#BioassaysService
Explore tagged Tumblr posts
Link
Assays are essentially tests. Bioassays are tests that are used to measure the potency of an active ingredient in the drug by measuring its effects on cells, tissues, or living animals. Cell-Based Assays, as the name suggests, are tests that are performed within a cell. Cell-Based Assays are conducted for several reasons. Sometimes the cytotoxicity of a substance (that is, whether the substance is toxic to the cells) is measured. Other times, as mentioned before, they are used to test at what concentration a substance produces the optimal effects in the test subject. A variety of parameters can be measured through a cell-based assay: radioactivity, luminescence, absorbance, fluorescence, etc. The key feature of a cell-based assay is that it must produce a detectable signal so that the parameter being measured can be determined. Cell-based assays employ a series of reagents for this purpose. What matters the most is the robustness of the signal. It is also important that the signal does not go off with the control experiment when the standard procedure is being tested. However, the same detectable signal must be produced when the same concentration of the substance is tested again. What this means is that the reproducibility of the assays is a necessary factor.

1 note
·
View note
Link
Detection of mutations arising in the genome of the SARS-CoV-2 virus responsible for COVID-19 have led to concerns that new forms of the virus might enable it to evade the protection conferred by recently approved mRNA vaccines. Two strains in particular, B.1.1.7 from England and B.1.351 from South Africa, are driving the unease. B.1.1.7, first reported in September 2020, has been shown to be more transmissible than the previous strain and is spreading globally. The South African mutant was reported in mid-December 2020 by health authorities there as becoming the chief variant, an indication of heightened transmissibility. This strain garnered additional attention because the portion of Novavax’s Phase 3 vaccine trial conducted in South Africa showed noticeably lower efficacy there than in the UK portion.

1 note
·
View note
Link
The successful development and use of cell-based assays can have a huge impact on the cost-effectiveness of pharmaceutical research. Evaluating the biological effects of chemical compounds requires multiple different types of approaches, including molecular, cellular, and biochemical. The first step towards such evaluation includes the successful execution of cell-based and in vitro assays. This article talks about the importance of high-quality cell-based assay development while highlighting six ways to make the process less time-consuming and more cost-effective.

1 note
·
View note
Link
Vaccine development is protracted and risky. On average, vaccines take more than 10 years to move from the preclinical phase to approval, and the probability of market entry for any given candidate has been just 6%. With 140 COVID-19 vaccines currently in development, 13 of which are already in human trials, it is extremely likely then that multiple candidates will eventually be approved, but the question is, how long will this take? Four basic vaccine strategies are being employed across these 140. Each approach has its strengths and weaknesses to activate the immune response to attack the SARS-CoV-2 virus when presented.

#VaccineDevelopmentStrategies#VaccineForSARSCoV2#BioassaysService#CellBasedAssays#ImmuneResponsesSystem#BioMedicalResearch
1 note
·
View note
Link
Every year, billions are invested by governments and businesses around the world for the clinical approval and testing of novel drug compounds. Despite this, the Food and Drug Administration (FDA) only approves a handful of new chemical entities for production and sale each year. For decades, cell-based assays have been used to streamline the process of drug discovery, testing, and development. These assays allow new drugs to be introduced into the market in a safe and efficient manner. Due to their usefulness, the technological evolution of cell-based assays is not slowing down.

1 note
·
View note
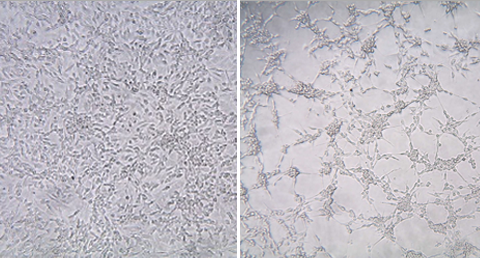
Link
Cell-based assays or bioassays for biological or biochemical activities are required for every lot of pharmaceutical drug or biopharmaceutical before release. Cell-based assays of small molecule pharmaceutical drugs or biologics are integral to and essential for the FDA drug approval process and commercialization.
0 notes