Peptide Legal? Regulatory Landscape for Clinical Use and Research
Target Keywords: GMP Retatrutide, Clinical Retatrutide Regulation, Retatrutide peptide legal status, research peptides FDA compliance Internal Link: 👉 Trusted Retatrutide Supplier with GMP Certification
Introduction: Why Regulatory Clarity Matters for Retatrutide Peptide
Retatrutide, a promising triple agonist peptide under clinical development, has gained global attention for its dramatic impact on obesity and metabolic disease. But before clinics, CROs, or pharmaceutical developers decide to purchase or administer it, a crucial question remains:
Is Retatrutide legal for research or clinical use in the U.S., EU, and other key regions?
In this article, we’ll break down the regulatory status of Retatrutide Peptide across major jurisdictions, and offer clear guidelines for sourcing and handling this compound within a compliant framework.
1. Retatrutide’s Current Development Status
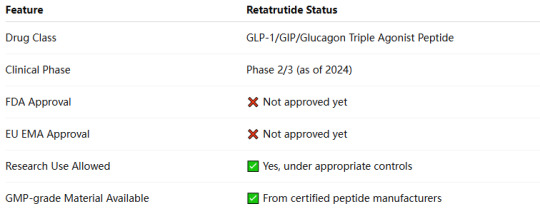
Although Retatrutide is not FDA- or EMA-approved for human therapeutic use, it can be legally used in non-human research and clinical trials under specific regulations.
2. Understanding Regulatory Terms: GMP, API, RUO

Procurement teams must ensure the Retatrutide they acquire:
Comes from a GMP-compliant source
Is labeled appropriately as RUO
Accompanied by COA, MSDS, and batch records
3. Is Retatrutide FDA-Compliant for Clinical Supply?
🧪 U.S. Regulatory Snapshot (FDA)
❌ Not approved for prescription or therapeutic use
✅ Allowed for use in:
Pre-IND studies
Clinical trials with IND exemption
Animal model research
Must be labeled: “For research use only. Not for human consumption.”
🇪🇺 EU EMA Regulatory Snapshot
❌ No marketing authorization granted
✅ Research material accepted under:
Clinical trial applications (CTAs)
Laboratory and formulation development
Requires adherence to EU GMP Directives (EudraLex Vol. 4)
🌏 Other Regions
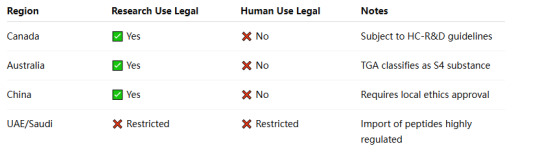
4. How to Stay Compliant When Sourcing Retatrutide
To avoid legal or safety issues, here’s a checklist for compliant Retatrutide procurement:
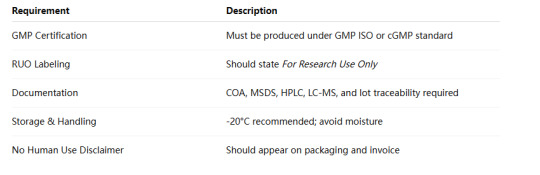
📌 Pro Tip: Always choose a peptide supplier who discloses GMP status, regulatory documentation, and origin of synthesis.
🔗 Explore GMP Retatrutide Peptide Options
5. Legal vs. Practical Use: What Clinics & Labs Must Know
Even though Retatrutide is not yet approved, many legitimate clinics and research labs are:
Using it in IRB-approved studies
Collaborating on pilot safety trials
Exploring combination therapy in pre-market formulations
🚫 However, selling or prescribing it for weight loss treatment outside of trials is prohibited in the U.S., EU, and most markets.
6. Final Recommendations for Labs, CROs, and Clinical Buyers
✅ Do:
Work with a GMP-certified Retatrutide Supplier
Keep clear documentation on file for import audits
Ensure staff is trained on handling, documentation, and labeling
❌ Don’t:
Use Retatrutide in human trials without IND or ethics board approval
Re-label research-grade product for off-label sale
Ignore local customs/import peptide restrictions
🔍 Summary Table: Legal Status of Retatrutide by Region
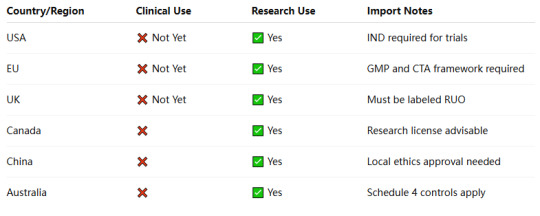
📌 Conclusion
Retatrutide is a cutting-edge metabolic peptide, but its legal use is strictly limited to preclinical and clinical trial contexts. It’s crucial to source GMP Retatrutide from trusted peptide suppliers and comply with regional regulations.
Whether you're a procurement lead at a CRO, clinical coordinator, or pharmaceutical buyer, understanding the legal status of Retatrutide Peptide protects your team—and your research integrity.
👉 Looking for a compliant Retatrutide Supplier? Explore GMP-certified options here: 🔗 https://retatrutidesupplier.com/retatrutide
📌 Conclusion
Retatrutide is a cutting-edge metabolic peptide, but its legal use is strictly limited to preclinical and clinical trial contexts. It’s crucial to source GMP Retatrutide from trusted peptide suppliers and comply with regional regulations.
Whether you're a procurement lead at a CRO, clinical coordinator, or pharmaceutical buyer, understanding the legal status of Retatrutide Peptide protects your team—and your research integrity.
👉 Looking for a compliant Retatrutide Supplier? Explore GMP-certified options here: 🔗 https://retatrutidesupplier.com/retatrutide
0 notes
Text
Top Generative AI Development Firms in the USA (2025)

AI is not helping you with your laundry as of yet, but can create new content and ideas, including conversations, stories, images, videos, and music!
The race to AI supremacy isn’t just heating up, it’s reshaping entire industries overnight. From automating customer service to revolutionizing content creation, generative AI has moved from experimental technology to business necessity in record time. Companies that once viewed AI as a “nice-to-have” are now scrambling to integrate intelligent solutions before competitors leave them behind.
As we navigate 2025, the question isn’t whether your business needs AI, it’s finding the right development partner to implement it effectively. The American AI space is packed with innovative firms, each offering unique expertise and competitive solutions. Choosing the wrong partner could mean wasted resources and missed opportunities, while the right choice can catapult your business ahead of the competition.
Before we reveal the list of the top Generative AI firms, let’s have an overview of how it helps your business reach higher peaks.
How Generative AI Facilitates Businesses?
Generative AI can be considered an umbrella service for businesses, covering all the necessities of an evolving business.
Content Creation & Marketing
Automated content generation for blogs, social media, and marketing campaigns
Personalized customer communications at scale across multiple channels
Visual asset creation including images, videos, and graphic designs without specialized teams
Customer Experience Enhancement
24/7 intelligent chatbots providing human-like customer support and problem resolution
Dynamic personalization of products, services, and user interfaces based on individual preferences
Predictive customer insights enabling proactive service and targeted recommendations
Operational Efficiency
Document automation for contracts, reports, and legal paperwork generation
Code generation and debugging accelerating software development cycles
Data analysis and reporting transforming complex datasets into actionable business intelligence
Innovation & Product Development
Rapid prototyping of new products and services through AI-assisted design
Market research synthesis analyzing trends and consumer behavior patterns
Competitive analysis generating strategic insights from vast amounts of market data
Cost Reduction & ROI
Reduced dependency on specialized creative and technical talent
Faster time-to-market for new initiatives and campaigns
Scalable solutions that grow with business needs without proportional cost increases
An overall development of business, eventually enhances automation and scalability. This guide includes companies with the following qualifications and are segregated to fit your business needs:
A portfolio of proven results with their clients as per their website.
Expertise in communication of what their brand stands for and what they bring on to the table.
Reviews and positioning on reliable platforms such as Clutch, DesignRush and GoodFirms and are listed under Top Generative AI Companies on BuiltIn.
Global partners that they have collaborated with.
Area/ sector/ industry that the company serves.
Valid statistics that prove their competency in the market.
We have curated a list of Top Generative AI Development Firms in the USA across the States. Before you shortlist your ideal tech provider, have a look at what they have to offer.
HubOps (Cincinnati, OH)
HubOps is a leading Ohio-based IT software development company delivering customized digital solutions that transform businesses. With 15+ years of experience, we specialize in crafting secure, scalable Generative AI tailored to your industry’s unique challenges and growth goals.
Key Services:
Generative AI Services
Custom SaaS Development
API Integration
Mobile & Web App Development
Artificial Intelligence & Machine Learning
Blockchain Solutions
AR/VR Applications
Digital Marketing
DaaS (Developers as a Service)
Industry-Specific IT Solutions (Healthcare, E-commerce, Real Estate, etc.)
Why Choose HubOps:
91% client satisfaction rate
672+ successful projects
End-to-end solution delivery
Expertise across cutting-edge technologies
2–3 months of post launch support and maintenance
USP:
The first free end-to-end analytics service for the site, designed to work with enterprises of various levels and business segments.
Monte Carlo (Dublin, IRL)
Monte Carlo is the #1 Data Observability Platform, dedicated to ensuring reliable data and AI. Their platform automatically detects anomalies, helps triage incidents, and resolves root causes, optimizing cost and performance. They provide solutions for data quality, ML/GenAI applications, and cloud migrations across various industries, emphasizing trusted data for AI readiness.
Key services offered:
Data Quality Monitoring & Testing: Proactive detection and resolution of data issues.
ML & GenAI Application Observability: Ensures reliable data for AI models.
Report & Dashboard Integrity: Maintains accuracy of business intelligence.
Cloud Migration Support: Facilitates seamless and reliable data transfer.
Why Choose Them?
Leading Platform: Recognized as the “#1 Data Observability Platform.”
Ensures Reliability: Guarantees trusted and reliable data for all applications, including AI.
Automated Solutions: Automatically detects issues and helps resolve root causes swiftly.
Comprehensive Coverage: Offers end-to-end observability across diverse data workloads.
Awards and Recognition:
Consistently recognized as the #1 Data Observability Platform and a leader in Data Quality, Database Monitoring, and DataOps Platforms by G2
Most Loved Workplaces list by Newsweek for 2024
BlackLine (New York, NY)
BlackLine offers an AI-enabled platform to modernize financial operations for over 4,400 companies. It accelerates record-to-report and invoice-to-cash processes, eliminating delays and strengthening controls. BlackLine’s focus is on digitally transforming the office of the CFO, delivering trusted financial data and enhancing efficiency.
What do they bring to the table?
Financial Close Automation: Streamlines and automates the record-to-report process.
Accounts Receivable Automation: Accelerates invoice-to-cash cycles.
Intercompany Financial Management: Simplifies complex intercompany transactions.
Journal Entry Automation: Automates and manages journal entries efficiently.
Specialization:
AI-Enabled Automation: Leverage AI for faster, more efficient financial processes.
Trusted Data & Controls: Ensures reliable financial data and strengthens internal controls.
Process Acceleration: Eliminates delays in record-to-report and invoice-to-cash cycles.
CFO Transformation: Modernizes the finance function with end-to-end digital solutions.
Notable Clients:
Ebay
Philips
Hersheys
Klaviyo (Boston, MS)
Klaviyo is a marketing platform with a B2C CRM, empowering businesses to build lasting customer relationships. It provides AI-enabled solutions for email, SMS, and mobile app marketing, coupled with analytics and a customer hub. Integrating with over 350 platforms, Klaviyo helps unify customer data, personalize experiences, and drive growth through smarter digital interactions.
Klaviyo’s key services include:
Email Marketing: Tools for creating and managing email campaigns.
SMS Marketing: Solutions for engaging customers via text messages.
Mobile App Marketing: Features to enhance interactions within mobile applications.
Reviews Management: Functionality to collect and leverage customer reviews.
Marketing Analytics: Insights and data-driven reporting to optimize campaigns.
Customer Hub: A centralized platform for managing customer interactions and data.
B2C CRM: A customer relationship management system specifically for business-to-consumer interactions.
AI Capabilities: Leverages artificial intelligence for enhanced personalization and engagement.
Why do they stand out?
Unified Platform: Consolidates email, SMS, and in-app marketing with a B2C CRM.
AI-Powered Personalization: Leverages AI for smarter, more effective customer engagement.
Data-Driven Growth: Helps businesses understand customer data to drive significant growth.
Extensive Integrations: Connects seamlessly with over 350 platforms for comprehensive marketing.
Partnered with:
Unilever
The Body Shop
Mattel
Capco (Charlotte, NC)
Capco is a global technology and management consultancy specializing in financial services and energy sectors. They drive transformation for clients in banking, capital markets, insurance, wealth management, and energy. Capco leverages deep domain expertise and a creative approach to solve complex challenges, delivering positive change for businesses and communities.
What do they serve?
Technology Consulting: Delivering solutions for digital transformation and innovation.
Digital Consulting: Helping clients embrace digital strategies and capabilities.
Management Consultancy: Providing strategic advice and operational improvements.
Why choose them?
Industry Specialization: Deep expertise in financial services and energy.
Transformative Approach: Drives significant business reinvention and change.
Disruptive Thinking: Leverages innovative and creative solutions.
Complex Problem Solvers: Addresses intricate challenges for positive outcomes.
Awards and Recognition:
Best ESG Data and Technology Consultancy for consecutive years
Top 10 Employers for Women in Technology
Best Recruitment Marketing Campaign
Hedra (San Francisco, LA)
Hedra is a next-gen multimodal content creation platform. It offers a personal AI studio, combining Character-3 with leading AI tools to streamline content generation. This enables creators, marketers, and businesses to efficiently produce engaging content, including generative images, videos, and audio.
Key Offerings:
Generative Content Creation: Offers tools for creating AI-generated images, videos, and audio.
Personal AI Studio: Provides a dedicated environment for creators to leverage AI.
Multimodal Capabilities: Supports creation across various content formats.
Streamlined Workflows: Designed to simplify content production for diverse users.
What sets them apart?
Advanced AI Integration: Leverages Character-3 and top AI tools for cutting-edge content generation.
Multimodal Output: Creates diverse content formats, including images, video, and audio.
Streamlined Workflow: Simplifies and accelerates the content creation process.
Targeted for Creators: Offers a personal AI studio tailored for various professionals.
Investment Story:
Hedra raised $32 million in Series A funding led by Andreessen Horowitz (a16z), a prominent venture capital firm, with participation from existing investors like a16z Speedrun, Abstract, and Index Ventures. This indicates significant investor confidence.
Qualtrics (Seattle, WA)
Qualtrics is recognized as the world’s leading Experience Management (XM) software platform. Used in over 100 countries, it helps organizations manage and improve customer, employee, product, and brand experiences. Qualtrics provides comprehensive solutions for understanding and acting on experience data to drive business growth and innovation.
Services by provide:
Customer Experience (CX): Managing and improving customer interactions.
Employee Experience (EX): Enhancing employee engagement and satisfaction.
Product Experience (PX): Optimizing product development and usage.
Brand Experience (BX): Building and maintaining brand perception.
Specializations:
Market Leader: Recognized as the world’s #1 Experience Management (XM) platform.
Comprehensive Platform: Offers solutions across customer, employee, product, and brand experiences.
Global Adoption: Trusted by organizations in over 100 countries worldwide.
Data-Driven Insights: Empowers businesses to act on experience data effectively.
Notable Clients:
BMW
Royal Caribbean Cruise Lines
Southwest Airlines
United States Census Bureau
FINAL WORD
The generative AI revolution is here, and choosing the right development partner will determine whether your business leads or follows. These top firms represent America’s finest AI expertise, each bringing unique strengths to transform your operations.
Don’t let competitors gain the AI advantage while you hesitate- the time for strategic AI implementation is now, not tomorrow!
0 notes
Text
Leading Chemical Manufacturer in India | Nikava Pharmaceutical Industries

Looking for a trusted chemical manufacturer in India that delivers consistent quality, innovative solutions, and regulatory compliance? Welcome to Nikava Pharmaceutical Industries — your dependable partner in the world of chemical and pharmaceutical manufacturing.
Who We Are
Nikava Pharmaceutical Industries is a well-established name in the Indian chemical manufacturing sector, known for its commitment to precision, purity, and performance. With decades of expertise, we specialize in producing pharmaceutical intermediates, active pharmaceutical ingredients (APIs), and specialty chemicals, catering to clients across pharmaceutical, biotech, and chemical industries worldwide.
Why Choose Nikava as Your Chemical Manufacturing Partner?
✅ High-Quality Manufacturing Standards
Our production facilities in India are GMP-compliant and equipped with advanced technology to handle small to bulk-scale chemical synthesis. From sourcing to packaging, every process is optimized for quality and consistency.
✅ Wide Product Range
As a leading chemical manufacturer in India, we offer:
Pharmaceutical intermediates
APIs (Active Pharmaceutical Ingredients)
Fine and specialty chemicals
Custom synthesis solutions
Our chemicals are developed to meet international pharmacopoeial standards such as IP, USP, BP, and EP.
✅ Custom Manufacturing & R&D Support
Need a unique formulation or custom chemical blend? Nikava offers flexible, end-to-end contract manufacturing and synthesis services. Our dedicated R&D team supports everything from pilot batches to full-scale production.
✅ Stringent Quality Control
Every batch is tested using in-house labs equipped with HPLC, GC, UV, IR, and other analytical instruments, ensuring your product meets the highest quality and safety standards.
✅ Sustainable & Ethical Practices
We believe in green chemistry. Our manufacturing practices are eco-conscious, with robust waste management, effluent treatment, and energy-efficient operations in place. We also uphold ethical sourcing and fair labor policies.
Industries We Serve
Nikava’s chemical solutions power progress across a wide spectrum:
Pharmaceuticals
Nutraceuticals
Agrochemicals
Personal Care & Cosmetics
Industrial Applications
Let’s Build a Cleaner, Smarter Future — Together
Whether you're a pharmaceutical giant, a growing cosmetics brand, or a lab seeking dependable intermediates, Nikava is the chemical manufacturer in India that brings professionalism, innovation, and value to every order.
Get in touch today to learn more about our product offerings, request a sample, or explore a custom solution tailored to your needs.
For more info visit Nikava Pharmaceutical Industries.
0 notes
Text
Ruxolitinib Intermediates: Enabling Targeted Therapy with Quality Manufacturing from Vidgastech
In the rapidly evolving world of pharmaceutical research and precision medicine, Ruxolitinib has gained significant attention as a JAK1/JAK2 inhibitor used to treat conditions like myelofibrosis, polycythemia vera, and other rare blood cancers. To ensure effective and scalable drug development, the demand for high-quality Ruxolitinib intermediates has surged — and that’s where Vidgastech leads the way.
🔬 What Are Ruxolitinib Intermediates?
Ruxolitinib intermediates are the essential chemical compounds used during the synthesis of the final active pharmaceutical ingredient (API), Ruxolitinib. These intermediates must be manufactured with strict quality standards to ensure:
Purity and consistency
Scalability for formulation
Compliance with global pharmacopeia
🏭 Vidgastech: A Trusted Source for Pharma Intermediates
Vidgastech has established itself as a reputable manufacturer and exporter of pharmaceutical intermediates in India, specializing in cutting-edge molecules like Ruxolitinib intermediates. With modern production facilities, a skilled R&D team, and commitment to regulatory standards, Vidgastech ensures:
GMP-compliant manufacturing
Customized synthesis on request
Prompt global delivery
💡 Why Choose Vidgastech for Ruxolitinib Intermediates?
High Purity Compounds: Ensuring effectiveness in the final API.
Regulatory Compliance: Following stringent quality checks.
Custom Solutions: Tailored synthesis as per client specifications.
Reliable Supply Chain: On-time delivery across the globe.
🌍 Applications in Oncology
Ruxolitinib is a vital component in the treatment of:
Myelofibrosis
Polycythemia Vera
Graft-Versus-Host Disease (GVHD)
Ongoing trials for autoimmune conditions
With the growth of targeted therapies, Ruxolitinib intermediates play a foundational role in pharmaceutical innovation.
🔗 Connect with Vidgastech
If you're a pharmaceutical manufacturer, researcher, or procurement manager looking for trusted sources of Ruxolitinib intermediates, Vidgastech offers scalable solutions tailored to your needs. 🌐 Website: https://www.vidgastech.com
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#API Manufacturing#JAK Inhibitor Intermediates#Ruxolitinib Suppliers India#Vidgastech#Oncology Intermediates#Drug Synthesis#Pharma Chemicals#GMP Manufacturing#Custom Synthesis#Myelofibrosis Treatment#Pharmaceutical Exporters#Life Science Chemicals#Pharma R&D India#High Purity Intermediates#Bulk Drug Intermediates#Specialty Chemicals Manufacturer#Healthcare Industry India#Pharmaceutical Industry News
0 notes
Text

As we evolve, we align with valued partners and clients who believe in our direction and actively support our momentum. Quality assurance and operational excellence are deeply ingrained in our work ethic. Our portfolio includes Pharmaceuticals, Agro-based products, Specialty Chemicals, and a complete range of CRO and CDMO offerings.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
BJ Madan: Trusted Channel Partner for Leading Peptide Manufacturers in India
BJ Madan – Connecting Research and Industry to the Best Peptide Manufacturers in India
In the rapidly growing fields of biotechnology, pharmaceuticals, diagnostics, and cosmetics, peptides have become essential components for drug development, therapeutic research, and innovative treatments. The demand for high-purity peptides has surged across India, and BJ Madan & Co. plays a pivotal role in fulfilling this need by partnering with reputed peptide manufacturers and making their products accessible to researchers, pharma companies, and healthcare institutions.
The Growing Role of Peptides
Peptides, which are short chains of amino acids, are used in:
Cancer immunotherapy
Hormone therapy
Vaccine development
Diagnostic kits
Skincare and anti-aging formulations
The complexity and sensitivity of peptide-based products demand that they be manufactured with rigorous quality standards and delivered under tightly controlled conditions.
BJ Madan: Your Link to Quality Peptide Supply
With over six decades of experience in medical and pharmaceutical distribution, BJ Madan serves as a trusted channel partner to leading peptide manufacturers, ensuring seamless delivery of:
Research-grade and clinical-grade peptides
Custom synthesized peptides
Peptide APIs for pharma formulations
Peptides for diagnostic and laboratory use
BJ Madan’s extensive industry network and logistical strength allow it to serve clients across India, from research institutes and CROs to biotech firms and diagnostic labs.
Why Choose BJ Madan?
Access to Certified Manufacturers: GMP- and ISO-compliant production facilities
Expert Procurement Support: Custom synthesis and technical consultation available
Regulatory Compliance: Documentation and certifications for R&D and clinical use
Reliable Distribution: Temperature-controlled shipping and prompt delivery across India
Enabling Innovation Across Healthcare and Life Sciences
BJ Madan continues to support India's innovation ecosystem by ensuring that high-quality peptide products are readily available to those who need them—whether for clinical research, product development, or advanced diagnostics.
Visit:- https://www.bjmadan.com/peptides.html
0 notes
Text
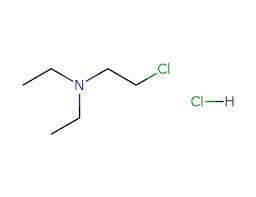
Best 2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines

Introduction
The pharmaceutical industry depends heavily on high-quality chemical intermediates for the production of life-saving medicines. One such essential compound is 2-Diethylaminoethyl Chloride Hydrochloride. It is widely used in the synthesis of antihistamines, local anesthetics, and other pharmaceutical drugs. With rising global demand, choosing the best 2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines ensures access to a consistent supply of this critical ingredient.
A trusted pharmaceutical drug manufacturer in Philippines focuses on providing superior-grade intermediates that meet global regulatory standards, ensuring safety and performance in drug development.
What is 2-Diethylaminoethyl Chloride Hydrochloride?
2-Diethylaminoethyl Chloride Hydrochloride is a chemical compound that plays a key role in the production of several pharmaceutical agents. It is mainly used as an intermediate in the synthesis of:
Antihistamines
Local anesthetics
Antispasmodic agents
Neurological medications
This compound is highly reactive and requires precise handling and manufacturing controls to maintain its purity and effectiveness.
Applications in the Pharmaceutical Industry
The primary use of 2-Diethylaminoethyl Chloride Hydrochloride is in drug synthesis. Pharmaceutical companies rely on its chemical properties to produce active pharmaceutical ingredients (APIs). Some important applications include:
Development of pain-relief and nerve-related medications
Production of drugs that control allergic reactions
Preparation of therapeutic compounds for gastrointestinal treatment
Using this compound in medicine manufacturing helps improve drug efficacy and patient outcomes.
Why Choose a Manufacturer from the Philippines?
1. High-Quality Standards
Manufacturers in the Philippines follow strict Good Manufacturing Practices (GMP) and adhere to global quality standards. Their production systems are audited regularly to maintain consistency and safety.
2. Advanced Technology
Facilities in the Philippines use advanced chemical synthesis and quality testing techniques. This ensures every batch of 2-Diethylaminoethyl Chloride Hydrochloride is reliable and meets global requirements.
3. Skilled Workforce
The Philippines has a skilled scientific workforce trained in chemical engineering, pharmaceutical manufacturing, and research. This helps ensure accurate production and quality control.
4. Affordable Manufacturing
The country offers cost-effective solutions without compromising product quality. This makes it ideal for companies seeking affordable but high-standard pharmaceutical intermediates.
5. Global Supply Chain Experience
A leading pharmaceutical drug manufacturer in Philippines is familiar with international logistics and export documentation. They can support clients worldwide with timely delivery and proper regulatory paperwork.
How to Select the Right Manufacturer?
When choosing a 2-Diethylaminoethyl Chloride Hydrochloride manufacturer in Philippines, look for the following:
Proven track record in pharmaceutical intermediate production
Certification in GMP, ISO, and other global standards
Modern facilities with in-house R&D and quality testing labs
Ability to supply custom quantities — from small to bulk orders
Transparent communication and customer support
Sustainability and Safety
Manufacturers in the Philippines also emphasize eco-friendly practices and workplace safety. They implement waste management systems, safe handling protocols, and energy-efficient production methods.
These initiatives make them reliable and responsible manufacturing partners.
Conclusion
If you're looking for a dependable supplier of 2-Diethylaminoethyl Chloride Hydrochloride, the Philippines stands out as a competitive and trustworthy location. With a focus on quality, affordability, and global supply capabilities, the country is home to some of the best pharmaceutical drug manufacturers in Philippines. These manufacturers not only offer top-grade products but also ensure you receive regulatory-compliant, timely deliveries worldwide.
#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) supplier in Philippines#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) exporter in Philippines
0 notes
Text
Thionyl Chloride Market Overview: Current Trends and Industry Outlook
The Thionyl Chloride Market has become an essential part of the global chemical industry, with growing demand across diverse applications such as agrochemicals, pharmaceuticals, dyes, batteries, and industrial synthesis. Known for its reactive properties and efficiency in chlorination processes, thionyl chloride (SOCl₂) plays a pivotal role as a chemical intermediate and reagent. As industries push toward innovation, efficiency, and sustainability, the market for thionyl chloride is poised for consistent growth in the forecast period from 2025 to 2032.

Market Overview and Chemical Profile
Thionyl chloride is an inorganic compound, typically colorless to pale yellow, with a pungent odor. It is widely used as a reagent in the synthesis of acyl chlorides and other organochlorine compounds. Its unique decomposition into gaseous by-products (sulfur dioxide and hydrogen chloride) makes it an effective choice in synthesis processes that require minimal residue. This characteristic provides a significant advantage in high-purity chemical applications, especially in the electronics and pharmaceutical sectors.
Current Market Trends
Several trends are currently shaping the Thionyl Chloride Market, influencing production, demand, and technological advancements:
Increased Use in Agrochemical Production The demand for crop protection products and herbicides has led to increased utilization of thionyl chloride in the agrochemical sector. Its role in synthesizing pesticides and herbicide intermediates is crucial for increasing crop yields and ensuring food security.
Growing Pharmaceutical Applications Thionyl chloride is widely used in pharmaceutical manufacturing, particularly in synthesizing active pharmaceutical ingredients (APIs). With the global healthcare sector experiencing steady growth, demand from this segment is rising accordingly.
Expansion in Lithium Battery Production Thionyl chloride is a key component in the production of lithium-thionyl chloride (Li-SOCl₂) batteries, which are known for their long shelf life and high energy density. These batteries are commonly used in defense, medical, and industrial applications.
R&D in Specialty Chemicals The increasing push for customized chemical formulations in industrial manufacturing and electronics is leading to higher usage of thionyl chloride in specialty chemicals and intermediates.
Industry Outlook
Looking ahead, the Thionyl Chloride Market is projected to register strong growth, driven by both demand-side and supply-side developments. The chemical industry’s gradual shift toward sustainable production methods is also expected to influence manufacturing processes of thionyl chloride.
Asia-Pacific remains the largest market due to the high concentration of chemical and pharmaceutical manufacturers in China and India. The growing need for agrochemicals in agricultural economies like India will further boost regional consumption.
North America and Europe are witnessing steady demand supported by pharmaceutical innovation, though strict environmental regulations may limit excessive growth.
Middle East and Africa are emerging as new markets due to rising investments in industrial zones and infrastructure development.
Challenges and Constraints
Despite promising growth, the Thionyl Chloride Market faces several challenges:
Environmental and Safety Concerns: Thionyl chloride is highly reactive and toxic. Its production and use are closely monitored by environmental authorities due to risks associated with handling and emissions.
Regulatory Compliance: Stringent compliance with chemical safety and disposal laws in Europe and North America can limit production flexibility and increase operational costs for manufacturers.
Supply Chain Vulnerabilities: The reliance on raw materials such as sulfur and chlorine, and the concentration of production facilities in a few geographic regions, make the market sensitive to geopolitical and logistic disruptions.
Key Players and Strategic Developments
Some of the major companies operating in the Thionyl Chloride Market include:
Lanxess AG
Shandong Kaisheng New Materials Co., Ltd.
Transpek Industry Limited
CABB Group GmbH
Jiangxi Selon Industrial Co., Ltd.
These companies are actively investing in research and development, capacity expansion, and international partnerships to enhance their competitive position. Mergers, acquisitions, and joint ventures are expected to be prominent strategies in the next few years.
Conclusion
The Thionyl Chloride Market is on a trajectory of robust growth, backed by rising demand across key industrial applications. While regulatory and environmental hurdles present challenges, technological advancements in production and handling, coupled with increased R&D spending, are creating new opportunities for market players. As the global economy shifts toward more sustainable and efficient chemical processes, thionyl chloride will continue to play a crucial role in enabling high-performance and high-purity solutions in both established and emerging sectors.
0 notes