#invitrodiagnostics
Explore tagged Tumblr posts
Text
In Vitro Diagnostics Market Drivers Shaping Industry Growth Dynamics Globally
The In Vitro Diagnostics market has emerged as a crucial segment within the healthcare industry, significantly influencing patient care and clinical decision-making. This market encompasses diagnostic tests performed on samples such as blood, urine, or tissue, outside the human body, providing vital information for disease diagnosis, treatment monitoring, and prevention. Understanding the drivers behind the rapid growth of the IVD market is essential to appreciate its evolving landscape and future potential.

1. Rising Prevalence of Chronic and Infectious Diseases One of the most prominent drivers of the IVD market is the escalating prevalence of chronic diseases such as diabetes, cancer, cardiovascular ailments, and infectious diseases like HIV/AIDS, hepatitis, and COVID-19. The growing global burden of these diseases demands early and accurate diagnosis, propelling the need for advanced diagnostic tools. IVD tests help in timely disease detection, enabling prompt treatment and better patient outcomes, which directly stimulates market growth.
2. Technological Advancements and Innovation Technological innovation is a critical driver fueling the expansion of the IVD market. Innovations such as molecular diagnostics, point-of-care testing (POCT), next-generation sequencing (NGS), and automated immunoassays have revolutionized diagnostics by enhancing test accuracy, reducing turnaround time, and enabling decentralized testing. These advancements make diagnostics more accessible and user-friendly, encouraging adoption in both developed and emerging markets.
3. Increasing Healthcare Expenditure and Infrastructure Development Global healthcare spending has increased steadily, supported by government initiatives and private investments aimed at improving healthcare infrastructure. Enhanced healthcare facilities and laboratories equipped with modern diagnostic technologies drive the demand for IVD products and services. Moreover, health insurance coverage expansion in many regions increases affordability and accessibility of diagnostic tests for a broader population base.
4. Growing Awareness and Focus on Preventive Healthcare The shift toward preventive healthcare is another significant driver for the IVD market. Patients and healthcare providers are increasingly focusing on early diagnosis and routine health screening to prevent disease progression. Awareness campaigns, health education programs, and government health policies emphasize regular diagnostic testing, thereby increasing market demand.
5. Aging Population and Increasing Geriatric Care The global population is aging rapidly, especially in developed nations. Older adults are more susceptible to chronic illnesses and require frequent diagnostic monitoring. This demographic trend amplifies the demand for in vitro diagnostic tests tailored for geriatric care, such as cardiovascular risk assessments and cancer screenings.
6. Expansion of Point-of-Care Testing (POCT) POCT has become a pivotal driver, offering rapid diagnostic results at or near the patient site. This approach reduces the dependency on centralized laboratories, shortens diagnosis time, and facilitates timely medical intervention. POCT devices are especially beneficial in remote and resource-limited areas, thus expanding the IVD market reach.
7. Regulatory Support and Reimbursement Policies Favorable regulatory frameworks and reimbursement policies encourage market growth by facilitating product approvals and reducing patient costs. Streamlined regulatory pathways accelerate the commercialization of innovative IVD products. Additionally, reimbursement from insurance companies for diagnostic tests makes them more accessible and affordable, driving adoption.
8. Integration of Artificial Intelligence and Digital Health Artificial Intelligence (AI) and digital health integration in IVD are transforming diagnostics by improving data analysis, accuracy, and personalized patient care. AI-powered algorithms assist in interpreting complex diagnostic data and predicting disease outcomes, fostering better clinical decisions. This technological synergy is creating new opportunities and accelerating market expansion.
9. Increasing Demand for Personalized Medicine Personalized medicine focuses on tailoring treatment based on individual genetic and molecular profiles. IVD tests are integral to this approach, enabling the identification of biomarkers and patient-specific therapeutic targets. The rising adoption of personalized medicine is thus directly propelling the demand for advanced IVD solutions.
10. Impact of COVID-19 Pandemic The COVID-19 pandemic significantly boosted the IVD market due to the urgent need for widespread testing to control the virus spread. The pandemic highlighted the importance of rapid, accurate diagnostics and accelerated the development and deployment of new testing technologies. This heightened awareness and investment in diagnostic infrastructure have long-term positive effects on the market.
Conclusion The In Vitro Diagnostics market is poised for robust growth driven by multiple factors ranging from technological innovation and demographic shifts to increasing disease burden and healthcare spending. As the demand for accurate, rapid, and accessible diagnostic testing continues to rise, the market will witness continuous evolution and expansion. Stakeholders in the healthcare ecosystem must recognize these drivers to strategically invest, innovate, and capitalize on emerging opportunities within the IVD space.
0 notes
Text
Regulatory Pathway for In vitro diagnostics (IVD)
Registration in India: IVDs have become integral components of modern healthcare across the globe to support the diagnosis, monitoring, and personalized treatment of diseases. IVDs are also in very high demand in India, where the IVD market was worth about USD 2.64 billion in 2023 and expected to grow at a CAGR of 7.17% from 2024 until 2030 by Scania Analytics. As a result, the demand, interest, and enthusiasm of foreign IVD manufacturers to register and enter the Indian market have peaked.
However, before IVD devices can be introduced to India, manufacturers and importers must navigate a complex regulatory pathway. Moreover, understanding the registration process is crucial for entry into the Indian marketplace. This blog will help manufacturer and importers understand the IVD registration process in India, discussing the critical components necessary obtain a market launch in India. Keep reading for details.
What are IVD Devices?
IVD Devices (in vitro diagnostics) are instruments employed to analyze biological samples (e.g., blood, tissue, urine, or other bodily fluids) outside of the human body. These tests are integral in providing valuable indications for the diagnosis of disease, monitoring a patient’s condition, quantifying certain substances, and identifying infections. More familiar examples of in vitro diagnostic devices are pregnancy test kits, COVID-19 test kits, blood glucose monitoring kits, immunoassays, and human genetic testing devices.
Which Authority Regulates IVDs in India?
The authority in charge of licensing and regulating IVDS in India is the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare.
How IVDs are classified?
IVDs are classified into four classes based on risk factors:
Class A: Low risk IVDs. Eg. Clinical chemistry analyzer (not near patient testing).
Class B: Low moderate risk IVDs. Eg. Pregnancy test strips, anti-nuclear antibody testing, urine test strips.
Class C: Moderate high risk IVDs. Eg. Blood glucose self-testing system, Human leukocyte antigen (HLA) typing, prostate-specific antigen (PSA) screening.
Class D: High risk IVDs. Eg. HIV blood donor screening, ABO/Rh(D) blood grouping analyser (near patient testing).
Central Licensing Authority’s Role in Regulating IVDs
The Central Licensing Authority is responsible for:
• Providing a license for the import of all classes of IVDs.
• Regulating the manufacture of Class C and Class D (high risk) IVDs.
• Providing the approval for clinical performance evaluation studies for new IVDs before they are allowed to be marketed in India.
• Working with State Licensing Authorities to ensure that there is consistent oversight of IVDs post-marketing.
Responsibilities of State Licensing Authority in Controlling IVDs
According to the Medical Devices Rules, 2017, the responsibilities of the State Licensing Authority are:
• To grant the licenses for the manufacture, sale or distribution of Class A or Class B (low risk) In Vitro Diagnostic medical devices in their state.
• To regulate the sale, stocking, exhibition and distribution of IVDs of all classes in their states.
How to register an IVD in India?

A manufacturer, importer or distributor can register an IVD in India by following these steps:
• Identify the Class of IVD: Identify the risk class for the device/personnel E or fQA Yo can more work.
• Designate the Authorized Indian Rep(s): Authorized agent if the applicant is a foreign manufacturer.
• Complete the Application Form: along with all supporting documents, for the developer or authorized agent on the SUGAM Portal:
Manufacturer: Applies for the Manufacturing license on Form MD-3orMD-4--Class A & B apply on Form MD-3 or MD-4. Class C and D manufacturers, MD-7 or MD-8.
More information about how to obtain a device manufacturing license can be read in our article, “5 Easy Steps To Obtain Your Medical Device Manufacturing License.”
Importers: Can apply for not only the manufacture device license but the device Import license using Form MD-14.The process of obtaining a licensing device can be perused at our blog, “Medical Devices Registration for Import.”
• Application for Review: The CDSCO will review the documents and may inspect overseas manufacturing. For example, review of importers data or evidence, while for manufacturers for India, will assign a State Licensing Authority (SLA)/ CLA a full site inspection process.
• License Issuance: Subject to all requirements being fulfilled, if the CDSCO is satisfied, a manufacturing or import license will be issued that is valid for five years unless otherwise noted.
How Regulatory Solutions India Can Help You?
If you have not registered your IVDs in India yet, RSI can assist you through the whole process. Having more than ten years in the medical device regulatory process, we have completed over 450 medical device registrations in all categories.
We have worked with clients in over 20 countries and can assist with everything from submission to regulatory approval in an effort to facilitate the approval process and secure your place in the market quicker. Let RSI help with gathering your IVD registration in India, contact us today!
#regulatory solutions india#regulatory consultancy in india#IVD#InVitroDiagnostics#MedicalDevices#FDA#RegulatoryAffairs#IVDR#MedTech#Biotech#HealthcareCompliance#QualityAssurance#MedicalDeviceRegulation#ClinicalDiagnostics#FDA510k#CEMarking#HealthcareInnovation
0 notes
Text
U.S. Point-of-Care Molecular Diagnostics: Adoption Trends, Regulatory Landscape, Market Potential
The U.S. point-of-care molecular diagnostics market size was estimated at USD 3.35 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 1.7% from 2024 to 2030. The growth of the market is driven by rise in end-user awareness, along with the increased efficiency of the testing methods and favorability for non-laboratory testing. For instance, in the U.S., approximately 1% infants are diagnosed with congenital heart diseases (CHDs) every year. However, advancements in prenatal testing have led to rapid detection of genetic biomarkers in embryos, thus leading to early diagnosis of CHDs and other genetic disorders.

Market Concentration & Characteristics
Degree of Innovation: Market growth stage is low, and pace of market growth is accelerating. The U.S. point-of-care molecular diagnostics market is characterized by a moderate-to-high degree of innovation owing to the advancements in technology, including the introduction of next-generation sequencing (NGS)-friendly applications. Previously, POC testing has seen barriers in NGS testing, and market players as well as academic institutions have focused on relentless research and development efforts to overcome these.
Players in the point-of-care molecular diagnostics market leverage market strategies such as mergers, acquisitions, collaborations, and partnerships to promote reach of their offerings and increase their product capabilities in the region. For instance, in 2022, Bio-Rad Laboratories acquires Curiosity Diagnostics, integrating Curiosity’s rapid PCR system, PCR|ONE, to expand its presence in near-patient molecular diagnostics labs.
Key U.S. Point-of-Care Molecular Diagnostics Company Insights
Some of the key players operating in the U.S. point-of-care molecular diagnostics market include Abbott Laboratories; QIAGEN; F. Hoffman-La Roche Ltd; bioMerieux; and BD. Numerous key players are undertaking strategic initiatives such as expansion, product introductions, mergers, and acquisitions, while also focusing on increasing product reach in the U.S. Several market participants are investing in research and development initiatives, making the market susceptible to further growth. For More Details or Sample Copy please visit link @:U.S. Point-of-Care Molecular Diagnostics Market
#PointOfCareDiagnostics#MolecularDiagnostics#POCT#HealthcareMarket#MedicalTechnology#InVitroDiagnostics#HealthcareTrends#MedTech#Biotech#HealthcareInnovation#MarketGrowth#InvestmentOpportunities
0 notes
Text
Application of Micro Diaphragm Pumps in Waste Liquid Disposal Systems of Fully Automated Biochemical Analyzers

In the field of modern medical diagnosis, fully automatic biochemical analyzers serve as the core equipment for in vitro diagnostic (IVD) technology. Their accuracy and reliability directly impact the precision of clinical decisions and the efficiency of patient treatments. The waste liquid disposal system is an indispensable component of fully automatic biochemical analyzers, and its performance significantly influences the overall operational efficiency of the instrument and the reliability of test results. TOPSFLO, as an outstanding manufacturer providing high-performance pump solutions for medical industry, has launched TF30 series diaphragm liquid pumps and TMD40 series dual-head diaphragm vacuum pumps. With their exceptional corrosion resistance, precise flow control, and superior airtightness, these pumps are specifically designed for the waste liquid disposal system of fully automatic biochemical analyzers, aiming to provide ideal solutions.

1. Corrosion resistance: Customized materials compatible with a variety of solvents
In fully automatic biochemical analysis, waste liquids may contain complex components such as various chemical reagents, serum, urine, etc., which are often highly corrosive. Special attention must be paid to the material selection of key components such as pump heads, diaphragms, and valves when selecting pumps. TOPSFLO offers customization services, selecting more corrosion-resistant materials based on the specific solvent characteristics used by customers, such as PPS pump heads, PTFE, EPDM, FPM, FFPM diaphragms, and valve materials. This ensures that the pumps are not affected by corrosion during long-term operation, extending their service life, reducing maintenance costs, and ensuring the continuous and stable operation of the system.
2. Efficient Flow Output: Enhancing Detection Efficiency
The stable and efficient flow output is a crucial prerequisite for the efficient operation and precise analysis of fully automated biochemical analyzers. In the process of waste liquid extraction and disposal, TF30 series diaphragm liquid pumps can achieve a maximum flow rate of 400ml/min, can efficiently extract waste liquid from the reaction cups and the entire piping system, effectively preventing the retention and accumulation of waste liquid, thereby ensuring the smooth and accurate flow of analytical process.

TOPSFLO vacuum pump TMD40 series has a maximum flow rate of 17L/min and a maximum vacuum of -95kPa. This extraction efficiency means that waste liquid inside the instrument can be rapidly and thoroughly removed in a very short time, greatly improving the efficiency of waste discharge and providing valuable time for subsequent testing steps. The indirect extraction operation ensures that the waste liquid never passes through the pump chamber, not only reducing the maintenance requirements for the pump but also significantly extending its service life, thus lowering long-term usage costs and maintenance burdens.

3. Outstanding Airtightness: Ensuring Consistent and Reliable Testing Results
In fully automatic biochemical analyzers, good airtightness of micro diaphragm pumps is crucial for ensuring the accuracy, reliability, and reproducibility of test results. TOPSFLO adopts advanced sealing technology and high-quality seals to ensure no leakage during pump operation, effectively preventing the intrusion of external contaminants and the leakage of internal media, thereby maintaining the purity and stability of the analysis environment. This not only enhances the overall performance of the instrument but also reduces test errors and waste liquid contamination caused by airtightness issues, providing a more reliable basis for clinical decisions.
Conclusion
TOPSFLO micro diaphragm pumps have become the ideal choice for pumps in the waste liquid disposal system of fully automatic biochemical analyzers due to their exceptional corrosion resistance, efficient flow output, and high airtightness. We are fully aware that every detail in the field of medical diagnosis is related to life and health. Therefore, we are committed to providing the highest quality pumping products to contribute to the continuous progress of in vitro diagnostic technology and the cause of human health. If you are looking for ideal pumping solutions to enhance the performance of in vitro diagnostic equipment, TOPSFLO will be your trustworthy partner.
TOPSFLO diaphragm pumps is the ideal choice for pumps in the waste liquid disposal system of fully automatic biochemical analyzers due to their exceptional corrosion resistance, efficient flow output, and high airtightness. We are fully aware that every detail in the field of medical diagnosis is related to life and health. Therefore, we are committed to providing the highest quality pumps to support the continuous advancement of in vitro diagnostic technologies and contribute to the cause of human health. If you are looking for ideal pump solutions to enhance the performance of in vitro diagnostic equipment, TOPSFLO will be your trustworthy partner.
Wanna to get custom pump service? Feel free to contact us now:
Email: [email protected]
Whatsapp/Wechat:+86-19376691419
Visit our Web: https://www.topstec.com/ | http://www.topsflo.com/
youtube
youtube
youtube
#diaphragm pump#diaphragmliquidpump#miniature diaphragm pump#diaphragmairpump#diaphragm air pump#InVitroDiagnostics#WasteLiquidDisposal#airpump#liquidpump#gaspumps
0 notes
Text
youtube
𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬 𝐨𝐟 𝐭𝐡𝐞 𝐈𝐧 𝐕𝐢𝐭𝐫𝐨 𝐃𝐢𝐚𝐠𝐧𝐨𝐬𝐭𝐢𝐜 𝐌𝐚𝐫𝐤𝐞𝐭
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐅𝐑𝐄𝐄 𝐒𝐚𝐦𝐩𝐥𝐞: https://www.nextmsc.com/in-vitro-diagnostics-ivd-market/request-sample
Welcome to our latest video on the 𝐈𝐧-𝐕𝐢𝐭𝐫𝐨 𝐃𝐢𝐚𝐠𝐧𝐨𝐬𝐭𝐢𝐜 (𝐈𝐕𝐃) 𝐌𝐚𝐫𝐤𝐞𝐭!
In this comprehensive analysis, we dive deep into the current trends, growth drivers, and future outlook of the IVD industry by 2030.
𝐖𝐡𝐚𝐭 𝐘𝐨𝐮'𝐥𝐥 𝐋𝐞𝐚𝐫𝐧:
𝐌𝐚𝐫𝐤𝐞𝐭 𝐎𝐯𝐞𝐫𝐯𝐢𝐞𝐰: Get a clear understanding of the IVD market landscape, including key segments and geographical insights.
𝐆𝐫𝐨𝐰𝐭𝐡 𝐓𝐫𝐞𝐧𝐝𝐬: Explore the factors driving growth, from technological advancements to rising demand for early disease detection.
𝐌𝐚𝐣𝐨𝐫 𝐏𝐥𝐚𝐲𝐞𝐫𝐬: Discover who the leading companies are and how they’re shaping the market.
𝐅𝐮𝐭𝐮𝐫𝐞 𝐏𝐫𝐞𝐝𝐢𝐜𝐭𝐢𝐨𝐧𝐬: What can we expect in the coming years? We provide forecasts and potential developments in the IVD field.
Whether you're an industry professional, a researcher, or just curious about the latest in medical diagnostics, this video offers valuable insights and up-to-date information.
Don't forget to like, comment, and subscribe for more content on market trends and industry analyses. Hit the notification bell so you never miss an update!
#invitrodiagnostics#healthcare#medicaldevices#marketresearch#markettrends#businessinsights#marketanalysis#marketgrowth#Youtube
0 notes
Text
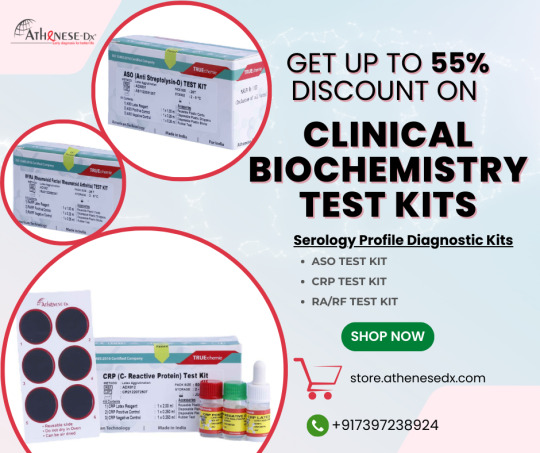
Most Popular IVD Biochemistry Reagents and Test Kits in June 2024
Hello there!
Athenese Dx - Early Diagnosis Medical Equipment & IVD Test Kits Provider. Athenese-Dx specializes in the research, development, manufacture, and marketing in vitro diagnostic tests and Instruments. We are developing high-quality IVD tests in Clinical Chemistry, Rapid Tests, ELISA, Fluorescence Immunoassay, Molecular Diagnostics, and Instruments. Our R&D Unit is recognized by the Department of Scientific and Industrial Research (DSIR).
Biochemistry Kits and Reagents:
Indigenous, highly stable, liquid-based clinical chemistry reagents with the brand name TRUEchemie. TRUEchemie kits are suitable for semi-automated analyzers and fully automated instruments. TRUEchemie Biochemistry Reagents.
Currently, we are selling following the disease categories and their biochemistry reagents,
Diabetic Profile
Lipid Profile
Cardiac Profile
Pancreatic Profile
Renal Profile
Immunoturbidimetry Products
Liver Profile
Electrolytes Profile
Serology Profile
Special Parameter
Diabetic Profile:
Diabetic Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Diabetic Profile helps monitor blood sugar levels and keep track of overall bodily functions. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Lipid Profile:
Lipid Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Lipid Profile looks at the levels of cholesterol and other fats in your blood. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Cardiac Profile:
Cardiac Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Cardiac Profile is used to check the overall functioning of the heart. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Pancreatic Profile:
Pancreatic Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Pancreatic Profile helps to measure the levels of tumour markers in the blood, urine, and other body fluids. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Renal Profile:
Renal Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Renal Profile Tests are used to determine how well the kidneys are functioning Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Immunoturbidimetry Products:
Immunoturbidimetry Products Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Immunoturbidimetry Products Test Kit Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Liver Profile:
Liver Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Liver Profile Test Kit helps to measure several substances made by your liver. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Electrolytes Profile:
Electrolytes Profile Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. Electrolytes Profile is a blood test that measures levels of the body’s main electrolytes. Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Serology Profile:
Serology profile diagnostic kits play a crucial role in detecting antibodies or antigens in blood serum, aiding in the diagnosis of infectious diseases. Our Biochemistry kits are Indigenous, highly stable, liquid-based clinical chemistry reagents with the brand name TRUEchemie.
TRUEchemie IVD Test Kits are suitable for semi-automated analyzers and fully automated instruments. Athenese-Dx offers simple, fast, and efficient serology testing for specific proteins or antibodies in human serum.
Here Athenese-Dx refers to some Biochemistry Reagents and Test Kits to diagnose a better life:
ASO Test Kit:
The TRUEchemie ASO Test Kit is for the qualitative and semi-quantitative determination of ASO in human serum.

https://store.athenesedx.com/product/aso-test-kit/
CRP Test Kit:
The TRUEchemie CRP (C-Reactive Protein) Test Kit (Latex Agglutination Method) is for the qualitative and semi-quantitative determination of CRP in serum.

https://store.athenesedx.com/product/crp-test-kit/
RA/RF Test Kit:
The TRUEchemie RF / RA (Rheumatoid Factor / Rheumatoid Arthritis) test Kit is for the qualitative and semi-quantitative determination of RF/RA in serum.
https://store.athenesedx.com/product/ra-rf-test-kit/
Buy Serology profile diagnostic kits with more than a 55% discount. Suitable for all laboratory settings. Click the link below to avail yourself of test kits at discounted rates at the Athenese-Dx Store.

Buy Now → https://store.athenesedx.com/
Special Parameter:
Special Parameter Diagnostic Kits | Clinical Biochemistry Test Kits | Athenese Dx. The special parameter of the ADA Test Kit is used for the direct quantitative determination of Adenosine Deaminase (ADA) in Human Serum/Plasma/CSF/Pleural Athenese Dx’s Biochemistry Test Kit products would help to find such diseases.
Limited Offer!!! End Soon!!!
#lab equipment#clinical chemistry#digital pathology market#hospital#pathologist#ivd#pathologylab#pathology#athenesedx#biochemistry#IVD#invitrodiagnostics#rapidtest#elisa#molecular#ivdinstruments#LabTech#labtests#labtesting#labtested#dengue#india#malaria#typhoid#typhoidfever#denguetestkit#rapidtestkit#rapidkit#rapid#medicaltestkit
0 notes
Text
#invitro#invitrodiagnostics#invitrofertilization#invitrofertilizationmicroscope#microscope#invitrofertilizationmarketreport#invitrofertilizationmarketresearch#invitrofertilizationmarketsize#invitrofertilizationmarketshare#invitrofertilizationmarketgrowth#invitrofertilizationmarketanalysis#invitrofertilizationmarketforecast#grandresearchstore
0 notes
Text
#invitrodiagnostics#invitro#bnw#infertilidad#ce#ar#nb#ivd#medicaldevice#europe#cemarking#ns#regulatoryaffairs#regulatory#medtech#equitment#eunews#compliance#meddev#manufacturer#safety#covid#cemark#regulation#qms#iso#eumarket#healthindustry#regulatorynews#devices
0 notes
Text
What is the meaning of sensitivity and linearity ?#anamol #healthcareresearch #medicineexplained
🔬 Decoding Sensitivity and Linearity in Quantitative and Qualitative Assays! 🔬
In today's video, we're describing the meaning of sensitivity and linearity in quantitative and qualitative assays.
WATCH FULL VIDEO: https://youtu.be/MH6wgM4I0Zc
💡 Who is this video for? 💡
This video is tailored for medical professionals, students, researchers, and anyone interested in the inner workings of diagnostic tests. Whether you're a seasoned healthcare expert or a curious individual eager to learn, this content will enrich your understanding of diagnostic testing. Do you have questions or specific topics you'd like me to cover in future videos? Leave a comment below, and we'll make sure to address them in upcoming content.
🌐 Follow us on social media for more insightful content:
Youtube:https://www.youtube.com/c/anamollaboratoriesprivatelimited/featured
Facebook: https://www.facebook.com/anamollabs
Instagram: https://www.instagram.com/anamol_biotechnology/ Twitter: https://twitter.com/anamollabs?lang=en
LinkedIn: https://www.linkedin.com/company/anamollabs
0 notes
Text
In Vitro Diagnostic (IVD) Reagents Market Size, Overview, Share and Forecast 2031
#InVitroDiagnostic(IVD)ReagentsMarket#InVitroDiagnostic(IVD)ReagentsMarketSize#InVitroDiagnostic(IVD)ReagentsMarketShare#InVitroDiagnostic(IVD)ReagentsMarketScope
0 notes
Text

In Vitro Diagnostic Market
The global 𝐈𝐧 𝐕𝐢𝐭𝐫𝐨 𝐃𝐢𝐚𝐠𝐧𝐨𝐬𝐭𝐢𝐜 (𝐈𝐕𝐃) 𝐦𝐚𝐫𝐤𝐞𝐭 is estimated at approximately ~USD 87 billion in 2023 and is projected to reach ~USD 120 billion by 2030, expected to grow at a CAGR of ~6% during the forecast period, 2024-2030. 𝐂𝐥𝐢𝐜𝐤 𝐭𝐡𝐞 𝐥𝐢𝐧𝐤 𝐭𝐨 𝐚𝐜𝐜𝐞𝐬𝐬 𝐭𝐡𝐞 𝐜𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐫𝐞𝐩𝐨𝐫𝐭: https://lnkd.in/gqz7EpwH 𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐂𝐮𝐬𝐭𝐨𝐦𝐢𝐳𝐞𝐝 𝐑𝐞𝐩𝐨𝐫𝐭: https://lnkd.in/d6q8Q-5a 𝐊𝐞𝐲 𝐏𝐥𝐚𝐲𝐞𝐫𝐬 • Roche Diagnostics • Abbott Diagnostics • Siemens • Johnson & Johnson • Beckman Coulter • BioMerieux 𝐈𝐕𝐃 𝐌𝐚𝐫𝐤𝐞𝐭 𝐎𝐩𝐩𝐨𝐫𝐭𝐮𝐧𝐢𝐭𝐢𝐞𝐬 • Growing adoption of Point-of-care testing • High growth potential of emerging markets • Advanced technology integration opens up new growth avenues • Rising targeted therapies are boosting demand for companion diagnostics 𝐔𝐩𝐜𝐨𝐦𝐢𝐧𝐠 𝐌𝐚𝐫𝐤𝐞𝐭 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐒𝐭𝐮𝐝𝐢𝐞𝐬 • Hepatitis Testing Market • Lateral Flow Assays Market 𝐅𝐨𝐫 𝐦𝐨𝐫𝐞 𝐝𝐞𝐭𝐚𝐢𝐥𝐬 𝐚𝐧𝐝 𝐩𝐞𝐫𝐬𝐨𝐧𝐚𝐥𝐢𝐳𝐞𝐝 𝐢𝐧𝐬𝐢𝐠𝐡𝐭𝐬, 𝐫𝐞𝐚𝐜𝐡 𝐨𝐮𝐭 𝐭𝐨 𝐮𝐬 𝐭𝐨𝐝𝐚𝐲! 𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐬 - https://lnkd.in/dcsjmSdc
#IVD #InVitroDiagnostics #MedicalDiagnostics #HealthcareInnovation #IVDMarket
0 notes
Text
Regulatory Pathway for In Vitro Diagnostics (IVDs) | FDA 510(k), EU IVDR, Compliance Guide
Are you working in MedTech or regulatory affairs and need clarity on how In Vitro Diagnostics (IVDs) are approved and brought to market?
In this post (and video), we explain the entire regulatory pathway for IVDs, covering:
🔹 What are IVDs? 🔹 Classification of IVDs (Class A, B, C, D) 🔹 FDA 510(k), PMA, and EUA pathways 🔹 EU IVDR and CE marking 🔹 Clinical evaluation, labeling, and compliance
Whether you're a beginner, student, or experienced professional, this breakdown will help you stay compliant and competitive in the medical device industry.

#regulatory solutions india#regulatory consultancy in india#IVD#InVitroDiagnostics#MedicalDevices#FDA#RegulatoryAffairs#IVDR#MedTech#Biotech#HealthcareCompliance#QualityAssurance#MedicalDeviceRegulation#ClinicalDiagnostics#FDA510k#CEMarking#HealthcareInnovation
0 notes
Text
Food Allergy Diagnostics And Therapeutics Market Insights: Detailed Overview of Market Size, Share, Projected Growth
The global food allergy diagnostics and therapeutics market size is expected to reach USD 13.28 billion by 2030, registering a CAGR of 8.0% during the forecast period, according to a new report by Grand View Research, Inc. The market is expected to grow in the coming years owing to increasing incidence of food allergies coupled with rising demand for products for management of these conditions.

Food Allergy Diagnostics And Therapeutics Market Report Highlights
Therapeutics segment dominated the market in 2023 due to growing demand for antihistamines and epinephrine auto-injector
Diagnostic segment includes instruments, consumables, and services, wherein consumables expected to grow at the fastest CAGR during the forecast period
The most common tests used to diagnose food allergies are skin prick tests and blood tests
Peanut allergen source segment is anticipated to register lucrative growth over the forecast period, owing to novel product launches coupled with rising incidence of peanut allergies worldwide.
Hospitals & clinics dominated the food allergy diagnostics & therapeutics market in 2023 owing to increase in number of hospitalizations due to severe allergic conditions
For More Details or Sample Copy please visit link @: Food Allergy Diagnostics And Therapeutics Market Report
Medical was the largest application for PEG, accounting for more than 40% of market share in 2022. Superior blending, hygroscopicity, and non-toxic properties of PEG have resulted in high demand for the chemical in numerous pharmaceutical products such as tablets and ointments. Increasing pharmaceutical expenditure, particularly in emerging economies of India, China, and Brazil, is expected to boost PEG market over the forecast period. Growing demand for paints & coatings coupled with the increasing use of PEG as a solvent due to its low VOC emissions is anticipated to fuel market growth over the next six years.
However, administering epinephrine using an auto-injector during the early stage of anaphylaxis might help avoid any serious complications. Treatment for food allergy costs around USD 25 billion annually in the U.S. Development of cost-effective in vitro diagnostic tests for detection of allergy is expected to fuel the demand for diagnostic products.
List of major companies in the Food Allergy Diagnostics And Therapeutics Market
Astellas Pharma, Inc.
Aimmune Therapeutics
Meridian Medical Technologies
ALK-Abelló Ltd
Medeca Pharma AB
bioMeriux SA
Omega Diagnostics Group PLC
HYCOR Biomedical
HOB Biotech Group Corp Ltd.
Cambridge Allergy Ltd
Medeca Pharma AB
For Customized reports or Special Pricing please visit @: Food Allergy Diagnostics And Therapeutics Market Analysis Report
We have segmented the global artificial food allergy diagnostics and therapeutics market based on product, allergen source, end-use, and region.
#FoodAllergyDiagnostics#FoodAllergyTherapeutics#AllergyTesting#AllergyTreatment#HealthcareInnovation#AllergyDiagnostics#FoodAllergyAwareness#Immunotherapy#Biotechnology#InVitroDiagnostics#ClinicalResearch#FoodSafety#AllergyCare#HealthAndWellness#DiagnosticMarket
0 notes
Photo

World Liver Day is a time to raise awareness about the importance of liver health and the impact of fatty liver disease. This year's theme "Be Vigilant, Do Regular Liver Check-Up, Fatty Liver Can Affect Anyone" emphasizes the need for regular check-ups to catch potential issues early. Let's all join hands to spread awareness and take steps towards a healthier liver and a healthier life.
#worldliverday#worldliverday2023#molecular#moleculardiagnostics#diagnostics#invitrodiagnostics#genes2me
0 notes
Photo
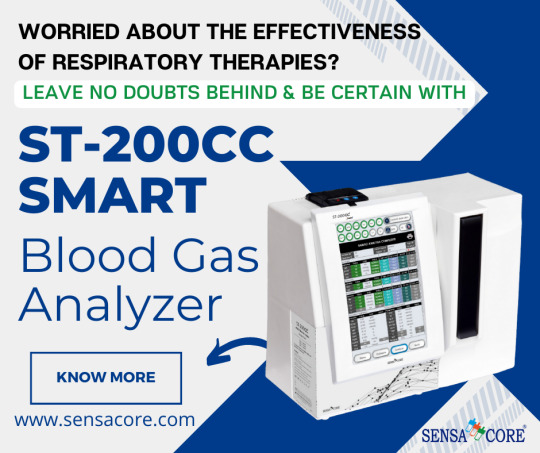
Sensa Core's ST-200CC Smart Blood Gas Analyzer is here to Make Respiratory Therapies More Effective by Delivering Precise Readings of Blood Gas, Electrolytes & Metabolites all in One
Explore Sensa Core’s ABG Machines
#bloodgasanalyzer#respiratorytherapy#st200#ivd#invitrodiagnostics#diagnostics#diagnosticdevice#medicalinstruments#MedicalDevices#medicaldevicemanufacturing#medicalequipment#medicaldiagnostics#healthcare#healthcaredevices#hospitalequipment#laboratoryequipment#makeinindia#sensacore
0 notes
Text

Most Popular IVD Instruments in June 2024 — Medical Laboratory Instruments
Hello there!
In vitro diagnostic (IVD) instruments are designed for clinical labs to obtain diagnostic information from human samples. These instruments play a crucial role in disease detection and monitoring.
Buy ALTA analytical instruments for Medical Lab purposes that are reliable and cost-effective to meet your in-vitro diagnostics needs and its Biochemistry Testing reagents from Athenese Dx.
Here Athenese-Dx includes some IVD clinical instruments to diagnose a better life:
ALTA Rapid Test Reader:
The ALTA Rapid Test Reader (RTR-1) is a highly precise reading device that avoids human error in the interpretation of results in all CTKs and Athenese-Dx Rapid cards. https://store.athenesedx.com/product/alta-rapid-test-reader/
ALTA ELISA Plate Reader:
The ALTA ELISA Plate Reader is a user-friendly microplate analyzer with special filters for up to 4 standard wavelengths and integrated analysis software. It is designed for in vitro diagnostic use to measure and interpret ELISA test results, both monochromatically and dichromatically. https://store.athenesedx.com/product/alta-elisa-plate-reader/
ALTA ELISA Washer:
The ALTA ELISA Washer is an easy-to-use, reliable washer for all microplate ELISA applications. It is intended to be used in laboratories, pathology departments, and hospitals for washing ELISA microplates. The instrument is to be used only by qualified lab technicians and doctors. https://store.athenesedx.com/product/alta-elisa-washer/
ALTA Semi-Auto Chemistry Analyzer:
The ALTA Semi Auto Chemistry Analyzer (ADX-CHEM-220) uses the Photometric principle for in vitro quantitative determination of clinical chemistries in serum, plasma, urine, or cerebrospinal fluid samples. It also helps to determine a few coagulation parameters. https://store.athenesedx.com/product/alta-semi-auto-chemistry-analyser/
ALTA Hematology Analyzer:
The ALTA 3-Part Hematology Analyzer (ADX-HEME-340) uses Electrical Impedance and Photometric (for HGB measurement) principles for detecting whole blood parameters like RBC, WBC, PLT, etc., for Complete Blood Count (CBC). https://store.athenesedx.com/product/alta-hematology-analyzer/
ALTA Fully Auto Chemistry Analyzer:
The ALTA Fully Automated Analyzer helps to measure in vitro quantitative determination of clinical chemistries in serum, plasma, urine, or cerebrospinal fluid samples. The machine consists of a tray where the sample and reagent are loaded. The tests are programmed and carried out in the device without or with minimum human interference. https://store.athenesedx.com/product/alta-fully-auto-chemistry-analyzer/
ALTA Real-Time PCR System RT48:
The ALTA Real-Time PCR Analyzer is fully integrated with a susceptible optical system and a uniform heating system provides quantitative PCR amplification, detection, and data analysis with a throughput of up to 48 samples/run. It delivers sensitive and reliable Detection of multiple targets in a single tube. https://store.athenesedx.com/product/alta-real-time-pcr-system-rt48/
ALTA Real-Time PCR System RT96:
The ALTA Real-Time PCR Analyzer is fully integrated with a susceptible optical system and a uniform heating system provides quantitative PCR amplification, detection, and data analysis with a throughput of up to 96 samples/run. It delivers sensitive and reliable Detection of multiple targets in a single tube. https://store.athenesedx.com/product/alta-real-time-pcr-system-rt96/
ALTA Nucleic Acid Extractor:
ALTA Nucleic Acid Extractor utilizes the proven magnetic-particle technology to extract highly purified nucleic acid from various sample types relevant to molecular diagnostics. Combining easy-to-use instruments, with pre-loaded magnetic particle-based kits ensures rapid nucleic acid extraction and highly purified product. https://store.athenesedx.com/product/alta-nucleic-acid-extractor/
Buy IVD clinical instruments with more than a 40% discount. Suitable for all laboratory settings. Click the link below to avail yourself of test kits at discounted rates at the Athenese-Dx Store.
Buy Now → https://store.athenesedx.com/product-category/instruments/
Limited Offer!!! End Soon!!!
IVD #invitrodiagnostics #athenesedx #rapidtest #elisa #biochemistry #molecular #ivdinstruments #LabTech #labtests #labtesting #labtested #jointogether #dengue #athenesedx #IVD #india #malaria #typhoid #typhoidfever #denguetestkit #rapidtestkit #rapidkit #rapid #medicaltestkit #bloodborne #bloodbornetestkit #hivtestkit #hbsagtestkit
#IVD#invitrodiagnostics#athenesedx#rapidtest#elisa#biochemistry#molecular#ivdinstruments#LabTech#labtests#labtesting#labtested#jointogether#dengue#india#malaria#typhoid#typhoidfever#denguetestkit#rapidtestkit#rapidkit#rapid#medicaltestkit#bloodborne#bloodbornetestkit#hivtestkit#hbsagtestkit#lab equipment#hospital#pathologylab
0 notes