#Clinical Chemistry Reagents Kits
Text

Celebrating 10 Years' Journey of Athenese-Dx Pvt. Ltd.
celebration #10years #athenesedx #Chennai #IVD #India #milestone
🎉 Celebrate a Decade of Innovation with Athenese-Dx! Join us in our 10th Anniversary Celebration as we continue to honor a decade of groundbreaking achievements at Athenese-Dx. This special video takes you on a journey through our most memorable moments, showcasing our commitment to excellence and innovation.
Highlights:
Milestone Moments: Relive our top achievements and how they've shaped the industry. From pioneering diagnostic technologies to life-changing breakthroughs, we've been at the forefront of medical advancements.Behind-the-Scenes: Get an exclusive look at the people and passion driving our success. Meet our brilliant scientists, dedicated researchers, and tireless support staff who have fueled our journey.Future Forward: Peek into the future as we unveil exciting plans for the next decade. Discover our vision for improving patient care, expanding global reach, and revolutionizing healthcare.
Thank You to Our Community:
We extend our heartfelt gratitude to our customers, partners, and employees who have been part of our story. Your unwavering support and trust have propelled us forward.
Stay Connected:
Don't miss out on any updates as we embark on the next chapter of our journey
diagnosticcenter #rapidtest #elisakit #biochemistry #ivdmanufacturing #clinicalchemistry #ivdinstrument #medicaldevices #reagents
Together, let's continue to redefine diagnostics and transform lives. Here's to the next ten years of excellence! 🥂
🎉 Cheers to many more amazing years! 🎉
ATHENESE-DX PRIVATE LIMITED
Address: Module No. 407 & 408, 4th Floor, TICEL Bio Park II, No. 5, CSIR Road, Taramani, Chennai, Tamil Nadu - 600113, India
For Inquires - WhatsApp +917397238924
Click here to chat: https://wa.me/917397238924
For Reviews & News, go to https://www.athenesedx.com/
Follow us on WhatsApp Channel: https://whatsapp.com/channel/0029Va8M7HQChq6HfUBMLj0X
🌎 Visit our office - https://qr.page/g/5lZXERbP8Av
🤝 Subscribe - https://youtube.com/@athenesedx Our early diagnosis products for a better life. Thank you for your support.
🔔 For more videos, products, reviews ↷
YouTube ▶ https://youtube.com/@athenesedx
🌐 For more information ↷
Website ▶ https://athenesedx.com
To Buy Athenese-Dx Products ▶ https://store.athenesedx.com
📱 Follow us on social media ↷
Facebook ▶ https://www.facebook.com/Athenese.Dx.Pvt.Ltd/
Instagram ▶ https://www.instagram.com/athenese_dx/
Pinterest ▶ https://www.pinterest.com/athenesedx/
X (Twitter) ▶ https://twitter.com/athenesedx
Tumblr ▶ https://www.tumblr.com/athenese-dx
LinkedIn ▶ https://www.linkedin.com/company/athenese-dx-private-limited/
Medium ▶ https://medium.com/@athenesedx01
#clinical chemistry#digital pathology market#pathologist#hospital#pathology#pathologylab#ivd#in vitro diagnostics market#in vitro diagnostics industry#reagent#biochemistry#rapid tests#elisa kits#lab equipment#lab supplies#celebration#10 years ago#10 year anniversary#athenesedx#india#milestone celebration
0 notes
Text
In Vitro Diagnostics Market: Revolutionizing Healthcare with Advanced Diagnostics
The In Vitro Diagnostics (IVD) market plays a pivotal role in the healthcare industry, offering essential tools for disease diagnosis, treatment monitoring, and overall patient care. With advancements in diagnostic technology and the rising demand for personalized medicine, the IVD market is experiencing rapid growth. This article provides a detailed overview of the market trends, segmentation, key drivers, and leading companies in the IVD industry, offering valuable insights for decision-makers.
Market Overview
According to SkyQuest's In Vitro Diagnostics Market report, the global market is currently valued at USD 87.93 Billion in 2023, with a projected CAGR of 5.3% over the forecast period. The market is driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and the growing demand for early and precise disease detection.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/in-vitro-diagnostics-market
Market Segmentation
By Product Type:
Reagents and Kits: Essential components used in diagnostic procedures across a range of diseases.
Instruments: Include advanced diagnostic tools like analyzers, molecular diagnostic machines, and point-of-care devices.
Software & Services: Diagnostic software for accurate test results and integrated solutions for laboratories.
By Technology:
Immunoassays: Widely used for infectious diseases and cancer diagnosis.
Molecular Diagnostics: Key in genetic testing and precision medicine applications.
Clinical Chemistry: Essential for routine testing and biomarker identification.
Microbiology: Used to identify pathogens and guide antibiotic therapies.
Hematology: Focuses on blood-related diagnostics such as complete blood count (CBC).
Others: Encompasses emerging technologies like proteomics and metabolomics.
By Application:
Infectious Diseases: Dominating the market due to the global rise in bacterial, viral, and fungal infections.
Oncology: Growing demand for early cancer diagnostics and targeted treatments.
Cardiology: Vital in diagnosing cardiovascular diseases and risk factors.
Diabetes: Includes blood glucose monitoring and HbA1c testing for diabetes management.
Other Applications: Includes diagnostics for autoimmune diseases, nephrology, and neurology.
By End-User:
Hospitals and Clinics: Major centers for diagnostic testing and patient care.
Diagnostic Laboratories: Specializing in high-volume testing across various disease areas.
Homecare Settings: Growing segment due to increasing demand for at-home diagnostic kits.
Academic and Research Institutes: Driving innovation in diagnostic tools and techniques.
Key Growth Drivers
Rising Prevalence of Chronic Diseases: Increasing cases of cancer, diabetes, and cardiovascular diseases fuel the demand for diagnostic tools.
Advancements in Technology: Development of rapid, accurate, and minimally invasive diagnostic methods is boosting the market.
Growing Demand for Personalized Medicine: The focus on tailored treatments and early diagnosis is driving the adoption of molecular diagnostics.
Expansion of Point-of-Care Testing (POCT): The shift towards decentralized testing is increasing the demand for portable and easy-to-use diagnostic devices.
Read More at: - https://www.skyquestt.com/report/in-vitro-diagnostics-market
Leading Companies in the Market
SkyQuest’s In Vitro Diagnostics Market report identifies key players that are shaping the market, including:
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
Danaher Corporation
Thermo Fisher Scientific
bioMérieux SA
Becton, Dickinson and Company
QIAGEN
Sysmex Corporation
Agilent Technologies
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/in-vitro-diagnostics-market
Challenges and Opportunities
The IVD market faces challenges such as stringent regulatory frameworks and high costs of diagnostic devices, especially in emerging markets. However, opportunities lie in the growing adoption of telemedicine, increased government funding for healthcare infrastructure, and technological advancements that enable faster and more precise diagnostic results.
Future Outlook
The In Vitro Diagnostics Market is expected to experience robust growth as healthcare providers increasingly rely on advanced diagnostics for effective patient care. Companies that invest in innovation and cater to the rising demand for point-of-care and home-based testing will have a competitive edge in this dynamic market.
As diagnostics play a critical role in healthcare, the In Vitro Diagnostics Market is poised for significant growth. Decision-makers should stay informed about emerging trends and technological advancements to leverage the full potential of this market. For more detailed insights and strategies, consult SkyQuest’s comprehensive In Vitro Diagnostics Market report.
0 notes
Text
The global demand for In-Vitro Diagnostics (IVD) was valued at USD 117545.8 Million in 2023 and is expected to reach USD 182352.1 Million in 2032, growing at a CAGR of 5.00% between 2024 and 2032.In-Vitro Diagnostics (IVD) is a critical segment of the healthcare industry, playing a vital role in the detection, diagnosis, and monitoring of diseases. These tests are conducted on samples such as blood, urine, or tissues, and they provide crucial information for making clinical decisions. The IVD market has witnessed substantial growth in recent years, driven by technological advancements, an aging population, and a rising prevalence of chronic diseases. This article delves into the current state of the IVD market, key trends, opportunities, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/in-vitro-diagnostics-ivd-market
Current Market Landscape
The global IVD market is experiencing robust growth, with projections estimating its value to reach USD 113.1 billion by 2025, growing at a CAGR of 4.7% from 2020 to 2025. North America holds the largest market share, followed by Europe and Asia-Pacific. The high prevalence of chronic and infectious diseases, advanced healthcare infrastructure, and strong R&D capabilities are key factors driving market growth in these regions.
Key Drivers of Growth
1. Technological Advancements: Innovations in molecular diagnostics, next-generation sequencing (NGS), and point-of-care testing (POCT) have revolutionized the IVD market. These technologies offer faster, more accurate, and less invasive diagnostic solutions, enhancing patient outcomes and streamlining clinical workflows.
2. Aging Population: The global population is aging, leading to an increase in age-related diseases such as cardiovascular diseases, diabetes, and cancer. The elderly population is more susceptible to these conditions, necessitating frequent diagnostic tests, thereby driving the demand for IVD products.
3. Rising Prevalence of Chronic Diseases: The increasing incidence of chronic diseases, including diabetes, cancer, and cardiovascular diseases, has significantly boosted the demand for IVD. Early and accurate diagnosis is crucial for effective disease management, making IVD an essential tool in healthcare.
4. Pandemic Impact: The COVID-19 pandemic has underscored the importance of diagnostic testing. The rapid development and deployment of COVID-19 tests have accelerated the growth of the IVD market, highlighting the need for robust diagnostic infrastructure to manage public health crises.
Key Market Segments
The IVD market is segmented based on product type, technology, application, and end-user.
1. Product Type: Reagents and kits dominate the market, followed by instruments and software. The continuous demand for reagents and kits for routine diagnostics and research purposes fuels this segment's growth.
2. Technology: Immunoassays, molecular diagnostics, clinical chemistry, microbiology, and hematology are the leading technologies in the IVD market. Molecular diagnostics, particularly PCR and NGS, are witnessing rapid growth due to their accuracy and speed.
3. Application: The market is categorized into infectious diseases, oncology, cardiology, endocrinology, and others. Infectious diseases hold the largest share, driven by the need for rapid and accurate diagnostic tests for diseases such as COVID-19, HIV, and tuberculosis.
4. End-User: Laboratories, hospitals, academic institutions, and point-of-care settings are the primary end-users of IVD products. Laboratories account for the largest share, attributed to the high volume of diagnostic tests conducted in these settings.
Opportunities and Challenges
The IVD market presents numerous opportunities, including the growing adoption of personalized medicine, the expansion of healthcare infrastructure in emerging economies, and the increasing focus on preventive healthcare. Personalized medicine, which tailors treatment to individual patients based on their genetic makeup, relies heavily on advanced diagnostic tests, offering significant growth potential for the IVD market.
However, the market also faces challenges. Regulatory hurdles, reimbursement issues, and the high cost of advanced diagnostic tests can hinder market growth. Additionally, the need for highly skilled professionals to operate sophisticated diagnostic equipment and interpret results poses a challenge in many regions.
Future Prospects
The future of the IVD market looks promising, with ongoing advancements in technology and a greater emphasis on early disease detection and prevention. Artificial intelligence (AI) and machine learning (ML) are set to play a pivotal role in diagnostics, enabling more accurate and efficient analysis of test results. The integration of digital health technologies and the increasing use of telemedicine are also expected to drive the demand for IVD products.
Key Players
Invitae Corporation
Freenome Holdings, Inc.
Natera, Inc.
F. Hoffmann-La Roche Ltd.
InterVenn Biosciences
Guardant Health
Agilent Technologies, Inc.
Exact Sciences Corporation
Illumina, Inc.
NeoGenomics Laboratories
Thermo Fisher Scientific, Inc.
Segmentation
By Product Type:
Reagents and Kits
Instruments
Data Management Software
By Technology:
Clinical Chemistry
Immunoassay/Immunochemistry
Molecular Diagnostics
Hematology
Coagulation and Hemostasis
Microbiology
Point-of-Care Testing (POCT)
Others
By Application:
Infectious Disease Testing
Oncology/Cancer Testing
Diabetes Testing
Cardiology Testing
Nephrology Testing
Autoimmune Disease Testing
Drug Testing/Pharmacogenomics
Others
By End-User:
Hospitals and Clinics
Diagnostic Laboratories
Academic and Research Institutes
Home Care
Others
By Usability:
Disposable IVD Devices
Reusable IVD Devices
By Type of Sample:
Blood Testing
Urine Testing
Cerebrospinal Fluid (CSF) Testing
Saliva Testing
Others
By Automation:
Fully Automated IVD Systems
Semi-Automated IVD Systems
By Point-of-Care Testing (POCT):
Glucose Monitoring
Pregnancy and Fertility Testing
Infectious Disease POCT
Cardiac Markers POCT
Others
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/in-vitro-diagnostics-ivd-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
In-Vitro Diagnostics Market Future Prediction Report By 2030
The Insight Partners recently announced the release of the market research titled In-Vitro Diagnostic Market Outlook to 2030 | Share, Size, and Growth. The report is a stop solution for companies operating in the In-Vitro Diagnostic market. The report involves details on key segments, market players, precise market revenue statistics, and a roadmap that assists companies in advancing their offerings and preparing for the upcoming decade. Listing out the opportunities in the market, this report intends to prepare businesses for the market dynamics in an estimated period.
Is Investing in the Market Research Worth It?
Some businesses are just lucky to manage their performance without opting for market research, but these incidences are rare. Having information on longer sample sizes helps companies to eliminate bias and assumptions. As a result, entrepreneurs can make better decisions from the outset. In-Vitro Diagnostic Market report allows business to reduce their risks by offering a closer picture of consumer behavior, competition landscape, leading tactics, and risk management.
A trusted market researcher can guide you to not only avoid pitfalls but also help you devise production, marketing, and distribution tactics. With the right research methodologies, The Insight Partners is helping brands unlock revenue opportunities in the In-Vitro Diagnostic market.
If your business falls under any of these categories – Manufacturer, Supplier, Retailer, or Distributor, this syndicated In-Vitro Diagnostic market research has all that you need.
What are Key Offerings Under this In-Vitro Diagnostic Market Research?
Global In-Vitro Diagnostic market summary, current and future In-Vitro Diagnostic market size
Market Competition in Terms of Key Market Players, their Revenue, and their Share
Economic Impact on the Industry
Production, Revenue (value), Price Trend
Cost Investigation and Consumer Insights
Industrial Chain, Raw Material Sourcing Strategy, and Downstream Buyers
Production, Revenue (Value) by Geographical Segmentation
Marketing Strategy Comprehension, Distributors and Traders
Global In-Vitro Diagnostic Market Forecast
Study on Market Research Factors
Who are the Major Market Players in the In-Vitro Diagnostic Market?
In-Vitro Diagnostic market is all set to accommodate more companies and is foreseen to intensify market competition in coming years. Companies focus on consistent new launches and regional expansion can be outlined as dominant tactics. In-Vitro Diagnostic market giants have widespread reach which has favored them with a wide consumer base and subsequently increased their In-Vitro Diagnostic market share.
Report Attributes
Details
Segmental Coverage
Product & Service
Reagents & Kits
Instruments
Software & Services
Technology
Immunoassay/ Immunochemistry
Clinical Chemistry
Molecular Diagnostics
Microbiology
Blood Glucose Self-Monitoring
Coagulation Hemostasis
Hematology
Urinalysis
Others
Application
Infectious Diseases
Diabetes
Oncology
Cardiology
Autoimmune Diseases
Nephrology
Others
End User
North America
Europe
Asia Pacific
the Middle East & Africa
South & Central America
and Geography
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
Abbott Laboratories?
F. Hoffmann-La Roche Ltd?
Danaher Corp?
Siemens AG?
Sysmex Corp?
Thermo Fisher Scientific Inc
Becton Dickinson and Co
bioMerieux SA?
Bio-Rad Laboratories Inc
Qiagen NV
Other key companies
What are Perks for Buyers?
The research will guide you in decisions and technology trends to adopt in the projected period.
Take effective In-Vitro Diagnostic market growth decisions and stay ahead of competitors
Improve product/services and marketing strategies.
Unlock suitable market entry tactics and ways to sustain in the market
Knowing market players can help you in planning future mergers and acquisitions
Visual representation of data by our team makes it easier to interpret and present the data further to investors, and your other stakeholders.
Do We Offer Customized Insights? Yes, We Do!
The The Insight Partners offer customized insights based on the client’s requirements. The following are some customizations our clients frequently ask for:
The In-Vitro Diagnostic market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
Author’s Bio:
Sunil Jadhav
Senior Market Research Expert at The Insight Partners
0 notes
Text
Nucleic Acid Sample Preparation Market Emerging Economies Expected to Influence Growth until (2023-2033)

In the intricate tapestry of life, nucleic acids—DNA and RNA—serve as the fundamental building blocks of genetic information. Nucleic acid sample preparation, therefore, forms the crucial initial step in a myriad of scientific endeavors, from unraveling the mysteries of the genome to diagnosing genetic diseases.
The global nucleic acid sample preparation market is projected to reach $5,615.9 million by 2033 from $2,922.8 million in 2023, growing at a CAGR of 6.75% during the forecast period 2023-2033.
Market Overview:
Nucleic acid sample preparation involves a series of steps aimed at extracting, isolating, and purifying DNA or RNA molecules from various biological sources such as cells, tissues, blood, or environmental samples. This process lays the foundation for downstream applications including sequencing, PCR (Polymerase Chain Reaction), cloning, and gene expression analysis.
Mainly includes various kits, reagents, instruments, and consumables used in laboratories around the world. With applications spanning genomics, diagnostics, drug discovery, and beyond, the market for nucleic acid sample preparation is both diverse and dynamic.
Grab a look at the free sample page for more understanding click here !
DNA/RNA sample preparation market
The DNA/RNA sample preparation market has witnessed robust growth, fueled by the increasing demand for personalized medicine, advancements in molecular diagnostics, and the rising prevalence of infectious diseases and genetic disorders.
DNA/RNA sample preparation is utilized across various applications, including research, clinical diagnostics, forensics, and agriculture
Key drivers of market growth include the growing focus on precision medicine, increasing investments in genomics research, rising awareness about genetic diseases, and technological innovations enhancing sample preparation workflows.
The DNA/RNA sample preparation market continues to evolve rapidly, driven by technological advancements, expanding applications, and increasing demand for genetic analysis across various sectors. As the market progresses, addressing challenges related to cost, automation, and standardization will be essential for sustained growth.
Key Market Players
Agilent Technologies, Inc.
Autogen, Inc.
Bio-Rad Laboratories, Inc.
Roche AG
Merck KGaA
and many others
The Nucleic Acid Sample Preparation market has made an impact in the following ways:
Emergence of Advanced Stabilization Products: The rapid growth of genomic research has resulted in an increasing need for the storage of biologic samples, including DNA and RNA. Stabilization products enable researchers to transport and store biological samples for long periods with complete and rapid sample recovery at affordable costs. These products are used to retain biological materials at room temperature without degradation.
Regulated vs. Multimodal Analysis: In order to cross-examine biological samples for changes in DNA and RNA efficiently, there have been considerable advances in NGS chemistries, platforms, and bioinformatics pipelines. Currently, the regulated approach requires the use of two separate workflows for library preparation from separate DNA and RNA isolates.
Visit our vertical page click here !
Kits Segment to Continue Dominating the Nucleic Acid Sample Preparation Consumables Market by Type Based on consumables market by type, the nucleic acid sample preparation market has been led by Kits, which held a 87.65% share in 2022.
Nucleic Acid Sample Preparation Market Segmentation:
Segmentation 1: by Product
Segmentation 2: Consumables Market by Type
Segmentation 3: Kits by Type
DNA/RNA Sample Isolation/Extraction/Purification Segment to Continue Dominating the Nucleic Acid Sample Preparation Consumables Market Kits by Type
Based on nucleic acid sample preparation consumables market Kits by Type, the nucleic acid sample preparation market has been led by DNA/RNA Sample Isolation/Extraction/Purification, which held an 83.93% share in 2022.
Segmentation 4: Consumables Market by Analyte
Segmentation 5: by Technology
Silica-Based Segment to Continue Dominating the Nucleic Acid Sample Preparation Consumable Market (by Consumable-Based Technology)
Based on consumable-based technology, the nucleic acid sample preparation consumable market has been led by silica-based technology, which held a 48.58% share in 2022.
Segmentation 6: by Application
Segmentation 7: by End User
Segmentation 8: by Region
China dominated the Asia-Pacific nucleic acid sample preparation market in 2022. China has maintained itself as an attractive market for life sciences solution providers capable of tapping the strong demand for nucleic acid extraction products.
Recent Developments in the Nucleic Acid Sample Preparation Market
Qiagen N.V. introduced two groundbreaking additions to its sample technologies portfolio, i.e., the TissueLyser III that facilitates high-throughput disruption of diverse biological samples and the RNeasy PowerMax Soil Pro Kit that isolates high-purity RNA from challenging soil samples using advanced Inhibitor Removal Technology.
Qiagen announced the launch of the QIAseq Normalizer Kits that give researchers a fast, convenient, and cost-effective method to pool different DNA libraries for best-quality results from next-generation sequencing (NGS) runs.
PerkinElmer introduced the CHEF Magnetic Bead Cleanup System, providing automated nucleic acid purification through advanced magnetic bead technology. This novel system would help automate the nucleic acid purification process efficiently.
PerkinElmer (Revvity, Inc.) rebranded its diagnostics and life sciences business as Revvity. This strategic move marked a new identity and focus for the company's ventures in these sectors.
Conclusion
In essence, nucleic acid sample preparation serves as the cornerstone of molecular biology and genetics research. By ensuring the integrity and purity of genetic material, scientists can unravel the complexities of the genome, diagnose genetic diseases, and unlock the secrets of life itself.
0 notes
Text
DNA Extraction Kits Market is Estimated to Witness High Growth Owing to Increasing Demand for Nucleic Acid-Based Molecular Tests

DNA extraction kits are essential tools used to isolate and purify DNA from a variety of biological samples in life science research and clinical diagnostics. DNA extraction kits can purify DNA from peripheral blood, tissue, cells, plasma, serum, saliva, stool, and other biological samples. Purified DNA is then utilized in various downstream applications including PCR, DNA sequencing, molecular testing, and synthetic biology. Common uses of DNA extraction kits in clinical applications include cancer diagnostics, forensic testing, prenatal testing, infectious disease testing, genetic testing, and personalized medicine.
The global DNA extraction kits market is estimated to be valued at US$1.2 Bn in 2023 and is expected to exhibit a CAGR of 5.9% over the forecast period 2023-2031, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics
Increasing demand for nucleic acid-based molecular diagnostic tests is expected to drive growth of the DNA extraction kits market size over the forecast period. Molecular diagnostic testing is gaining immense popularity over traditional diagnostics due to its accuracy, high sensitivity, and specificity. Growing prevalence of cancer and infectious diseases is propelling demand for rapid and accurate molecular testing which require purified DNA obtained from extraction kits. According to WHO, cancer burden is expected to grow to 27.5 million new cancer cases and 16.3 million cancer deaths by 2040. Technological advancements in extraction methods allowing purification of DNA even from complex biological samples is another factor fueling the DNA extraction kits market growth. Automated liquid handling workstations integrated with magnetic bead-based DNA extraction techniques have simplified workflow and increased throughput of purification process.
SWOT Analysis
Strength: DNA extraction kits provide an easy and efficient way to isolate and purify DNA from various biological samples like blood, saliva, and tissues. These kits use optimized procedures and reagents to reliably extract high yields of pure DNA. They minimize hands-on time and produce consistent results. Some kits are automated and high-throughput, allowing labs to extract DNA from large numbers of samples simultaneously in an easy and standardized manner.
Weakness: DNA extraction kits rely on consumables like columns, plates and reagents that need regular replenishment, increasing long-term costs. Additionally, automation requires larger initial investments. The quality of extracted DNA can vary between kits depending on their purification chemistry and protocols.
Opportunity: Growth in genomic researchareas like precision medicine, cancer research and genetic testing is driving increased demand for DNA extraction. Rising focus on pharmacogenomics and companion diagnostics also presents commercialization opportunities. Point-of-care extraction devices and portable battery-operated systems allow decentralized testing and expand the addressable market.
Threats: Competition from in-house extraction methods developed by research institutes poses a challenge. Stringent regulatory approvals for diagnostic-grade kits increase barriers. Intellectual property disputes between manufacturers also affect commercialization strategies.
Key Takeaways
The global DNA extraction kits market is expected to witness high growth over the forecast period driven by increasing genomics funding and expanding application areas.
The market is dominated by North America currently due to extensive R&D spending and presence of leading life science companies. Asia Pacific is poised to be the fastest growing regional market due to growing genomics research in countries like China and India as well as increasing healthcare investments.
Key players operating in the DNA extraction kits market are Qiagen N.V., Thermo Fisher Scientific, Hoffmann-La Roche AG, Merck KGaA, Agilent Technologies, Bio-Rad Laboratories Inc., Illumina, Inc., Promega Corporation, LGC Ltd., and Takara Bio, Inc. Companies are focused on portfolio expansion through new product launches. For example, in 2021 Thermo Fisher launched the MagMAX Cellular RNA Isolation Kit designed for high-throughput extraction of total RNA from cultured cells.
Get more insights on this topic: https://www.newswirestats.com/dna-extraction-kits-market-size-and-outlook/ Explore more information, Please visit:https://masstamilan.in/savoring-life-the-essential-role-and-health-benefits-of-spices-in-everyday-cuisine/
#DNA Extraction Kits#DNA Extraction Kits Market#DNA Extraction Kits Market size#DNA Extraction Kits Market share#Coherent Market Insights
0 notes
Text
Unleash the Power of Precision and Efficiency with Trivitron Healthcare's Chemistry Analyzer Solutions

Revolutionize your clinical diagnostics with Trivitron Healthcare's cutting-edge Chemistry Analyzers, offering a comprehensive range of solutions tailored to your laboratory's needs. From Clinical Chemistry Reagents Kits to Elite, Labmate, Labmate NXT, Labmate PLUS, Nanolab 200, and Nanolab 500, Trivitron's analyzers deliver unparalleled accuracy, efficiency, and ease of use.
0 notes
Text
In Vitro Diagnostics Market Competitive Landscape: 2030
In Vitro Diagnostics Market By Product And Services (Reagents, Instruments, Software And Services), by Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, Clinical Chemistry, Others), by Application (Infectious Diseases, Cancer, Cardiac Diseases, Immune System Disorders, Nephrological Diseases, Gastrointestinal Diseases, Others), by End User (Standalone Laboratories, Hospitals, Academic And Medical Schools, Point Of Care, Others) and region (North America, Europe, Asia-Pacific, Middle East and Africa and South America).
Market Overview
The global In-Vitro Diagnostics market size was estimated at USD 120.7 billion in 2023 and is projected to reach USD 161.2 billion in 2030 at a CAGR of 4.2% during the forecast period 2023-2030.
In May 2021, the esteemed University of California accomplished a remarkable feat in the field of medical science by developing a highly sensitive molecular test. This particular test capitalizes on cutting-edge chip technology and is capable of detecting the presence of both influenza A and SARS-CoV-2 antigens.

It is currently undergoing further scrutiny and investigation for potential conversion into a Point-of-Care (POC) test. The industry dynamics have witnessed a significant shift, with an ever-growing number of players directing their focus towards the development and launch of tests for at-home use. Furthermore, in the year 2021, the Food and Drug Administration (FDA) has demonstrated an increased proclivity towards prioritizing home-based molecular diagnostic tests. Specifically in the month of March, the renowned BATM Advanced Communications Ltd. announced the launch of its revolutionary molecular diagnostics self-test kit, designed for the sole purpose of detecting COVID-19.
Request for the Sample Copy: https://www.delvens.com/get-free-sample/in-vitro-diagnostics-market-trends-forecast-till-2030
The ascension of the elderly demographic and the expansion of acumen concerning initial screening has brought about a notable escalation in the quantity of routine medical examinations, given that a majority of fatalities as a result of infections and persistent pathologies come to pass in the populace exceeding 75 years of age. Based on the Office for Budget Responsibility in the United Kingdom, healthcare expenses have undergone a sharp upturn, which could generate economic strain on nations that are aging rapidly. Nonetheless, it is anticipated that this disbursement will yield a favorable outcome for the in vitro diagnostics industry, propelling the advancement of the market.
The unprecedented and devastating COVID-19 pandemic has brought about a significant and much-needed emphasis on in vitro diagnostics (IVD) in light of the rapidly growing and exigent necessity for IVD kits and reagents geared towards the prompt and precise diagnosis of SARS-CoV2 virus infection among the world's populace. The outbreak of this highly contagious virus has had a salutary effect on the market, as in vitro diagnostics encompass a diverse range of tests on a multitude of biological samples, thereby facilitating the diagnosis of an array of infectious diseases.
Delvens Industry Expert's Standpoint
The expansion of the in-vitro diagnostics (IVD) market is predominantly propelled by the escalation in the number of occurrences of enduring and communicable infirmities. In the current commercial milieu, there has been a surge in the incidence of persistent ailments such as tuberculosis (TB), cancer, cardiovascular disorders, and diabetes. Moreover, there has been a momentous upsurge in the number of patients afflicted with communicable maladies, such as gastrointestinal, respiratory, and sexually transmitted diseases (STDs). The upswing in the prevalence of these diseases is expected to augment the requirement for diagnostic devices, thereby boosting the IVD market. Furthermore, the technological progressions such as rapid test kits for the IVD sector have proliferated at an astounding pace in recent years and are foreseen to persist in the forthcoming times. The application of in-vitro diagnostics is extensive in various aspects of healthcare. Additionally, there has been a growth in the utilization of in vitro diagnostic testing across the globe.
Make an Inquiry Before Buying: https://www.delvens.com/Inquire-before-buying/in-vitro-diagnostics-market-trends-forecast-till-2030
Key Findings
On the basis of product, the segment pertaining to reagents was found to have secured the most substantial market share in the in vitro diagnostic market in the year 2020, and it is anticipated that it shall continue to maintain its preeminent position during the projected period, all due to the irrefutable fact that reagents in this field are an indispensable and integral component of every diagnostic test conducted in vitro.
On the basis of Technique, the immunodiagnostics sector, which specializes in the identification of antibodies or antigens within a biological sample, was found to have the largest proportion of market share during the year 2020. Furthermore, it is anticipated to maintain its dominant status throughout the forecasted period due to its exceptional sensitivity analysis, superior throughput, minimal cost, and inherent specificity. This is a result of its ability to precisely identify and quantify the presence of specific proteins or other biomolecules, which are integral in the analysis of biological samples.
On the basis of application, The segment of infectious diseases, which pertains to the study of diseases caused by pathogenic microorganisms such as bacteria, viruses, parasites, and fungi, held the largest market share in the field of in vitro diagnostics (IVD) in the year 2020. Furthermore, it is expected to maintain its dominance in the market during the forecast period. This can be attributed to the notable increase in the prevalence of infectious diseases such as tuberculosis, human immunodeficiency viruses (HIV), and sexually transmitted diseases (STDs) among the population.
The market is also divided into various regions such as North America, Europe, Asia-Pacific, South America, and Middle East and Africa. The North American region, is experiencing noteworthy expansion in the market for In Vitro Diagnostics (IVDs), with projections indicating that it will sustain its preeminent position for a few additional years. In the future, this particular region is anticipated to augment its market share, thereby solidifying its position as a dominant player in the IVDs market.
Regional Analysis
North America to Dominate the Market
The market dominance of the United States in the North American region can be attributed to the escalation of healthcare expenditure and the expeditious implementation of point-of-care testing. This notion is supported by the Centers for Disease Control and Prevention, which has provided data on chronic diseases.
According to the American Cancer Society, in January 2022, cancer continues to be the second most common cause of death in the United States
Get the Direct Order for the Research Report: https://www.delvens.com/checkout/in-vitro-diagnostics-market-trends-forecast-till-2030
Competitive Landscape
Abbott
bioMérieux SA
Quidel Corp.
Siemens Healthineers
Bio-Rad Laboratories, Inc.
Qiagen
Sysmex Corp.
Charles River Laboratories
Quest Diagnostics
Agilent Technologies, Inc.
Danaher Corporation
Becton Dickinson and Company
F. Hoffmann-La Roche Ltd.
Thermo Fisher Scientific
Illumina
Hologic
Devyser
PerkinElmer
Chembio Diagnostics
Surmodics. Inc
Menarini Silicon
Recent Developments
In November 2022, Thermo Fisher Scientific, launched the rapid RT-PCR Accula Flu A/Flu B Test Designed to enable healthcare providers to detect and differentiate influenza A and B in about 30 minutes.
In October 2022, Thermo Fisher Scientific entered a definitive areement to acquire the biding site group a global leader in specially diagnostics. The binding site provides specially diagnostics assays and instruments to improve the diagnosis and management of blood cancers and immune system disorders.
Reasons to Acquire
Increase your understanding of the market for identifying the most suitable strategies and decisions based on sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends, and factors.
Gain authentic and granular data access for the In-Vitro Diagnostics Market to understand the trends and the factors involved in changing market situations.
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future.
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns.
Browse for More Reports:
Report Scope
The In-Vitro Diagnostics Market is segmented into various segments such as Product And Services, Technique, Application, End-User, and region:
Based on Product And Services
Reagents
Instruments
Software and Services
Based on the Technique
Immunodiagnostics
Enzyme-linked Immunosorbent assay
Chemiluminescence Immunoassay
Fluoresence Immunoassay
Colorimetric Immunoassay
Rapid Tests
Enzyme-linked Immunospot
Radioimmunoassay
Western blot
Hematology
Molecular Diagnostics
Polymerize Chain Reaction
Isothermal Nucleic acid Amplification Technology
Hybridization
DNA diagnostics
Microarray
Tissue Diagnostics
Clinical Chemistry
Basic Metabolic Panel
Liver Panel
Renal Profile
Lipid Profile
Thyroid Function Panel
Electrolyte Panel
Specialty Chemicals
Based on the Application
Infectious Diseases
Cancer
Cardiac Diseases
Immune System Disorders
Nephrological Diseases
Gastrointestinal Diseases
Based on End-User
Standalone laboratories
Hospitals
Academic And Medical Schools
Point Of Care
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
+44-20-8638-5055
0 notes
Text
Africa IVD Market to be Worth $1.78 Billion by 2029
According to a new market research report titled, ‘Africa In Vitro Diagnostics Market by Product & Services, Technology (Immunoassay, Point of Care, Molecular Diagnostics, Coagulation), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), and Customer Type - Forecast to 2029,’ published by Meticulous Research®, the Africa in vitro diagnostics market is expected to reach $1.78 billion by 2029, at a CAGR of 4.8% from 2022 to 2029.
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In vitro diagnostics (IVD) are tests performed on samples of blood or tissue taken from the human body to detect a wide range of diseases. These tests help monitor overall health to prevent and treat various diseases. IVD involves the use of various reagents, assays, and kits based on technologies such as immunoassay, whole blood glucose monitoring, molecular diagnostics, point of care, clinical chemistry, hematology, coagulation & hemostasis, critical care, and urinalysis.
High Prevalence of Infectious Diseases Driving the Growth of the Africa IVD Market
Diagnostic tests play a crucial role in the medical care of patients with infections. The demand for IVD kits used to diagnose infectious diseases is high in Africa due to the increasing awareness regarding emerging and re-emerging infectious diseases and the adoption of advanced molecular testing and lab-on-chip technology. The burden of diseases such as HIV, tuberculosis, malaria, viral hepatitis, and neglected tropical diseases (NTDs) is large in Africa. For example, according to WHO, in 2020, 25.6 million people were living with HIV in the African Region. The annual number of new HIV infections also increased in the Middle East and North Africa (source: UNAIDS data 2021).
In addition, there is a frequent outbreak of endemic diseases in Africa. For instance, Lassa fever is endemic in Nigeria and continues to be reported throughout the country. According to Nigeria Centre for Disease Control and Prevention (NCDC). In January 2022, 981 suspected and 211 confirmed cases of Lassa fever were reported in Nigeria. There were 40 deaths reported among confirmed cases, indicating a case fatality rate of 19%. Thus, the high burden of infectious diseases increases the demand for IVD tests in Africa.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5415
Africa IVD Market: Future Outlook
The Africa IVD market is segmented based on Product Category (Reagents, Assays, and Kits, Instruments, and Software & Services), Technology (Immunoassay, Whole Blood Glucose Monitoring, Point-of-Care Diagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, Coagulation & Hemostasis, Critical Care, Urinalysis, and Other IVD Technologies), Application (Infectious Diseases, Oncology, Diabetes, Endocrinology, Cardiology, and Other Applications), Diagnostic Approach (Laboratory Testing, Point-of-Care Testing, and OTC/Self Testing), and Customer Type (Hospital Labs, Private Labs, Home Care/Self Testing, Government, and Other Customer Types), and Geography (South Africa, Egypt, Nigeria, Kenya, Algeria, Tanzania, Morocco, Tunisia, and the rest of Africa). The study also evaluates industry competitors and analyzes their market shares.
Based on product category, the reagents, assays, and kits segment is slated to register the fastest growth rate during the forecast period. In Africa, infectious diseases cause chronic illnesses, premature deaths, and loss of productivity, affecting the region's economic growth. Thus, the emerging threats of infectious diseases drive the demand for IVD in Africa. The frequent use of reagents for diagnosing diseases and the easy availability & accessibility of these tests are the major factors driving the growth of this segment.
Based on technology, the point of care segment is slated to register the fastest growth rate during the forecast period. The rising geriatric population, the growing prevalence of chronic diseases, and the rising incidence of infectious diseases are driving the growth of this segment. The increasing burden of chronic diseases and pathogen outbreaks has significantly increased the demand for PoC diagnostics in Africa. The increased need for early and accurate diagnosis, precise confirmation of clinical findings, and immediate & informed decision-making on treating diseases contributes to the growth of the PoC segment.
Based on application, the oncology segment is slated to register the fastest growth rate during the forecast period. Cancer involves a complex set of genetic alternations and subsequent changes in gene expression, leading to the uncontrolled proliferation of cells. In cancer diagnostics, early diagnosis and knowing the exact nature of the tumor are of prime importance. Early cancer detection and access to effective anti-cancer treatment can result in higher rates of survival and a better quality of life. According to GLOBOCAN 2020, 1.1 million new cases and 711,429 deaths were recorded in Africa. The growing demand for personalized medicines in cancer therapy, the increasing prevalence of cancer, and the rising demand for rapid cancer diagnosis tests drive the growth of the IVD market for oncology applications.
Based on diagnostic approach, the OTC/self-testing segment is slated to register the fastest growth rate during the forecast period. In Africa, over-the-counter (OTC)/self-testing is promoted for diseases, such as HIV and COVID-19, due to the high prevalence of infectious diseases. Self-testing reduces the burden on healthcare workers and the system. The OTC/self-testing kits are diagnostic tests that can be purchased from any pharmacy store and used by the patient. These tests can give rapid results and can be taken from anywhere.
Quick Buy – Africa IVD Market Research Report: https://www.meticulousresearch.com/Checkout/47708335
Based on customer type, the home care/self-testing segment is slated to register the fastest growth rate during the forecast period. The growth of this segment is attributed to the increasing need to monitor and manage chronic diseases, the growing awareness about home healthcare testing products, and the reduced waiting time and healthcare costs offered by self-testing kits.
This research report analyzes the market across major African countries and provides a comprehensive analysis of South Africa, Algeria, Nigeria, Kenya, Morocco, Tunisia, Tanzania, Egypt, and the rest of Africa.
Key companies operating in the Africa IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), and Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/africa-ivd-market-5415
Scope of the Report
Africa IVD Market, by Product Category
Reagents, Assays, and Kits
Instruments
Software & Services
Africa IVD Market, by Technology
Immunoassay
Whole Blood Glucose Monitoring
Point-of-Care Diagnostics
Clinical Chemistry
Molecular Diagnostics
Hematology
Coagulation & Hemostasis
Critical Care
Urinalysis
Other IVD Technologies
Note: Other technologies include microscopy, hybridization, and loop-mediated amplification.
Africa IVD Market, by Application
Infectious Diseases
Oncology
Diabetes
Endocrinology
Cardiology
Other Applications
Note: Other applications include nephrology, toxicology, gastroenterology, neonatal, genetic, and neurological disorders.
Africa IVD Market, by Diagnostic Approach
Laboratory Testing
Point-of-Care Testing
OTC/Self-testing
Africa IVD Market, by Customer Type
Hospital Labs
Private Labs
Home Care/Self Testing
Government
Other Customer Types
Note: Other customer types include long-term care facilities, academic & research institutes, ambulatory care centers, and transfusion laboratories.
Africa IVD Market, by Country
South Africa
Egypt
Nigeria
Kenya
Algeria
Tanzania
Morocco
Tunisia
Rest of Africa
Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5415
In Vitro Diagnostic (IVD) Reagents Market - https://www.meticulousresearch.com/product/in-vitro-diagnostic-reagents-market-5110
0 notes
Text
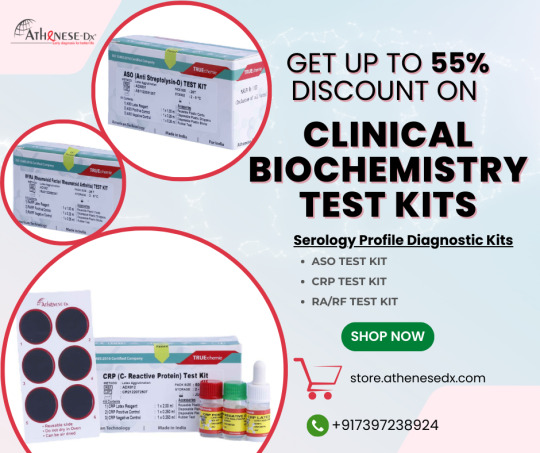
Most Popular IVD Biochemistry Reagents and Test Kits in June 2024
Hello there!
Athenese Dx - Early Diagnosis Medical Equipment & IVD Test Kits Provider. Athenese-Dx specializes in the research, development, manufacture, and marketing in vitro diagnostic tests and Instruments. We are developing high-quality IVD tests in Clinical Chemistry, Rapid Tests, ELISA, Fluorescence Immunoassay, Molecular Diagnostics, and Instruments. Our R&D Unit is recognized by the Department of Scientific and Industrial Research (DSIR).
Biochemistry Kits and Reagents:
Indigenous, highly stable, liquid-based clinical chemistry reagents with the brand name TRUEchemie. TRUEchemie kits are suitable for semi-automated analyzers and fully automated instruments. TRUEchemie Biochemistry Reagents.
Currently, we are selling following the disease categories and their biochemistry reagents,
Diabetic Profile
Lipid Profile
Cardiac Profile
Pancreatic Profile
Renal Profile
Immunoturbidimetry Products
Liver Profile
Electrolytes Profile
Serology Profile
Special Parameter
Diabetic Profile:
Diabetic Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Diabetic Profile helps monitor blood sugar levels and keep track of overall bodily functions. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Lipid Profile:
Lipid Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Lipid Profile looks at the levels of cholesterol and other fats in your blood. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Cardiac Profile:
Cardiac Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Cardiac Profile is used to check the overall functioning of the heart. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Pancreatic Profile:
Pancreatic Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Pancreatic Profile helps to measure the levels of tumour markers in the blood, urine, and other body fluids. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Renal Profile:
Renal Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Renal Profile Tests are used to determine how well the kidneys are functioning Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Immunoturbidimetry Products:
Immunoturbidimetry Products Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Immunoturbidimetry Products Test Kit Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Liver Profile:
Liver Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Liver Profile Test Kit helps to measure several substances made by your liver. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Electrolytes Profile:
Electrolytes Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Electrolytes Profile is a blood test that measures levels of the body’s main electrolytes. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Serology Profile:
Serology profile diagnostic kits play a crucial role in detecting antibodies or antigens in blood serum, aiding in the diagnosis of infectious diseases. Our Biochemistry kits are Indigenous, highly stable, liquid-based clinical chemistry reagents with the brand name TRUEchemie.
TRUEchemie IVD Test Kits are suitable for semi-automated analyzers and fully automated instruments. Athenese-Dx offers simple, fast, and efficient serology testing for specific proteins or antibodies in human serum.
Here Athenese-Dx refers to some Biochemistry Reagents and Test Kits to diagnose a better life:
ASO Test Kit:
The TRUEchemie ASO Test Kit is for the qualitative and semi-quantitative determination of ASO in human serum.

https://store.athenesedx.com/product/aso-test-kit/
CRP Test Kit:
The TRUEchemie CRP (C-Reactive Protein) Test Kit (Latex Agglutination Method) is for the qualitative and semi-quantitative determination of CRP in serum.

https://store.athenesedx.com/product/crp-test-kit/
RA/RF Test Kit:
The TRUEchemie RF / RA (Rheumatoid Factor / Rheumatoid Arthritis) test Kit is for the qualitative and semi-quantitative determination of RF/RA in serum.
https://store.athenesedx.com/product/ra-rf-test-kit/
Buy Serology profile diagnostic kits with more than a 55% discount. Suitable for all laboratory settings. Click the link below to avail yourself of test kits at discounted rates at the Athenese-Dx Store.

Buy Now → https://store.athenesedx.com/
Special Parameter:
Special Parameter Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. The special parameter of the ADA Test Kit is used for the direct quantitative determination of Adenosine Deaminase (ADA) in Human Serum/Plasma/CSF/Pleural Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Limited Offer!!! End Soon!!!
#lab equipment#clinical chemistry#digital pathology market#hospital#pathologist#ivd#pathologylab#pathology#athenesedx#biochemistry#IVD#invitrodiagnostics#rapidtest#elisa#molecular#ivdinstruments#LabTech#labtests#labtesting#labtested#dengue#india#malaria#typhoid#typhoidfever#denguetestkit#rapidtestkit#rapidkit#rapid#medicaltestkit
0 notes
Text
Global In Vitro Diagnostics (IVD) Market Report 2022| Research Analysis with Trends, Segmentation, Share and Forecast by 2030| By R&I

The report is titled as ‘In Vitro Diagnostics Market: Opportunity Analysis and Future Assessment 2020-2028’. An overview of conceptual frameworks, analytical approaches of the In Vitro Diagnostics market is the main objective of the report, which further consists the market opportunity and insights of the data involved in the making of the respective market. In Vitro Diagnostics market is expected to grow with significant rate in the near future.
The global In Vitro Diagnostics (IVD) market in 2020 is estimated for more than US$ 70,561.9 Mn and expected to reach a value of US$ 1,03,310.9 Mn by 2028 with a significant CAGR of 4.9%.
Request a Sample Copy of this Report @: https://reportsandinsights.com/sample-request/1256
In Vitro Diagnostics Market Dynamics
In-vitro diagnostics are tests performed in laboratories on biological samples such as blood, urine, tissue, etc. In vitro diagnostic are not only used for diagnosis but also provides useful information on monitoring of disease and treatment. Two-thirds of today’s clinical diagnoses involve in vitro diagnostics, hence are referred to as the backbone of healthcare.
In-vitro diagnostics market has witnessed substantial growth in last decade owing to technological advancements in testing methods as well as in analyzer instruments. According to the EPRS report, there are over 40,000 different IVD products available in the European market and per capita annual spending on IVD is approximately US$ 26. In-vitro diagnostics help to reduce the healthcare cost by making treatments more efficient and precise.
Evolving regulations for in-vitro diagnostics is the major driving force for market growth over the forecast period. For instance, regulation of medical devices in the European Union (EU) is undergoing a significant transformation with expanding the scope for in vitro diagnostics, which is expected to impact the increased product approvals for IVD in the region. Significant progress in point-of-care testing is another factor driving the growth of the global in-vitro diagnostics market. Introduction of molecular based assays and identification of range of biomarkers has opened up huge new opportunity for growth of global in-vitro diagnostics market.
MMC Overview on In Vitro Diagnostics Market Report
The non-identical approach of Reports and Insights stands with conceptual methods backed up with the data analysis. The novel market understanding approach makes up the standard of the assessment results that give better opportunity for the customers to put their effort.
A research report on the In Vitro Diagnostics market by Reports and Insights is an in-depth and extensive study of the market based on the necessary data crunching and statistical analysis. It provides a brief view of the dynamics flowing through the market, which includes the factors that supports market and the factors that are acting as impedance for the growth of the market.
Furthermore, the report includes the various trends and opportunities in the respective market in different regions for a better understanding of readers that helps to analyze the potential of the market.
Wish to Know More About the Study? Click here to get a Report Description: https://reportsandinsights.com/pressrelease/global-in-vitro-diagnostics-market
In Vitro Diagnostics Market Segmentation
The global in vitro diagnostics market is segmented on the basis of product type, technology, end user and region. On the basis of product type, in vitro diagnostics market is segmented as reagents and kits, analyze, and consumables. Based on technology, global market is segmented as immunochemistry, molecular diagnostics, hematology, point-of-care diagnostics, tissue diagnostics and clinical chemistry. On the basis of end user, global in vitro diagnostics market is segmented into hospitals, diagnostic centers, clinics, home care settings and others.
By Products & Services
Reagents & Kits
Instruments
Fully Automated Instruments
Semi-Automated Instruments
Other Instruments
Data Management Software
Services
By Technology
mmunoassay/Immunochemistry
Enzyme-Linked Immunosorbent Assay
hemiluminescence Immunoassays
Fluorescence Immunoassays
Colorimetric Immunoassays
Radioimmunoassay
Rapid Test
Western Blotting
Enzyme-Linked Immunospot Assays
Clinical Chemistry
Basic Metabolic Panel
Electrolyte Panel
Liver Panel
Lipid Profile
Renal Profile
Thyroid Function Panel
Specialty Chemical Tests
Molecular Diagnostics
Polymerase Chain Reaction
Isothermal Nucleic Acid Amplification Technology
Microarray
Hybridization
DNA Sequencing & Next-Generation Sequencing
Other Mdx Technologies
Microbiology
Hematology
Coagulation and Hemostasis
Urinalysis
Other IVD Technologies
By Application
Infectious Diseases
Diabetes
Oncology
Cardiology
Autoimmune Diseases
Nephrology
Drug Testing/Pharmacogenomics
HIV/Aids
Other Applications
By End User
Hospitals
Laboratories
Large/Reference Laboratories
Medium-Sized Laboratories
Small Laboratories
Point-Of-Care Testing
Patient Self-Testing
Academic Institutes
Other End Users
By Region
North America
Latin America
Europe
Asia Pacific
Middle East
Africa
In Vitro Diagnostics Market Competitive Landscape
Global market for in vitro diagnostics is dominated by Abbott Laboratories, Merck KGaG, F. Hoffman-La Roche AG, Becton Dickinson & Company, Danaher Corporation, Thermo Fisher Scientific Inc., Qiagen N.V., Diasorin S.P.A., biomerieux SA, Alere Inc., Bayer AG, Bio-Rad Laboratories Inc., Sysmex Corporation, etc. to name a few.
To view Top Players, Segmentation and other Statistics of In Vitro Diagnostics (IVD) Industry, Get Sample Report @: https://reportsandinsights.com/sample-request/1256
About Reports and Insights:
Reports and Insights is one of the leading market research companies which offers syndicate and consulting research around the globe. At Reports and Insights, we adhere to the client needs and regularly ponder to bring out more valuable and real outcomes for our customers. We are equipped with strategically enhanced group of researchers and analysts that redefines and stabilizes the business polarity in different categorical dimensions of the market.
Contact Us:
Neil Jonathan
1820 Avenue M, Brooklyn
NY 11230, United States
+1-(718) 312-8686
Find Us on LinkedIn: www.linkedin.com/company/report-and-insights/
View Latest Market Updates At: https://marketsresearchanalytics.com
#In Vitro Diagnostics (IVD) Market Research#In Vitro Diagnostics (IVD) Market Report#In Vitro Diagnostics (IVD) Market Share 2021#In Vitro Diagnostics (IVD) Market Size 2022#In Vitro Diagnostics (IVD) Market Trends#In Vitro Diagnostics (IVD) Market Key Players#Global In Vitro Diagnostics (IVD) Market Analysis#In Vitro Diagnostics (IVD) Industry News#In Vitro Diagnostics (IVD) Industry Analysis#In Vitro Diagnostics (IVD) Market Forecast#In Vitro Diagnostics (IVD) Market CAGR#USA In Vitro Diagnostics (IVD) Market#Japan In Vitro Diagnostics (IVD) Market#In Vitro Diagnostics (IVD) Market Demand#Argentina In Vitro Diagnostics (IVD) Market#Australia In Vitro Diagnostics (IVD) Market#Belgium In Vitro Diagnostics (IVD) Market#Brazil In Vitro Diagnostics (IVD) Market#Canada In Vitro Diagnostics (IVD) Market#Chile In Vitro Diagnostics (IVD) Market#China In Vitro Diagnostics (IVD) Market#Columbia In Vitro Diagnostics (IVD) Market#Egypt In Vitro Diagnostics (IVD) Market#France In Vitro Diagnostics (IVD) Market#Germany In Vitro Diagnostics (IVD) Market#Global In Vitro Diagnostics (IVD) Market#India In Vitro Diagnostics (IVD) Market#Indonesia In Vitro Diagnostics (IVD) Market#In Vitro Diagnostics (IVD) Applications#In Vitro Diagnostics (IVD) Industry
0 notes
Text
In Vitro Diagnostics Market: Growth, Development and Forecast Report 2017 – 2027

Global In-Vitro Diagnostic Market, by Product & Services (Reagents & Kits, Instruments, Data Management Software, Services). By Technology (Immunoassay/Immunochemistry, Clinical Chemistry, Microbiology, Hematology, Coagulation and Hemostasis, Urinalysis, Other IVD Technologies). By End User (Hospitals, Laboratories, Point-Of-Care Testing, Patient Self-Testing, Academic Institutes, Other End Users). By Application (Infectious Diseases, Diabetes, Oncology, Cardiology, Autoimmune Diseases, Nephrology, Drug Testing/Pharmacogenomics, HIV/Aids, & Other Applications) and By Region (North America, Europe, Asia Pacific, Latin America, Middle East, & Africa) is expected to gain traction over the forecast period between 2019 and 2027.
For requesting a free sample to our latest study on Global In-Vitro Diagnostic Market, visit: https://meridianmarketconsultants.com/requst?rid=1694
In-Vitro Diagnostic Market Dynamics
Increase in the prevalence of autoimmune diseases and cancer is expected to drive the growth of the market in nearby future. According to the statistics published by the American Autoimmune Related Diseases Association, in 2019, around 50 million of the U.S. population is suffering from autoimmune disease. Autoimmune disease is considered in top 10 leading causes of death in women and girls up to the age of 64 years in the U.S.
Also, an increase in the government expenditure towards treatment of cancer is expected to be the one of the major factors that will drive the growth of market. For instance, according to the Insititute of Medicine report published in 2018, the U.S. is considered way behind in terms of research and development into immune system. In the year 2018, the National Institute of Health (NIH) contribute US$ 591 Mn on autoimmune disease research and development programs, which is very low in comparison to their spending on cancer research and development which is around US$ 6.1 billion.
Furthermore, an increase in the prevalence of cancer owing to the rapid growth in the consumption rate of alcohol and tobacco, sedentary lifestyle, and etc. is expected to act as a major factor that will drive the growth of the market around the globe. For instance, According to National Cancer Institute published data in 2018, it is estimated that 1,735,350 new cases of cancer will be diagnosed in the U.S. in the year 2018 and more than 600k people died due to the cancer. Also, on the basis of 2011-2015 cases, the incidence of cancer is 432.2/100,000 men and women. NCI also estimated that in 2017, a national expenditure for cancer care in the U.S. was more than 147 billion and it is expected to increase in nearby future owing to an increase in the geriatric population and prevalence of cancer. The cost for the treatment will also increase due to the advancement in technology and new treatments likely to be adopted as standards of care.
Some of the other factors such as advancement in technology and increasing demand of point of care devices and an increase in the number of laboratory tests due to the increasing burden of many diseases and increasing research facility are likely to propel the growth of the market mainly in the developing economies.
The global In-Vitro diagnostic market in 2018 is accounted for more than US$ xx Bn and expected to reach a value of US$ XX Bn by 2027 with a significant CAGR of around 4.7 % over the forecast period of 2019-2027.
To know the latest insights, qualitative data, trends, quantitative data, and more related to Global In-Vitro Diagnostic Market, visit: https://meridianmarketconsultants.com/global-in-vitro-diagnostics-market
Global In-Vitro Diagnostic Market Regional Segmentation
On the basis region, the global in-vitro diagnostics market is segmented into six regions namely North America, Latin America, Asia Pacific, Europe, Middle East, and Africa. North America is accounted for the largest market share of more than 45% in the year 2018, and expected to maintain its dominance throughout the forecast period (2019-2027). It is due to the favorable government regulations and availability of funds from government mainly in the U.S. and also due to the presence of market-dominating players in the region.
Asia Pacific region is expected to grow at the highest annual growth rate in comparison to other regions during the forecast period, owing to the increasing incidence and prevalence in various countries like China and India.
Global In-Vitro Diagnostic Market Competitive Landscape
Some of the key participating players in the global In-Vitro Diagnostics market are Roche Diagnostics, Siemens Healthineers, Danaher Corporation, Abbott Laboratories, Thermo Fisher Scientific, Johnson & Johnson, Becton, Dickinson and Company, Bio-Rad Laboratories, Sysmex Corporation, bioMérieux, DiaSorin, Ortho Clinical Diagnostics, Agilent Technologies, QIAGEN, and among others.
Read Detailed News: https://meridianmarketconsultants.com/pressrelese/global-in-vitro-diagnostics-market
About Meridian Market Consultants:
Meridian Market Consultants (MMC) is committed to providing deep insights that serve as a creative tool for the client that enables it to perform confidently in the market. At MMC we adhere to the client's needs and regularly ponder to bring out more valuable and real outcomes for our customers. We are equipped with a strategically enhanced group of researchers and analysts that redefines and stabilizes the business polarity in different categorical dimensions of the market.
Contact Us:
Meridian Market Consultants (MMC)
13121, Louetta Road,
#440, Cypress, Texas 77429
United States
Tel: +1-(281)-619-8646
For Sales Queries: [email protected]
#In Vitro Diagnostics Market share#In Vitro Diagnostics Market size#In Vitro Diagnostics Market analysis#In Vitro Diagnostics Market News#In Vitro Diagnostics Market in USA#health care market report#Health management market report
1 note
·
View note
Text
Automated Microbial Identification Systems Clinical Chemistry Reagents Kits Newborn Screening System
Programmed immunoassay analyzers
IMMUNOLOGY
Immunology tests can decide if the insusceptible framework is sufficiently solid to appropriately battle off such ailments as titers, lupus, varicella and others. Immunology packs help exploring issues with the invulnerable framework, for example, immune system illnesses and immunodeficiency issue. They are additionally used to decide organ, tissue and liquid similarity for transplantation.
Programmed immunology analyzers Completely Automated Microbial Identification SystemsChemiluminescence Immunoassay Analyzers
Highlights :
Greatest menu Productive from the begin Most extreme adaptability Arranged for all consequences For viable utilization of lab assets Clinical Chemistry Reagents Kits
Contact XL :
Highlights :
Xlight-All-in-one framework - Small impression empowers simple establishment Programmed immunoassay analyzers also, keep away from lab reconfiguration Newborn Screening System
XLerate-Improved execution, higher throughput, long leave time and enhanced dependability
XLellence-security quality, similarity with persistent observing of all basic examines
XLarge-Higher number of Tests on board and new tests accessibility
XLogic-Remote help
#Automated Microbial Identification Systems#Clinical Chemistry Reagents Kits#Newborn Screening System
0 notes
Text
In Vitro Diagnostics Market Share, Industry Growth, Trend, Key Companies by 2028
The Global in vitro diagnostics industry Market report published by Reports and Data is a concise summary on the in vitro diagnostics industry industry and offers deep insights into the industry’s core structure and mechanism. The report digs into the key segments and sub-segments of the industry and offers a thorough study of the industry’s leading regional markets, competitive scenario, product and application segments, technology landscape, sales & distribution networks, and key industry statistics. Market insights included in the report have been compiled through extensive research, detailed market surveys, and expert interviews.
Key market dynamics illustrated in the report include market share, market size, revenue growth drivers, restraints, opportunities, threats, challenges, emerging market trends, product innovations, and industry revenue growth rate. Other imperative factors highlighted in the report are volatility in demand and supply graphs, production & consumption patterns, paradigm shifts in consumer preferences, import/export analysis, and a multitude of macro-economic and micro-economic growth indicators. Going ahead, the report elaborates on the highly competitive environment of the in vitro diagnostics industry industry and discusses the strategic initiatives undertaken by each market player, including partnerships & collaborations, mergers & acquisitions, joint ventures, government & corporate deals, and new product launches.
Get a sample of the report @ https://www.reportsanddata.com/sample-enquiry-form/4520
Some Key Factors Contributing to the Global Pharma & Healthcare Market Growth
Unprecedented revenue growth of the global pharma & healthcare industry is attributed to factors such as rising prevalence of chronic and acute diseases worldwide, increasing geriatric population, rising awareness of health & wellness among consumers, and growing demand for more advanced healthcare services. Increasing demand for advanced drugs and therapeutics, growing availability of next-generation diagnostics and treatment options – especially in developing countries like India and China – rise in R&D activities and clinical trials in the pharmaceutical and biotechnology sectors, increasing public and private investments in healthcare research projects, and rising consumer expenditure on healthcare are among the other significant factors contributing to the industry revenue growth.
Top Players in the Global in vitro diagnostics industry Market:
Company 1
Company 2
Company 3
Company 4
Company 5
Company 6
Company 7
Company 8
Company 9
Company 10
Company 11
Company 12
Others
The coronavirus pandemic has had a drastic impact on the global healthcare industry, with rising cases of COVID-19 worldwide, substantially growing hospital admission and readmission rates, and rising demand for telehealth and telemedicine services for remote patient monitoring. Furthermore, rising focus on development of rapid COVID-19 diagnostics such as the RT-PCR test kits, increased government funding for vaccine development, stringent regulatory norms and protocols for COVID-19 safety, and increasing sales of COVID-19 safety equipment, such as N-95 masks, face shields, PPE kits, and hand sanitizers, have driven the global pharma & healthcare industry revenue growth over the recent past.
To know more about the report @ https://www.reportsanddata.com/report-detail/in-vitro-diagnostics-ivd-market
in vitro diagnostics industry Market Segmentation:
Product Type Outlook (Revenue, USD Billion; 2018-2028)
Reagents
Instruments
Software and Services
Others
Technology Type Outlook (Revenue, USD Billion; 2018-2028)
Immunoassay/ Immunochemistry
Enzyme-linked Immunosorbent Assay (ELISA)
Radioimmunoassay
Rapid Tests
Western Blotting
Enzyme-linked Immunospot Assays
Biochemistry/Clinical Chemistry
Molecular Diagnostics
Polymerase Chain Reaction (PCR)
Isothermal Nucleic Acid Amplification Technology
Microarray
Hybridization
DNA Sequencing & Next-generation Sequencing
Microbiology
Hematology
Coagulation/Hemostasis
Urinalysis
Others
Application Outlook (Revenue, USD Billion; 2018-2028)
Infectious Diseases
Oncology
Endocrinology
Diabetes
Cardiology
Nephrology
Others
End-use Outlook (Revenue, USD Billion; 2018-2028)
Laboratories
Large/Reference Laboratories
Medium-sized Laboratories
Small Laboratories
Hospitals
Academics
Point-Of-Care Testing
Patient Self-Testing
Others
Global in vitro diagnostics industry Market Report: Regional Segmentation
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
Request customization of the report @ https://www.reportsanddata.com/request-customization-form/4520
Frequently Asked Questions Answered in the Report:
What is the estimated revenue growth rate of the global in vitro diagnostics industry market over the forecast period?
What are the major factors driving the global market revenue growth?
Which are the leading manufacturers and suppliers in the global in vitro diagnostics industry market?
Which regional market is expected to lead in terms of revenue share in the global in vitro diagnostics industry market over the forecast years?
What are the key outcomes of SWOT analysis and Porter’s Five Forces analysis of the market?
Thank you for reading our report. Please connect with us to know more about the report and its customization feature. Our team will ensure the report is well suited to meet your requirements.
About Reports and Data
Reports and Data is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyze consumer behavior shifts across demographics, across industries, and help clients to make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Products, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Reports and Data has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
0 notes
Text
In Vitro Diagnostics (IVD) and Laboratory Developed Tests for Autoimmune Diseases Market Analysis Geography Trends, Demand and Forecasts 2030
In Vitro Diagnostics (IVD) and Laboratory Developed Tests for Autoimmune Diseases Market: Snapshot
In vitro diagnostics and laboratory developed tests for autoimmune diseases are a key part of the global healthcare sector and are likely to account for a significant share in the same in the coming years due to the rising investment in the healthcare sector from various channels. In vitro diagnostics refers to testing performed on human tissues such as blood, urine, and other tissues in order to diagnose various diseases. The growing threat of autoimmune diseases, due to the rising prevalence of unhealthy lifestyles, is likely to remain a steady driver for the global in vitro diagnostics and laboratory developed tests for autoimmune diseases market in the coming years.
Download PDF Brochure: https://www.tmrresearch.com/sample/sample?flag=B&rep_id=1268
By technology, the global in vitro diagnostics and laboratory developed tests for autoimmune diseases market is segmented into clinical chemistry, molecular diagnostics, clinical microbiology, immunochemistry or immunoassay, coagulation and hemostasis, hematology, and others. The molecular diagnostics segment is likely to remain a leading contender in the global in vitro diagnostics and laboratory developed tests for autoimmune diseases market in the coming years due to the increasing attention being paid to development of genetic therapeutics in the global healthcare sector.
The growing research in human genomics is likely to play a key role in the development of the global in vitro diagnostics and laboratory developed tests for autoimmune diseases market in the coming years. Genetic components play a role in the causation of autoimmune diseases, making the development of genetic research important for the in vitro diagnostics and laboratory developed tests market.
Global In Vitro Diagnostics (IVD) and Laboratory Developed Tests for Autoimmune Diseases Market: Overview
IVDs are medical equipment that are used to test human samples of blood, urine, and tissue, and help in detecting infections, diagnosing a medical condition, and to prevent the disease. As the healthcare industry touches new peaks in the coming years, gaining from the prevalence of several chronic diseases and aging population, the demand for in vitro diagnostics as well as other laboratory developed tests (LDT) are expected to expand at a healthy growth rate during the forecast period of 2017 to 2025.
This report on global IVD and LTD for autoimmune diseases studies all the macro and micro factors that are expected to impact the demand over the course of next few years and evaluates their implications. The report also estimates the revenue available in the global market and forecasts the future scenario. The report contains a dedicated section on company profiles, wherein a number of leading players in the industry have been analyzed for their market share, financial performance, product benchmarking, and recent strategic initiatives. The report aims to serve as an informational guide to IVD product manufacturers, original equipment manufacturers, hospitals and clinics, and research institutes.
By product and services, the global in vitro diagnostics and laboratory developed tests for autoimmune diseases market can be segmented into reagents and kits, instruments, services, and data management software. In terms of technology, the market can be bifurcated into clinical chemistry, immunochemistry or immunoassay, hematology, coagulation and hemostasis, clinical microbiology, molecular diagnostics, and other technologies. The segment of clinical chemistry can be further sub-segmented into basic metabolic panel, electrolyte panel, liver panel, lipid profile, renal profile, thyroid function panel, and specialty chemical tests. The technology segment of molecular diagnostics can also be sub-bifurcated into polymerase chain reaction, isothermal nucleic acid amplification technology (INAAT), microarrays, hybridization, DNA sequencing and next-generation sequencing, and other technologies. By application, the global market for IVD and LTD can be categorized into diabetes, infectious diseases, oncology or cancer, cardiology, nephrology, autoimmune diseases, drug testing and HIV or Aids. Hospitals, laboratories, point-of-case testing, academic institutes, and patient self-testing can be the segments of the market in terms of end user. Geographically, the report studies the growth opportunities in regions such as North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa.
Global In Vitro Diagnostics and Laboratory Developed Tests for Autoimmune Diseases Market: Trends and Opportunities
According to the report, some of the factors that are favoring the market expansion are: growing prevalence of autoimmune diseases, increasing demand for personalized medicine, and technological advancements in the field which has considerably increased the efficiency and accuracy of these diagnostic devices. However, low research budget for autoimmune diseases is expected to hinder the growth rate to a certain extent during the forecast period.
In terms of products and services, the segment of reagents and kits currently has the maximum demand, which can be attributed to the growing number of IVD tests now accessible to a wide range of reagents. Application-wise, infectious diseases are the most in-demand segment, although oncology or cancer segment is anticipated to expand at the most prominent rate during the forecast period.
Global In Vitro Diagnostics and Laboratory Developed Tests for Autoimmune Diseases Market: Regional Outlook
North America is the most lucrative regional market by a country mile, followed by Europe. This is a reflection of easy accessibility to technologically advanced products and robust healthcare infrastructure in these two regions. However, Asia Pacific, which homes nearly half of the world’s population, is expected to increase the demand exponentially in coming years, especially from emerging economies such as India, China, South Korea, Japan, and Australia.
Companies mentioned in the research report
Some of the leading companies in the global IVD and LDT market are Qiagen N.V., Roche Diagnostics, Becton, Dickinson and Company, Abbott Laboratories, Siemens Healthineers, Danaher Corporation, Bio-Rad Laboratories, Inc., bioMérieux, Johnson and Johnson, Thermo Fisher Scientific Inc., Ortho-Clinical Diagnostics, Inc., and Diasorin S.P.A.
About Us:
TMR Research is a premier provider of customized market research and consulting services to business entities keen on succeeding in today’s supercharged economic climate. Armed with an experienced, dedicated, and dynamic team of analysts, we are redefining the way our clients’ conduct business by providing them with authoritative and trusted research studies in tune with the latest methodologies and market trends.
Contact Us:
Rohit Bhisey
Head Internet Marketing
Tel: +1-415-520-1050
Website: https://www.tmrresearch.com/
0 notes
Text
7 Tips to purchase the best ultrasound machine in india
7 Tips to purchase the best ultrasound machine
In the event that you are in the market to supplant your obsolete imaging hardware and need to purchase best convenient ultrasound machines, it is imperative for you to pick gear that is reasonable to your financial plan and your workplace. The ordinary ultrasound machines are very overwhelming and massive with a typical introduce base being healing facilities. Subsequently, to make the things more straightforward for the little healing facilities or centers the convenientultrasound scanners for sale are gainful so you can bear them the premises adding to the comfort of both the patients and experts.
7 Tips to purchase the best ultrasound machine
Versatile ultrasound machines are very feasible as they can be conveyed effortlessly the restorative foundation premises to guarantee opportune and quick treatment from ultra-convenient to little truck based portability. They are mostly utilized as a part of vascular, cardiovascular, endocrinology, radiology, veterinary, OB/GYN and pediatric cases. A portion of the benefits of versatile ultrasound machine incorporate straightforwardness to patients, prompt determination, and Automatic immunoassay analyzers diminished finding cost.
On the off chance that you are wanting to purchase new ultrasound machines, here are seven focuses that you should remember to settle on an educated choice:
Cost
Regardless of whether you purchase Samsung ultrasound machines or purchase Philips new ultrasound machine, the cost is a central point. Aside from the underlying expense likewise, consider its support. Consider what amount consumable and new parts cost? Consider how your price tag thinks about to other comparable machines in the market? As an expression of alert, don't go for the least expensive alternative unless choices/components or extreme life span are not as essential; rather search for the compact ultrasound machine that is very much outfitted with the most recent elements.
Distinguish the key elements
When purchasing the ultrasound machine you have to search for certain specialized determinations. A portion of the components to search for are:
Recurrence: The recurrence of the ultrasound machine is conversely proportionate to how profound will the waves infiltrate. A general guideline, the higher the recurrence (10-16MHz), the more shallow imaging, the lower the recurrence (1 or 2MHz), the more profound the entrance.
Control yield: It is an essential component, and it is recommended to utilize machines with lower control settings as higher power yield can make consumes the tissues or epidermis.
Different elements that you should pay special mind to are imaging, CW Doppler, DICOM framework, 3D/4D alternative, and shading Doppler, among numerous more up to date includes like HDLive, Matrix, Live 3D Echo.
Use
When you purchase versatile ultrasound gear, regardless of whether revamped or new, consider how regularly it will be utilized. Additionally, pay notice to nature in which it will be utilized. The conditions in which the machine will be utilized will decide the result of elements you require to take care of business successfully. This is the place talking with your professionals makes a difference. They can furnish you with important knowledge into item highlights that are useful to address patient's issues or better encourage their own efficiency.
End Users
Consider the expertise levels of every one of your specialists before you purchase new compact ultrasound machines.Clinical Chemistry Reagents Kits On the off chance that you are thinking about putting resources into cutting edge CT machines, for example, consider the required extra preparing that is important to appropriately utilize the hardware. You likewise need to calculate how to advance in clinical conventions. Inquire as to whether they give on the web, disconnected preparing, and if on location preparing is accessible likewise at an additional cost.
Benefit Contract
When you purchase ultrasound scanners, take a gander at the length of the producer's guarantee. This differs from maker to producer. At the end of the day, when you purchase Samsung ultrasound machines or when you purchase Philips new ultrasound machines, the guarantee length may fluctuate for both. After the lapse of the guarantee, consider the administration conditions and contract terms and at what cost these are given. Take a gander at the term of the administration contract and additionally for auto restoration statements. What is the cancelation charges, ensured reaction times, parts costs and so forth.? It is best to converse with a specialist before marking the agreement. On the off chance that you discover something not ideal, you can simply arrange. Invest as much energy as you need in light of the fact that once the administration contract is marked, you will lose your arranging influence.
Specialized Support/System Updates
Both are packaged into terms and conditions when you buy an expensive administration contract. Associations dependably have better contrasting options to run of the mill benefit contracts; in this manner it arranges other options to Automated Microbial Identification Systems framework updates and specialized support while you are buying exorbitant analytic hardware. You may approach specialized support through a without toll number. On the off chance that restored ultrasound gear is being considered, inquire as to whether they can run diagnostics or investigate.
Accessibility of Parts or Consumables
When you need to purchase ultrasound machine parts, consider if the consumables and parts are effortlessly accessible. In the event that the Radiation Protection Aprons consumables are excessively costly or hardly accessible, there is no point purchasing the machine from that maker. Likewise, on the off chance that the organization has no extra parts strategy or the parts are exceptionally costly, at that point it is best to search for reasonable choices. Ensure that the essential parts like screens, upper control board, control supplies, casters, consoles, boards, keys and catches are promptly accessible.
Your tirelessness and research when purchasing any ultrasound machine will enable you to spare cash and locate the best ultrasound machine in the market. We give compact ultrasound machines from respectable brands like Edan, Sonosite, Toshiba, Philips, Siemens, Mindray, GE and Sonoscape. We will comprehend your necessities and spending plan and likewise make proposals and guide you to take the correct choice. Our point is to give client benefit situated administrations along these lines we will dependably guarantee that your inquiries and issues are tended to at the earliest opportunity. Our group of specialists with expert learning and aptitude set will address the issues and guarantee smooth working of the machine to guarantee prosperity of the patients.
#ultrasound scanners for sale#Automatic immunoassay analyzers#Clinical Chemistry Reagents Kits#Radiation Protection Aprons#Automated Microbial Identification Systems
0 notes