#Anisomycin
Text
Hydrogen alleviates osteoarthritis via JNK signaling pathway
New Post has been published on https://depression-md.com/hydrogen-alleviates-osteoarthritis-via-jnk-signaling-pathway/
Hydrogen alleviates osteoarthritis via JNK signaling pathway

Introduction
As an irreversible condition of arthritic degeneration that can result in seriously unsteady joints, osteoarthritis (OA) is pathologically characterized by degenerative articular cartilage, progressive subchondral sclerosis, genesis of osteophytes, as well as inflammatory synovium.1 Being the most widespread type of chronic arthritic condition, its impact on everyday activities is especially prominent in elderly populations.2,3 Plentiful studies have identified a range of influencing factors of OA development, including inflammation, aging, obesity, trauma, deformed joints, osteoporosis, among others.4 In spite of these, the pathogenesis of OA remains hardly understood. Oxidative stress and inflammation have been proven as crucial risk factors for the OA progression.5 Besides, a large number of OA factors are capable of causing oxidant-antioxidant level imbalance, which facilitates chondrocyte stimulation to result in inflammatory cytokine generation.6–8 The current clinical therapies for OA mainly aim at improving joint functions and relieving pain. However, this treatment regime does not alleviate the progression of OA. Therefore, it is essential to explore new strategies for OA treatment.
Hydrogen (H2) is a colorless and odorless gas. It exhibits diverse biological activities, including anti-inflammatory and anti-oxidative properties.9–13 In 1975, Dole et al found that 97.5% H2 could be used as therapy for cancer.14 Ohta S found that a 2% concentration of H2 could ameliorate mitochondrial diseases due to its rapid diffusion into the tissues and cells.15 Ohsawa et al observed that the inhalation of 1–4% H2 could significantly alleviate cerebral ischemia-reperfusion injury by selectively reducing cytotoxic oxygen radicals.16
Previous studies revealed that the JNK signaling pathway can regulate inflammatory and apoptosis. When initiated by certain stimuli such as TBHP (tert-butyl hydroperoxide), the activation of JNK causes the phosphorylation of c-Jun. The c-Jun then decreases proteoglycan synthesis and enhances the production of matrix metalloproteinase-13 (MMP-13).17 Gao et al found that LncRNA MALAT-1 can suppress apoptosis and cartilage matrix degradation via the JNK signaling pathway.18 Jiang et al revealed that Nesfatin-1 inhibited the IL-1β-induced activation of NF-κB, the mitogen-activated protein kinase (MAPK), and the Bax/Bcl-2 signal pathway in chondrocytes.19 The activation of the JNK pathway stimulates the production of apoptosis mediators like Bax, Cytochrome c, cleaved caspase-3, etc.20,21 Therefore, inhibiting the activation of the JNK pathway might be a promising therapeutic strategy for the treatment of OA. A recent study indicated that H2 could suppress oxidative damage by decreasing MMP-13 and increasing collagenase type II (Col II) expression.22 However, it is unclear whether H2 mediates its protective effect in OA by utilizing the JNK pathway.
In this study, we found that H2 exerted a chondroprotective effect in OA and explored the potential mechanism under in vivo as well as in vitro conditions. Here, chondrocytes were exposed to tert-butyl hydroperoxide (TBHP) for the in vitro induction of oxidative stress. We found that H2 may suppress TBHP-induced apoptosis and inflammation in chondrocytes through the JNK pathway. Moreover, the activation of JNK by Anisomycin eliminated the anti-apoptotic and anti-inflammatory effects of H2. Thus, H2 suppressed apoptosis and inflammation and attenuated OA through the JNK signaling pathway. Our study highlighted the therapeutic potential of H2 in OA and explored the mechanism of anti-apoptotic and anti-inflammatory effects of H2 in the chondrocytes.
Materials and Methods
Ethics Statement and Experimental Animals
All Experimental procedures involving animal care strictly followed the guidelines for the Animal Care and Use outlined by the Committee of Wenzhou Medical University and approved by the Animal Care and Care Committee of Wenzhou Medical University. Tissue collection and experiments involving human OA were approved by the Second Affiliated Hospital of Ethics Committee of Wenzhou Medical University (ethic cord: LCKY-2019–67) and Yuying unknown Children’s Hospital and followed the guiding principles of the Helsinki Declaration. Informed consent was obtained from the human participants of this study.
Reagents
Anisomycin, Collagenase-II, Safranin-O/Fast Green, and dimethylsulfoxide (DMSO) were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). Primary antibodies of Collagenase II, Aggrecan, ADAMTS-5, and MMP-13 were gained from Abcam (Cambridge, MA, USA). Primary antibodies directed against P-JNK and JNK were procured from Cell Signaling Technology (MA, USA). Primary antibodies against Bcl-2 and GAPDH were procured from ProteinTech Group (Wuhan, China). Primary antibodies against cleaved caspase-3 and Bax were procured from Affinity Biosciences (Cincinnati, OH, USA). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM)/F12 were obtained from Gibco (Grand Island, NY, USA). Tert-Butyl hydroperoxide solution (TBHP) was procured from Sigma-Aldrich (St Louis, MO, USA). The secondary antibodies of Goat Anti-Mouse IgG, Goat Anti-Rabbit IgG, Alexa Fluor®488 and labeled Alexa Fluor®594 were obtained from bioWORLD (OH, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). The BCA protein assay kit was procured from Beyotime Biotechnology (Shanghai, China). Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan).The In Situ Cell Death Detection Kit was purchased from Roche (San Francisco, CA, USA). Caspase-3 colorimetric assay kit was obtained from Keygen Biotech Co., Ltd. (Nanjing, China).
Development of Mice OA Models
For the experiment, forty-five 10-week-old C57BL/6 female mice were obtained from the Animal Center of Chinese Academy of Sciences Shanghai. All mice procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Wenzhou Medical University and approved by the Animal Ethics Committee of Wenzhou Medical University (ethics code: wydw2019–0808). After injection with 2% pentobarbital sodium (40 mg kg−1) intraperitoneally for anesthesia, we established OA model by the method described in the previous study.23 The tibial ligament of the medial meniscus was cut with microsurgical scissors. An operation of arthrotomy without the transaction of medial meniscus ligament was also performed in the left knee joint of the mice in the sham operation group. After the operation, the mice were randomly divided into three groups (n = 15): sham group, an OA group (DMM), and an OA group treated with H2 (DMM + 75% H2). The gas was inhaled for 1 h per day.
X-Ray Imaging Assay
The mice were subjected to radiographic assessment at 8 weeks after surgery. Kubtec Model XPERT.8 X-ray machine (KUB Technologies Inc.) was utilized to determine the formation of osteophyte, joint clearance, and calcification of the cartilage surface. The settings of the machine were as follows: 160 µA and 50 kV.
Histopathologic Analysis
Safranin-O/Fast Green was used to measure the articular cartilage destruction. A light microscope was utilized to assess the morphological changes in the mice chondrocytes and the surrounding tissues. The destruction of articular cartilage was evaluated by the Osteoarthritis Research Society International (OARSI) scoring system for the medial tibial condyle and medial femoral plateau.
TUNEL Staining
After fixing the chondrocytes or cartilage sections, TUNEL staining was performed in a dark, humidified room using an In Situ Cell Death Detection kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Next, DAPI (4′,6-diamidino-2-phenylindole) was used to stain the cell nuclei. Positive staining of DNA strand breaks in the apoptotic cells was detected under a fluorescence microscope.
Immunohistochemical Assay
As a first step, the knee joints were subjected to 4% paraformaldehyde (PFA) fixation, decalcification, paraffin-embedment, as well as cutting into 7 µm sections for deparaffinization and rehydration processes. Then, 30 min treatment of histological sections was carried out at 37 °C using hydrogen peroxide (3% v/v) and trypsin-EDTA solution (0.25%). After a further 60 min incubation of these sections at 37 °C in BSA (10%), they were treated with the primary antibodies at 4 °C against the cleaved caspase-3 and P-JNK for a 24 h duration. Afterwards, 1 h section incubation was performed on the 2nd d, at 4 °C using an HRP-conjugated second antibody. Aided by the Image-Pro Plus version 6.0 (Media Cybernetics, MD, USA), image analysis was accomplished, while in the quantitative analysis, five sections from every group were used.
Primary Human Chondrocyte Isolation and Culture
Initially, from 10 patients with OA (half males and half females, age of 54 ± 8 years), who had received complete knee arthroplasty at the Second Hospital Affiliated to Wenzhou Medical University, we collected the human cartilage tissues, which were cut into pieces (1×1×1 mm3) and thrice washed using PBS. Then, 4 h incubation of the cut pieces was performed at 37 °C using collagenase II (2 mg/mL). After 6 min centrifugation at 800 rpm, suspension of the digested cartilage tissues was carried out, which was followed by plating into flasks for tissue cultures. At 37 °C, chondrocyte incubation was achieved under a 5% CO2 atmosphere using DMEM/F12 medium involving 10% FBS. Medium replacement was implemented every alternate day. We utilized trypsin-EDTA (0.25%) to subculture the human cells till reaching 80 to 90% confluences.
The Application of H2
H2 was stored in gas cylinders before the experiment. Then, hydrogen, oxygen, and nitrogen were mixed, and the H2 gas concentration (6.25, 12.5, 25, 50, and 75% (vol/vol)) was adjusted through a three-way connection and measured with TRACE GC Ultra gas chromatography (Thermo Fisher, MA, USA). After a volume of H2 was removed in each group, the remaining volume was mixed according to the ratio of nitrogen and oxygen in the air, that is, 0.78:0.21. Chondrocytes were pretreated with 25 µM TBHP for 24 h, and H2 was introduced into the cultured cells for 4 h.
CCK-8 Assay
The cytotoxicity of H2 on chondrocytes was detected with CCK-8 kits by following the manufacturer’s instruction. Firstly, cells were plated in 96-well plates at a density of 50,000 cell per cm2 for 24 h and incubated with various concentrations of H2 (0, 12.5, 25, 50, and 75%) for 4 h. Later, the chondrocytes were rinsed thrice in PBS. Finally, 10 mol/L CCK-8 solution was added to each well for 2 h, and the optical density was observed at 450 nm with a spectrophotometer (Thermo Fisher Scientific).
qRT-PCR
After stimulation with TBHP (25 µM) and treatment with H2 (75%), total RNA was extracted with the aid of TRIzol (Invitrogen) from the human chondrocytes. Regarding the RT-qPCR procedure, the CFX96 PCR System (Bio-Rad Laboratories, California, USA) was utilized under the following PCR cycling conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The reaction mixture comprised, in a 10 µL total volume, 5 µL of 2 X SYBR Master Mix, 4.5 µL of diluted cDNA, as well as each 0.25 µL of primer. Collection and normalization of cycle threshold (Ct) values were carried out towards the expression levels of GAPDH. To achieve computation of relative mRNA levels for every target gene, the 2−ΔΔCt method was employed. The primer creation for Bax, Bcl-2 and GAPDH was accomplished via the NCBI Primer-Blast program. In Table 1, the sequences of forward and reverse primers are detailed.

Table 1 Primers Used in the Studies
Western Blotting
The expression level of the proteins was measured by Western blotting. The proteins were separated with the RIPA lysis buffer, sonicated on ice for 10 min, and then centrifuged at 12,000 rpm for 15 min at 4 °C. The BCA protein detection kit was used to estimate the protein concentration. After protein isolation by gel electrophoresis with sodium dodecyl sulfate-polyacrylamide (SDS-PAGE), the isolated 40 mg of proteins were shifted to the polyvinylidene difluoride (PVDF) membranes (Millipore), and subjected to 3 h blockage using non-fat milk (5%). Incubation of the resulting membranes was performed against such primary antibodies as JNK (1:2000), P-JNK (1:2000), Bax (1:2000), Bcl-2 (1:2000), GAPDH (1:2000), cleaved caspase-3 (1:2000), MMP-13 (1:2000), ADAMTS-5 (1:2000), Collagenase II (1:2000), as well as Aggrecan (1:2000). Next, an additional 2.5 h incubation was carried out at room temperatures against secondary antibodies. For blots visualization, the Image Lab Touch 3.0 (Bio-Rad, Hercules, CA, USA) was utilized, followed by washing thrice using TBST.
Analysis of Immunofluorescence
The cells were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min. Next, the chondrocytes were incubated with 0.1% Triton X-100 at room temperature. Further, 10% goat serum solution was used to block in 37 °C water bath for 30 minutes and incubated with primary antibodies against Collagenase II (1:300) for the entire night at 4 °C. The next day, the cells were exposed to Alexa Fluor® 488-labeled conjugated secondary antibodies (1:400) for 1.5 h. Finally, the cells were exposed to DAPI (Beyotime) for 1 min. Ultimately, the cell samples were detected on the Olympus fluorescence microscope (Tokyo, Japan). The fluorescence intensity was observed by using the Image J software.
Statistical Analysis
The experiments were performed at least three times. The data obtained were expressed as the mean ±SEM. The data were analyzed via GraphPad Prism (United States). Inter-group comparisons were performed using a one-way ANOVA followed by the Tukey’s test. Probability values of P < 0.05 were considered statistically significant.
Results
H2 Inhibits the Cartilage Deterioration in a Murine DMM Model
A murine OA model was created through surgical operation, which was achieved by making the medial meniscus unstable In order to figure out whether H2 exerted protective functions on the progression of OA in vivo, we examined the cartilage histology in OA mice based on Safranin–O (S–O) staining in conjunction with X-radioscopy. As a result of the surgery, the density of cartilage surface was elevated and the joint space was narrowed. In the H2 group, on the contrary, improvements in the above impairments were noticed (Figure 1A). Based on the S–O staining outcomes presented in Figure 1B, the H2 therapy reduced the erosion of superficial cartilage and the substantial loss of proteoglycan. Plus, both the synovitis and OARSI scores agreed with the S–O staining outcomes. As shown in Figure 1C and D, the two types of scores were lower in the group treated with H2 than the untreated OA group.

Figure 1 H2 inhibits the progression of OA in the mouse DMM model. The digital X-ray images of mouse knee joints. (A) The white arrow indicates the narrowing of the joint space; the black arrow implies the calcification of the cartilage surface. Typical Safranin O staining of the cartilage and subchondral cortical bone (n = 15, scale bar: 200 µm and 50 µm) (B). Synovitis scores. H2 reduced synovitis scores compared to DMM. (n = 15) (C). Diagrams indicate the cartilage OARIS scores (n = 15) (D). All data are presented as mean ±SEM. ###P < 0.001 vs the sham group; ***P < 0.001 vs the DMM group; n = 15.
H2 Attenuates the Expression of Cytokines and Apoptosis-Related Proteins Following DMM in Mice
The apoptosis of chondrocytes was measured by TUNEL staining and cleaved caspase-3 immunohistochemical staining. As shown in Figure 2A and B, a higher proportion of cellular apoptosis was observed in the DMM group than in the sham group. However, the H2 group significantly reversed this pathological phenomenon. Besides, immunohistochemical staining of cleaved caspase-3 was performed to detect the in vivo protective effect of H2. As shown in Figure 2C and E, H2 decreased the expression of cleaved caspase-3 in mouse articular cartilage. Furthermore, immunohistochemical staining of P-JNK demonstrated increased levels of P-JNK in the DMM group than in the sham group. Again, the H2 treatment significantly reduced the expression of P-JNK (Figure 2C and D). These data indicated that H2 was a potential agent of treatment of OA under in vivo conditions.
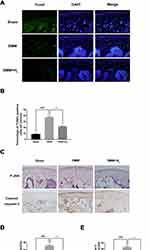
Figure 2 H2 suppresses the apoptosis of cartilage in OA mice. TUNEL staining assay in the mouse cartilage (n = 15) (A and B). Immunohistochemistry of cleaved caspase-3 and P-JNK identified the effect of H2 on the degradation of cartilage matrix in OA mice (n = 15) (C). Quantification of cleaved caspase-3 and P-JNK-positive cells in the cartilage samples (D and E). All data are presented as mean ±SEM. ###P < 0.001 vs the sham group; ***P < 0.001 vs the DMM group; n = 15.
H2 Protects Chondrocytes from TBHP Treatment
We used toluidine blue, immunofluorescence, and Safranin O staining to characterize the isolated human chondrocytes. (Figure 3A). The chondrocytes were stained red by Safranin O staining, while their cytoplasm was stained purple by toluidine blue. The collagen II in the cytoplasm of human chondrocytes was stained green by immunofluorescence without any positive staining in the nucleus (Figure 3B and C). Both stains demonstrated that the cells extracted from the articular cartilage were chondrocytes. The cytotoxic effects of H2 on human chondrocytes were detected by the CCK-8 assay. The cells were treated with a concentration gradient (0,12.5, 25, 50, and 75%) of H2 for 4 h. The CCK-8 analysis revealed that H2 did not show any obvious cytotoxicity toward human chondrocytes (Figure 3D). Furthermore, the chondrocytes were treated with different concentrations of H2 (0,12.5, 25, 50, and 75%) for 4 h after stimulation with TBHP for 24 h. As shown in Figure 3E, the viability of the chondrocytes was reduced in the TBHP group whereas H2 significantly increased the viability of the cells in a dose-dependent manner. Thus, we adopted 75% H2 as the concentration to conduct subsequent experiments.
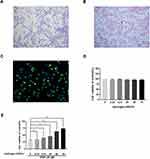
Figure 3 Effect of H2 on human chondrocyte viability. Chondrocytes were stained by toluidine blue, and proteoglycans in chondrocytes were stained purple (n = 3) (A). Safranin O staining of human primary chondrocytes (n = 3) (B). Collagen II immunofluorescence staining of human primary chondrocytes (n = 3) (C). The cytotoxicity and the cell viability of H2 on the TBHP-induced chondrocytes by using the CCK-8 assay (n = 3, 24 h) (D and E). All data are presented as mean ±SEM. *P < 0.05, **P < 0.01, ***P < 0.001, vs the control group, n = 3.
H2 Exerts Anti‑Apoptotic Effects in TBHP-Induced Human Chondrocytes
To identify the effects of TBHP on the human chondrocytes, different concentrations (0, 12.5, 25, and 50 µM) were used for stimulation. As pictured in Figure 4A and B, the apoptosis-related protein expression of cleaved-caspase 3 upregulated with increasing concentrations of TBHP. Moreover, TBHP induced the apoptosis of chondrocytes (Figure 4C and D). Therefore, the TUNEL assay was used to detect the anti-apoptotic effect of H2 on human chondrocytes. As shown in Figure 4E and F, a greater incidence of apoptosis was recorded in the chondrocytes in the TBHP (25 µM) group compared with the control group; the H2 treatment significantly reversed this trend. The activation of caspase-3 was evaluated by the caspase colorimetric assay kit. As shown in Figure 4G, the activation of caspase-3 was significantly upregulated after stimulation with TBHP. However, H2 treatment downregulated this activation of caspase-3.
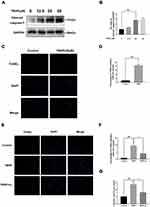
Figure 4 Effects of H2 on TBHP-induced apoptosis in human chondrocytes. The protein expression of cleaved caspase-3 in chondrocytes treated with/without TBHP was detected by Western blotting and TUNEL assay (A–D). H2 exerts the anti-apoptosis effect in TBHP-induced chondrocytes as seen from the TUNEL assay (scale bar: 50 µm) and the quantification of apoptotic positive cells (E and F). The activity of caspase-3 was determined using the caspase colorimetric assay kit (n = 3) (G). All data are presented as mean ±SEM. ###P < 0.001 vs the control group; ***P < 0.001 vs the TBHP group; n = 3.
H2 Decreases the Expression of Cytokines and Apoptosis‑Related Proteins in TBHP-Induced Human Chondrocytes
To determine the effect of H2 on apoptosis in TBHP-induced human chondrocytes, the expression of cleaved caspase-3, Bcl-2, and Bax was evaluated by Western blot. As pictured in Figure 5A–E, TBHP increased the expression of Bax, cytochrome c, and cleaved caspase-3 and downregulated the expression of Bcl-2. On the contrary, H2 suppressed the expression of Bax, cytochrome c, and cleaved caspase-3 and improved the Bcl-2 expression. In addition, the results of qRT-PCR showed that H2 suppressed the generation of Bax, which had increased after TBHP stimulation (Figure 5F and G). Moreover, the immunofluorescence of cleaved caspase-3 also indicated that H2 suppressed the expression of cleaved caspase-3 in TBHP-induced chondrocytes (Figure 5H). Therefore, these data demonstrated that H2 was capable of preventing the TBHP-induced apoptosis of chondrocytes.

Figure 5 Effects of H2 on the TBHP-induced expression of cytokines and apoptosis-related proteins in human chondrocytes. The levels of cleaved caspase-3, Bcl-2, cytochrome c, and Bax were evaluated by Western blotting (A–E). The mRNA expression levels of Bcl-2 and Bax were assessed by qRT-PCR (F and G). Immunofluorescence of cleaved caspase-3 was observed with a fluorescence microscope (OLYMPUS)(Scale bar: 50 µm) and assayed by Image J (H). All data are presented as mean ±SEM. ##P < 0.01, ###P < 0.001, vs the control group; **P < 0.01, ***P < 0.001, vs the TBHP group; n = 3.
H2 Exerts Anti‑Inflammatory Effects in TBHP-Induced Human Chondrocytes
To investigate the role of H2 in inhibiting the degradation of extracellular matrix (ECM) in TBHP-induced human chondrocytes, Western blotting was utilized. We, respectively, assessed the protein levels of Collagen II, Aggrecan, ADAMTS-5, and MMP-13 in the chondrocytes. As pictured in Figure 6A–E, chondrocytes exerted an apparent downregulation of the expression of collagen II and Aggrecan following TBHP-induction. In contrast, an upregulation of the expression of ADAMTS-5 and MMP-13 was observed. Moreover, H2 treatment could reverse this trend. Further, the immunofluorescence outcomes demonstrated that H2 attenuated the degradation of collagen II (Figure 6F and G). Therefore, these results indicated that H2 can inhibit inflammation induced by TBHP in human chondrocytes.
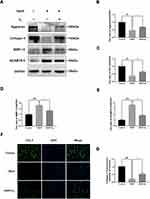
Figure 6 Effects of H2 on influencing ECM synthesis on TBHP-induced chondrocytes. The levels of aggrecan, ADAMTS5, Collagen II, and MMP13 were evaluated by Western blotting (A–E). A representative image of immunofluorescence staining of collagen II in the chondrocytes was detected by a fluorescence microscope (OLYMPUS) (Scale bar: 50 µm) and assayed by the Image J software (F and G). All data are presented as mean ±SEM. ##P < 0.01, ###P < 0.001, vs the control group; *P < 0.05, **P < 0.01, ***P < 0.001, vs the TBHP group; n = 3.
H2 Exerts Anti-Apoptotic and Anti‑Inflammatory Effects in the TBHP-Induced Human Chondrocytes by Suppressing the JNK Pathway
Western blot was used to investigate the effect of H2 on the JNK signaling pathway. The expression of P-JNK significantly increased in the chondrocytes after stimulation with TBHP. Conversely, H2 notably suppressed the TBHP-induced activation of JNK in the human chondrocytes (Figure 7A–C).
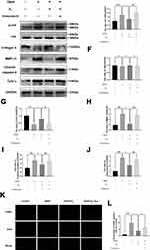
Figure 7 H2 inhibits the TBHP–induced JNK signaling pathway in the chondrocytes. The levels of JNK, P-JNK, Collagen II, MMP-13, cytochrome c, and cleaved caspase-3 in the human chondrocytes were assayed by Western blotting (A–J). The results of the TUNEL assay of the above-treated chondrocytes (scale bar: 50 µm) (K) and the quantification of apoptotic positive cells (L). All data are presented as mean ±SEM. ##P < 0.01, ###P < 0.001 vs the control group; *P < 0.05, **P < 0.01, and ***P < 0.001, vs the TBHP group; &P < 0.05, &&P < 0.01, and &&&P < 0.001 vs the TBHP + H2 group; n = 3.
However, treatment with Anisomycin (a JNK activator) inhibited the dephosphorylation of JNK. Moreover, H2 mediated a reduction in the expression of MMP-13, cytochrome c, and cleaved caspase-3, and this reduction was abolished by the addition of Anisomycin in the H2+Anisomycin group (Figure 7D–J). Moreover, the TUNEL assay showed that H2 decreased the incidence of apoptosis in TBHP-induced chondrocytes compared to the TBHP group, whereas the Anisomycin treatment could significantly reverse this trend (Figure 7K and L). These results revealed that H2 suppressed the apoptosis induced by TBHP in human chondrocytes via the JNK signaling pathway.
Discussion
Osteoarthritis (OA) is a chronic joint degradation disease that is characterized by long-term pain and joint limitation.2 The current options for the treatment of OA are an early application of non-steroidal anti-inflammatory drugs (NSAIDs), but they only relieve the clinical symptoms.24,25 Moreover, they also result in a series of side effects such as heart attack and stroke.26 Artificial joint replacement remains the only choice for the treatment of the final stages of OA.27 Therefore, an agent that prevents the progress of OA and is accompanied by fewer side effects is a potential therapeutic strategy for OA. Recently, increasing attention has been focused on anti-apoptosis compounds that may be capable of treating OA in the absence of harmful side effects.28 Biochemical and biomechanical events induce apoptosis and imbalance between the catabolism and anabolism of the ECM in the chondrocytes.29 Several pharmacological treatments for the genetic regulation of apoptosis have shown therapeutic effects against OA development in cellular and animal models.30 Therefore, studying the underlying mechanism of apoptosis in the chondrocytes may result in a potential therapy for OA.
Oxidative stress is the main process in chondrocyte apoptosis. When chondrocytes are stimulated with TBHP, they generate ROS like superoxide anion (O2−), H2O2, and hydroxyl radical (OH); this leads to mitochondrial damage and eventually apoptosis.31 An increasing number of studies have found that excessive ROS leads to the degradation of cartilage and apoptosis of chondrocytes.32,33 Therefore, decreasing the production of ROS may be an effective strategy for OA treatment. Interestingly, hydrogen is an anti-oxidative compound.
H2 is the lightest element and characteristic of the simplest and abundant elements in nature.16 Many studies have reported the anti-inflammatory, anti-apoptotic, and anti-oxidative effects of H2 in several diseases.10,34 They demonstrated that inhalation of 4% H2 was a safe and efficient treatment for human diseases. Hydrogen has aroused the attention of researchers due to its simple preparation, high safety, strong permeability, and ease of carrying it.16 Peng Guan et al found that H2 improved chronic intermittent hypoxia (CIH)-induced kidney injury via the JNK signaling pathway.35 Zhao et al revealed that H2 alleviated ER stress and apoptosis of cardiac myocytes by blocking the c-Jun N-terminal kinase(JNK)-MAPK.36 Wu et al uncovered that H2 attenuated the hypoxic-ischemic brain damage by suppressing apoptosis and inflammation in neonatal rats.37 Chen et al found that Inhalation of hydrogen could ameliorate apoptosis and oxidative stress of spinal cord neurons in spinal cord injury in mice.38 The JNK pathway is a classical pro-apoptosis pathway and has been demonstrated to play a pivotal role in the regulation of apoptosis of chondrocytes with OA development. Previous studies have demonstrated that some drugs such as Urolithin A, Kinsenoside, and Luteolin protected the chondrocytes by inhibiting the activation of the JNK signaling pathway.39–41 Hence, targeted downregulation of phosphorylation of JNK may be deemed to cure OA effectively.
Therefore, in the present study, we explored the anti-apoptotic capability of H2 on human chondrocytes with the JNK signaling pathway. In the TBHP-treated chondrocytes of humans, remarkable inhibition of JNK phosphorylation by H2 was demonstrated. Due to the inhibited activity of JNK pathway, the production of such apoptosis factors as Bax and cleaved caspase-3 was downregulated, which in turn led to improvements in the OA progression. Collectively, our data somewhat suggested that the H2 possessed an anti-apoptotic activity during OA development, which was demonstrated on THBP-treated chondrocytes, and that the JNK pathway might be involved in its mechanism of action. The potential value of H2 was thus apparent. Moreover, shrinking space of joint, degradative ECM, seriously eroded cartilage and vastly deficient proteoglycan were noticed in the DMM groups in contrast to the sham group. Upon the H2 therapy, the aforementioned symptoms all got improved, and the murine DMM model also exhibited lower grading score of OARSI. According to the TUNEL stain of cartilage and IHC stain of cleaved caspase-3, the apoptosis of cartilage was mitigated by H2. Furthermore, the OA progression got improved by H2, which involved the signaling pathway of JNKs. Collectively, these outcomes and the preparatory findings suggest the ameliorating activity of H2 against the OA development, which was achieved by suppressing the JNK pathway activation (Figure 8).
Figure 8 Schematic reveals the involvement of H2 via the JNK pathway and the potential protective effects in the progression of osteoarthritis.
Interestingly, hydrogen has been studied for its therapeutic effects on various diseases at different concentrations. In this study, high H2 concentration (6.23–75%) was used to study the protective effect of H2 on osteoarthritis. However, the role of H2 in osteoarthritis still needs to be investigated as this study has some limitations. For example, we do not know whether a high concentration of H2 has adverse effects on other systems of the body. Moreover, the effects of long-term low concentration of hydrogen on osteoarthritis have not been studied; these need to be further studied in the future.
In conclusion, we uncovered that H2 alleviates the apoptotic reaction and degradative ECM by deactivating the signaling pathway of JNKs in chondrocytes of humans. In the meantime, a vital function of H2 therapy is also found against the OA development in the murine model of surgically-induced DMM. Our data collectively imply an anti-OA protective activity exerted by H2.
Funding
This study is supported by Zhejiang Provincial Natural Science Foundation of China (LY16H060010).
Disclosure
The authors have declared that no competing interests exists.
References
1. FrenchH P, Galvin R, Horgan NF, et al. Prevalence and burden of osteoarthritis amongst older people in Ireland: findings from The Irish Longitudinal Study on Ageing (TILDA). Osteoarthr Cartil. 2015;23(1):A188. doi:10.1016/j.joca.2015.02.967
2. Thysen S, Luyten FP, Lories RJ. Targets, models and challenges in osteoarthritis research. Dis Model Mech. 2015;8(1):17–30. doi:10.1242/dmm.016881
3. Aicher WK, Rolauffs B. The spatial organization of joint surface chondrocytes: a review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2013.
4. Shen CL, Smith BJ, Lo DF. Dietary polyphenols and mechanisms of osteoarthritis. J Nutr Biochem. 2012;23(11):1367–1377. doi:10.1016/j.jnutbio.2012.04.001
5. Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–642. doi:10.1136/bmj.332.7542.639
6. Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1beta in primary osteoarthritis influences the expression of mediators implicated in pathogenesis. Osteoarthr Cartil. 2012;20:1610–1618. doi:10.1016/j.joca.2012.08.011
7. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5–15. doi:10.1016/j.berh.2014.01.004
8. Goldring MB, Fukuo K, Birkhead JR, et al. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. Cell Biochem. 1994;54:85–99. doi:10.1002/jcb.240540110
9. Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi:10.1016/j.ijcard.2010.08.058
10. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi:10.1016/j.bbrc.2007.07.088
11. Wang F, Yu G, Liu S, Li J. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167:339–344. doi:10.1016/j.jss.2010.11.005
12. Chen H, Sun Y, Hu P, et al. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res. 2011;167:316–322. doi:10.1016/j.jss.2009.07.045
13. Huang Y, Xie K, Li J, et al. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2011;1378:125–136. doi:10.1016/j.brainres.2010.12.071
14. Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190(4210):152–154. doi:10.1126/science.1166304
15. Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 2012;1820(5):586–594. doi:10.1016/j.bbagen.2011.05.006
16. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi:10.1038/nm1577
17. Ge H-X, Zou F-M, Li Y, et al. JNK pathway in osteoarthritis: pathological and therapeutic aspects. J Recept Signal Transduct Res. 2017;37(5):431–436. doi:10.1080/10799893.2017.1360353
18. Gao GC, Cheng XG, Wei QQ, Chen WC, Huang W-Z. Long noncoding RNA MALAT-1 inhibits apoptosis and matrix metabolism disorder in interleukin-1β-induced inflammation in articular chondrocytes via the JNK signaling pathway. J Cell Biochem. 2019;120(10):17167–17179. doi:10.1002/jcb.28977
19. Jiang L, Kai X, Jin L, et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging. 2020;12(2):1760–1777. doi:10.18632/aging.102711
20. Dai X, Li Y, Zhang R, et al. Effects of sodium selenite on c-Jun N-terminal kinase signalling pathway induced by oxidative stress in human chondrocytes and c-Jun N-terminal kinase expression in patients with Kashin-Beck disease, an endemic osteoarthritis. Br J Nutr. 2016;115(9):1547–1555. doi:10.1017/S0007114516000362
21. Zhang H-B, Zhang Y, Chen C, et al. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-κB. Apoptosis. 2016;21(10):1082–1093. doi:10.1007/s10495-016-1280-z
22. Cheng S, Peng L, Xu B, et al. Protective effects of hydrogen-rich water against cartilage damage in a rat model of osteoarthritis by inhibiting oxidative stress, matrix catabolism, and apoptosis. Med Sci Monit. 2020;12(26):e920211.
23. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the mouse. Osteoarthr Cartil. 2007;15:1061–1069. doi:10.1016/j.joca.2007.03.006
24. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. doi:10.1016/S0140-6736(17)31744-0
25. Sun MM, Beier F, Pest MA. Recent developments in emerging therapeutic targets of osteoarthritis. Curr Opin Rheumatol. 2017;29:96–102. doi:10.1097/BOR.0000000000000351
26. Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi:10.1016/j.joca.2009.03.002
27. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi:10.1016/j.coph.2016.02.009
28. Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res. 2001;50:19–23. doi:10.1007/s000110050719
29. Sutipornpalangkul W, Morales NP, Harnroongroj T. Free radicals in primary knee osteoarthritis. J Med Assoc Thai. 2009;92(Suppl. 6):S268eS274.
30. Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13.
31. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi:10.3390/ijms161125943
32. Hosseinzadeh A, Kamrava SK, Joghataei MT. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016;61(4):411–425. doi:10.1111/jpi.12362
33. Ansari MY, Khan NM, Ahmad I, et al. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097. doi:10.1016/j.joca.2017.07.020
34. Ohta S. Recent progress toward hydrogen medicine: the potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011;17(22):2241–2252. doi:10.2174/138161211797052664
35. Guan P, Sun Z-M, Luo L-F, et al. Hydrogen protects against chronic intermittent hypoxia-induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi:10.1016/j.lfs.2019.04.005
36. Zhao Y-S, Ji-Ren A, Yang S, et al. Hydrogen and oxygen mixture to improve cardiac dysfunction and myocardial pathological changes induced by intermittent hypoxia in rats. Oxid Med Cell Longev. 2019;2019:7415212. doi:10.1155/2019/7415212
37. Wu G, Chen Z, Wang P, et al. Hydrogen inhalation protects hypoxic-ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats. Exp Biol Med. 2019;244(12):1017–1027. doi:10.1177/1535370219855399
38. Chen X, Cui J, Zhai X, et al. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell Physiol Biochem. 2018;47(1):176–190. doi:10.1159/000489764
39. Ding S-L, Pang Z-Y, Chen X-M, et al. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J Inflamm. 2020;17:13. doi:10.1186/s12950-020-00242-8
40. Zhou F, Mei J, Han X, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF- κ B/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9(5):973–985. doi:10.1016/j.apsb.2019.01.015
41. Xue J, Ye J, Xia Z, et al. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell Mol Biol. 2019;65(6):91–95. doi:10.14715/cmb/2019.65.6.15
Source link
0 notes
Text
Antibiotics produced by Bacteria activate Human Oocytes, creating Healthy Babies: AMPK links the Creation of Human Life with HIV, Progeria, & Cancer
CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons;By De Wood, Pooley, USDA, ARS, EMU. [Public domain], via Wikimedia Commons
A recent study published online in the journal Fertility and Sterility in September of 2017 systematically reviewed for the first time evidence for the effect of two compounds, ionomycin and A23187 (also known as calcimycin), on fertilization rates and pregnancy outcomes in infertile couples undergoing an in vitro fertilization procedure known as intracytoplasmic sperm injection (ICSI) [1]. ICSI involves the direct deposition of sperm into the oocyte cytoplasm, which typically leads to high rates of fertilization. However, fertilization failure despite repeated ICSI is likely caused by a failure of the oocyte to activate [1]. Physiological oocyte activation is accomplished by the delivery of a sperm-borne oocyte activating factor called phospholipase C zeta (PLCζ). PLCζ activates human oocytes by inducing an intracellular signaling cascade that ultimately results in increased calcium (Ca2+) oscillations in the oocyte, which drives oocyte activation to completion [1]. As oocyte activation is an indispensable prerequisite for the creation of all human life, every human being alive today and any human being that has ever lived began their existence as an activated oocyte [2]. Ionomycin and A23187 increase the levels of intracellular Ca2+ and are thus commonly known as Ca2+ ionophores [1]. The authors of the Fertility and Sterility study showed that over a total of 1,521 ICSI cycles, calcium ionophores including ionomycin and A23187 led to a statistically significant improvement in fertilization, cleavage, blastulation, implantation rates, overall pregnancy, and live-birth rates [1]. Ionomycin and A23187 have also been shown in several independent studies to effectively induce human oocyte activation, leading to the birth of normal, healthy children [3,4].
Strikingly, as described further below, both ionomycin and A23187 are antibiotics that are naturally produced by certain species within the bacterial genus Streptomyces [5,6]. Other structurally distinct compounds and methods have also been shown to induce human oocyte activation, including ethanol, puromycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces alboniger), as well as mechanical manipulation and electrical stimulation, both of with have been reported to result in the creation of normal children [7-11]. Interestingly, as mouse oocytes are considered models for human oocytes, ionomycin, A23187, anisomycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces griseolus), mycophenolic acid (an immunosuppressant produced by the fungus Penicillium brevicompactum), cycloheximide (a protein synthesis inhibitor produced by Streptomyces griseus), carvacrol (a secondary plant metabolite produced by Origanum vulgare{oregano}), and phorbol 12-myristate 13-acetate (PMA, a secondary plant metabolite produced by Croton tiglium) each induce activation of mouse oocytes [12-22]. Ionomycin, A23187, PMA, and reactive oxygen species (ROS) also induce the acrosome reaction in human sperm, a process characterized by the release of hydrolytic enzymes from the head of sperm which is necessary for oocyte penetration and thus indispensable for the creation of all human life outside of a clinical setting (ICSI bypasses the need for oocyte penetration) [23,24]. Additionally, although an over-production of ROS, similar to Ca2+, may lead to deleterious effects including cell death/apoptosis, low levels of ROS have been shown to act as signaling molecules and ROS is significantly increased on or immediately following mouse oocyte activation [25,26].
Furthermore, the master metabolic regulator AMPK is critical for oocyte meiotic resumption and maturation (a process that precedes and is essential for oocyte activation), is found located across the entire acrosome in the head of human sperm, and is activated by increases in ROS and Ca2+ [27-29]. Ionomycin, A23187, ethanol, puromycin, mechanical force, electrical stimulation, anisomycin, mycophenolic acid, carvacrol, and PMA also induce AMPK activation, indicating that a common mechanism of action links chemically distinct compounds with the creation of human life [30-39]. This common mechanism of action likely centers on the induction of cellular stress, mediated by indirect increases in intracellular Ca2+, ROS, and/or the AMP(ADP)/ATP ratio, etc. as I originally proposed in 2016 [40]. Because the bacterial-derived antibiotics ionomycin and A23187 induce both the acrosome reaction in human sperm and human oocyte activation, producing normal, healthy children, it can be said that “non-human organisms have the power to create human life or the power to end life.” As explained below, the beneficial effects of cellular stress induction (i.e. a “shock”) crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution.
A number of bacterial species residing within the genus Streptomyces have proven to be extremely important and medicinally valuable as approximately 70% of clinically useful antibiotics are derived from Streptomyces [41]. The antibiotics ionomycin and A23187 are naturally produced by Streptomyces conglobatus and Streptomyces chartreusensis, respectively [5,6]. Other important examples include the antibiotic tetracycline (produced by Streptomyces aureofaciens), the immunosuppressant rapamycin (produced by Streptomyces hygroscopicus), and the anti-helminthic avermectins (produced by Streptomyces avermitilis) [42]. Many soil and aquatic-dwelling species of Streptomyces can be found in harsh environments and are characterized by a unique life cycle, including spore germination followed by vegetative mycelium production, aerial hyphae formation, sporulation (i.e. spore formation), and antibiotic production [43,44]. Curiously, just as cellular stress induction leads to the creation of human life and other beneficial effects in human cells (see below), stress induction also promotes the induction of aerial hyphae formation, sporulation, and antibiotic production in many Streptomyces species (spp.). Indeed, a decrease in the levels of ATP and bacterial growth is associated with sporulation, aerial hyphae formation, and antibiotic production [42,45]. A reduction in glucose/nutritional deprivation, the preferred sugar/carbon source for many Streptomyces spp., also significantly increases antibiotic production [46]. An increase in intracellular ROS and Ca2+ is associated with spore germination, aerial hyphae formation, and antibiotic production [47-49]. Other cellular stressors, including heat shock and ethanol, also significantly increase antibiotic production, provocatively indicating that the effects of cellular stress crosses species boundaries, enhancing bacterial survival and facilitating the creation of human life [50,51].
The beneficial effects of low-level cellular stress induction also extends to plants, as many plants produce secondary metabolites partly for the purpose of self-defense, analogous to antibiotics. Similar to the harsh, stressful environments often inhabited by Streptomyces spp., the Great Basin Bristlecone Pine (Pinus Longaeva), considered the oldest living non-clonal organism on the planet ( >5000 years old), thrives in an exceptionally harsh environment, characterized by increased elevations and exposure to UV radiation, nutritionally-deprived soils, harsh temperatures, and mechanical stress due to wind variances, leading early researchers to conclude that it’s longevity is intimately associated with adversity [52-54]. Conversely, Pinus Longaeva species that are located in less stressful environments (i.e. lower elevations) are strongly associated with younger age classes (<875 years) [55]. Similarly, the Creosote bush (Larrea tridentate), considered one of the oldest living clonal organisms on the planet (>11,000 years old), also thrives in harsh environments including the Mohave Desert [56]. AMPK, which increases lifespan and healthspan in several model organisms, is the primary sensor of cellular stress in eukaryotic organisms (e.g. plants and humans) and the plant AMPK orthologue SnRK1 as well as Ca2+ and ROS are critical for seed germination, fertilization, root gravitropism, and secondary metabolite production [57-64]. The secondary plant metabolites PMA (which activates mouse oocytes and promotes the acrosome reaction in human sperm) and artemisinin (an anti-malarial drug) both activate AMPK and the antibiotic A23187 also increases production of the secondary metabolite resveratrol in grape cell cultures, again indicating that exposure to low-level stressors may promote extension of lifespan and initiate the creation of human life [17,23,39,65,66].
Organismal exposure to beneficial levels of stress may also play a critical role in evolution. As first noted by Charles Darwin, evolution is driven by natural selection, a process characterized by environmentally-induced phenotypic changes that may lead to inheritable survival and reproductive advantages [67]. From “On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life”, Darwin explained that “if there be, owing to the high geometrical powers of increase of each species, at some age, season, or year, a severe struggle for life, and this certainly cannot be disputed;……But if variations useful to any organic being do occur, assuredly individuals thus characterised will have the best chance of being preserved in the struggle for life;” [67]. This “struggle for life” Darwin spoke of is embodied by selective pressures which may be abiotic (i.e. light, wind, temperature, etc.) or biotic (predation, disease, competition, etc.) [68,69]. As alluded to above, such selective pressures are indeed sources of cellular stress, sensed by both prokaryotes and eukaryotes, that induce beneficial responses (at appropriate levels), leading to the acquisition of phenotypes conducive for continued survival. Both biotic (e.g. infection) and abiotic (e.g. heat) stressors/selective pressures activate AMPK (which is evolutionarily conserved among eukaryotes) in human cells [70,71]. A phenomenon often cited as an example of natural selection on a readily observable timescale is the development of bacterial resistance to antibiotics, resulting in problematic mutant strains that may be life-threatening for some individuals (i.e. the elderly and immunocompromised) [72]. Intriguingly, lethal levels of bactericidal antibiotics have been shown to kill microorganisms via the induction of ROS, sub-lethal levels of bactericidal antibiotics however increase mutagenesis and bacterial resistance via induction of lower levels of ROS, and heat as well as nutritional stress increase bacterial resistance to antibiotics, providing compelling evidence that continuous exposure to low levels of stress likely plays a significant role in natural selection and evolution [73-75].
Moreover, gravity itself likely functions as a cellular stressor/selective pressure that has influenced the development of organisms on Earth since the emergence of the very first lifeform. Gravity exerts its effects on living organisms via the application of force, which is experienced by human cells in the form of mechanical loading or stress [76]. The application of force or a mechanical load has recently been shown to activate AMPK and simulated microgravity (i.e. hind limb unloading in mice) significantly decreases AMPK activation [77,78]. Spaceflight also inhibits the activation of T cells (immune cells essential for adaptive immunity), whereas the application of force and AMPK activation promote T cell activation [79-81]. Interestingly, spaceflight has recently been shown to decrease the levels of the master antioxidant transcription factor Nrf2 and the heat shock-inducible protein HSP90a but increase the levels of the growth-promoting kinase mTOR in mice [82]. AMPK however inhibits mTOR but increases the phosphorylation, nuclear retention, and transcriptional activity of Nrf2 [57,83,84]. Also, HSP90 interacts with and maintains AMPK activity and HSP90 is necessary for progesterone-induced human sperm acrosome reaction [85,86]. Interestingly, rapamycin, an immunosuppressant produced by Streptomyces hygroscopicus, extends lifespan in genetically heterogeneous mice, activates AMPK in vivo in normal aged mice, and increases human sperm motility [42,87,88]. Simulated microgravity via the use of NASA-designed rotating wall vessels (RWVs) however drastically reduces rapamycin production (~90%) whereas the antibiotic gentamycin increases rapamycin production by Streptomyces hygroscopicus, providing further evidence that cellular stress, in the form of mechanical loading induced by gravity, is essential for development, function, and survival of Earth-bound organisms [89,90].
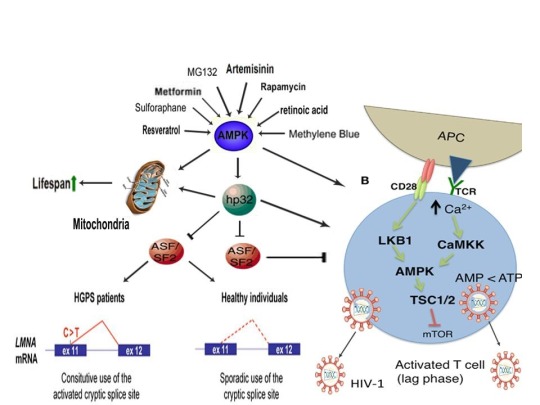
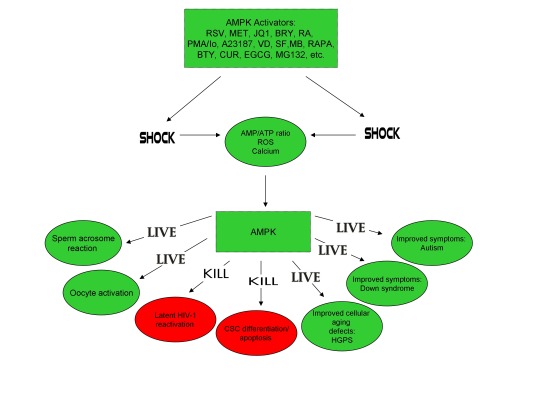
The induction of cellular stress also links seemingly dissimilar physiological and pathological states with the activation of AMPK. As discussed above, both ionomycin and ROS activate AMPK and promote oocyte meiotic resumption, a process that is AMPK-dependent and is essential for efficient oocyte activation [27,30,91]. ROS is also critical for ovulation, PMA and ionomycin both activate mouse oocytes, and ionomycin is extensively used during ICSI procedures, creating normal healthy children, suggesting that cellular stress-induced AMPK activation is also essential for oocyte activation [3,4,12,17,92]. The activation of oocytes and T cells share strikingly similar intracellular signaling mechanisms (e.g. PLC-PIP2-DAG-PKC-IP3-Ca2+) and ionomycin combined with PMA are extremely effective in activating T cells and are often used as positive controls in HIV-1 latency reversal studies [93-95]. Reactivating latent/dormant HIV-1 in CD4+ T cells, potentially facilitating immune system detection and virus destruction, is currently being pursued as a method for the potential eradication of HIV-1 (called the “shock and kill” approach) [96]. Similar to oocyte activation, both Ca2+ and ROS are critical for T cell activation (and hence latent HIV-1 reactivation) and other cellular stress-inducing compounds, including NDGA derived from the Creosote bush, butyrate derived from bacteria, as well as ROS and HSP90 have been shown to reactivate latent HIV-1 [26,93,94,97-101]. Interestingly, AMPK inhibition leads to cell death on T cell activation, knockdown of AMPK significantly decreases HIV-1 replication, and metformin (a well-studied AMPK activator derived from the French Lilac plant) increases butyrate production in human diabetic patients [81,102,103]. Perhaps most convincingly, early preliminary data showed that metformin significantly reduced several markers preferentially associated with cells latently infected with HIV-1 (e.g. PD-1, TIGIT, TIM-3) and also destabilized the latent HIV-1 reservoir in chronically-infected HIV patients, indicating that cellular-stress induced AMPK activation likely links the creation of human life with the potential eradication of HIV-1, as I originally proposed in 2016 [40,104,105].
AMPK activation may also link the disparate disease states of HIV-1 latency and Hutchinson-Gilford progeria syndrome (HGPS). HGPS is a genetic disorder caused by aberrant alternative splicing of the LMNA gene, generating a toxic protein called progerin that induces an accelerated aging phenotype and premature death at approximately 14 years of age [106]. Excessive activity of the gene splicing factor SRSF1 has been shown to prevent reactivation of latent HIV-1 and contribute to aberrant splicing of the LMNA gene in HGPS [107-109]. Metformin however has recently been shown to ameliorate the accelerated aging phenotype in cells derived from children with HGPS by reducing the levels of both SRSF1 and progerin and activating AMPK, as I first proposed in 2014 [110-112]. Interestingly, both Ca2+ and ROS induce autophagy (a process of disposing of damaged/toxic proteins and organelles) and rapamycin, which activates AMPK in vivo and increases intracellular Ca2+ levels, improves accelerated aging in progeria cells by inducing autophagic degradation of progerin [87,113-116]. Temsirolimus, an analog of rapamycin, also alleviated accelerated aging defects in progeria cells but also increased the levels of ROS and superoxide within the first hour of treatment [117]. Such evidence strongly suggests that cellular stress-induced AMPK activation links the reversal of HIV-1 latency and alleviation of accelerated cellular aging defects in HGPS.
Cellular stress-induced AMPK activation also links the potential elimination of cancer stem cells (CSCs) with HIV-1 latency reversal and viral eradication. CSCs, which are largely resistant to chemoradiation therapy, are a subpopulation of cancer cells that exhibit characteristics similar to embryonic stem cells (ESCs), including self-renewal, multi-lineage differentiation, & the ability to initiate tumorigenesis [118,119]. Mechanisms that sustain quiescence & promote self-renewal in adult stem cells (ASCs) & CSCs likely also function to maintain latency of HIV-1 in CD4+ memory T cells. Indeed, HIV-1 has been found to establish long-lasting latency in a recently discovered subset of CD4+ T cells that exhibit stem cell-like properties known as T memory stem (TSCM) cells and increases in Ca2+, ROS, and AMPK activation have been shown to promote T cell activation and ESC, ASC, and CSC differentiation [119,120]. Additionally, A23187 and PMA have been shown to promote CSC differentiation (causing CSCs to become more susceptible to chemoradiation) and metformin induces CSC differentiation and/or apoptosis in an AMPK-dependent manner in the deadliest of cancers, including glioblastoma and pancreatic cancer, providing support for my publication in 2017 in which I first proposed that CSC differentiation and/or apoptosis and HIV-1 latency reversal/viral eradication may be linked by cellular stress-induced AMPK activation [119,121-124].
In conclusion, the ability of non-human organisms including certain Streptomyces spp. to initiate the creation of human life is predicated on the induction of cellular stress, mediated by increases in intracellular ROS, Ca2+, AMP(ADP)/ATP ratio increase, etc. The beneficial effects of transient cellular stress induction, which may be likened to selective pressures, crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution, as evidenced by stress-induced increases in antibiotic production by Streptomyces spp. and stress-induced mutagenesis and antibiotic resistance in various bacterial strains. Because AMPK, a primary sensor of cellular stress in eukaryotic cells that increases lifespan and healthspan, plays a critical role in oocyte meiotic resumption/maturation, T cell activation, and stem cell differentiation, the creation of human life, the potential eradication of HIV-1, amelioration of accelerated aging in HGPS cells, and CSC differentiation/apoptosis are likely linked by a “Shock to Live”, or a “Shock to Kill”.
https://www.linkedin.com/pulse/antibiotics-produced-bacteria-activate-human-oocytes-creating-finley/
References:
Murugesu S, Saso S, Jones BP, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017 Sep;108(3):468-482.e3.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994 Mar;9(3):511-8.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015 Mar;22(3):322-8.
Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: A randomized clinical trial. Iran J Reprod Med. 2013 Nov;11(11):875-82.
Liu WC, Slusarchyk DS, Astle G, Trejo WH, Brown WE, Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo). 1978 Sep;31(9):815-9.
Reed PW, Lardy HA. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970-7.
Zhang Z, Wang T, Hao Y, et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology. 2017 Feb;74:43-49.
De Sutter P, Dozortsev D, Cieslak J, Wolf G, Verlinsky Y, Dyban A. Parthenogenetic activation of human oocytes by puromycin. J Assist Reprod Genet. 1992 Aug;9(4):328-37.
Sankaran L, Pogell BM. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721-32.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002 Sep;78(3):619-24.
Mansour R, Fahmy I, Tawab NA, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009 Jan;91(1):133-9.
Nikiforaki D, Vanden Meerschaut F, de Roo C, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016 Mar;105(3):798-806.e2.
Liu H1, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod. 2000 Sep;15(9):1997-2002.
Downs SM. Stimulation of parthenogenesis in mouse ovarian follicles by inhibitors of inosine monophosphate dehydrogenase. Biol Reprod. 1990 Sep;43(3):427-36.
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E. Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol. 1978 Feb;43:157-66.
Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013 Dec 12;5(5):1375-86.
Colonna R, Tatone C, Malgaroli A, Eusebi F, Mangia F. Effects of protein kinase C stimulation and free Ca2+ rise in mammalian egg activation. Gamete Res. 1989 Oct;24(2):171-83.
Tang Z, Xing F, Chen D, et al. In vivo toxicological evaluation of Anisomycin. Toxicol Lett. 2012 Jan 5;208(1):1-11.
Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 2011 May;77(9):3035-43.
K'ominek LA. Cycloheximide production by Streptomyces griseus: control mechanisms of cycloheximide biosynthesis. Antimicrob Agents Chemother. 1975 Jun;7(6):856-6.
Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000 May;63(5):620-4.
Pal PK, Nandi MK, Singh NK. Detoxification of Croton tiglium L. seeds by Ayurvedic process of Śodhana. Anc Sci Life. 2014 Jan;33(3):157-61.
Rotem R, Paz GF, Homonnai ZT, et al. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992 Nov;131(5):2235-43.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998 Sep-Oct;19(5):585-94.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015 Dec;6:260-71.
Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991 Oct;113(2):551-60.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006 Mar;74(3):585-92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2016 Sep 27.
Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011 Sep;31(17):3531-45.
Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006;203(7):1665–70.
Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005 Jul;2(1):9-19.
Nammi S, Roufogalis BD. Light-to-moderate ethanol feeding augments AMPK-α phosphorylation and attenuates SREBP-1 expression in the liver of rats. J Pharm Pharm Sci. 2013;16(2):342-51.
Koh W, Jeong SJ, Lee HJ, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5'-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J Pineal Res. 2011 May;50(4):367-73.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997 Feb;272(2 Pt 1):E262-6.
Ohsaka Y, Nishino H, Nomura Y. Induction of phospho-Thr-172 AMPK in 3T3-L1 adipocytes exposed to cold or treated with anisomycin, mithramycin A, and ionic compounds. Cryo Letters. 2010 May-Jun;31(3):218-29.
Fernández-Ramos AA, Marchetti-Laurent C, Poindessous V, et al. A comprehensive characterization of the impact of mycophenolic acid on the metabolism of Jurkat T cells. Sci Rep. 2017 Sep 5;7(1):10550.
Kim E, Choi Y, Jang J, Park T. Carvacrol Protects against Hepatic Steatosis in Mice Fed a High-Fat Diet by Enhancing SIRT1-AMPK Signaling. Evid Based Complement Alternat Med. 2013;2013:290104.
Zogovic N, Tovilovic-Kovacevic G, Misirkic-Marjanovic M, et al. Coordinated activation of AMP-activated protein kinase, extracellular signal-regulated kinase, and autophagy regulates phorbol myristate acetate-induced differentiation of SH-SY5Y neuroblastoma cells. J Neurochem. 2015 Apr;133(2):223-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Kitani S1, Miyamoto KT, Takamatsu S, et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16410-5.
Challis GL, Hopwood DA. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2:14555-61.
Yagüe P, López-García MT, Rioseras B, Sánchez J, Manteca A. Pre-sporulation stages of Streptomyces differentiation: state-of-the-art and future perspectives. FEMS Microbiol Lett. 2013 May;342(2):79-88.
Seipke RF, Kaltenpoth M, Hutchings MI. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev. 2012 Jul;36(4):862-76.
Meng L, Li M, Yang SH, Kim TJ, Suh JW. Intracellular ATP levels affect secondary metabolite production in Streptomyces spp. Biosci Biotechnol Biochem. 2011;75(8):1576-81.
Sánchez S, Chávez A, Forero A, et al. Carbon source regulation of antibiotic production. J Antibiot (Tokyo). 2010 Aug;63(8):442-59.
Wang SL, Fan KQ, Yang X, Lin ZX, Xu XP, Yang KQ. CabC, an EF-hand calcium-binding protein, is involved in Ca2+-mediated regulation of spore germination and aerial hypha formation in Streptomyces coelicolor. J Bacteriol. 2008 Jun;190(11):4061-8.
Wang D, Wei L, Zhang Y, Zhang M, Gu S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl Microbiol Biotechnol. 2017 Jul 28. doi: 10.1007/s00253-017-8440-0. [Epub ahead of print].
Wei ZH, Bai L, Deng Z, Zhong JJ. Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2011 Jan;102(2):1783-7.
Doull JL, Ayer SW, Singh AK, Thibault P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock. J Antibiot (Tokyo). 1993 May;46(5):869-71.
Zhou WW1, Ma B, Tang YJ, Zhong JJ, Zheng X. Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2012 Jun;114:616-21.
Flanary BE, Kletetschka G. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Rejuvenation Res. 2006 Spring;9(1):61-3.
R. S. BeasleyJ. O. Klemmedson. Recognizing site adversity and drought-sensitive trees in stands of bristlecone pine (Pinus longaeva). January 1973, Volume 27, Issue 1, pp 141–146. doi: 10.1007/BF02862228.
Schulman E. Longevity under Adversity in Conifers. Science. 1954 Mar 26;119(3091):396-9.
Hiebert, R. D.; Hamrick, J. L. 1984. An ecological study of bristlecone pine (Pinus longaeva) in Utah and eastern Nevada. The Great Basin Naturalist. 44(3): 487-494.
Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE. Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol. 2012 Nov;64(4):1008-17.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 Apr;11(2):230-41.
Leymarie J, Vitkauskaité G, Hoang HH, et al. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012 Jan;53(1):96-106.
Pang X, Halaly T, Crane O, et al. Involvement of calcium signalling in dormancy release of grape buds. J Exp Bot. 2007;58(12):3249-62.
Duan Q, Kita D, Johnson EA, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5:3129.
Gao XQ, Liu CZ, Li DD, et al. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016 Jul 29;12(7):e1006228.
Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001 Jul;126(3):1055-60.
Lee JS, Mulkey TJ, Evans ML. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375-6.
Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005 Jun;23(4):283-333.
Wang H, Sharma L, Lu J, Finch P, Fletcher S, Prochownik EV. Structurally diverse c-Myc inhibitors share a common mechanism of action involving ATP depletion. Oncotarget. 2015 Jun 30;6(18):15857-70.
K.V. Kiselev, O.A. Shumakova, A.Y. Manyakhin, A.N. Mazeika. Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regulation. December 2012, Volume 68, Issue 3, pp 371–381. DOI: 10.1007/s10725-012-9725-z.
Darwin, Charles (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: Murray. [1st ed.].
Sobral M, Veiga T, Domínguez P, Guitián JA, Guitián P, Guitián JM. Selective Pressures Explain Differences in Flower Color among Gentiana lutea Populations. PLoS One. 2015 Jul 14;10(7):e0132522.
Lefebvre V, Kiani SP, Durand-Tardif M. A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int J Mol Sci. 2009 Aug 13;10(8):3547-82.
Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8(4):e1002661.
Goto A, Egawa T, Sakon I, et al. Heat stress acutely activates insulin-independent glucose transport and 5'-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015 Nov;3(11). pii: e12601.
Maclean RC, Hall AR, Perron GG, Buckling A. The evolution of antibiotic resistance: insight into the roles of molecular mechanisms of resistance and treatment context. Discov Med. 2010 Aug;10(51):112-8.
Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014 May 20;111(20):E2100-9.
Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010 Feb 12;37(3):311-20.
Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012 Sep;67(9):2069-89.
Ito M, Arakawa T, Okayama M, Shitara A, Mizoguchi I, Takuma T. Gravity loading induces adenosine triphosphate release and phosphorylation of extracellular signal-regulated kinases in human periodontal ligament cells. J Investig Clin Dent. 2014 Nov;5(4):266-74.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Zhong G, Li Y, Li H, et al. Simulated Microgravity and Recovery-Induced Remodeling of the Left and Right Ventricle. Front Physiol. 2016 Jun 29;7:274.
Chang TT, Walther I, Li CF, et al. The Rel/NF-jB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J Leukoc Biol 2012;92(6):1133–45.
Li YC, Chen BM, Wu PC, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol 2010;184(11):5959–63.
Ouyang Z, Wang X, Meng Q, et al. Suppression of adenosine monophosphate-activated protein kinase selectively triggers apoptosis in activated T cells and ameliorates immune diseases. Biochem Biophys Res Commun. 2017 May 27;487(2):223-229.
Blaber EA, Pecaut MJ, Jonscher KR. Spaceflight Activates Autophagy Programs and the Proteasome in Mouse Liver. Int J Mol Sci. 2017 Sep 27;18(10). pii: E2062.
Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014 Feb 1;20(4):574-88.
Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016 Jun 29;36(14):1931-42.
Zhang L, Yi Y, Guo Q, et al. Hsp90 interacts with AMPK and mediates acetyl-CoA carboxylase phosphorylation. Cell Signal. 2012 Apr;24(4):859-65.
Sagare-Patil V, Bhilawadikar R, Galvankar M, Zaveri K, Hinduja I, Modi D. Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet. 2017 Apr;34(4):495-503.
Lesniewski LA, Seals DR4, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017 Feb;16(1):17-26.
Aparicio IM, Espino J, Bejarano I, et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep. 2016 Sep 16;6:33647.
Fang A, Pierson DL, Mishra SK, Demain AL. Growth of Steptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl Microbiol Biotechnol. 2000 Jul;54(1):33-6.
Cheng YR, Huang J, Qiang H, Lin WL, Demain AL. Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot (Tokyo). 2001 Nov;54(11):967-72.
Heindryckx B, Lierman S, Combelles CM, Cuvelier CA, Gerris J, De Sutter P. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011 Apr;26(4):791-800.
Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1462-7.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLC ζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53(3):292–308.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Marsden MD, Zack JA. HIV/AIDS eradication. Bioorg Med Chem Lett 2013;23(14):4003–10.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013 Feb 21;38(2):225-36.
Barquero AA, Dávola ME, Riva DA, Mersich SE, Alché LE. Naturally occurring compounds elicit HIV-1 replication in chronically infected promonocytic cells. Biomed Res Int. 2014;2014:989101.
Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009 Mar 15;182(6):3688-95.
Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem Biol Interact. 1994 Jun;91(2-3):79-89.
Anderson I, Low JS, Weston S, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):E1528-37.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008 Nov 13;4(5):495-504.
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858.
G.M. Chew, D.C. Chow, S.A. Souza, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. Journal of Virus Eradication 2017; 3 (Supplement 1): 6–19.
http://viruseradication.com/supplement-details/Abstracts_of_the_IAS_HIV_Cure_and_Cancer_Forum_2017/
Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249-64.
Berro R, Kehn K, de la Fuente C, et al. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J Virol 2006;80(7):3189–204.
Paz S, Lu ML, Takata H, Trautmann L, Caputi M. SRSF1 RNA Recognition Motifs Are Strong Inhibitors of HIV-1 Replication. J Virol. 2015 Jun;89(12):6275-86.
Lopez-Mejia IC, Vautrot V, De Toledo M, et al. A conserved splicing mechanism of the LMNA gene controls premature aging. Hum Mol Genet. 2011 Dec 1;20(23):4540-55.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017 Oct;26(10):889-895.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis. 2016 Nov 10;2:16026.
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Decuypere JP, Welkenhuyzen K, Luyten T, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011 Dec;7(12):1472-89.
Cao Y, Fang Y, Cai J, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. 2016 Dec;21(10):613-618.
Decuypere JP, Kindt D, Luyten T, et al. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS One. 2013;8(4):e61020.
Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011 Jun 29;3(89):89ra58.
Gabriel D, Gordon LB, Djabali K. Temsirolimus Partially Rescues the Hutchinson-Gilford Progeria Cellular Phenotype. PLoS One. 2016 Dec 29;11(12):e0168988.
Moncharmont C, Levy A, Gilormini M, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett 2012;322(2):139–47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014;20(2):139–42.
Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014 Dec 22;9(12):e115698.
Hayun M, Okun E, Hayun R, Gafter U, et al. Synergistic effect of AS101 and Bryostatin-1 on myeloid leukemia cell differentiation in vitro and in an animal model. Leukemia 2007;21(7):1504–13.
Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med 2012;1(11):811–24.
Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res 2014;182(1):50–9.
#ampk#gut bacteria#metformin#oocyte#HIV/AIDS#progeria#cancer stem cell#evolution#charles darwin#sperm#acrosome reaction#fertilization#reproduction
1 note
·
View note
Text
Antibiotics produced by Bacteria activate Human Oocytes, creating Healthy Babies: AMPK links the Creation of Human Life with HIV, Progeria, & Cancer
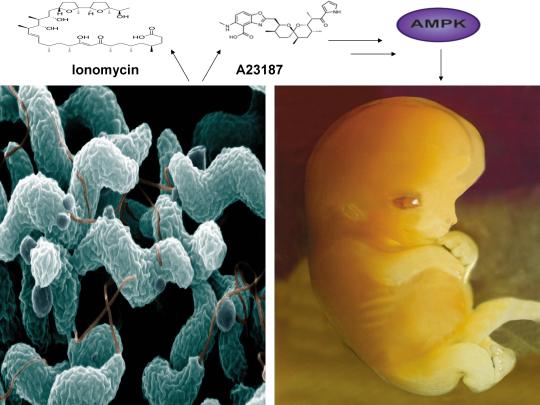
CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons;By De Wood, Pooley, USDA, ARS, EMU. [Public domain], via Wikimedia Commons
A recent study published online in the journal Fertility and Sterility in September of 2017 systematically reviewed for the first time evidence for the effect of two compounds, ionomycin and A23187 (also known as calcimycin), on fertilization rates and pregnancy outcomes in infertile couples undergoing an in vitro fertilization procedure known as intracytoplasmic sperm injection (ICSI) [1]. ICSI involves the direct deposition of sperm into the oocyte cytoplasm, which typically leads to high rates of fertilization. However, fertilization failure despite repeated ICSI is likely caused by a failure of the oocyte to activate [1]. Physiological oocyte activation is accomplished by the delivery of a sperm-borne oocyte activating factor called phospholipase C zeta (PLCζ). PLCζ activates human oocytes by inducing an intracellular signaling cascade that ultimately results in increased calcium (Ca2+) oscillations in the oocyte, which drives oocyte activation to completion [1]. As oocyte activation is an indispensable prerequisite for the creation of all human life, every human being alive today and any human being that has ever lived began their existence as an activated oocyte [2]. Ionomycin and A23187 increase the levels of intracellular Ca2+ and are thus commonly known as Ca2+ ionophores [1]. The authors of the Fertility and Sterility study showed that over a total of 1,521 ICSI cycles, calcium ionophores including ionomycin and A23187 led to a statistically significant improvement in fertilization, cleavage, blastulation, implantation rates, overall pregnancy, and live-birth rates [1]. Ionomycin and A23187 have also been shown in several independent studies to effectively induce human oocyte activation, leading to the birth of normal, healthy children [3,4].
Strikingly, as described further below, both ionomycin and A23187 are antibiotics that are naturally produced by certain species within the bacterial genus Streptomyces [5,6]. Other structurally distinct compounds and methods have also been shown to induce human oocyte activation, including ethanol, puromycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces alboniger), as well as mechanical manipulation and electrical stimulation, both of with have been reported to result in the creation of normal children [7-11]. Interestingly, as mouse oocytes are considered models for human oocytes, ionomycin, A23187, anisomycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces griseolus), mycophenolic acid (an immunosuppressant produced by the fungus Penicillium brevicompactum), cycloheximide (a protein synthesis inhibitor produced by Streptomyces griseus), carvacrol (a secondary plant metabolite produced by Origanum vulgare{oregano}), and phorbol 12-myristate 13-acetate (PMA, a secondary plant metabolite produced by Croton tiglium) each induce activation of mouse oocytes [12-22]. Ionomycin, A23187, PMA, and reactive oxygen species (ROS) also induce the acrosome reaction in human sperm, a process characterized by the release of hydrolytic enzymes from the head of sperm which is necessary for oocyte penetration and thus indispensable for the creation of all human life outside of a clinical setting (ICSI bypasses the need for oocyte penetration) [23,24]. Additionally, although an over-production of ROS, similar to Ca2+, may lead to deleterious effects including cell death/apoptosis, low levels of ROS have been shown to act as signaling molecules and ROS is significantly increased on or immediately following mouse oocyte activation [25,26].
Furthermore, the master metabolic regulator AMPK is critical for oocyte meiotic resumption and maturation (a process that precedes and is essential for oocyte activation), is found located across the entire acrosome in the head of human sperm, and is activated by increases in ROS and Ca2+ [27-29]. Ionomycin, A23187, ethanol, puromycin, mechanical force, electrical stimulation, anisomycin, mycophenolic acid, carvacrol, and PMA also induce AMPK activation, indicating that a common mechanism of action links chemically distinct compounds with the creation of human life [30-39]. This common mechanism of action likely centers on the induction of cellular stress, mediated by indirect increases in intracellular Ca2+, ROS, and/or the AMP(ADP)/ATP ratio, etc. as I originally proposed in 2016 [40]. Because the bacterial-derived antibiotics ionomycin and A23187 induce both the acrosome reaction in human sperm and human oocyte activation, producing normal, healthy children, it can be said that “non-human organisms have the power to create human life or the power to end life.” As explained below, the beneficial effects of cellular stress induction (i.e. a “shock”) crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution.
A number of bacterial species residing within the genus Streptomyces have proven to be extremely important and medicinally valuable as approximately 70% of clinically useful antibiotics are derived from Streptomyces [41]. The antibiotics ionomycin and A23187 are naturally produced by Streptomyces conglobatus and Streptomyces chartreusensis, respectively [5,6]. Other important examples include the antibiotic tetracycline (produced by Streptomyces aureofaciens), the immunosuppressant rapamycin (produced by Streptomyces hygroscopicus), and the anti-helminthic avermectins (produced by Streptomyces avermitilis) [42]. Many soil and aquatic-dwelling species of Streptomyces can be found in harsh environments and are characterized by a unique life cycle, including spore germination followed by vegetative mycelium production, aerial hyphae formation, sporulation (i.e. spore formation), and antibiotic production [43,44]. Curiously, just as cellular stress induction leads to the creation of human life and other beneficial effects in human cells (see below), stress induction also promotes the induction of aerial hyphae formation, sporulation, and antibiotic production in many Streptomyces species (spp.). Indeed, a decrease in the levels of ATP and bacterial growth is associated with sporulation, aerial hyphae formation, and antibiotic production [42,45]. A reduction in glucose/nutritional deprivation, the preferred sugar/carbon source for many Streptomyces spp., also significantly increases antibiotic production [46]. An increase in intracellular ROS and Ca2+ is associated with spore germination, aerial hyphae formation, and antibiotic production [47-49]. Other cellular stressors, including heat shock and ethanol, also significantly increase antibiotic production, provocatively indicating that the effects of cellular stress crosses species boundaries, enhancing bacterial survival and facilitating the creation of human life [50,51].
The beneficial effects of low-level cellular stress induction also extends to plants, as many plants produce secondary metabolites partly for the purpose of self-defense, analogous to antibiotics. Similar to the harsh, stressful environments often inhabited by Streptomyces spp., the Great Basin Bristlecone Pine (Pinus Longaeva), considered the oldest living non-clonal organism on the planet ( >5000 years old), thrives in an exceptionally harsh environment, characterized by increased elevations and exposure to UV radiation, nutritionally-deprived soils, harsh temperatures, and mechanical stress due to wind variances, leading early researchers to conclude that it’s longevity is intimately associated with adversity [52-54]. Conversely, Pinus Longaeva species that are located in less stressful environments (i.e. lower elevations) are strongly associated with younger age classes (<875 years) [55]. Similarly, the Creosote bush (Larrea tridentate), considered one of the oldest living clonal organisms on the planet (>11,000 years old), also thrives in harsh environments including the Mohave Desert [56]. AMPK, which increases lifespan and healthspan in several model organisms, is the primary sensor of cellular stress in eukaryotic organisms (e.g. plants and humans) and the plant AMPK orthologue SnRK1 as well as Ca2+ and ROS are critical for seed germination, fertilization, root gravitropism, and secondary metabolite production [57-64]. The secondary plant metabolites PMA (which activates mouse oocytes and promotes the acrosome reaction in human sperm) and artemisinin (an anti-malarial drug) both activate AMPK and the antibiotic A23187 also increases production of the secondary metabolite resveratrol in grape cell cultures, again indicating that exposure to low-level stressors may promote extension of lifespan and initiate the creation of human life [17,23,39,65,66].
Organismal exposure to beneficial levels of stress may also play a critical role in evolution. As first noted by Charles Darwin, evolution is driven by natural selection, a process characterized by environmentally-induced phenotypic changes that may lead to inheritable survival and reproductive advantages [67]. From “On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life”, Darwin explained that “if there be, owing to the high geometrical powers of increase of each species, at some age, season, or year, a severe struggle for life, and this certainly cannot be disputed;……But if variations useful to any organic being do occur, assuredly individuals thus characterised will have the best chance of being preserved in the struggle for life;” [67]. This “struggle for life” Darwin spoke of is embodied by selective pressures which may be abiotic (i.e. light, wind, temperature, etc.) or biotic (predation, disease, competition, etc.) [68,69]. As alluded to above, such selective pressures are indeed sources of cellular stress, sensed by both prokaryotes and eukaryotes, that induce beneficial responses (at appropriate levels), leading to the acquisition of phenotypes conducive for continued survival. Both biotic (e.g. infection) and abiotic (e.g. heat) stressors/selective pressures activate AMPK (which is evolutionarily conserved among eukaryotes) in human cells [70,71]. A phenomenon often cited as an example of natural selection on a readily observable timescale is the development of bacterial resistance to antibiotics, resulting in problematic mutant strains that may be life-threatening for some individuals (i.e. the elderly and immunocompromised) [72]. Intriguingly, lethal levels of bactericidal antibiotics have been shown to kill microorganisms via the induction of ROS, sub-lethal levels of bactericidal antibiotics however increase mutagenesis and bacterial resistance via induction of lower levels of ROS, and heat as well as nutritional stress increase bacterial resistance to antibiotics, providing compelling evidence that continuous exposure to low levels of stress likely plays a significant role in natural selection and evolution [73-75].
Moreover, gravity itself likely functions as a cellular stressor/selective pressure that has influenced the development of organisms on Earth since the emergence of the very first lifeform. Gravity exerts its effects on living organisms via the application of force, which is experienced by human cells in the form of mechanical loading or stress [76]. The application of force or a mechanical load has recently been shown to activate AMPK and simulated microgravity (i.e. hind limb unloading in mice) significantly decreases AMPK activation [77,78]. Spaceflight also inhibits the activation of T cells (immune cells essential for adaptive immunity), whereas the application of force and AMPK activation promote T cell activation [79-81]. Interestingly, spaceflight has recently been shown to decrease the levels of the master antioxidant transcription factor Nrf2 and the heat shock-inducible protein HSP90a but increase the levels of the growth-promoting kinase mTOR in mice [82]. AMPK however inhibits mTOR but increases the phosphorylation, nuclear retention, and transcriptional activity of Nrf2 [57,83,84]. Also, HSP90 interacts with and maintains AMPK activity and HSP90 is necessary for progesterone-induced human sperm acrosome reaction [85,86]. Interestingly, rapamycin, an immunosuppressant produced by Streptomyces hygroscopicus, extends lifespan in genetically heterogeneous mice, activates AMPK in vivo in normal aged mice, and increases human sperm motility [42,87,88]. Simulated microgravity via the use of NASA-designed rotating wall vessels (RWVs) however drastically reduces rapamycin production (~90%) whereas the antibiotic gentamycin increases rapamycin production by Streptomyces hygroscopicus, providing further evidence that cellular stress, in the form of mechanical loading induced by gravity, is essential for development, function, and survival of Earth-bound organisms [89,90].
The induction of cellular stress also links seemingly dissimilar physiological and pathological states with the activation of AMPK. As discussed above, both ionomycin and ROS activate AMPK and promote oocyte meiotic resumption, a process that is AMPK-dependent and is essential for efficient oocyte activation [27,30,91]. ROS is also critical for ovulation, PMA and ionomycin both activate mouse oocytes, and ionomycin is extensively used during ICSI procedures, creating normal healthy children, suggesting that cellular stress-induced AMPK activation is also essential for oocyte activation [3,4,12,17,92]. The activation of oocytes and T cells share strikingly similar intracellular signaling mechanisms (e.g. PLC-PIP2-DAG-PKC-IP3-Ca2+) and ionomycin combined with PMA are extremely effective in activating T cells and are often used as positive controls in HIV-1 latency reversal studies [93-95]. Reactivating latent/dormant HIV-1 in CD4+ T cells, potentially facilitating immune system detection and virus destruction, is currently being pursued as a method for the potential eradication of HIV-1 (called the “shock and kill” approach) [96]. Similar to oocyte activation, both Ca2+ and ROS are critical for T cell activation (and hence latent HIV-1 reactivation) and other cellular stress-inducing compounds, including NDGA derived from the Creosote bush, butyrate derived from bacteria, as well as ROS and HSP90 have been shown to reactivate latent HIV-1 [26,93,94,97-101]. Interestingly, AMPK inhibition leads to cell death on T cell activation, knockdown of AMPK significantly decreases HIV-1 replication, and metformin (a well-studied AMPK activator derived from the French Lilac plant) increases butyrate production in human diabetic patients [81,102,103]. Perhaps most convincingly, early preliminary data showed that metformin significantly reduced several markers preferentially associated with cells latently infected with HIV-1 (e.g. PD-1, TIGIT, TIM-3) and also destabilized the latent HIV-1 reservoir in chronically-infected HIV patients, indicating that cellular-stress induced AMPK activation likely links the creation of human life with the potential eradication of HIV-1, as I originally proposed in 2016 [40,104,105].
AMPK activation may also link the disparate disease states of HIV-1 latency and Hutchinson-Gilford progeria syndrome (HGPS). HGPS is a genetic disorder caused by aberrant alternative splicing of the LMNA gene, generating a toxic protein called progerin that induces an accelerated aging phenotype and premature death at approximately 14 years of age [106]. Excessive activity of the gene splicing factor SRSF1 has been shown to prevent reactivation of latent HIV-1 and contribute to aberrant splicing of the LMNA gene in HGPS [107-109]. Metformin however has recently been shown to ameliorate the accelerated aging phenotype in cells derived from children with HGPS by reducing the levels of both SRSF1 and progerin and activating AMPK, as I first proposed in 2014 [110-112]. Interestingly, both Ca2+ and ROS induce autophagy (a process of disposing of damaged/toxic proteins and organelles) and rapamycin, which activates AMPK in vivo and increases intracellular Ca2+ levels, improves accelerated aging in progeria cells by inducing autophagic degradation of progerin [87,113-116]. Temsirolimus, an analog of rapamycin, also alleviated accelerated aging defects in progeria cells but also increased the levels of ROS and superoxide within the first hour of treatment [117]. Such evidence strongly suggests that cellular stress-induced AMPK activation links the reversal of HIV-1 latency and alleviation of accelerated cellular aging defects in HGPS.
Cellular stress-induced AMPK activation also links the potential elimination of cancer stem cells (CSCs) with HIV-1 latency reversal and viral eradication. CSCs, which are largely resistant to chemoradiation therapy, are a subpopulation of cancer cells that exhibit characteristics similar to embryonic stem cells (ESCs), including self-renewal, multi-lineage differentiation, & the ability to initiate tumorigenesis [118,119]. Mechanisms that sustain quiescence & promote self-renewal in adult stem cells (ASCs) & CSCs likely also function to maintain latency of HIV-1 in CD4+ memory T cells. Indeed, HIV-1 has been found to establish long-lasting latency in a recently discovered subset of CD4+ T cells that exhibit stem cell-like properties known as T memory stem (TSCM) cells and increases in Ca2+, ROS, and AMPK activation have been shown to promote T cell activation and ESC, ASC, and CSC differentiation [119,120]. Additionally, A23187 and PMA have been shown to promote CSC differentiation (causing CSCs to become more susceptible to chemoradiation) and metformin induces CSC differentiation and/or apoptosis in an AMPK-dependent manner in the deadliest of cancers, including glioblastoma and pancreatic cancer, providing support for my publication in 2017 in which I first proposed that CSC differentiation and/or apoptosis and HIV-1 latency reversal/viral eradication may be linked by cellular stress-induced AMPK activation [119,121-124].
In conclusion, the ability of non-human organisms including certain Streptomyces spp. to initiate the creation of human life is predicated on the induction of cellular stress, mediated by increases in intracellular ROS, Ca2+, AMP(ADP)/ATP ratio increase, etc. The beneficial effects of transient cellular stress induction, which may be likened to selective pressures, crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution, as evidenced by stress-induced increases in antibiotic production by Streptomyces spp. and stress-induced mutagenesis and antibiotic resistance in various bacterial strains. Because AMPK, a primary sensor of cellular stress in eukaryotic cells that increases lifespan and healthspan, plays a critical role in oocyte meiotic resumption/maturation, T cell activation, and stem cell differentiation, the creation of human life, the potential eradication of HIV-1, amelioration of accelerated aging in HGPS cells, and CSC differentiation/apoptosis are likely linked by a “Shock to Live”, or a “Shock to Kill”.
https://www.linkedin.com/pulse/antibiotics-produced-bacteria-activate-human-oocytes-creating-finley/

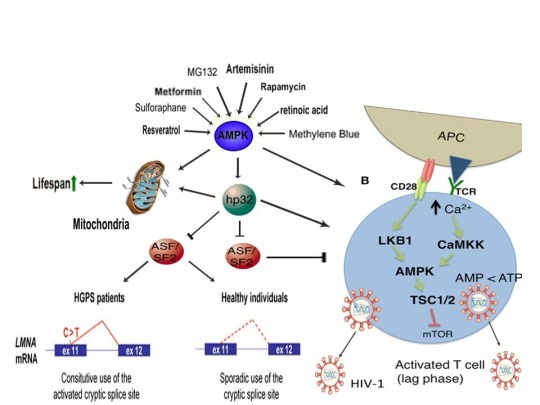
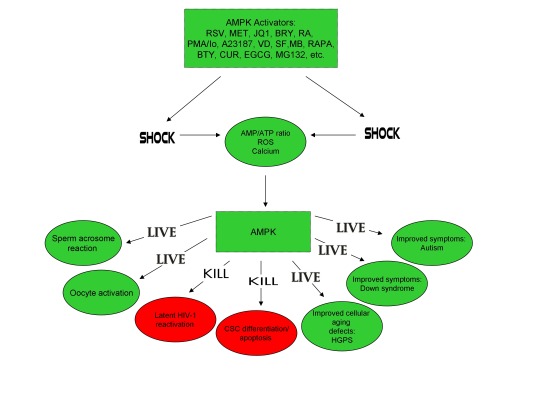
References:
Murugesu S, Saso S, Jones BP, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017 Sep;108(3):468-482.e3.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994 Mar;9(3):511-8.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015 Mar;22(3):322-8.
Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: A randomized clinical trial. Iran J Reprod Med. 2013 Nov;11(11):875-82.
Liu WC, Slusarchyk DS, Astle G, Trejo WH, Brown WE, Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo). 1978 Sep;31(9):815-9.
Reed PW, Lardy HA. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970-7.
Zhang Z, Wang T, Hao Y, et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology. 2017 Feb;74:43-49.
De Sutter P, Dozortsev D, Cieslak J, Wolf G, Verlinsky Y, Dyban A. Parthenogenetic activation of human oocytes by puromycin. J Assist Reprod Genet. 1992 Aug;9(4):328-37.
Sankaran L, Pogell BM. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721-32.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002 Sep;78(3):619-24.
Mansour R, Fahmy I, Tawab NA, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009 Jan;91(1):133-9.
Nikiforaki D, Vanden Meerschaut F, de Roo C, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016 Mar;105(3):798-806.e2.
Liu H1, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod. 2000 Sep;15(9):1997-2002.
Downs SM. Stimulation of parthenogenesis in mouse ovarian follicles by inhibitors of inosine monophosphate dehydrogenase. Biol Reprod. 1990 Sep;43(3):427-36.
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E. Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol. 1978 Feb;43:157-66.
Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013 Dec 12;5(5):1375-86.
Colonna R, Tatone C, Malgaroli A, Eusebi F, Mangia F. Effects of protein kinase C stimulation and free Ca2+ rise in mammalian egg activation. Gamete Res. 1989 Oct;24(2):171-83.
Tang Z, Xing F, Chen D, et al. In vivo toxicological evaluation of Anisomycin. Toxicol Lett. 2012 Jan 5;208(1):1-11.
Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 2011 May;77(9):3035-43.
K'ominek LA. Cycloheximide production by Streptomyces griseus: control mechanisms of cycloheximide biosynthesis. Antimicrob Agents Chemother. 1975 Jun;7(6):856-6.
Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000 May;63(5):620-4.
Pal PK, Nandi MK, Singh NK. Detoxification of Croton tiglium L. seeds by Ayurvedic process of Śodhana. Anc Sci Life. 2014 Jan;33(3):157-61.
Rotem R, Paz GF, Homonnai ZT, et al. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992 Nov;131(5):2235-43.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998 Sep-Oct;19(5):585-94.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015 Dec;6:260-71.
Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991 Oct;113(2):551-60.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006 Mar;74(3):585-92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2016 Sep 27.
Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011 Sep;31(17):3531-45.
Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006;203(7):1665–70.
Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005 Jul;2(1):9-19.
Nammi S, Roufogalis BD. Light-to-moderate ethanol feeding augments AMPK-α phosphorylation and attenuates SREBP-1 expression in the liver of rats. J Pharm Pharm Sci. 2013;16(2):342-51.
Koh W, Jeong SJ, Lee HJ, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5’-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J Pineal Res. 2011 May;50(4):367-73.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997 Feb;272(2 Pt 1):E262-6.
Ohsaka Y, Nishino H, Nomura Y. Induction of phospho-Thr-172 AMPK in 3T3-L1 adipocytes exposed to cold or treated with anisomycin, mithramycin A, and ionic compounds. Cryo Letters. 2010 May-Jun;31(3):218-29.
Fernández-Ramos AA, Marchetti-Laurent C, Poindessous V, et al. A comprehensive characterization of the impact of mycophenolic acid on the metabolism of Jurkat T cells. Sci Rep. 2017 Sep 5;7(1):10550.
Kim E, Choi Y, Jang J, Park T. Carvacrol Protects against Hepatic Steatosis in Mice Fed a High-Fat Diet by Enhancing SIRT1-AMPK Signaling. Evid Based Complement Alternat Med. 2013;2013:290104.
Zogovic N, Tovilovic-Kovacevic G, Misirkic-Marjanovic M, et al. Coordinated activation of AMP-activated protein kinase, extracellular signal-regulated kinase, and autophagy regulates phorbol myristate acetate-induced differentiation of SH-SY5Y neuroblastoma cells. J Neurochem. 2015 Apr;133(2):223-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Kitani S1, Miyamoto KT, Takamatsu S, et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16410-5.
Challis GL, Hopwood DA. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2:14555-61.
Yagüe P, López-García MT, Rioseras B, Sánchez J, Manteca A. Pre-sporulation stages of Streptomyces differentiation: state-of-the-art and future perspectives. FEMS Microbiol Lett. 2013 May;342(2):79-88.
Seipke RF, Kaltenpoth M, Hutchings MI. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev. 2012 Jul;36(4):862-76.
Meng L, Li M, Yang SH, Kim TJ, Suh JW. Intracellular ATP levels affect secondary metabolite production in Streptomyces spp. Biosci Biotechnol Biochem. 2011;75(8):1576-81.
Sánchez S, Chávez A, Forero A, et al. Carbon source regulation of antibiotic production. J Antibiot (Tokyo). 2010 Aug;63(8):442-59.
Wang SL, Fan KQ, Yang X, Lin ZX, Xu XP, Yang KQ. CabC, an EF-hand calcium-binding protein, is involved in Ca2+-mediated regulation of spore germination and aerial hypha formation in Streptomyces coelicolor. J Bacteriol. 2008 Jun;190(11):4061-8.
Wang D, Wei L, Zhang Y, Zhang M, Gu S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl Microbiol Biotechnol. 2017 Jul 28. doi: 10.1007/s00253-017-8440-0. [Epub ahead of print].
Wei ZH, Bai L, Deng Z, Zhong JJ. Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2011 Jan;102(2):1783-7.
Doull JL, Ayer SW, Singh AK, Thibault P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock. J Antibiot (Tokyo). 1993 May;46(5):869-71.
Zhou WW1, Ma B, Tang YJ, Zhong JJ, Zheng X. Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2012 Jun;114:616-21.
Flanary BE, Kletetschka G. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Rejuvenation Res. 2006 Spring;9(1):61-3.
R. S. BeasleyJ. O. Klemmedson. Recognizing site adversity and drought-sensitive trees in stands of bristlecone pine (Pinus longaeva). January 1973, Volume 27, Issue 1, pp 141–146. doi: 10.1007/BF02862228.
Schulman E. Longevity under Adversity in Conifers. Science. 1954 Mar 26;119(3091):396-9.
Hiebert, R. D.; Hamrick, J. L. 1984. An ecological study of bristlecone pine (Pinus longaeva) in Utah and eastern Nevada. The Great Basin Naturalist. 44(3): 487-494.
Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE. Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol. 2012 Nov;64(4):1008-17.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 Apr;11(2):230-41.
Leymarie J, Vitkauskaité G, Hoang HH, et al. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012 Jan;53(1):96-106.
Pang X, Halaly T, Crane O, et al. Involvement of calcium signalling in dormancy release of grape buds. J Exp Bot. 2007;58(12):3249-62.
Duan Q, Kita D, Johnson EA, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5:3129.
Gao XQ, Liu CZ, Li DD, et al. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016 Jul 29;12(7):e1006228.
Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001 Jul;126(3):1055-60.
Lee JS, Mulkey TJ, Evans ML. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375-6.
Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005 Jun;23(4):283-333.
Wang H, Sharma L, Lu J, Finch P, Fletcher S, Prochownik EV. Structurally diverse c-Myc inhibitors share a common mechanism of action involving ATP depletion. Oncotarget. 2015 Jun 30;6(18):15857-70.
K.V. Kiselev, O.A. Shumakova, A.Y. Manyakhin, A.N. Mazeika. Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regulation. December 2012, Volume 68, Issue 3, pp 371–381. DOI: 10.1007/s10725-012-9725-z.
Darwin, Charles (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: Murray. [1st ed.].
Sobral M, Veiga T, Domínguez P, Guitián JA, Guitián P, Guitián JM. Selective Pressures Explain Differences in Flower Color among Gentiana lutea Populations. PLoS One. 2015 Jul 14;10(7):e0132522.
Lefebvre V, Kiani SP, Durand-Tardif M. A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int J Mol Sci. 2009 Aug 13;10(8):3547-82.
Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8(4):e1002661.
Goto A, Egawa T, Sakon I, et al. Heat stress acutely activates insulin-independent glucose transport and 5’-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015 Nov;3(11). pii: e12601.
Maclean RC, Hall AR, Perron GG, Buckling A. The evolution of antibiotic resistance: insight into the roles of molecular mechanisms of resistance and treatment context. Discov Med. 2010 Aug;10(51):112-8.
Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014 May 20;111(20):E2100-9.
Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010 Feb 12;37(3):311-20.
Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012 Sep;67(9):2069-89.
Ito M, Arakawa T, Okayama M, Shitara A, Mizoguchi I, Takuma T. Gravity loading induces adenosine triphosphate release and phosphorylation of extracellular signal-regulated kinases in human periodontal ligament cells. J Investig Clin Dent. 2014 Nov;5(4):266-74.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Zhong G, Li Y, Li H, et al. Simulated Microgravity and Recovery-Induced Remodeling of the Left and Right Ventricle. Front Physiol. 2016 Jun 29;7:274.
Chang TT, Walther I, Li CF, et al. The Rel/NF-jB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J Leukoc Biol 2012;92(6):1133–45.
Li YC, Chen BM, Wu PC, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol 2010;184(11):5959–63.
Ouyang Z, Wang X, Meng Q, et al. Suppression of adenosine monophosphate-activated protein kinase selectively triggers apoptosis in activated T cells and ameliorates immune diseases. Biochem Biophys Res Commun. 2017 May 27;487(2):223-229.
Blaber EA, Pecaut MJ, Jonscher KR. Spaceflight Activates Autophagy Programs and the Proteasome in Mouse Liver. Int J Mol Sci. 2017 Sep 27;18(10). pii: E2062.
Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014 Feb 1;20(4):574-88.
Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016 Jun 29;36(14):1931-42.
Zhang L, Yi Y, Guo Q, et al. Hsp90 interacts with AMPK and mediates acetyl-CoA carboxylase phosphorylation. Cell Signal. 2012 Apr;24(4):859-65.
Sagare-Patil V, Bhilawadikar R, Galvankar M, Zaveri K, Hinduja I, Modi D. Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet. 2017 Apr;34(4):495-503.
Lesniewski LA, Seals DR4, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017 Feb;16(1):17-26.
Aparicio IM, Espino J, Bejarano I, et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep. 2016 Sep 16;6:33647.
Fang A, Pierson DL, Mishra SK, Demain AL. Growth of Steptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl Microbiol Biotechnol. 2000 Jul;54(1):33-6.
Cheng YR, Huang J, Qiang H, Lin WL, Demain AL. Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot (Tokyo). 2001 Nov;54(11):967-72.
Heindryckx B, Lierman S, Combelles CM, Cuvelier CA, Gerris J, De Sutter P. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011 Apr;26(4):791-800.
Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1462-7.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLC ζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53(3):292–308.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Marsden MD, Zack JA. HIV/AIDS eradication. Bioorg Med Chem Lett 2013;23(14):4003–10.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013 Feb 21;38(2):225-36.
Barquero AA, Dávola ME, Riva DA, Mersich SE, Alché LE. Naturally occurring compounds elicit HIV-1 replication in chronically infected promonocytic cells. Biomed Res Int. 2014;2014:989101.
Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009 Mar 15;182(6):3688-95.
Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem Biol Interact. 1994 Jun;91(2-3):79-89.
Anderson I, Low JS, Weston S, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):E1528-37.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008 Nov 13;4(5):495-504.
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858.
G.M. Chew, D.C. Chow, S.A. Souza, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. Journal of Virus Eradication 2017; 3 (Supplement 1): 6–19.
http://viruseradication.com/supplement-details/Abstracts_of_the_IAS_HIV_Cure_and_Cancer_Forum_2017/
Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249-64.
Berro R, Kehn K, de la Fuente C, et al. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J Virol 2006;80(7):3189–204.
Paz S, Lu ML, Takata H, Trautmann L, Caputi M. SRSF1 RNA Recognition Motifs Are Strong Inhibitors of HIV-1 Replication. J Virol. 2015 Jun;89(12):6275-86.
Lopez-Mejia IC, Vautrot V, De Toledo M, et al. A conserved splicing mechanism of the LMNA gene controls premature aging. Hum Mol Genet. 2011 Dec 1;20(23):4540-55.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017 Oct;26(10):889-895.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis. 2016 Nov 10;2:16026.
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Decuypere JP, Welkenhuyzen K, Luyten T, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011 Dec;7(12):1472-89.
Cao Y, Fang Y, Cai J, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. 2016 Dec;21(10):613-618.
Decuypere JP, Kindt D, Luyten T, et al. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS One. 2013;8(4):e61020.
Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011 Jun 29;3(89):89ra58.
Gabriel D, Gordon LB, Djabali K. Temsirolimus Partially Rescues the Hutchinson-Gilford Progeria Cellular Phenotype. PLoS One. 2016 Dec 29;11(12):e0168988.
Moncharmont C, Levy A, Gilormini M, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett 2012;322(2):139–47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014;20(2):139–42.
Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014 Dec 22;9(12):e115698.
Hayun M, Okun E, Hayun R, Gafter U, et al. Synergistic effect of AS101 and Bryostatin-1 on myeloid leukemia cell differentiation in vitro and in an animal model. Leukemia 2007;21(7):1504–13.
Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med 2012;1(11):811–24.
Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res 2014;182(1):50–9
#ampk#progeria#cancer stem cell#HIV/AIDS#oocyte#sperm#acrosome reaction#fertilization#metformin#gut bacteria#evolution#reproduction#charles darwin
0 notes
Text
Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin
Renal cell carcinoma (RCC) is one of the most drug-resistant malignancies, and an effective therapy is lacking for metastatic RCC. Anisomycin is known to inhibit protein synthesis and induce ribotoxic stress. The aim of this study was to explore whether anisomycin enhances the cytotoxic effects of mapatumumab, a human agonistic monoclonal antibody specific for death receptor 4 (DR4), in human RCC cells. We examined the cytotoxicity of anisomycin alone and in combination with mapatumumab in human RCC cell lines and primary RCC cell cultures. RCC cells treated with anisomycin showed cytotoxicity in a dose-dependent manner. Anisomyin in combination with mapatumumab showed a synergistic effect not only in two human RCC cell lines but also in five primary RCC cell cultures. The synergy between anisomycin and mapatumumab for cytotoxicity was also observed for apoptosis. Interestingly, anisomycin significantly increased DR4 expression at both the mRNA and the protein level. Furthermore, the combination-induced cytotoxicity was significantly suppressed by a human recombinant DR4:Fc chimeric protein. The combination of anisomycin and mapatumumab also enhanced the activity of caspases 8 and 3, the downstream molecules of death receptors. These findings indicate that anisomycin sensitizes RCC cells to DR4-mediated apoptosis through the induction of DR4, suggesting that combinational treatment with anisomycin and mapatumumab might represent a novel therapeutic strategy for the treatment of RCC.
http://ift.tt/2jOe0PB
0 notes
Text
Möss och människor
Ett minnesknep kognitionsvetenskapens tappra krigare har lärt mig på sistone (förutom normala saker som att moffa socker, pumpa upp adrenalinet, göra saken jag ska komma ihåg innan läggdags, alltid plugga på femton olika ställen, vara aggresiv när jag pluggar om jag planerar att bråka med bordsgrannen under tentan) är att relatera all information till den egna jaget.
Nu i efterhand är det bara att konstatera att vissa saker har varit svårare att än andra.
Exempelvis anisomycin som är giftigt för människor, men hindrar möss från att konsolidera minnen.
Men vill man så kan man.*
Plugga i poolen, någon?
Relevanta teorier
*Om jag en bister kväll (i en vild jakt på anis) skulle råkade äta en söt liten skogsmus, hade jag önskat att den stackars musen inte hade kommit ihåg det - och att jag hade dött.
Det mänskliga minnet är komplext, och slutar aldrig att förvåna - ska man komma ihåg en sak, så är det att minnen alltid kodas in kontextuellt. Det spelar roll var du var, hur glad du var, vad du hade ätit och vem du var i närheten av.
Moffa socker och/eller locka fram adrenalin refererar till teorierna kring Sweet memories - som utgår ifrån att adrenalinet påverkar levern till att frigöra glukos, vilket i sin tur påverkar hur väl ett minne kodas in. Det här är en del av mekanismen bakom posttraumatisk stress, när en patient plågas av outplåneliga minnen från en rad traumatiska händelser. På försök har man gett patienter på akutmottagningar betablockerare, som till naturen stoppar adrenalinet från att binda sig till receptorer och i slutändan frigöra glukos. Utan glukos, inga (okej, några) extremt inkodade minnen = ingen PTS.
Att göra saker innan läggdags refererar till tanken att konsolidering av minnen görs under REM-sömn. Ju närmare sömnen du gör något, ju större är chansen att smita förbi i konsolideringskön.
Plugga på femton olika ställen baseras på teorin encoding specificity principle, som menar att information kodas in i en kontext - och för att dekodningstillfället ska flyta på på bästa sätt bör informationen från inkodningstillfället finnas närvarande även då.
Har man pluggat på femton olika ställen är sannolikheten större att den kontextuella informationen från något av tillfällena finns med när det väl kommer till det kritiska minnesögonblicket. Då kanske du tänker - men plugga i tentasalen då? Jag säger - GO!
Att plugga aggresiv baseras på State dependent learning, teorin som menar att en glad student borde vara glad på tentan för att uppnå bästa möjliga dekodningsläge (eller, var full när du pluggar - full på tentan). Visst är den lite lika encoding specificity principle?
Grattis, du tog din igenom teoridelen!
0 notes