#CTM Machine Features
Explore tagged Tumblr posts
Text
https://heicoin.com/blog/ctm-machine-features-and-benefits-for-industrial-testing/
A "CTM Machine" or Compression Testing Machine plays a very significant role in industrial testing. This testing equipment assesses the strength and durability of materials. More importantly, industrial testing uses precise measurements.

#CTM Machine Features#CTM Features#CTM Machine#CTM#Benefits of CTM#CTM Machine Price#Compression Testing Machines#Compression Testing Machines Manufacturer
0 notes
Text
Global Risk-based Monitoring Software Market: Analysis Of Market Segmentation And Trends
The global Risk-Based Monitoring (RBM) Software Market is on a high-growth trajectory, with the market size valued at USD 359.32 million in 2023 and projected to reach USD 1004.32 million by 2031, expanding at a Compound Annual Growth Rate (CAGR) of 13.71% during the forecast period from 2024 to 2031.
Get Free Sample Report on Risk-based Monitoring Software Market
This notable surge in market value reflects the healthcare industry’s transition toward more efficient, data-driven, and patient-centric clinical trial practices. Risk-based monitoring software is at the forefront of this transformation, playing a critical role in enhancing data quality, improving patient safety, and optimizing clinical operations.
What Is Risk-Based Monitoring Software?
Risk-Based Monitoring (RBM) software enables sponsors, contract research organizations (CROs), and research teams to focus on potential risks in clinical trials, rather than applying uniform monitoring processes across all sites. By leveraging real-time data analytics, predictive modeling, and centralized monitoring dashboards, RBM tools allow for proactive detection and mitigation of issues, streamlining trial management and significantly reducing costs.
Driving Forces Behind Market Growth
Several key factors are fueling the rapid expansion of the RBM software market:
Rising Costs and Complexity of Clinical Trials: As drug development becomes increasingly sophisticated, so do the logistics and costs associated with clinical trials. RBM software helps streamline operations by targeting resources more effectively, reducing site visits, and automating compliance checks.
Adoption of Decentralized Clinical Trials (DCTs): The post-COVID era has brought a lasting shift toward decentralized and hybrid trial models. RBM software supports these models by enabling remote oversight and continuous monitoring, aligning with evolving regulatory frameworks and patient needs.
Regulatory Push for Risk-Based Approaches: Global regulatory agencies such as the FDA, EMA, and ICH have issued guidance encouraging the use of RBM strategies to improve data integrity and trial efficiency. This has significantly boosted market adoption.
Technology Advancements in AI and Analytics: Modern RBM platforms integrate artificial intelligence (AI), machine learning (ML), and real-time data visualization, offering robust insights that help clinical teams make informed, timely decisions. These capabilities are particularly crucial for detecting anomalies, protocol deviations, or patient safety concerns before they escalate.
Focus on Patient Safety and Data Integrity: As sponsors and regulators place greater emphasis on clinical trial transparency and patient outcomes, RBM software offers a valuable tool for maintaining high data quality standards throughout the trial lifecycle.
KEY MARKET SEGMENTS:
By Type
By Component
By Delivery Mode
By End-User
Competitive Landscape
The Risk-Based Monitoring Software Market is highly competitive and technology-driven, with companies focusing on strategic innovations, partnerships, and platform integrations to gain market share. Leading vendors are investing in AI-driven analytics, customizable dashboards, mobile-enabled monitoring, and seamless integration with EDC (Electronic Data Capture) and CTMS (Clinical Trial Management System) platforms.
Key players in the market include:
Medidata Solutions (Dassault Systèmes)
Oracle Corporation
Veeva Systems
Parexel International
IBM Watson Health
Covance (Labcorp Drug Development)
IQVIA
Clario
ArisGlobal
These companies continue to expand their software offerings with features that support real-time monitoring, risk scoring, site performance analysis, and compliance tracking—tools that are now essential in a rapidly evolving clinical trial ecosystem.
Challenges and Opportunities
Despite strong market momentum, certain challenges remain:
Data integration issues across disparate platforms
Resistance to adoption due to organizational inertia or lack of training
Concerns about data privacy and regulatory compliance, especially in cross-border trials
Nonetheless, the market offers immense opportunities, especially with the rise of AI in clinical trials, increasing demand for real-world evidence, and the growing need for digital trial oversight tools in low- and middle-income countries.
Conclusion
The Risk-Based Monitoring Software Market is entering a phase of sustained expansion, propelled by innovation, regulation, and the increasing complexity of clinical trials. As the industry embraces digital transformation, RBM platforms are quickly becoming indispensable for trial sponsors, CROs, and regulators seeking to ensure safety, efficiency, and compliance.
Make Enquiry about Risk-based Monitoring Software Market
With the market projected to grow from USD 359.32 million in 2023 to USD 1004.32 million by 2031, organizations that invest early in robust RBM solutions stand to gain a strategic advantage in the next generation of clinical research.
About US
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us:
Jagney Dave - Vice President Of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Risk-based Monitoring Software Market#Risk-based Monitoring Software Market Trend#Risk-based Monitoring Software Market Share#Risk-based Monitoring Software Market Growth#Healthcare Data Storage Market.
0 notes
Text
Software Tools Used in the Pharmaceutical Industry: A Closeup on CRM Solutions

The pharmaceutical industry is rapidly evolving, with software tools playing a critical role in improving efficiency, ensuring regulatory compliance, and enhancing patient outcomes. From drug development and manufacturing to sales and marketing, pharmaceutical companies rely on various specialized software solutions. In this article, we’ll explore the key software tools used in the pharmaceutical industry, with a particular focus on how Closeup CRM can revolutionize customer relationship management in this highly regulated space.
1. Enterprise Resource Planning (ERP) Software
ERP systems help pharmaceutical companies manage day-to-day operations, such as manufacturing, supply chain logistics, and financials. With features tailored to the pharmaceutical industry, these tools allow businesses to streamline their processes, optimize inventory management, and ensure regulatory compliance. Popular ERP solutions include SAP, Oracle NetSuite, and Microsoft Dynamics.
2. Clinical Trial Management Systems (CTMS)
Clinical trials are at the heart of pharmaceutical product development. CTMS solutions manage the complexities of clinical trials, from patient recruitment to data collection and analysis. Tools like Medidata and Veeva Vault CTMS enable efficient trial management, regulatory compliance, and data integrity, while improving collaboration across research teams.
3. Regulatory Compliance Software
Given the stringent regulations in the pharmaceutical industry, compliance software is essential for tracking, documenting, and ensuring adherence to global standards such as FDA regulations, GMP (Good Manufacturing Practices), and EU regulations. Tools like Veeva Vault QMS and MasterControl streamline the management of quality assurance, regulatory reporting, and audits.
4. Pharmaceutical Sales and Marketing Software
Sales and marketing software help pharmaceutical companies create and execute targeted campaigns, engage healthcare professionals, and promote their products effectively. By 2025, AI-driven solutions will provide deep insights into customer behaviors and enable real-time adjustments to marketing strategies. CRM systems, such as Closeup CRM, are particularly beneficial in this regard.
Closeup CRM: Transforming Pharmaceutical Sales and Marketing
Closeup CRM is a cutting-edge Customer Relationship Management software specifically designed for the pharmaceutical industry. As pharmaceutical companies face increasing competition and more demanding regulatory standards, Closeup CRM offers a suite of tools that help organizations enhance customer engagement, streamline sales operations, and achieve compliance—all in one platform. Here's how Closeup CRM is transforming the pharmaceutical industry:
Key Features of Closeup CRM for the Pharmaceutical Industry
Advanced Customer Segmentation Closeup CRM leverages AI and machine learning to analyze vast amounts of customer data, segmenting healthcare providers (HCPs), patients, and other stakeholders based on behaviors, prescribing patterns, and needs. This segmentation allows sales and marketing teams to deliver personalized communications, increasing engagement and improving conversion rates.
Personalized Marketing Campaigns With Closeup CRM, pharmaceutical companies can create targeted, compliant marketing campaigns tailored to specific healthcare professionals or patient groups. Automated, data-driven insights help marketers optimize their messaging and approach, ensuring the right message reaches the right audience at the right time.
Compliance-First Features Compliance is critical in the pharmaceutical industry, and Closeup CRM is built with this in mind. It ensures all interactions with healthcare providers and patients are compliant with regulatory standards like HIPAA, GDPR, and FDA guidelines. The platform includes features such as audit trails, real-time reporting, and automated alerts for compliance breaches.
Salesforce Automation Sales reps in the pharmaceutical industry often struggle with time-consuming administrative tasks. Closeup CRM automates many of these processes, such as tracking meetings, managing follow-ups, and generating sales reports. This allows reps to focus on what truly matters—building relationships with healthcare providers and driving sales.
Analytics and Reporting One of the standout features of Closeup CRM is its powerful analytics and reporting tools. Pharmaceutical companies can track the performance of their marketing campaigns, sales activities, and customer interactions in real-time. This data-driven approach enables teams to make faster, more informed decisions and optimize their strategies for better results.
Mobile Accessibility Sales representatives in the pharmaceutical industry are often on the go, meeting healthcare professionals and attending events. Closeup CRM is fully mobile-optimized, allowing sales teams to access essential customer data, update records, and track their activities from anywhere—boosting productivity and responsiveness.
Seamless Integration Closeup CRM integrates easily with other pharmaceutical software, such as ERP systems, clinical trial management tools, and regulatory compliance platforms. This seamless integration ensures that all data is synchronized and accessible, allowing for more efficient workflows and data sharing across departments.
5. Supply Chain Management Software
Pharmaceutical companies need robust software solutions to manage their complex and highly regulated supply chains. Supply chain management software helps track raw materials, ensure timely delivery, and maintain compliance with safety regulations. Leading tools in this area include SAP Integrated Business Planning (IBP) and Kinaxis RapidResponse.
6. Pharmacovigilance Software
Pharmacovigilance software helps pharmaceutical companies monitor the safety of their products once they’re on the market. These tools collect and analyze adverse event reports, track product complaints, and ensure that any risks are addressed quickly. Platforms like Oracle Argus and Veeva Vault QMS are commonly used to ensure safety and regulatory reporting.
7. Artificial Intelligence and Data Analytics Tools
AI and machine learning are becoming increasingly important in the pharmaceutical industry, enabling predictive analytics, drug discovery, and patient outcome modeling. Tools like IBM Watson for Drug Discovery and Veeva Vault Analytics are enhancing the capabilities of pharmaceutical companies by providing actionable insights from vast datasets.
Conclusion
The pharmaceutical industry is increasingly adopting software solutions to streamline operations, improve patient outcomes, and ensure regulatory compliance. Among the key tools, Closeup CRM stands out as a transformative platform for managing customer relationships in the pharmaceutical space. By offering advanced features like customer segmentation, personalized marketing, compliance tracking, and sales automation, Closeup CRM helps pharmaceutical companies boost sales, maintain regulatory standards, and ultimately, deliver better healthcare outcomes. As the industry continues to evolve, AI-powered CRMs like Closeup CRM will be at the forefront of this transformation, shaping the future of pharmaceutical sales and marketing.
1 note
·
View note
Text
The Clinical Trial Management System Market is projected to grow from USD 2075 million in 2024 to an estimated USD 5981.71 million by 2032, with a compound annual growth rate (CAGR) of 14.15% from 2024 to 2032.The Clinical Trial Management System (CTMS) market has witnessed remarkable growth in recent years, driven by advancements in healthcare technology and the increasing complexity of clinical trials. CTMS is a software solution that supports the planning, tracking, and management of clinical trials, enabling pharmaceutical companies, Contract Research Organizations (CROs), and academic research institutions to streamline their processes. As the demand for more efficient drug development and research intensifies, CTMS has become an essential tool for optimizing clinical trial operations.
Browse the full report at https://www.credenceresearch.com/report/clinical-trial-management-systems-market
Market Overview
The CTMS market is projected to experience substantial growth, driven by an increasing number of clinical trials and the expanding pharmaceutical and biotechnology industries. According to recent industry reports, the market is expected to grow at a compound annual growth rate (CAGR) of around 13-14% from 2023 to 2030. This rapid expansion is attributed to the growing adoption of CTMS solutions, technological advancements, and the rising need to manage large volumes of clinical data effectively.
Key Drivers of Market Growth
1. Increase in Clinical Trials The rise in the number of clinical trials, especially in emerging markets, is a significant driver for the CTMS market. As more pharmaceutical and biotechnology companies aim to bring new drugs to market, they require systems that can manage complex trial data, regulatory requirements, and patient recruitment. CTMS platforms offer robust solutions to these challenges by ensuring compliance and data integrity while minimizing operational inefficiencies.
2. Technological Advancements With advancements in artificial intelligence (AI), machine learning (ML), and cloud-based solutions, CTMS systems have become more sophisticated, providing better integration, real-time data tracking, and predictive analytics. These innovations allow sponsors and researchers to make informed decisions more quickly and efficiently, reducing trial costs and timelines.
3. Regulatory Compliance The increasing stringency of regulatory guidelines globally has made compliance management crucial for clinical trials. CTMS platforms are equipped with features that ensure adherence to regulatory standards such as Good Clinical Practice (GCP), the International Council for Harmonisation (ICH) guidelines, and the Food and Drug Administration (FDA) regulations. Automated monitoring and audit trail capabilities offered by these systems help sponsors and CROs maintain regulatory compliance seamlessly.
4. Rising Adoption of Decentralized Trials The COVID-19 pandemic accelerated the trend toward decentralized clinical trials, where patient data is collected remotely. CTMS platforms are playing a key role in supporting decentralized trials by enabling remote monitoring, patient data capture, and virtual collaboration between stakeholders. This shift has not only enhanced the reach of clinical trials but has also improved patient retention and engagement.
### **Regional Insights**
The CTMS market is globally segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America holds the largest market share due to the presence of a robust pharmaceutical and biotechnology industry, as well as supportive regulatory frameworks. However, the Asia Pacific region is anticipated to witness the fastest growth due to increasing R&D activities, growing healthcare infrastructure, and government initiatives to boost clinical trials.
Challenges Facing the CTMS Market
Despite the strong growth trajectory, the CTMS market faces several challenges:
1. High Implementation Costs The initial cost of setting up and implementing a CTMS can be substantial, especially for small- and medium-sized enterprises (SMEs). This can hinder widespread adoption, particularly in developing regions where cost sensitivity is high.
2. Data Integration Issues Clinical trials generate large amounts of data from various sources, including electronic health records (EHRs), wearable devices, and laboratory information management systems (LIMS). Integrating these data streams into a single CTMS platform can be challenging, leading to data silos and inefficiencies.
3. Data Security and Privacy Concerns The growing reliance on cloud-based solutions has raised concerns about data security and privacy. As clinical trial data often includes sensitive patient information, ensuring the security of this data is a significant challenge for CTMS vendors and users alike.
Future Outlook
The future of the CTMS market looks promising, with increasing investments in research and development, advancements in AI and machine learning, and the growing adoption of decentralized trial models. As the demand for more efficient clinical trial management continues to rise, CTMS platforms are poised to play a critical role in optimizing trial operations, improving patient outcomes, and accelerating the drug development process.
Key Player Analysis:
Calyx (formerly Parexel Informatics)
Clario
DATATRAK International, Inc.
IQVIA, Inc.
Laboratory Corporation of America Holdings
Medidata (Dassault Systèmes)
Oracle
PHARMASEAL International Ltd.
RealTime Software Solutions, LLC
SimpleTrials
Veeva Systems
Wipro Limited
Segmentation:
By Type
Enterprise
Site
By Component
Software
Services
By Delivery Mode
Web & Cloud Based
On Premise
By End-user
Pharmaceutical and Biotechnology Firms
Medical Device Firms
CROs & Others
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/clinical-trial-management-systems-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
E-Clinical Solution Software Market Dynamics: Trends, Innovations, and Future Prospects| GQ Research
The E-Clinical Solution Software market is set to witness remarkable growth, as indicated by recent market analysis conducted by GQ Research. In 2023, the global E-Clinical Solution Software market showcased a significant presence, boasting a valuation of US$ 10.91 billion. This underscores the substantial demand for E-Clinical Solution Software technology and its widespread adoption across various industries.
Get Sample of this Report at: https://gqresearch.com/request-sample/global-e-clinical-solution-software-market/

Projected Growth: Projections suggest that the E-Clinical Solution Software market will continue its upward trajectory, with a projected value of US$ 26.64 billion by 2030. This growth is expected to be driven by technological advancements, increasing consumer demand, and expanding application areas.
Compound Annual Growth Rate (CAGR): The forecast period anticipates a Compound Annual Growth Rate (CAGR) of 13.60%, reflecting a steady and robust growth rate for the E-Clinical Solution Software market over the coming years.
Technology Adoption:
In the E-Clinical Solution Software market, technology adoption is shaped by the diverse range of applications these solutions offer. E-Clinical solutions encompass a variety of software applications designed to streamline and enhance various aspects of clinical trials and healthcare research. These applications include electronic data capture (EDC) systems, clinical trial management systems (CTMS), electronic patient-reported outcomes (ePRO) tools, and electronic clinical outcome assessment (eCOA) platforms, among others. The adoption of technology in this market is driven by its ability to improve efficiency, accuracy, and compliance throughout the clinical trial process, ultimately accelerating the development of new medical treatments and therapies.
Consumer Preferences:
Consumer preferences in the E-Clinical Solution Software market are influenced by several key factors. Healthcare organizations, pharmaceutical companies, contract research organizations (CROs), and academic institutions, among others, are the primary consumers of these solutions. Their preferences are guided by the need for user-friendly interfaces, robust functionality, seamless integration with existing systems, and regulatory compliance. Additionally, factors such as data security, scalability, and support for mobile devices may also influence purchasing decisions. Ultimately, consumers seek E-Clinical solutions that not only meet their specific requirements but also enhance overall efficiency and data quality in clinical trials and research studies.
Technological Advancements:
Technological advancements play a significant role in driving innovation and evolution within the E-Clinical Solution Software market. Emerging technologies such as artificial intelligence (AI), machine learning, blockchain, and cloud computing are being increasingly integrated into E-Clinical solutions to enhance data analysis, improve decision-making processes, and optimize trial operations. For example, AI algorithms can analyze large datasets to identify patterns and insights, while blockchain technology can ensure the integrity and security of clinical trial data. These advancements enable greater automation, efficiency, and transparency in clinical research, paving the way for more effective and reliable healthcare interventions.
Market Competition:
Competition in the E-Clinical Solution Software market is intense, with numerous vendors vying for market share and industry leadership. Established players, as well as niche providers and startups, compete to offer comprehensive and innovative solutions that address the diverse needs of healthcare organizations and research institutions. Competitive factors include product features, pricing, reliability, customer support, and regulatory compliance. Strategic partnerships, mergers, and acquisitions are common strategies employed by companies to expand their market presence, enhance their product portfolios, and gain a competitive edge in this rapidly evolving market landscape.
Environmental Considerations:
Environmental considerations are becoming increasingly relevant in the E-Clinical Solution Software market, albeit indirectly. While the focus of this market is primarily on improving clinical trial efficiency, data quality, and patient outcomes, there is growing recognition of the environmental impact of healthcare practices, including clinical research activities. Efforts to digitize and streamline clinical trial processes through E-Clinical solutions can contribute to reducing paper usage, minimizing waste, and lowering energy consumption associated with traditional paper-based methods. Additionally, the use of cloud-based technologies in E-Clinical solutions can support remote collaboration and reduce the need for physical infrastructure, further aligning with environmental sustainability goals.
Regional Dynamics: Different regions may exhibit varying growth rates and adoption patterns influenced by factors such as consumer preferences, technological infrastructure and regulatory frameworks.
Key players in the industry include:
Oracle Corporation
Medidata Solutions
ERT
Parexel International Corporation
Bioclinica
IBM Corporation
Datatrak International, Inc
CRF Health
Merge Healthcare Solutions Inc.
Anju Software
The research report provides a comprehensive analysis of the E-Clinical Solution Software market, offering insights into current trends, market dynamics and future prospects. It explores key factors driving growth, challenges faced by the industry, and potential opportunities for market players.
For more information and to access a complimentary sample report, visit Link to Sample Report: https://gqresearch.com/request-sample/global-e-clinical-solution-software-market/
About GQ Research:
GQ Research is a company that is creating cutting edge, futuristic and informative reports in many different areas. Some of the most common areas where we generate reports are industry reports, country reports, company reports and everything in between.
Contact:
Jessica Joyal
+1 (614) 602 2897 | +919284395731
Website - https://gqresearch.com/
0 notes
Text
Why is a Clinical Trial Management System Essential for Success?
Clinical trials are fundamental in advancing healthcare and bringing new treatments to patients. Effective management of these trials is crucial for success. Clinical Trial Management streamlines this process, enhancing efficiency and aiding in better decision-making.
Understanding the Clinical Trial Management System (CTMS)
Clinical Trial Management System, often abbreviated as CTMS, is a centralized software solution designed to streamline and manage every aspect of clinical trials. From planning and tracking to reporting and analyzing, a CTMS system is a comprehensive tool used by researchers, sponsors, and stakeholders to ensure the smooth execution of clinical trials.
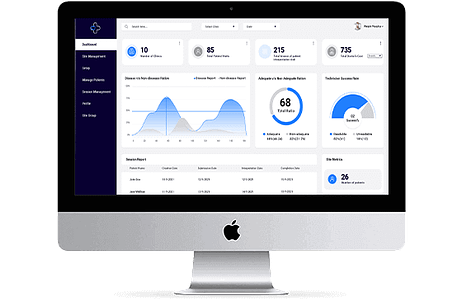
The Key Components of a CTMS System
Trial Planning and Design: Creating a blueprint for the trial, including protocols, endpoints, and participant criteria.
Subject Management: Managing participant information, recruitment, and enrollment into the trials.
Document Management: Organizing and storing trial-related documents and essential paperwork.
Data Collection and Analysis: Collecting, organizing, and analyzing trial data to derive meaningful insights.
Budget and Financial Management: Monitoring and controlling trial-related expenses and budgets.
Advantages of Implementing a Clinical Trial Management System
Efficient Data Management: Streamlining data collection, reducing errors, and enabling real-time access to data for all stakeholders.
Enhanced Compliance and Regulatory Adherence: Assisting in maintaining compliance with regulatory standards and guidelines throughout the trial.
Improved Communication and Collaboration: Facilitating seamless communication among trial stakeholders, promoting collaboration and efficiency.
Cost and Time Efficiency: Optimizing clinical trial software solutions processes, resulting in cost savings and faster development of treatments.
The Evolving Landscape of Clinical Trial Management Clinical trial management has come a long way from paper-based systems to advanced CTMS solutions. These systems now integrate features like Artificial Intelligence (AI) and Machine Learning (ML) to predict outcomes, optimize resource allocation, and enhance decision-making.
Emerging Trends in Clinical Trial Management
Decentralized Clinical Trials: Leveraging technology for remote monitoring and data collection, minimizing the need for physical site visits. Patient-Centric Approach: Placing more emphasis on patient experience and involvement in the trial process. Real-world Evidence Integration: Incorporating real-world data to complement traditional clinical trial data, providing a more comprehensive understanding of treatment outcomes.
How To Choose the Right Clinical Trial Management Software?
When selecting clinical trial software, organizations should consider factors such as ease of use, scalability, integration capabilities, compliance with industry standards, and the specific needs of their trials.
Conclusion:
The Evolving Landscape of Clinical Trial Management
Clinical trial management has come a long way from paper-based systems to advanced CTMS solutions. These systems now integrate features like Artificial Intelligence (AI) and Machine Learning (ML) to predict outcomes, optimize resource allocation, and enhance decision-making.
Emerging Trends in Clinical Trial Management
Decentralized Clinical Trials: Leveraging technology for remote monitoring and data collection, minimizing the need for physical site visits.
Patient-Centric Approach: Placing more emphasis on patient experience and involvement in the trial process.
Real-world Evidence Integration: Incorporating real-world data to complement traditional clinical trial data, providing a more comprehensive understanding of treatment outcomes.
How To Choose the Right Clinical Trial Management Software?
When selecting clinical trial software, organizations should consider factors such as ease of use, scalability, integration capabilities, compliance with industry standards, and the specific needs of their trials.
Conclusion:
Clinical Trial Management Systems are transforming the landscape of healthcare trials. By streamlining processes, enhancing collaboration, and leveraging cutting-edge technologies, clinical trial management software plays a pivotal role in expediting the development and approval of life-changing treatments.
0 notes
Text
The Digital Edge: How Clinical Trial Management Systems Are Shaping the Healthcare Industry
Introduction
The Clinical Trial Management System (CTMS) market has witnessed substantial growth and transformation in recent years. This dynamic sector plays a pivotal role in the pharmaceutical and biotechnology industries by streamlining and optimizing the complex process of conducting clinical trials. CTMS solutions have become indispensable tools for managing clinical research, offering features such as patient recruitment, data management, regulatory compliance, and real-time monitoring. This article delves into the current state of the Clinical Trial Management System market, its driving factors, challenges, and future prospects.
Market Overview
The global Clinical Trial Management System market has experienced robust expansion, driven by the increasing need for efficient and cost-effective drug development processes. As pharmaceutical companies face mounting pressure to bring new drugs to market faster, CTMS solutions have become essential for enhancing clinical trial productivity and ensuring data accuracy. In 2021, the CTMS market was valued at approximately $1.7 billion, and it is projected to grow at a compound annual growth rate (CAGR) of around 12% from 2021 to 2028.
Key Drivers
1. Growing Clinical Trial Complexity: Clinical trials are becoming more complex due to the rise of personalized medicine, targeted therapies, and the inclusion of diverse patient populations. CTMS software simplifies the management of these intricate trials, leading to increased demand.
2. Regulatory Compliance: Stringent regulatory requirements in the healthcare and pharmaceutical sectors necessitate robust data management and reporting. CTMS platforms help organizations adhere to these regulations, reducing the risk of non-compliance and penalties.
3. Cost Efficiency: CTMS solutions can significantly reduce operational costs associated with clinical trials. By automating various processes and improving resource allocation, they contribute to substantial cost savings.
4. Globalization of Clinical Trials: The globalization of clinical trials has increased the need for efficient communication and collaboration among geographically dispersed research teams. CTMS systems facilitate real-time data sharing and project coordination across borders.
Challenges
1. Integration Issues: Integrating CTMS software with existing systems can be challenging, leading to compatibility issues and data transfer problems. Ensuring seamless integration remains a hurdle for many organizations.
2. Data Security and Privacy: With the sensitive nature of patient data involved in clinical trials, data security and privacy concerns are paramount. Ensuring compliance with data protection regulations adds complexity to CTMS implementation.
3. Limited Adoption in Smaller Organizations: While larger pharmaceutical companies have readily adopted CTMS solutions, smaller organizations often face budget constraints and resource limitations, hindering their adoption.
4. Changing Regulatory Landscape: The regulatory environment for clinical trials is constantly evolving. CTMS systems must adapt to these changes, requiring ongoing updates and customization.
Future Prospects
The Clinical Trial Management System market is poised for further growth and innovation in the coming years. Several trends and developments will shape its future:
1. Artificial Intelligence (AI) and Machine Learning: AI and machine learning will play a crucial role in CTMS, automating data analysis, predictive analytics, and decision-making processes. This will enhance trial efficiency and reduce manual errors.
2. Cloud-Based Solutions: Cloud-based CTMS solutions will become increasingly popular, offering scalability, flexibility, and remote access. They will also address integration challenges and provide real-time collaboration capabilities.
3. Patient-Centric Trials: The focus on patient-centric trials will continue to grow, and CTMS platforms will incorporate patient engagement and feedback mechanisms, improving recruitment and retention rates.
4. Blockchain Technology: Blockchain technology has the potential to enhance data security and transparency in clinical trials. Its adoption may increase as regulatory bodies seek to improve data integrity.
5. Virtual Clinical Trials: The COVID-19 pandemic accelerated the adoption of virtual clinical trials. CTMS systems will need to adapt to support remote monitoring, telemedicine, and decentralized trials effectively.
Conclusion
The Clinical Trial Management System market is undergoing a profound transformation, driven by the need for more efficient, cost-effective, and compliant clinical trials. As the pharmaceutical and biotechnology industries continue to innovate and expand, CTMS solutions will play an increasingly vital role in bringing new therapies and medications to market. The market's future is bright, with technological advancements, increased adoption, and a commitment to improving patient outcomes at its core. Organizations that embrace CTMS technology and navigate its challenges effectively are well-positioned to thrive in this ever-evolving landscape.
0 notes
Note
...you have opened my eyes to a vast universe of VintageBeef lore that I was unaware of. I knew about the New Hermit Order, of course, and the UHC invention, and I've watched a few of his CTM things but -- I will take all the info and lore you feel like giving out because Beef is amazing and my knowledge is so small.
Vintagebeef my beloved <3
So the thing is, right, until about 2016 I only watched two (2) youtubers- Vintagebeef for Minecraft and aDrive for Pokemon (and funnily enough both of them are named Dan irl). So I've watched most of Beef's videos over the years and have a general knowledge of most of his stuff, except because it's been like a decade I don't remember where most of the lore comes from XD
The thing with him is that he doesn't do Lore tm the way other mcyters often do lore- he doesn't have an extensive RP series to draw from like Grian, doesn't have a solo world with steadily increasing amounts of lore like Etho or Zisteau, and while he's played on SMPs and been involved in storylines before it's not really the focus of his episodes unlike with Evo or Legacy or Empires
So where does that leave us?
IRL, Beef always has multiple series running at the same time. Often he's playing on an smp while doing a singleplayer, often modded, series as well as a CTM or modpack with a group of friends. For example, right now he's playing on Hermitcraft, doing weekly Pixelmon and Building a Zoo episodes, and a CTM map with Slip. And to me, this translates to one thing: Beef is an adventurer. He travels frequently- he explores a world and when he decides he's done, he leaves for the next one. That's the basis of my personal interpretation of his series and his character for my writing.
Ok so reading this back, this got extremely long and didn't explain much in the way of lore, somehow? If anyone has any additions to add please do so, I am very definitely leaving out a lot and would love to see what other lore people remember and are using for Beef! I didn’t include the Hermitcraft stuff since my memory of season 4 is blurry (his base was themed after the Martian, that much I know, and he and Iskall were buddies :D) and most of the s5 NHO lore is best watched from Bdub’s perspective from what I remember, and the only s6 stuff is a single line in Hermitgang and then the Area 77 arc with its possibility of an NHO reunion which we did not get rip. And s7 of course had the cloning machine and also the Podzol Party as the main lore. So all the original rambling is still below the cut though it is very long, and I'm gonna bullet point the main stuff here instead:
Actual canonical things:
Invented UHC and was the only survivor of the first ever uhc (Mindcrack UHC s1)
Married to an ender dragon (one of the UHCs I think), later father to a different dragon (Mindcrack season 3? I think?)
Might not have legs if you choose to take that joke as canon (Mindcrack s2)
Was a wizard (RAD)
is a zookeeper (Building a Zoo)
Had a wife and kids (Sims in Minecraft)
Part of the Trial of the B Team court case (Mindcrack)
NHO founder, founder of the Podzol Party (Hermitcraft)
Created a cloning machine that sort of works (Hermitcraft)
Played the Forest which is I believe the first time he and Keralis played together (look up the trigger warnings for this one, it's a horror game)
Was the creator/owner of Sourceblock SMP (featuring some familiar faces if you know Legacy, Empires, or MCC) and there is literal magic from a mysterious sourceblock of water that teleports people and summons mobs and probably more stuff that I haven't seen yet since I'm still watching it myself
Things you can infer:
Good with animals (Life in the Woods, Pixelmon, Ark)
Is a car nerd (irl and all of the car games he's played)
Is a highly experienced adventurer who has traveled through dozens of worlds both vanilla and modded, across multiple dimensions (Twilight Forest, the Aether, the Betweenlands, Limbo), completed dozens of monuments, fought in blood sports, survived apocalypse after apocalypse, tamed dinosaurs, and played a lot of prop hunt and golf with your friends
If you're looking for what to watch for lore purposes, I'd say the Mindcrack UHCs and Team Canada's RAD series are pretty good, definitely Sourceblock and HC s5, plus the Diversity CTM maps and Ruins of the Mindcrackers maybe? And Mindcrack Prank Wars for the chaos and the origin of Team Canada. And if you can handle horror than the Forest is fun and if you don't do horror you can watch the Pojkband play golf or prop hunt they're hilarious I love them sm I want a Pojkband reunion So Bad
Beef's first series was a singleplayer series in beta 1.4_01 though he had played the game extensively before that, and was a big fan of Guude, having watched his own Minecraft videos. The series was functionally a hardcore one where if he died Beef would delete the world and start again! I haven't actually Watched this series so idk if he died or how often lmao. When Guude made Mindcrack, which was btw one of the very first Minecraft SMPs, he also hosted a competition for people to join, and Beef submitted a video (which is still viewable on his channel I believe!) and won, and was added to Mindcrack in season 2 :D (fun fact, Guude said that even if Beef hadn’t won he would have added him anyway)
Two running jokes emerged from Mindcrack- pulling a Vintagebeef and Beef doesn't have legs. The first is a reference to Beef dying of fall damage (I believe the exact instance was him trying to jump into his swimming pool and failing spectacularly) and after the incident, every time someone died of fall damage they were pulling a Vintagebeef. The second joke comes from Guude, who joked that the reason Beef wasn't going to a convention was because he didn't have legs, and then he pranked Beef's base by building a giant pair of legs at the entrance to his castle so you had to walk between them to get into the base. This joke has long since died and both Beef and Guude feel pretty bad about it iirc because there were people who genuinely thought Beef was disabled and were emailing him supportive messages and stuff oops. So if you go looking on the Salad or find old Mindcrack fics, you might see references to Beef having prosthetic legs!
Mindcrack also brought about the creation of several Player groups- Team Nancy Drew, Team Canada, and GOB to name a few relevant to Beef. Team Nancy Drew consists of Beef, Pauseunpause, Guude, and Baj, who formed to investigate a prank on one of the members but I forget who. They're named Nancy Drew after the detective! Team Canada also formed in retaliation to pranks, with it consisting of Beef, Etho, and Pause, the three Canadian members on the server (not including Adlington who moved to Canada but never joined the group). There was also a Team America who pranked them with American flags everywhere. GOB is Guude, OMGChad, and Beef, who played stuff like the Ragecraft, Pantheon, and Monstrosity ctms together but that's way down the line lol
Team Nancy Drew is also notable for inventing UHC. It was Beef's brainchild but it was the four of them who first played it! The first UHC had the four of them working to kill the dragon with no natural regen, with everyone dying but Beef, who "won" the UHC. The second uhc was still dragon focused and iirc is where Beef married the dragon? Memories are hazy but they do kill the dragon in this one I think. UHC was then revamped as a pvp event and became a regular Mindcrack game every few months, featuring most of the Mindcrackers and several special guests, including Dinnerbone, who as we know Thanos-snapped Doc's arm out of existence as a result of Doc killing him in one of them
In one of the seasons of Mindcrack, Beef invited swedish Mindcracker and good friend Anderzel to go caving with him and invented ABBA Rules caving, where the winner takes it all. ABBA Rules is a game where each ore (and also dungeon loot like nametags) is assigned a point value and the person with the most points at the end wins and gets to keep all the stuff collected from the game.
In Mindcrack season 3?, Beef punched the ender dragon in an... awkward area, so when the dragon died and left the egg behind, Guude said Beef was the father of the egg XD I don't remember if I watched s3 so I have no idea if anything Happened with this concept but *history of the world voice* you could make lore out of this!
So Team Canada has played a Lot of CTM maps (which fun fact were pretty much invented by another Mindcrack member, Vechs, with his Super Hostile series! Super Hostile has a bunch of things called "Zistonian", which are references to another Mindcrack member Zisteau, who has a very wild singleplayer series with even wilder lore but I digress). In Ruins of the Mindcrackers, they had a running joke that Beef was Etho and Pause's mom, which is a joke we can leave in the past actually /lh. They also played all the Diversity maps, Sky Factory, Terra Restore, Uncharted Territory uhhh and a couple more ctms and adventure maps! Each map kinda has its own story so in Diversity 3 for example they were trapped in a simulation? I think? Team Canada also recently played the Roguelike Adventures and Dungeons modpack, aka RAD, in which Beef was a wizard with a magic staff that could do anything from summon lightning to control hostile mobs.
Sourceblock SMP is a vanilla survival 1.14 series that ran for one season and the series starts with each of the Players being drawn to a strange sparkling water source that, once they touch it, brings them to the Sourceblock world. It also summons a giant zombie at one point. There's probably more lore for this series but like I said I haven't watched it all the way through yet
He has a Patreon server called VintageCraft and has done a series or two on there as well, and played a few UHCs with them, so lore that how you will!
Beef also played a few popular mods, notably Pixelmon, Life in the Woods, and Feed the Beast, with LitW being singleplayer and the other multiplayer. He's also recently played the Zoo and Wild Animals mod a lot. He did a short series with the Minecraft Comes Alive mod where he married one of the villagers and had two children, so that's canon now :D he’s played a Lot of Pixelmon starting when the mod first came out iirc (he chose Turtwig in his first series and built a Grass gym, then made a Normal gym in another series in uhh 2016) and he still plays to this day. Quite a few Hermits played on his Pixelmon servers with him, like Wels, Etho, Iskall, Stress, Slip, Zueljin, and also Guude and Phedran (a Mindcrack adjacent player and creator of the LitW modpack) and a few Mindcrackers on the older servers
Mindcrack and friends played a lot of other games too- 7 Days to Die, Ark Survival Evolved, Unturned, to name a few, so you can pull a lot of lore out of these as well. Speaking of friends and non-Minecraft games, Beef teamed up with Pause, Keralis, and Slip (a former Hermit) to play the horror game the Forest, which saw them stuck on an island trying to survive against terrifying mutated human... things. They played it a few times as the game updated but as afaik it's the first time Beef played with Keralis and possibly Slip and since the game starts with the Player's airplane crashing, that could totally be how Beef first met them in-universe
I... think? that’s everything I mentioned in the tags? There is probably way more stuff I’ve forgotten that stems from inside jokes and things that happen within each series, but I hope that was a) helpful and b) at least somewhat comprehensible lmao
#hermitcraft#mindcrack#vintagebeef#mcyt#long post#asks#redwinterrises#that was so many words#kudos to anyone who reads the whole thing lmao
211 notes
·
View notes
Text
CTM Machine is particularly essential because these machines allow cutting metal sheets into plates, bars, or coiled strips with high precision and at tremendous speed, which meets customer’s requirements.
0 notes
Text
CTM Labeling Systems Can Help You Maximize Your Productivity

CTM Labeling Systems builds labeling automation equipment that can be used in a variety of industries, including automotive, pharmaceutical, food processing/packaging, health and beauty, and e-Commerce. They offer a complete line of labeling machines, including Wrap, Bottle, Tabletop Wrap, Front/Back, Horizontal and Vertical Trunnion, Clamshell, Hugger, Pail, Top/Bottom, and custom designed systems to meet your most demanding labeling needs. Print Engines for High Production Environments
These printers are designed to handle the printing speed and operating requirements of your labeling system. They use thermal transfer technology to heat a ribbon and melt ink onto the label, resulting in a permanent mark that won't fade with time. They're ideal for long-term labeling applications, such as shipping labels and product IDs.
They're also able to keep up with high volume throughput, as they have the ability to process multiple labels at once. They're perfect for businesses that want to save time and money on labor while increasing productivity. The Labeling Machine That's Right for Your Business
A labeling machine is a system that prints, dispenses, and applies labels to products, including individual packages, cartons, cases, and pallet loads. They're used for product identification, product traceability, and branding. They can print on a wide variety of substrates, including clear and opaque labels. Read more great facts on Labeling Systems, click here.
Typically, these systems are designed with a photoeye to ensure accurate label placement. If a label isn't placed correctly, it can lead to problems with tracking and shipping, which can negatively affect your customers. These machines are programmable to save the format of your labels, so they can be changed in real time. Automatic Labeling Machines are Industrial Grade and Backward Compatible
The equipment in a labeling machine is designed and built to last several times as long as commercial components, ensuring that it will continue to perform properly in harsh conditions. Its industrial-grade support is backed by a local distributor that can make repairs, adjustments, and modifications to meet new manufacturing needs quickly and efficiently. For more useful reference regarding CTM, have a peek here.
For more information on how a labeling machine can help you maximize your productivity, reach out to one of our Packaging Specialists today! Automatic Labeling Machines Are the Best Investment for Your Business
The smallest of all the labeling machines, intermediate models aren't floor-mounted like their industrial counterparts. These machines are often used in small and medium-sized businesses that don't need as much capacity. They still print, dispense, and apply labels, but they don't have the heavy duty industrial parts found on their larger brothers.
This makes them more affordable and easier to maintain than their larger industrial brethren, but they have a few drawbacks. They're usually less expensive than their larger industrial cousins, but they aren't able to withstand extreme temperatures, and can be more susceptible to mechanical breakdowns.
They're also prone to human error, and they don't have the flexibility of changing label formats in real time, so they can cost you more in the long run.
A rotary labeling system is a type of automated labeling system that attaches labels to a variety of containers, including clamshells, tub-style and boxes, cartons, and cases. These systems feature a rotating, circular base that can present the labeling head to the product as it passes through the applicator. When the labeling head senses a container, it sticks the PSA to the product and then transports it along the conveyor to the next step in the labeling process. Please view this site https://www.ehow.com/how_4859509_make-cosmetic-labels.html for further details.
0 notes
Note
3, 4, 5? :)
3. Favourite Album of the Year?
aaaah this is so hard bc 2018 was full of INCREDIBLE albums but here r 6 of my favourites (spotify links included!!):
twenty one pilots’ Trench
TWRP’s Together Through Time
Idles’ Joy As An Act of Resistance
Mitski’s Be The Cowboy
Hippo Campus’ Bambi
Louis Cole’s Time
Hozier’s Nina Cried Power EP
4. Favourite song from that favourite album?
LMFAO I’ve made a mistake i didn’t read ahead, so I’ll choose Trench for my favourite album.
the entire album is my favourite I think it’s a tie between Morph, Legend and Pet Cheetah. Morph is a Big BPD bop imo, Legend reminds me of my grandfather every damn time I hear it, and Pet Cheetah has an INCREDIBLE live vibe and I cannot unhear the smoke machines exploding whenever I listen to it now.
5. Favourite 5 songs of the year?
ok here i’ll do a song from each of the albums above bc i don’t have any other songs i really enjoyed i think (and so it’s 6 now rip sorry!!)
Life Party! This is literally my favourite song: it’s funky, it’s joyful, and it’s exactly what I needed in a year that kicked my fucking ass.
Colossus and Cry to Me: v good songs with very powerful messages. I love CtM’s message of men being there for one another to be a shoulder to cry on. It’s lovely.
GEYSER. GEYSER GEYSER GEYSER. Geyser is an INCREDIBLE fucking song and I get so hype every time. The build up, the bizarre almost white noise-y intro, the power in her vocals!!! god. mitski is a goddess honestly
The song the album is named after, Bambi. I feel like it’s really relatable and that’s kinda something I extremely needed in 2018. I love how sad and calm it is. It’s also a bop, lmao
Trying Not To Die (featuring Dennis Hamm!): this is Another Big Mood song, and it’s definitely tied with When You’re Ugly, but I think I prefer TNTD. Louis Cole is another rare artist where I can listen to a whole album and just be super impressed by the entire thing & love every song so this was difficult, lmao.
Shrike! Shrike is uncontested champion of the EP for me. It’s kinda like a spiritual successor to Cherry Wine, which is an incredibly bittersweet and depressing song, and Shrike has… I don’t know. It’s got that same sort of bittersweet vibe, except it feels more hopeful to me? Ugh.
send me an end of year/2018 music ask!
1 note
·
View note
Text
5 Essential Features to Consider in a Compression Testing Machine

When selecting a Compression Testing Machine (CTM) for your industrial or laboratory needs, several critical features demand your attention. A CTM serves as the backbone of material strength assessment, ensuring reliability and accuracy in testing various materials. To make an informed decision, it's crucial to consider specific pivotal features integral to these machines.
Robust Frame Design for Precision Testing
The foundational aspect of any CTM is its frame design. Look for a robustly engineered frame, typically constructed from high-quality materials like steel, capable of withstanding substantial loads without deformation. This ensures the machine's stability during compression tests, providing accurate and consistent results.
Accurate Load Measurement Systems
The accuracy of a CTM hinges on its load measurement system. Opt for machines equipped with advanced load cells or hydraulic systems, enabling precise and reliable measurement of the applied force. Calibration and accuracy in load measurement are paramount, ensuring consistent and error-free testing.
Control and Data Acquisition Software
A CTM's efficiency heavily relies on its control and data acquisition software. Seek machines integrated with user-friendly software allowing seamless control of the testing process. Features like real-time data acquisition, graphical representations, and customizable test parameters enhance the overall testing experience.
Safety Features for Operator Protection
Prioritize CTMs equipped with robust safety features to safeguard operators and ensure a secure working environment. Emergency stop buttons, overload protection, interlocking mechanisms, and safety shields are indispensable components to prevent accidents during testing procedures.
Versatile Testing Capabilities
Consider the machine's versatility in conducting various tests on diverse materials. A CTM offering flexibility to perform compression tests on an array of specimens and accommodating different sizes and shapes expands its utility across different industries and research domains.
In conclusion, the selection of a Compression Testing Machine demands meticulous consideration of pivotal features. A robust frame, precise load measurement systems, advanced software, comprehensive safety measures, and versatile testing capabilities constitute the quintessence of an efficient and reliable CTM. Prioritizing these aspects ensures optimal performance, accuracy, and safety in material strength assessments.
#Compression Testing Machine#Compression Testing Machine in Delhi#Best Compression Testing Machine#Compression Testing Machine in india
0 notes
Text
CTM Machine Manufacturers at Best Cost
Peak Technology is pioneer for CTM Machine Manufacturers and this is designed by using top quality materials and the latest techniques. Our offered CTM Machine is mostly used to test the compressive strength of concrete and different other building materials. This is highly demanded in the market due to its best features like precisely engineered, quality assured, high tensile strength, etc.

Click Here: - https://peaktest.in
0 notes
Text
Cosmetics Labeling Systems

Cosmetic labeling systems are essential for the accurate display of ingredients on a product. For example, if the cosmetics product is a shaving cream, it's important to list the ingredients in a legible manner. If the container is ornamental, the information might not be visible. Fortunately, there are a number of options for cosmetics labeling systems. Here's a quick overview of the various types of labels and their requirements:
Most cosmetics labeling systems will feature a machine that can quickly convert to accommodate new product dimensions. Many of these machines will also be easy to operate and simplify the task of the operating personnel. These machines have been designed for maximum speed and versatility. They can accommodate various types of products, including tubes, containers, and bottles. They can be configured to incorporate other features such as ejection devices, printers, and cameras. And because they are modular, they're easy to maintain and customize. Read more great facts on CTM Labeling Systems, click here.
A flexible cosmetic labeling system can be easily modified to suit the needs of the manufacturer. The machine's features and functionality can quickly adapt to new product dimensions, making them ideal for changing product shapes. The machine's timesaving features ensure that the labeling process is fast and error-free. Because cosmetics products are always marketed to attract the attention of the consumer, the cosmetics labels play a key role in marketing. A flexible cosmetic labeling system can reach different target groups and react quickly to changing trends. For more useful reference regarding CTM, have a peek here.
Another option is to purchase an automatic cosmetics labeling system. These machines are highly flexible and can adapt to new product dimensions. They also feature an advanced modular design that makes them easy to adjust and reconfigure. In addition to labeling systems, the automatic cosmetics packaging line can speed up the entire packaging line. They can also be customized to include cameras, ejection devices, and printers. With this flexible approach, VKPAK labeling systems are flexible enough to suit your needs.
Cosmetic labeling systems are a crucial component of the packaging process. The labeling process requires the correct information for the product to be safe for the consumer. The FDA regulates the cosmetics labeling system and sets the standards for the products. In addition to these, the FDA also has a website with lists of necessary warning text and customary ingredients. A labeling system will ensure that the cosmetics packaging is compliant with the laws of its country.
Cosmetic labeling systems are required by law to provide information about the ingredients and their application. The labeling system should be able to display all the relevant information. The food and drug administration, which is in charge of cosmetics labeling, has created standards for the labels. However, this does not mean that the FDA's regulations are irrelevant if the right system is in place. If your packaging line is not speedy, the labeling system must be able to accommodate the changes in the market. Please view this site https://www.wikihow.com/Start-Your-Own-Cosmetics-Line for further details.
0 notes
Text
Labeling System Basics

Labeling systems are generally used to apply standard pre-printed labels over items, packages, cases, and Pallet loads for better product identification and better traceability. The CTM Labeling Systems employ laser-based label printers that produce crisp, legible labels and are ideal for use over a wide variety of materials. They are extensively used in the retail and distribution sectors for custom label applications. Moreover, they can be effectively applied on items like books, folders, CDs, DVDs, CDs/DVDs, VHS tapes, and even clothing to customize them for better product recognition. Also, these labels are ideal for addressing different issues related to product identification like shelf life, return, and security.
In addition, labeling systems make use of computer-based software to add, update, or delete information about the inventory. They can be used for product inventory control, product recall, inventory control, and for periodic product analysis. In fact, they can also be utilized to print barcodes on products for better customer service. The flexibility of these systems is widely seen in the form of user-friendly software that allows users to create, update and manage inventory using simple forms. Users can also add, delete, and edit human-readable information using barcode software.
Among the popular labeling systems used today are desktop label dispensers, handheld label dispensers, stand-alone labeling machines, automatic dispenser machines, and wireless label dispensers. All these dispensers have the following basic features: user-friendly features such as labels can be easily wiped off; multiple labeling trays can accommodate different types of products; labels can be printed in single or multiples; and an integrated design that enables convenient and fast operation. Furthermore, a wide range of labeling equipment is available. These include automatic label applicators, label printers, lamination machines, garment rollers, and many more. This makes it possible for companies to have complete control over their labeling process.
Label applicators are among the most important dispenser-type labeling system used today. This is because they provide for a quick, easy way of applying labels on containers. Applying labels in this way does not only help improve the quality of the products and make them easily recognizable by consumers but also improves storage container security. Automatic applicators for round containers can come with a variety of features including built-in screw threads, removable parts that can be secured to the bottom part of the container, and a threaded peg attached to the side. see here to get a detailed overview of this topic: https://en.wikipedia.org/wiki/Packaging_and_labeling.
Some other popular labeling systems include hazard communication systems and bio hazard communication systems. Hazard communication involves automatic triggering of safety devices when exposed to specific hazardous materials. These safety devices can be used in workplaces, including hospitals and manufacturing facilities, to warn employees of hazardous conditions or materials before an accident takes place.
Meanwhile, bio hazard communication involves using special labels that are affixed to the inside surface of round containers. These labels warn of the presence of certain substances or materials that are harmful to the user. When the product label for a round container is used in this manner, it is easy to identify the correct product. The CTM labeling systems can improve chemical identification. They also help in reducing injuries as a result of misidentification of chemicals and other dangerous substances.
0 notes
Text
Best Insulation Compound for Fiber Glass Coating by New Manthan Industries

Introduction:
New Manthan Industries is a pioneer when it involves trading in chemicals and their compounds. As a result, it has earned its reputation of being the topmost manufacturing, supplying, producing, and delivering industrial chemicals and construction chemicals. Our wide array of products includes concrete admixture, surface treatment, grouts, industrial flooring products, structure repair products, adhesives, and bonding agents designed to bond or fix construction material. We even focus on allied products. We are accredited as ISO 9001:2015 CERTIFIED company.
Our motto has been to deliver and produce a non-compromised quality from the time we exist in this field. We have got a team of experienced supervisors who are involved in quality checks constantly. We conduct few tests on various testing parameters to ensure the performance and quality consistency of the required. We never compromise on our tests and products, which is why we are the best in business.
Our products are safely packaged to make sure that these products are free from damage while transferring. We've got a listing of prestigious clients like government, semi-government, private institutions, builders and contractors, etc. we offer the top-selling insulation compounds for Fiberglass coating. One such product is Coatman Thermocoat 155 HT.
Fiberglass and Coatman thermocoat 155 HT
Fiberglass
Insulation materials come from different sources like minerals, vegetable fibers, animal products, and artificial compounds. Like in many engineering decisions, each material has advantages and drawbacks that must be considered when selecting insulation for anything.
This article outlines the fiberglass options within the market and the way they perform in actual projects. Fiberglass is an insulation material that's one among the modern inventions but could also be found in older constructions - one example is insulation with an asbestos content, which has been outlawed.
(side note: ensure your building has the proper insulation and reduces your energy costs.)
Fiberglass is one of the foremost popular insulation materials, made by weaving fine strands of glass. It's manufactured mostly from recycled glass.
The characteristics are the self-explanatory reasons why fiberglass is preferred. Characteristics like Minimal heat transfer, Non-flammability, the R-values range from R-2.9 to R-3.8 per inch, is Low cost yet Environmentally friendly. It doesn't absorb water.
IT are often dangerous for installers; they require special protection equipment. the tiny particles of glass can damage the eyes, lungs, and skin if one has not been careful. Loose-fill insulation is applied using an insulation blowing machine. It's available in Blankets (batts and rolls): fiberglass batt are often found as medium or high-density, with higher R-values than standard batts, it's loose-fill and blown-in, Blow-in Blanket System (BIBS): a variation of loose-fill insulation that's blown dry, and tests have proven a better insulation level than other sorts of fiberglass. It are often utilized in rigid boards, Duct insulation, Rigid fibrous insulation, etc.
Coatman Thermocoat 155 HT
Coatman Thermocoat 155 HT is a compound that can be applied for things like Electrical-Wire Sleeving Insulation Compound, fabric coating and is also suitable for food contact. The benefits of using Coatman Thermocoat 155 HT is that it has Good Clarity, is Easily Pigmentable and is Formulated to meet BfR, XV and FDA21 CFR 177.2600 and is capable of Unprimed adhesion to glass fabric.
It can also be used as an Electrical-Wire Sleeving Insulation Compound. The components are supplied strained and might be used as plenty of matched kits. Also, keep in mind that the mixture must be thoroughly de-gassed under a vacuum. It is often used for Mixing and de-airing, Pot Life, Cleaning, Curing, Pigmentation.
The product is this product is supplied in 20 Ltr. & 200 ltr. and also, the usable Life And Storage is as follows:
When stored at or below 32°C (90°F) within the original unopened containers, this product features a usable lifespan of 15 months from the date of production.
The product is tested as per ASTM (American Society for Testing end Materials). They're tested consistent with Dam Corporate Test Methods (CTM); in most cases, they are almost like the ASTM standard listed above. During the tests, Properties obtained on 2 mm, thick slabs were Press cured for 10 minutes at 120.0 (24B.F), and Viscosity measured on the Brookfield HAF Viscometer, spindle 7, 10 rpm C
Conclusion:
During winter and everyone, summer and heat loss are often taken care of by Coatman Thermocoat 155 HT.
When effective insulation like Coatman Thermocoat 155 HT is combined with a high-efficiency HVAC design, your building achieves a drastic reduction of heating and cooling costs. Applying insulation is cheaper and simpler in new buildings since there's no got to disturb an existing construction. Developers who are planning a replacement project should keep this in mind.
0 notes