#Endoscope Reprocessing Device Market Analysis
Explore tagged Tumblr posts
Text
Trocars Market : Size, Trends, and Growth Analysis 2032
The Trocars Market was valued at US$ 820.90 million in 2024 and is projected to grow at a CAGR of 5.90% from 2025 to 2032. This growth is propelled by the global shift toward minimally invasive surgeries (MIS), which offer significant benefits such as faster recovery times, reduced patient trauma, and lower hospital costs. Trocars — key components in laparoscopic, thoracoscopic, and endoscopic procedures — play a vital role in enabling this transformation in surgical care.
What Are Trocars?
Trocars are specialized surgical instruments that serve as gateways into body cavities during minimally invasive procedures. A typical trocar assembly includes three parts:
Cannula: The hollow tube inserted into the body, allowing access for instruments.
Seal: Maintains insufflation pressure and prevents fluid or gas leakage.
Obturator: A pointed or blunt-tipped device used to puncture tissue and facilitate cannula insertion.
Trocars are designed to safely introduce surgical tools like cameras, scissors, graspers, and energy devices into the operative field without requiring large incisions, supporting enhanced surgical precision and patient outcomes.
Key Market Drivers
1. Rising Adoption of Minimally Invasive Surgery (MIS)
The growing preference for MIS in treating gastrointestinal, gynecological, urological, and cardiovascular conditions is a key growth factor. These procedures use fewer and smaller incisions, leading to shorter hospital stays and lower risk of complications — advantages that have made them a gold standard in many surgical disciplines.
2. Technological Advancements in Trocar Design
The market has seen continuous innovation, including bladeless and optical trocars that reduce insertion-related injuries. Newer models offer integrated valves, ergonomic handles, and anti-leakage seals, improving surgeon control and patient safety.
3. Increased Surgical Volumes Worldwide
An aging population, rising prevalence of chronic conditions like obesity and cancer, and the growing availability of healthcare services are leading to higher surgical volumes — particularly in Asia-Pacific and Latin America. This directly boosts the demand for reliable and efficient trocar systems.
4. Surge in Outpatient and Ambulatory Surgeries
With healthcare systems encouraging cost-effective treatments, many laparoscopic procedures are shifting to outpatient settings. Trocars enable these surgeries to be performed efficiently and safely with minimal infrastructure.
5. Surgeon Training and MIS Education
Medical education has increasingly integrated laparoscopic techniques into curricula, and professional bodies are encouraging adoption. The resulting increase in trained surgeons is expanding the global MIS footprint.
Product Types and Features
Bladed vs. Bladeless Trocars: Bladed trocars are sharp-tipped and used for easier penetration, while bladeless (dilating tip) trocars minimize tissue trauma and bleeding.
Optical Trocars: Equipped with a clear pathway for endoscope insertion, these allow real-time visualization during entry, reducing the risk of accidental injury.
Disposable vs. Reusable: Disposable trocars ensure sterility and eliminate reprocessing costs. Reusable trocars, while more expensive upfront, are preferred in cost-sensitive facilities.
Insufflation Trocars: These allow the introduction of CO₂ gas to expand the operative field, especially in laparoscopic procedures.
Radially Expanding Trocars: Designed to stretch tissue instead of cutting it, reducing the incidence of hernia formation at the insertion site.
Key Application Areas
General Surgery: Includes appendectomy, hernia repair, and gallbladder removal. These procedures often rely on multiple trocar ports for effective access.
Gynecology: Laparoscopic hysterectomy, ovarian cyst removal, and endometriosis treatment rely heavily on trocars.
Urology: Trocars are essential in prostatectomy and nephrectomy surgeries.
Bariatric Surgery: In weight-loss procedures like sleeve gastrectomy, multiple access points created by trocars facilitate organ resection and stapling.
Pediatric Surgery: Smaller trocar sizes are tailored for child patients, ensuring minimal impact and faster recovery.
Regional Insights
North America: Leads the market due to the high volume of minimally invasive procedures, advanced healthcare systems, and early adoption of innovative surgical technologies.
Europe: Follows closely with strong demand driven by government-funded healthcare, supportive regulatory policies, and the presence of leading manufacturers.
Asia-Pacific: The fastest-growing region, attributed to rising medical tourism, healthcare infrastructure development, and increasing awareness about MIS benefits in countries like China, India, and Japan.
Latin America and Middle East & Africa: These are emerging markets with increasing surgical intervention rates, though limited by affordability and access to advanced medical devices in certain regions.
Competitive Landscape
Global trocar manufacturers are focusing on R&D, product innovation, and strategic partnerships to expand market reach. Key players include:
Medtronic A leader in MIS equipment, Medtronic offers a wide portfolio of bladed, bladeless, and optical trocars with advanced sealing technology for enhanced surgical outcomes.
Johnson & Johnson (Ethicon Division) Offers innovative trocar systems under the Endopath brand, known for ergonomic designs, secure seals, and intuitive insertion mechanisms.
B. Braun Melsungen AG Provides high-precision trocar systems that integrate seamlessly with B. Braun's comprehensive laparoscopic surgery suite.
Teleflex Incorporated Offers disposable trocar products that ensure sterility and efficiency in high-volume surgical centers.
CONMED Corporation Supplies a range of access devices, including optical and radially expanding trocars, emphasizing surgeon comfort and patient safety.
The Cooper Companies, Inc. Through its medical device subsidiary, CooperSurgical, offers gynecology-focused trocar systems designed for precision and safety.
GENICON, INC. Specializes in cost-effective and intuitive laparoscopic access systems that cater to both developed and emerging markets.
LaproSurge and Purple Surgical UK-based manufacturers known for customizable, single-use trocar systems with competitive pricing.
Applied Medical Resources Corporation A well-known brand for its Kii® trocar line, combining high flow, low profile, and safe entry systems.
Trocar Site Closure Systems Focuses on post-procedure closure solutions that complement trocar usage and reduce postoperative complications such as hernias.
Browse more Report:
Translation Management Systems Market
Smart Implants Market
Small Molecule Sterile Injectable Drugs Market
Respiratory Syncytial Virus Therapeutics Market
Pulmonary Fibrosis Biomarkers Market
0 notes
Text
Disposable Endoscopes Market: Emerging Trends Shaping Future Medical Practices
The disposable endoscopes market is rapidly gaining traction within the medical device industry, driven by the increasing demand for cost-effective, safe, and infection-free diagnostic and surgical procedures. Unlike traditional reusable endoscopes, disposable variants offer single-use functionality, eliminating the risk of cross-contamination and reducing the need for complex reprocessing. This market has witnessed significant evolution due to technological innovations, rising healthcare awareness, and growing regulatory support, positioning itself as a transformative force in modern medical diagnostics and treatment.

Market Overview
Disposable endoscopes are being widely adopted across various medical specialties including urology, pulmonology, gastroenterology, and otolaryngology. With healthcare providers under constant pressure to enhance patient safety and streamline operational efficiency, single-use devices have emerged as a practical solution. The demand is particularly high in outpatient settings, emergency rooms, and intensive care units, where rapid turnaround and infection prevention are top priorities.
The global market size for disposable endoscopes has been growing steadily and is expected to continue on an upward trajectory. This growth is largely attributed to the increasing prevalence of hospital-acquired infections, the rising geriatric population, and a broader shift toward value-based healthcare models.
Key Emerging Trends
1. Integration of Smart Technologies
One of the most influential trends in the disposable endoscopes market is the integration of smart and digital technologies. Many new devices now feature advanced imaging capabilities such as high-definition visualization, real-time data transmission, and AI-assisted diagnostics. These innovations are enhancing clinical outcomes by improving diagnostic accuracy and procedure efficiency.
2. Expansion into New Specialties
Initially concentrated in urology and bronchoscopy, disposable endoscopes are now finding applications in other fields such as gastrointestinal endoscopy and ENT (ear, nose, and throat) diagnostics. As manufacturers refine designs to suit the unique needs of each specialty, the scope of application is expanding, bringing with it a broader customer base.
3. Regulatory Momentum and Guidelines
Regulatory agencies around the world are increasingly acknowledging the safety advantages of disposable medical devices. Guidelines promoting single-use endoscopes to reduce infection risks have bolstered their adoption. This regulatory backing has encouraged hospitals to invest more in these devices, especially in high-risk procedures and vulnerable patient populations.
4. Cost-Benefit Analysis Favoring Single-Use
While disposable endoscopes might seem costlier per unit compared to reusable ones, they eliminate hidden costs associated with cleaning, maintenance, repair, and sterilization. Studies and internal hospital audits have begun to reveal the long-term economic advantages of adopting single-use endoscopes, especially when factoring in litigation risks and costs of treating infections.
5. Sustainability Concerns and Eco-Friendly Initiatives
A growing concern with disposable medical devices is environmental sustainability. However, recent advancements have seen the development of recyclable materials and eco-friendly disposal solutions. Companies are increasingly focusing on designing biodegradable or low-impact devices to align with healthcare institutions' environmental goals.
Competitive Landscape
The disposable endoscopes market is highly competitive, with both established medical device giants and innovative startups vying for market share. Leading players are investing heavily in R&D to improve device performance, miniaturize components, and enhance user ergonomics. Strategic partnerships, mergers, and acquisitions are also common, as companies seek to broaden their product portfolios and gain competitive advantages.
Startups are contributing significantly by offering niche solutions with specialized functionalities, catering to underserved segments of the market. This has resulted in a dynamic ecosystem that fosters rapid innovation and responsiveness to clinical needs.
Regional Insights
North America currently holds a significant share of the global disposable endoscopes market, driven by stringent infection control protocols and advanced healthcare infrastructure. Europe follows closely, supported by favorable regulatory frameworks and rising healthcare expenditures. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare access, a growing middle-class population, and expanding hospital networks in countries like China and India.
Future Outlook
The future of the disposable endoscopes market looks promising, with continuous improvements in materials science, imaging technology, and AI integration likely to revolutionize diagnostic and surgical procedures. As healthcare systems worldwide strive for improved outcomes, patient safety, and operational efficiency, the demand for single-use endoscopic devices is expected to rise further.
In the coming years, expect to see greater standardization, cost reduction through mass production, and deeper penetration into low- and middle-income countries. These trends, combined with a proactive regulatory environment and shifting clinical preferences, position the disposable endoscopes market as a pivotal component of the evolving global healthcare landscape.
0 notes
Text
Clean & Critical: Endoscope Reprocessing Market Reaches $4.8B Goal 🔁🧼

As hospitals and clinics strive to reduce the risk of healthcare-associated infections (HAIs), the need for reliable endoscope reprocessing systems — ranging from automated reprocessors and disinfectants to drying cabinets and tracking solutions — has intensified. The goal is clear: prevent contamination, ensure compliance, and boost patient trust in clinical environments.
Market Dynamics
The surge in demand for endoscope reprocessing is being powered by several intertwined dynamics. First, the increasing prevalence of chronic gastrointestinal disorders and cancers is fueling endoscopic procedure volumes. In parallel, global infection control standards are becoming more rigorous, compelling healthcare providers to invest in high-quality reprocessing solutions.
Click to Request a Sample of this Report for Additional Market Insights: https://www.globalinsightservices.com/request-sample/?id=GIS10277
Among the product categories, automated endoscope reprocessors (AERs) are leading the charge due to their efficiency and consistency, helping reduce human error. The trend toward automation is transforming the market, ensuring standardization and compliance across facilities. Complementary technologies like UV-C disinfection and hydrogen peroxide vaporization are also gaining traction.
On the flip side, challenges like high equipment costs, skilled labor shortages, and regulatory complexities pose hurdles. Additionally, the rise of single-use endoscopes could potentially disrupt the market by reducing the need for reprocessing altogether. Nevertheless, the drive toward sustainable and eco-friendly solutions presents fresh opportunities, with companies innovating to meet environmental and economic goals.
Key Players Analysis
The competitive landscape is vibrant, with leading firms like Olympus Corporation, Steris PLC, and Cantel Medical Corporation dominating the market. These companies continue to invest in advanced technologies, R&D, and strategic partnerships to stay ahead. Innovations in endoscope tracking, energy-efficient devices, and smart integration with hospital systems have set them apart.
In addition to established giants, emerging players such as Medi Clean Innovations, Scope Care Systems, and Endo Pure Solutions are making waves with cost-effective, specialized, and eco-conscious offerings. This diversity of players contributes to a dynamic marketplace brimming with innovation and adaptability.
Regional Analysis
North America leads the global market, underpinned by robust healthcare infrastructure, high awareness levels, and strict regulatory oversight. The United States is a standout performer, investing heavily in infection control technologies.
Europe follows closely, with countries like Germany and the UK playing key roles due to their emphasis on healthcare quality and technological innovation. The continent benefits from harmonized standards that support consistent market growth.
Browse Full Report : https://www.globalinsightservices.com/reports/endoscope-reprocessing-market/
Meanwhile, Asia-Pacific is emerging as a powerhouse. Nations like China and India are investing in healthcare modernization, driven by a growing middle class and medical tourism. With improved accessibility and infrastructure, this region is likely to witness exponential growth.
Latin America and the Middle East & Africa show moderate but steady development. While challenges remain in terms of economic and logistical constraints, the growing awareness of infection risks is pushing healthcare providers to upgrade their reprocessing practices.
Recent News & Developments
The past year has seen a flurry of activity in the endoscope reprocessing market. Pricing dynamics are shifting, influenced by the integration of smart features, energy-efficient systems, and compliance-focused design. Costs for AERs now range from $10,000 to $50,000, depending on their capabilities and sophistication.
Regulatory frameworks from agencies like the FDA and EU MDR have become more stringent, demanding robust compliance and spurring innovation in sterilization protocols. Key players are aligning with these regulations by enhancing automation, traceability, and sustainability in their products.
Additionally, strategic mergers and acquisitions are reshaping the landscape. Companies are joining forces to expand global footprints, strengthen product portfolios, and explore untapped regional markets. This consolidation trend is expected to intensify as the market matures.
Scope of the Report
This report offers an in-depth exploration of the endoscope reprocessing market, analyzing trends across product types, technologies, services, applications, and end-users. It maps out the historical journey and provides accurate forecasts from 2025 to 2034, highlighting growth drivers, restraints, competitive strategies, and technological innovations.
The report evaluates key development strategies, including product launches, partnerships, and acquisitions. It provides actionable insights into market segmentation, regional performance, and regulatory landscapes. Stakeholders can leverage this information to navigate challenges, identify growth hotspots, and capitalize on emerging trends.
Whether you’re a manufacturer, healthcare provider, or investor, this comprehensive outlook equips you with the knowledge to make informed decisions in an increasingly complex and competitive market.
Discover Additional Market Insights from Global Insight Services:
Outpatient Oncology Infusion Market : https://www.globalinsightservices.com/reports/outpatient-oncology-infusion-market/
Neurorehabilitation Devices Market : https://www.globalinsightservices.com/reports/neurorehabilitation-devices-market/
Aquaculture Vaccines Market : https://www.globalinsightservices.com/reports/aquaculture-vaccines-market/
Bioanalytical Testing Services Market : https://www.globalinsightservices.com/reports/bioanalytical-testing-services-market/
Biodegradable Implant Devices Market : https://www.globalinsightservices.com/reports/biodegradable-implant-devices-market/
#endoscopereprocessing #healthtech #infectioncontrol #patientcare #medicaldevices #hospitaltech #disinfectiontech #aers #uvdisinfection #hydrogenperoxide #healthcareinfrastructure #automatedreprocessing #cleaningprotocols #gastrotech #pulmonologycare #urologytech #medicalstandards #hospitalequipment #diagnostictech #surgicalsafety #globalhealth #medinnovation #sterileprocessing #regulatorycompliance #healthtechinnovation #medicaltourism #asiahealthcare #northamericahealth #europehealthcare #latamhealth #middleeasthealthcare #reprocessingtech #singleusevsreusable #biomedicaldevices #greenhealthcare #sustainablehospitals #ecofriendlytech #medtechstartups #infectionprevention #endoscopytech #futureofhealthcare
About Us:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC 16192, Coastal Highway, Lewes DE 19958 E-mail: [email protected] Phone: +1–833–761–1700 Website: https://www.globalinsightservices.com/
0 notes
Text
Disposable Endoscopes Market Future Outlook Shows Increased Adoption Due To Infection Control And Cost Efficiency
The global healthcare industry continues to evolve, driven by the need for safer, more efficient, and cost-effective solutions. Among the innovations transforming modern medical practices is the increasing adoption of Disposable Endoscopes Market. These single-use devices are steadily gaining traction across various clinical applications, including bronchoscopy, urology, gastrointestinal (GI) procedures, and ENT (ear, nose, and throat) diagnostics.

Market Drivers and Growth Factors
The shift toward disposable endoscopes is largely driven by infection control concerns and the limitations of traditional reusable endoscopes. In recent years, several outbreaks of infection have been linked to improperly reprocessed endoscopes, despite rigorous cleaning protocols. Disposable endoscopes eliminate this risk by offering a sterile, ready-to-use option for each patient.
Additionally, technological advancements in materials, optics, and manufacturing have made disposable devices increasingly viable alternatives to their reusable counterparts. Today’s single-use endoscopes are more advanced, offering high-definition visualization, greater maneuverability, and ergonomic designs—all at lower costs.
Other key growth factors include:
Increasing number of minimally invasive procedures
Rising demand for outpatient and ambulatory care
Expanding aging population with chronic and degenerative diseases
Growing investments from medical device companies and venture capital firms
Segmental Analysis and Applications
The disposable endoscopes market is segmented based on type, application, end user, and geography. By type, bronchoscopes and urologic endoscopes are among the fastest-growing segments due to high procedure volumes and the critical need for sterility. In terms of applications, diagnostic procedures dominate due to the high frequency of use in detecting abnormalities and infections.
From an end-user perspective, hospitals and ambulatory surgical centers (ASCs) are leading adopters. ASCs, in particular, are pushing the demand for cost-effective, portable, and easily managed equipment that does not require specialized sterilization infrastructure.
Regional Trends
North America currently holds the largest market share, attributed to the region’s well-established healthcare infrastructure, favorable reimbursement policies, and heightened awareness of hospital-acquired infections (HAIs). Europe is also showing steady growth, particularly in countries like Germany and the UK, where healthcare regulations are increasingly emphasizing infection control.
However, Asia-Pacific is projected to experience the fastest growth, fueled by rising healthcare expenditure, government initiatives for modernizing medical equipment, and the expansion of private hospitals and diagnostic centers.
Challenges and Limitations
Despite strong growth potential, the disposable endoscopes market is not without challenges. Concerns around environmental sustainability are beginning to emerge, as the single-use nature of these devices contributes to medical waste. Manufacturers and healthcare providers are exploring recyclable materials and more sustainable disposal methods.
Another concern is the cost perception. While disposable endoscopes eliminate expenses related to reprocessing and maintenance, their upfront cost per use is still higher compared to amortized reusable devices. Educating hospitals and clinicians on the total cost of ownership (TCO)—including potential savings from infection prevention—is critical to increasing adoption.
Future Outlook
Looking ahead, the disposable endoscopes market is expected to witness robust CAGR through 2030. This growth will be shaped by continued innovations, growing preference for outpatient procedures, and regulatory shifts favoring infection prevention. As hospitals and healthcare systems seek greater efficiency without compromising patient safety, disposable endoscopes will increasingly become standard practice.
To sustain this growth, industry players must focus on affordability, environmental responsibility, and clinical performance. The future holds promise not just in high-income regions but also in emerging economies where healthcare access and infrastructure are rapidly advancing.
0 notes
Text
Medical Device Reprocessing Market: Market Trends and Competitive Analysis 2024-2032

The Medical Device Reprocessing Market was estimated at USD 2.69 billion in 2023 and is projected to reach USD 9.63 billion by 2032, growing at a compound annual growth rate (CAGR) of 15.29% during the forecast period of 2024-2032. Get Free Sample Report @ https://www.snsinsider.com/sample-request/3485 Regional Analysis North America: Dominates the market due to advanced healthcare infrastructure and stringent regulatory standards promoting reprocessing practices. Europe: Exhibits significant growth driven by cost-containment measures in healthcare and increasing adoption of reprocessed devices. Asia-Pacific: Anticipated to witness rapid expansion owing to rising healthcare expenditures, growing awareness about reprocessing benefits, and the presence of a large patient pool. Market Segmentation By Type: Reprocessing Support & Services Reprocessed Medical Devices By Device Category: Critical Devices Semi-Critical Devices Non-Critical Devices By Application: Surgical Instruments Endoscopy Equipment Respiratory Care Devices Others Key Players The major key players are Stryker, Innovative Health, NEScientific, Inc., Medline Industries, LP, Arjo, Vanguard AG, Cardinal Health, SureTek Medical, Soma Tech Intl, Johnson & Johnson MedTech and other players. Key Points Reusable medical devices, such as surgical forceps and endoscopes, are essential in healthcare for their cost-effectiveness and utility across multiple patients. Proper reprocessing of these devices is crucial to eliminate contaminants and prevent infections, thereby ensuring patient safety. The increasing prevalence of chronic diseases, like asthma and COPD, underscores the importance of reprocessed surgical instruments in managing healthcare costs and sustainability efforts. Adherence to industry standards and best practices in medical device reprocessing significantly reduces infection risks and enhances patient outcomes. Supply chain challenges, including material shortages and manufacturing complexities, can impact the availability and cost of reprocessed devices. Future Scope The future of the medical device reprocessing market appears promising, with technological advancements enhancing the efficiency and safety of reprocessing methods. Innovations in sterilization technologies and the development of more durable medical devices suitable for multiple reprocessing cycles are expected to drive market growth. Additionally, increasing environmental concerns and the push for sustainable healthcare practices will likely encourage the adoption of reprocessed devices, further propelling the market forward. Conclusion The medical device reprocessing market is set for substantial growth, driven by the need for cost-effective healthcare solutions, environmental sustainability, and stringent infection control measures. As healthcare systems worldwide strive to balance quality patient care with economic and environmental considerations, the role of medical device reprocessing becomes increasingly vital. Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Other Related Reports: Fertility Services Market Medical Power Supply Market Post Traumatic Stress Disorder Treatment Market MRI Guided Neurosurgical Ablation Market
#Medical Device Reprocessing Market#Medical Device Reprocessing Market Share#Medical Device Reprocessing Market Trends#Medical Device Reprocessing Market Size
0 notes
Text
Automated Endoscope Reprocessing Market Analysis: Growth Opportunities and Challenges in 2024 and Beyond
The automated endoscope reprocessing (AER) market is experiencing significant growth as healthcare institutions seek more efficient and effective methods for cleaning and disinfecting endoscopic equipment. With increasing concerns over infection control, technological advancements, and regulatory pressures, the market is poised to grow substantially in 2024. However, several challenges and constraints remain that could impact the pace of this growth.

Growth Drivers
Increased Demand for Endoscopic Procedures The rising number of minimally invasive surgeries, driven by an aging population and advancements in diagnostic techniques, has created an increased demand for endoscopes. Proper cleaning and disinfection of these instruments is pivotal in preventing healthcare-associated infections (HAIs), further driving the need for automated reprocessing systems.
Technological Advancements Innovation in AER technologies has made these systems more efficient, user-friendly, and capable of providing better outcomes regarding time-saving, quality control, and reducing human error. Smart features such as automated tracking, integrated reporting, and sterilization protocols are boosting the demand for these systems in hospitals and clinics worldwide.
Stringent Regulatory Guidelines As healthcare facilities face mounting pressure to adhere to infection control standards, regulatory bodies like the FDA and CDC have implemented stricter guidelines for the reprocessing of endoscopic equipment. Automated systems offer a more reliable and consistent way to comply with these regulations, further driving the market growth.
Focus on Patient Safety Patient safety remains a priority in healthcare settings, and automated endoscope reprocessing systems help minimize the risk of cross-contamination and infection transmission. This increased focus on infection control has made AER systems a vital component in healthcare institutions.
Challenges and Restraints
High Initial Investment Costs One of the primary barriers to the widespread adoption of automated endoscope reprocessing systems is the high upfront costs associated with purchasing and installing these machines. This can be a significant obstacle for smaller healthcare providers or facilities operating on tight budgets.
Technical Limitations Although advancements in AER technology are notable, certain technical limitations still persist. These include challenges related to the reprocessing of complex or delicate endoscopes and the need for ongoing maintenance and calibration to ensure the systems remain efficient and reliable.
Shortage of Skilled Workforce Despite the automation of reprocessing systems, skilled personnel are still required to operate and maintain these devices. The shortage of trained professionals in healthcare settings poses a challenge, particularly in regions with a limited workforce in the medical device field.
Regulatory Compliance and Standards Variability The lack of uniform global standards for automated endoscope reprocessing can create confusion and difficulties for manufacturers, healthcare providers, and end-users. Variability in regulatory requirements across different regions complicates market entry for some companies and may limit the growth of the market in certain areas.
Opportunities
Emerging Markets The market for automated endoscope reprocessing systems is expanding rapidly in emerging economies, particularly in Asia-Pacific and Latin America. These regions are experiencing growing healthcare infrastructure and an increasing demand for better infection control measures, presenting significant growth opportunities for AER manufacturers.
Integration with Hospital Information Systems (HIS) The integration of automated endoscope reprocessing systems with hospital information systems offers a tremendous opportunity for improving workflow efficiency, ensuring better data tracking, and providing a holistic approach to infection control and patient safety.
Sustainability and Eco-Friendly Innovations There is increasing demand for environmentally friendly products in the healthcare industry. Manufacturers are working towards developing automated reprocessing systems that use less water, energy, and chemicals, thereby promoting sustainability while still ensuring effective sterilization.
Post-Pandemic Surge in Healthcare Investment The COVID-19 pandemic has underscored the importance of infection control measures in healthcare. As a result, hospitals and healthcare institutions are expected to increase investment in automated reprocessing systems to improve patient safety and prevent the spread of infections.
Future Outlook
The future of the automated endoscope reprocessing market looks promising as the healthcare sector continues to prioritize patient safety and infection control. The growing emphasis on minimally invasive procedures, technological advancements, and stricter regulatory requirements will drive demand for AER systems. However, challenges such as high costs, workforce shortages, and regulatory variability must be addressed to fully realize the market's potential.
The market is expected to witness significant growth, with key players focusing on innovations to overcome existing barriers and capitalize on emerging opportunities. The integration of AI and machine learning into AER systems, as well as the continued expansion into emerging markets, will be key factors that shape the future landscape of the market.
Conclusion
The automated endoscope reprocessing market is poised for substantial growth in 2024 and beyond, driven by technological advancements, a focus on patient safety, and increasing regulatory pressures. While challenges such as cost, technical limitations, and workforce shortages persist, the market presents significant opportunities for growth and innovation. Manufacturers and healthcare providers must work together to overcome these barriers and unlock the full potential of automated reprocessing systems, paving the way for a safer and more efficient healthcare system.
Request Sample PDF Report: https://www.pristinemarketinsights.com/get-free-sample-and-toc?rprtdtid=NjE2&RD=Automated-Endoscope-Reprocessing-Market-Report
#AutomatedEndoscopeReprocessingMarket#AutomatedEndoscopeReprocessingMarketGrowth#AutomatedEndoscopeReprocessingMarketInsights#AutomatedEndoscopeReprocessingMarketOpportunities#AutomatedEndoscopeReprocessingMarketAnalysis#AutomatedEndoscopeReprocessingMarketDynamics
0 notes
Text
Exploring the Algae Supplements Market: Key Drivers and Emerging Opportunities
An Automated Endoscopy Reprocessor (AER) is a specialized machine designed to disinfect and sterilize endoscopes and other flexible medical instruments, providing an efficient and reliable alternative to manual cleaning. As the demand for endoscopic procedures has increased due to rising cases of gastrointestinal, respiratory, and urinary diseases, the importance of safe, efficient reprocessing methods like Automated Endoscopy Reprocessors has grown. AERs ensure that the devices are disinfected according to high medical standards, reducing the risk of cross-contamination and infection between procedures. These systems offer consistent, controlled cleaning cycles that minimize human error, helping healthcare facilities adhere to strict infection control guidelines.
The Automated Endoscopy Reprocessor Market Size was projected to reach 1.36 billion USD in 2022, according to MRFR analysis. By 2032, the market for automated endoscopy reprocessors is projected to have grown from 1.47 billion USD in 2023 to 2.8 billion USD. During the projected period (2024-2032), the Automated Endoscopy Reprocessor Market is anticipated to grow at a CAGR of approximately 7.45%.
Automated Endoscopy Reprocessor Size and Market Share
The Automated Endoscopy Reprocessor market has seen steady growth in recent years, reflecting the expanding usage of endoscopic procedures globally. Market size is influenced by several factors, including healthcare providers’ focus on infection prevention, advancements in healthcare infrastructure, and the growing awareness about patient safety. In terms of market share, leading manufacturers such as Olympus, Medivators, and Getinge have dominated the market due to their advanced product offerings and established distribution networks. However, the market also includes new entrants bringing innovative solutions to Automated Endoscopy Reprocessor technology, contributing to the market’s size and competitive landscape. The demand for compact, space-saving models has also increased, particularly in smaller healthcare facilities where space constraints are a concern.
Automated Endoscopy Reprocessor Analysis
Analyzing the Automated Endoscopy Reprocessor market involves looking at current trends, regulatory factors, technological advancements, and the regional demands that shape its growth. Technological advancements such as touchscreen interfaces, automated reporting, and reduced cycle times are significant drivers. Additionally, compliance with regulatory bodies like the FDA and ISO is crucial, as these organizations set stringent standards for disinfection processes to ensure patient safety. Regional factors also play a role; for instance, the North American market has seen a high adoption rate due to stringent healthcare regulations, while emerging markets in Asia-Pacific are expected to see growth due to increased healthcare investments and rising awareness of infection control practices.
Automated Endoscopy Reprocessor Trends
Several key trends are currently shaping the Automated Endoscopy Reprocessor market. Firstly, there is a growing demand for eco-friendly and energy-efficient systems as healthcare providers look to reduce their environmental impact. Secondly, automated data tracking and digital connectivity have become more prevalent, allowing facilities to monitor and document disinfection cycles for compliance purposes. Thirdly, the shift toward single-use devices in some regions impacts the AER market, although reusable devices remain prevalent in many areas due to cost efficiency. Additionally, there is an increased focus on user-friendly interfaces and quick turnaround times, which help improve workflow efficiency in busy healthcare settings. These trends indicate a movement toward smarter, safer, and more efficient disinfection systems.
Reasons to Buy the Reports
Gain comprehensive insights into the Automated Endoscopy Reprocessor market, including market size, share, and key growth drivers.
Understand the latest Automated Endoscopy Reprocessor trends, from technological advancements to regulatory factors that influence purchasing decisions.
Access detailed Automated Endoscopy Reprocessor analysis, helping stakeholders make informed investment and strategy decisions.
Learn about recent developments and innovations in the AER market, including environmentally friendly options and smart connectivity features.
Benchmark leading players and assess potential new entrants to understand competitive dynamics in the Automated Endoscopy Reprocessor landscape.
Recent Developments in the Automated Endoscopy Reprocessor Market
Recent advancements in the Automated Endoscopy Reprocessor market include the introduction of devices that support rapid cycle times, allowing for quicker patient turnover in busy healthcare environments. Companies are also focusing on the development of AER systems with integrated data logging and remote monitoring capabilities, which allow facilities to track reprocessing data in real-time and ensure compliance with regulations. Additionally, manufacturers have responded to market demands for compact models that fit smaller facilities without compromising on performance. Sustainability has been another area of focus, with companies like Getinge releasing AER models that use lower amounts of water and chemicals, reducing the overall environmental impact of the reprocessing process. These advancements indicate an ongoing trend toward efficient, eco-friendly, and data-centric reprocessing solutions that cater to the needs of modern healthcare facilities.
In summary, the Automated Endoscopy Reprocessor market is evolving to meet the increasing demand for safe, effective, and efficient disinfection solutions, driven by trends in digital connectivity, environmental sustainability, and enhanced performance.
Relate reports:
pelvic floor stimulator market
pharmaceutical solvent market
plastic surgery instrument market
Top of Form
Bottom of Form
0 notes
Text
Endoscopy Devices Market Competitive Analysis and Forecast 2023-2033
Market Definition
An endoscopy is a medical procedure that involves inserting a thin, flexible tube called an endoscope into the body. The endoscope is equipped with a light and a camera, which allows the doctor to see inside the body. Endoscopy is used to diagnose and treat a variety of conditions.
Market Outlook
There are several key trends in endoscopy devices technology.
One is the miniaturization of endoscopes. This has led to the development of new, smaller devices that can be used for a variety of procedures.
Another trend is the use of new imaging technologies, such as 3D imaging, that provide more detailed images of the inside of the body.
There is also a trend towards the use of more sophisticated devices that can be controlled remotely, which allows for more precise procedures.
The main drivers of the endoscopy devices market are the increasing prevalence of gastrointestinal diseases, the growing geriatric population, the rising number of minimally invasive surgical procedures, and the increasing adoption of endoscopy devices.
The rising prevalence of gastrointestinal diseases is one of the key drivers of the endoscopy devices market. The rising prevalence of gastrointestinal diseases is attributable to the growing geriatric population, the changing lifestyle, and the increasing incidence of obesity.
The growing geriatric population is another key driver of the endoscopy devices market. The elderly population is more susceptible to gastrointestinal diseases.
The rising number of minimally invasive surgical procedures is another key driver of the endoscopy devices market. Minimally invasive surgery is associated with shorter hospital stays, less pain, and faster recovery times.
The increasing adoption of endoscopy devices is another key driver of the endoscopy devices market. Endoscopy is a minimally invasive procedure that allows the visualization of the digestive tract.
To Know More: https://www.globalinsightservices.com/reports/endoscopy-devices-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21225/
Market Segments
The Endoscopy Devices Market is segmented by product, hygiene, application, end user, and region. On the basis of product, the market is categorized into endoscope, mechanical endoscopic equipment, accessories, and other endoscopy equipment. On the basis of hygiene, the market is segmented into single-use, reprocessing, and sterilization. By application, it is classified into bronchoscopy, arthroscopy, laparoscopy, and others. By end user, it is segmented into hospitals, ambulatory surgery centers & clinics, and others. Region-wise the market is divided into North America, Europe, Asia-Pacific, and the Rest of the World.
Request Customization: https://www.globalinsightservices.com/request-customization/GIS21225/
Key Players
The Endoscopy Devices Market includes players such as HOYA Corporation, Olympus Corporation, Stryker Corporation, Boston Scientific Corporation, Fujifilm Holdings Corporation, CONMED Corporation, Medtronic Plc, Karl Storz GmbH & Co. KG, Johnson & Johnson, and Medrobotics Corporation.
Request Discounted Pricing: https://www.globalinsightservices.com/request-special-pricing/GIS21225/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
0 notes
Text
Driving Forces and Future Outlook: Navigating the Competitive Landscape of the Disposable Endoscopes Market
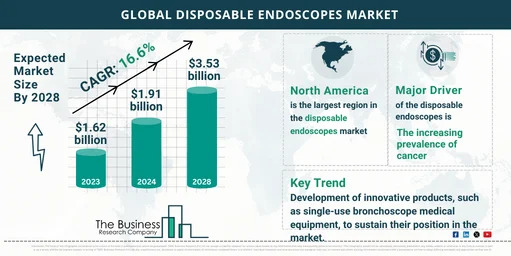
Overview and Scope Disposable endoscopes refer to medical devices designed used on a patient for a single procedure and discarded after use. It is used by physicians to access, visualize and perform procedures in the urinary system and kidney to visualize and examine internal organs, tissues and structures. Sizing and Forecast The disposable endoscopes market size has grown rapidly in recent years. It will grow from $1.62 billion in 2023 to $1.91 billion in 2024 at a compound annual growth rate (CAGR) of 18.1%. The disposable endoscopes market size is expected to see rapid growth in the next few years. It will grow to $3.53 billion in 2028 at a compound annual growth rate (CAGR) of 16.6%. To access more details regarding this report, visit the link: https://www.thebusinessresearchcompany.com/report/disposable-endoscopes-global-market-report Segmentation & Regional Insights The disposable endoscopes market covered in this report is segmented – 1) By Product: Gastroscopes; Bronchoscopes; Duodenoscopes; Laryngoscopes; Colonoscopes; Ureteroscopes; Other Endoscopes 2) By Patient Type: Adult; Pediatric 3) By End User: Hospitals; Ambulatory Surgical Centers; Diagnostic Centers North America was the largest region in the disposable endoscope market in 2023. The regions covered in disposable endoscopes market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. Intrigued to explore the contents? Secure your hands-on sample copy of the report: https://www.thebusinessresearchcompany.com/sample.aspx?id=13009&type=smp Major Driver Impacting Market Growth The increasing prevalence of cancer is expected to propel the growth of the disposable endoscopes market. Cancer is a complex group of diseases characterized by the uncontrolled growth and division of abnormal cells. Disposable endoscopes can provide high-quality imaging capabilities, allowing for more precise observations during endoscopic examinations. This medical equipment aids in detecting cancerous lesions or abnormalities within the gastrointestinal tract. Key Industry Players Major players in the disposable endoscopes market are Medtronic PLC, Fujifilm Holdings Corporation, Baxter International Inc., Boston Scientific Corporation, Olympus Corporation, Steris Corporation, Karl Storz SE & Co. KG, PENTAX Medical, Ambu A/S, Cooper Surgical Inc. The disposable endoscopes market report table of contents includes: 1. Executive Summary 2. Market Characteristics 3. Market Trends And Strategies 4. Impact Of COVID-19 5. Market Size And Growth 6. Segmentation 7. Regional And Country Analysis . . . 27. Competitive Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis Explore the trending research reports from TBRC:
Endoscopy Devices And Equipment Global Market Report 2024 Endoscope Reprocessing Global Market Report 2024
Dermatology Endoscopy Devices Global Market Report 2024 Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected] Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Single Use Medical Device Reprocessing Market is Estimated to Witness High Growth Owing to Opportunity of Cost Savings

Single-use medical devices are medical devices meant for one-time use on a single patient during a single procedure. Reprocessing of such single-use medical devices involves cleaning, disinfecting, inspecting, sterilizing and repackaging these devices to allow their reuse on multiple patients without compromising the safety. This saves significant costs as compared to the purchase and disposal of new single-use devices for each procedure. Reprocessed single-use medical devices are mostly implants such as orthopedic and laparoscopic devices along with other devices such as endoscopes and accessories. Reprocessing provides an inexpensive option with respect to new single-use devices, thereby increasing access to healthcare. The global Single Use Medical Device Reprocessing Market is estimated to be valued at US$ 645.43 Mn in 2023 and is expected to exhibit a CAGR of 15% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights. Market Opportunity: Cost savings offered by reprocessed single-use medical devices present a major opportunity for growth of the single use medical device reprocessing market. Reprocessing of single-use devices can save up to 70% as compared to the prices of new single-use devices. This provides significant costs benefits to hospitals and healthcare facilities especially in emerging economies. The opportunity to reuse expensive implantable devices multiple times at a lower cost helps in improving access to healthcare for more number of patients globally. Also, reprocessing reduces medical waste generation and has environmental benefits which is promoting market growth. Thus, the ability to offer large cost savings to the healthcare system acts as the key growth driver for single use medical device reprocessing market. Porter's Analysis: Threat of new entrants: The threat of new entrants is moderate. The single use medical device reprocessing market requires high capital investment and stringent regulatory norms which makes entry difficult for new players. Bargaining power of buyers: The bargaining power of buyers is high. Large medical facilities and hospitals have significant influence over suppliers in this market due to the high volume of demand. Bargaining power of suppliers: The bargaining power of suppliers is moderate. The market has the presence of both global as well as domestic/regional suppliers. The differentiation in products and services offered also impacts the bargaining power.
SWOT Analysis: Strengths: Offer cost savings to healthcare facilities compared to single use devices. Reprocessing is more eco-friendly and sustainable option versus direct disposal. Weaknesses: Stricter regulatory environment and compliance requirements increase operations costs. Quality concerns and perceived risks regarding reprocessed devices. Opportunities: Growth in medical device consumption and surgical volumes in emerging markets provide new opportunities. Rising environmental awareness and need for sustainability drives the market. Threats: Introduction of stringent regulations around quality and safety of reprocessed devices. Increased preference towards purchase of original single use devices or potential shift to more reusable products. Key Takeaways: The global single use medical device reprocessing market is expected to witness high growth over the forecast period driven by increasing volumes of surgical procedures and rising healthcare costs globally. Regional analysis: Asia Pacific region is anticipated to witness substantial growth during the forecast period. Countries such as China, India, South Korea and Indonesia are major growth engines of the regional market. The growth is attributed to expanding medical infrastructure, improving access to healthcare across rural areas, and growing medical tourism industry in these countries. Key players: Key players operating in the single use medical device reprocessing market are Stryker Corporation, Medline ReNewal, Centurion Medical Products Corporation, Midwest Reprocessing Center, SterilMed Inc., Vanguard AG and Hygia Health Services Inc, among others. Stryker Corporation and Medline ReNewal are the market leaders with extensive service portfolio and global presence.
0 notes
Text
Infection Control Market Emerging Technologies, Developments And Future Plans
Infection Control Market Analysis
Generally, infections result from microorganisms namely fungi, virus and bacteria and is categorized into different types. Some of the most common forms of infections include STDs (sexually transmitted diseases), skin infections, respiratory and lung infections, ear infections, eye infections, common childhood infections, intestinal and stomach infections and HAIs (Hospital Acquired Infections). There are various steps that can control and prevent such infections such as washing hands thoroughly, immunization and using protective clothing mainly gloves and masks while sneezing and coughing. The global infection control market is prognosticated to have a promising growth at an alluring 7.5% CAGR over the forecast period (2017-2023) following the increasing awareness in healthcare sector and burgeoning infection outbreaks.
There are several factors that is fueling the growth of the infection control market. These factors according to the Market Research Future (MRFR) report include increasing incidence of hospital acquired infections, rising surgical procedures, rapidly growing medical device, life science and pharmaceutical industry, growing demand for food sterilization, growing geriatric population, increasing cases of dental caries as well as other periodontal diseases, increasing awareness on infection risks that are healthcare associated, infection control programs for preventing disease transmission, advancements in technology in equipment utilized in sterilization procedures, various initiatives undertaken by the governmental organizations for controlling infections, increasing mergers and acquisitions amid different healthcare companies and rapid product launches. On the contrary, imposition of strict regulations for approval of equipment utilized in sterilization may hamper the growth of the infection control market.
Infection Control Market Segmentation
As per the Market Research Future (MRFR) report, the infection control market is segmented on the basis of type and end-user.
Based on type, it is segmented into sterilization services and products, disinfection products and others. The disinfection products are further segmented into endoscope re-processors, disinfectors, medical nonwovens and disinfectants. Disinfectants are sub-segmented into type, formulation and EPA classification. By type, disinfectants are segmented into surface disinfectants, instrument disinfectants, skin disinfectants and hand disinfectants. By formulation, disinfectants are segmented into disinfectant sprays, disinfectant liquids and disinfectant wipes. By EPA classification, disinfectants are segmented into high-level disinfectants, intermediate-level disinfectants and low-level disinfectants. Medical nonwovens are again segmented into face masks, sterilization wraps, surgical gowns and surgical drapes. The disinfectors are segmented into UV ray disinfectors, flusher disinfectors and washer disinfectors. Endoscope re-processors are segmented into endoscope tracking systems, automated endoscope re-processors and other forms of endoscopic reprocessing products. The sterilization services and products are segmented into contract sterilization services, sterilization equipment by type, consumables and accessories. The sterilization equipment is segmented into radiation sterilization, filtration sterilization, low-temperature sterilization and heat sterilization equipment. The heat sterilization equipment segment is segmented into dry heat sterilizers and heat sterilizers. Low-temperature sterilization is segmented into formaldehyde sterilizers, ozone sterilizers, hydrogen peroxide sterilizers, Ethylene Oxide Sterilizers (EtO) and other forms of low-temperature sterilization. The contract sterilization services segment by type is segmented into steam sterilization services, e-beam sterilization services, gamma sterilization services, Ethylene Oxide Sterilization (EtO) and other forms of contract sterilization services. The consumables and accessories segment are segmented into sterilant cassettes, sterilization indicators and other forms of consumables and accessories.
Based on end-user, the infection control market is segmented into food industry, pharmaceutical companies, medical device companies, life sciences industry, hospitals and clinics and others.
Infection Control Market Regional Analysis
Based on region, the infection control market covers latest trends and growth opportunities across North America, Europe, Asia Pacific and Middle East and Africa. Of these, North America will lead the infection control market over the forecast period due to rising surgical procedures, growing need for the infection control products as well as services to reduce the incidences of HAIs, and growing competition amid key players in the market for proper disinfection and sterilization. The infection control market in Europe will have the second position owing to use of novel therapies, accessibility of advanced and innovative treatment facilities and skilled treatment facilities. In the APAC region, the infection control market will have a promising growth owing to penetration of leading players in this region, aging population and awareness of infections as well as other diseases. Moreover, the growing initiatives taken by WHO (World Health Organization) in countries namely Japan, China and India are anticipated to drive the market growth in the future. The infection control market in the Middle East and Africa will have a steady growth owing to the rising need to diagnose infections in the remote areas coupled with the prevalence of various infectious diseases.
Infection Control Market Key Players
Leading players profiled in the infection control market include Honeywell International, Inc. (U.S.), Ahlstrom Corporation (Finland), Nordion, Inc. (U.S.), Pal Internation (U.K.), Reckitt Benckiser (U.K.), Metrex Research (U.S.), Halyard Health (U.S.), Belimed AG (Switzerland), Matachana (Spain), MMM Group (Germany), Advanced Sterilization Products (U.S.), Sotera Health (U.S.), 3M Healthcare Company (U.S.), Ecolab (U.S.), Cantel Medical Corporation (U.S.), Getinge Group (Sweden), STERIS Corporation (U.K.), and others.
Get Premium Research Report, Inclusive of COVID-19 Impact Analysis, Find more information @ https://www.marketresearchfuture.com/reports/infection-control-market-5901
Dec 2018- America’s the Society for Healthcare Epidemiology has recently issued an expert guidance regarding how healthcare providers and hospitals can reduce infections related to anesthesiology equipment and procedures in an operating room. It suggests steps in improving prevention of infection with the help of continuous improvement plans, environmental disinfection and increased hand hygiene.
#Infection Control Market#Infection Control Market Size#Infection Control Market Share#Infection Control Market Growth#Infection Control Market Analysis
1 note
·
View note
Text
Endoscope Reprocessing Device Market Professional Survey Report 2019
Endoscope Reprocessing Device Market Professional Survey Report 2019
This report focuses on top manufacturers in global market, Involved the assessment of Sales, price, revenue and market share for each manufacturer, covering
Olympus
Medivators
Steris
ANIOS Laboratoires
Wassenburg?Medical
Shinva?Medical
Getinge Infection Control
Belimed
Miele
Choyang Medical
Arc Healthcare
BHT
Medonica
Steelco
Jin Nike
Download FREE Sample of this Report @ https://www.grandresearc…
View On WordPress
#Endoscope Reprocessing Device Market#Endoscope Reprocessing Device Market 2019#Endoscope Reprocessing Device Market Analysis#Endoscope Reprocessing Device Market Growth#Endoscope Reprocessing Device Market Industry#Endoscope Reprocessing Device Market Trends#Global Endoscope Reprocessing Device Business Overview#Global Endoscope Reprocessing Device Industry Overview#Global Endoscope Reprocessing Device Market Analysis
0 notes
Text
Disposable Endoscopes Market Growth Factors, Opportunities, Ongoing Trends and Key Players 2032
As per Future Market Insights’ latest analysis, valuation of the global disposable endoscopes market was around US$ 909.9 Million in 2021. The market is projected to exhibit a CAGR of close to 17.6% from 2022 to 2032 and reach an estimated valuation of around US$ 5.3 Billion in 2032.
Endoscope-related infections can be differentiated into endogenous and exogenous. Presence of microbes that have the ability to form biofilms or adhere to medical devices can possess the ability to cause cross-contamination in healthcare settings. Hence, disposable endoscopes are a better option that allows healthcare facilities to skip the steps of reprocessing and also reduce the chances of acquiring infections. Between 2019 and 2020, in the U.S., there was a 24% increase in central line-associated bloodstream infection (CLABSI), with the largest increase observed in ICU (50%), as per the Centers for Disease Control and Prevention (CDC).
Growing investments in research and development activities & healthcare settings to develop novel products with an emphasis on low-cost product offerings and lesser infection exposure, are also expected to propel the disposable endoscopes market during the forecast period. Emerging startups are focusing on improving features of endoscopes to attract more customers.
The use of ultra-thin polymer fiber allows reaching anatomical sites that were formerly unreachable. Increasing preference among physicians and patients for the detection of cancer & tumors and reducing the risk of infection due to the safety and efficacy of endoscopic procedures would also favor growth in the disposable endoscopes market.
In August 2021, for instance, a customized peregrine endoscope from 3NT was launched to provide clinicians with a close-up view of the difficult-to-reach regions with sinuses to aid in the diagnosis and treatment of conditions, including polyps and cancer.
Key Takeaways: Disposable Endoscopes Market
Disposable bronchoscopes are the leading product type and held approximately 30.1% of the global market share in 2021.
By end user, hospitals are dominating the global disposable endoscopes market and are expected to exhibit a CAGR of 17.5% during the forecast period.
China is one of the leading manufacturers of disposable endoscopes and it held a market share of around 6.7% in 2021.
Germany exhibited a share of around 7.5% in the Europe disposable endoscopes market in 2021.
North America is considered to be the leading region and held a share of 36.8% in 2021.
For More Information : https://www.futuremarketinsights.com/reports/disposable-endoscopes-market
“Growing emphasis on technological advancement and development of cost-effective disposable medical equipment are set to propel sales of disposable endoscopes across the globe,” says an analyst of Future Market Insights.
Competitive Landscape: Disposable Endoscopes Market
The market for disposable endoscopes is highly fragmented with the presence of several local, emerging, as well as established players. Key players are focusing on new product launches with additional features, obtaining regulatory approvals, and increasing their manufacturing capabilities to compete with their rivals.
For instance,
In April 2022, Ambu launched a single-use endoscope and accompanying mA scope, known as Ascope 4 Rhinolaryngo Slim and Aview 2 Advance HD monitor, which would enable fiberoptic endoscopic evaluation of swallowing.
In May 2021, Boston Scientific Corporation attained CE approval for its EXALT Model B. It is a single-use bronchoscope, a device made for bedside operations in the ICU and operating room.
0 notes
Text
Infection Control Market size 2023 to 2031
Newark, New Castle, USA - Growth Plus Reports' most recent study examines the Global Infection Control Market's production, prospective uses, demand, key companies, and SWOT analysis.
The Infection Control Market Report will help you determine the best distribution strategies for specific products and identify potential markets. In addition, the report examines the purchasing and supply patterns that impact the market. The Infection Control market research report provides insights into the limitations, market trends, prospects, drivers, and competition in the Infection Control sector.
You may get insights into the TOC, and Statistics for essential facts, information, trends, and competitive landscape information.
Download Free Sample Report Now @ https://www.growthplusreports.com/inquiry/request-sample/infection-control-market/8187
The following are the leading companies in the Global Infection Control market:
3M Company
Belimed AG
Halyard Health, Inc.
Getinge Group
Cantel Medical Corporation
Advanced Sterilization Products
Matchana Group
Reckitt Benckiser
Sterigenics International
MMM Group
Honeywell International, Inc.
Cantel Medical Corporation
STERIS Corporation
Ecolab
Growth Plus Reports studies the key trends in each category and sub-segment of the Infection Control market, along with Global and regional projections from 2023 to 2031. Our research splits the market into product type and application segments.
SEGMENTATION
GLOBAL INFECTION CONTROL MARKET - ANALYSIS & FORECAST, BY PRODUCT
Sterilization
Steam
Radiation
Sterilant
Indicators
Disinfection
Washers
UV Disinfection
Disinfectants
Endoscope Reprocessing
Protective Barriers
GLOBAL INFECTION CONTROL MARKET - ANALYSIS & FORECAST, BY END-USER
Hospitals & Clinics
Pharmaceutical & Medical Device Companies
Others
For More Information or Query or Customization visit: https://www.growthplusreports.com/inquiry/customization/infection-control-market/8187
Companies may utilize Infection Control market report to get insights on market variables and any restraints that may affect the manufacturing of their product. Companies that are expanding abroad require thorough Global market research that includes real market data to assist with their marketing strategy. This Global market Infection Control industry study analyzes important market dynamics and provides in-depth information and statistics to help companies flourish. This research report on the Infection Control market takes advantage of advanced and professional approaches such as SWOT analysis and GRG Health's unique GrowthMIX strategy.
This report is useful in addressing various essential issues for market participants, while also supporting them in making investments and leveraging the market opportunities.
Infection Control Market TOC: https://www.growthplusreports.com/report/toc/infection-control-market/8187
Market segment by Region/Country including: –
-North America (United States, Canada and Mexico) -Europe (Germany, UK, France, Italy, Russia and Spain etc.) -Asia-Pacific (China, Japan, Korea, India, Australia and Southeast Asia etc.) -South America (Brazil, Argentina and Colombia etc.) -Middle East and Africa (South Africa, UAE and Saudi Arabia etc.)
QUICK BUY: https://www.growthplusreports.com/checkout-8187
Browse Latest Healthcare Reports:
Aortic Valve Replacement Devices Market Industry Analysis report to 2031
Gastrostomy Tube Feeding Devices Market Size, Share & growth report to 2031
Bioabsorbable Stents Market Size, Share, growth & Trends to 2031
Peripheral Nerve Repair Market Size, Share, Demand & Trends to 2031
PARP Inhibitors Market Size, Share & Trends to 2031
Central Venous Catheters Market size, share & growth 2031
Aortic Valve Replacement Devices Market Industry Analysis report to 2031
Bioabsorbable Stents Market Size, Share, growth & Trends to 2031 Peripheral Nerve Repair Market Size, Share, Demand & Trends to 2031
Gastrostomy Tube Feeding Devices Market Size, Share & growth report to 2031
PARP Inhibitors Market Size, Share & Trends to 2031
0 notes
Text
Endoscope Reprocessing Device Market: Key Players, Applications, Outlook, SWOT Analysis And Forecasts to 2032
Robust expansion of biotechnology and pharmaceutical industries across emerging countries is expected to pave significant opportunities for the development and advancements in molecular biology enzymes, kits & reagents. In addition, accelerating adoption of personalized medicines will further boost demand for molecular biology enzymes, kits & reagents. The molecular biology enzymes kits reagents market is predicted to grow at a 15.8% CAGR through 2032.
Adoption of these enzymes and their kits & reagents is expected to witness proliferation with technological advancements dedicated toward the development of innovative and more efficient products, in tandem with surging research and development investments being made by leading pharmaceutical and biotechnology companies.Very recently, there has been an increase in the occurrence of genetic illnesses, which has prompted more people to use molecular diagnostics. Epigenetics and polymerase chain reaction (PCR), two crucial molecular diagnostics procedures, call for molecular biology enzymes, kits, and reagents. The demand for these enzymes and kits & reagents is anticipated to significantly increase in the near future due to rising genetic disorder occurrences and an ageing population.
Future Market Insights has published a new comprehensive research report titled, “Molecular Biology Enzymes, Kits & Reagents Market: Global Industry Analysis (2012-2016) & Opportunity Assessment (2017-2026)”. The report covers present market scenario as well as imparts future growth prospects of the molecular biology enzymes, kits & reagents market for the period between 2017 and 2026.
Request a Sample of this Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-6385
The report also engulfs key drivers, hindrances, opportunities and trends that are affecting expansion of the global molecular biology enzymes, kits & reagents market. The report offers an overall picture of the global molecular biology enzymes, kits & reagents market, in order to help businesses seeking opportunities for making investments in the market.
Structure of the Report
The report provides an exhaustive synopsis of the global molecular biology enzymes, kits & reagents market, engulfing an executive summary that elucidates the core trends influencing the market expansion. This chapter also sheds light on impacts that the dynamics are likely to pose on growth of the market in the long run. The report also imparts figures appertaining to CAGRs from a historical and forecast point of view. An overview of the global molecular biology enzymes, kits & reagents market follows the executive summary, and issues a clear picture of the market’s scope to the report readers.
The overview includes a concise market introduction succeeded by a formal definition of “molecular biology enzymes, kits & reagents”. Chapters subsequent to the overview elaborates several dynamics including driving factors, limitations and prospects being observed in the market through the forecast period. Meanwhile these chapter also inundate detailed insights related to the bottom line of enterprises, global economy and fiscal stimulus.
Request Customization @ https://www.futuremarketinsights.com/customization-available/rep-gb-6385
Market Taxonomy
A segmentation analysis offered in the report propounds forecasts on global molecular biology enzymes, kits & reagents market. Categorizing the market in terms of application, end-user, product type, and region. Analysis on Y-o-Y growth comparison, the market share comparison, and the revenue comparison coupled with relevant market numbers is offered in this chapter. Global market for molecular biology enzymes, kits & reagents has been regionally divided into Japan, Middle East & Africa, Europe, Asia-Pacific excluding Japan, North America, and Latin America.
Region
North America
Latin America
Europe
Japan
APEJ
MEA
Product Type
Kits & Reagents
Modifying Enzymes
Restriction Enzymes
Other Product Types
End User
Pharmaceutical and Biotechnology Companies
Hospitals and Diagnostic Centers
Academic and Research Institutes
Other End Users
Application
Sequencing
Cloning
Polymerase Chain Reaction
Restriction Digestion
Synthetic Biology
Epigenetics
Other
Competition Landscape
This analytical research report on the global molecular biology enzymes, kits & reagents market is a complete package, which includes intelligence on key participants underpinning the market expansion. In the last chapter of the report, which elucidates the competitive scenario of the market, strategies implemented by the market players, along with their product overview, company overview, key financials, key developments and SWOT analysis has been rendered exhaustively. In addition, region-wide spread of these market players, their future expansion plans, market shares, revenues, and mergers & acquisition activities between them have been described in detail in this concluding chapter of the report. An intensity map has been employed in the report to profile the market players situated across geographies.
Request for Report Ask A Question @ https://www.futuremarketinsights.com/ask-question/rep-gb-6385
Research Methodology
Credibility of the researched statistics and data is backed by the unique research methodology employed by the analysts at FMI, which ensures higher accuracy. FMI’s research report on the global molecular biology enzymes, kits & reagents market can assist its readers in gaining detailed insights on many different aspects governing the market around key regional segments included in the report.
The report readers can further slate key strategies for tapping into vital revenue pockets and gaining benefits over the intensifying competition in the market. Information presented in the report has been scrutinized and monitored thoroughly by FMI’s industry experts. Figures and numbers offered in the report have also been validated by the analysts in order to facilitate strategic decision making for the report readers.
0 notes
Text
Rising prevalence of colorectal cancer and other endoscopy-related diseases to boost the growth of endoscope reprocessing device market.

Market Overview:
Endoscope reprocessing devices are those which are made to kill the organisms in the reusable endoscopes using high disinfectants or liquid chemical solutions to expose the exterior surfaces and the interior channels. Manual cleaning, sterilization, documentation, leak testing and pre –cleaning are some of the steps performed in endoscopy reprocessing.
Competitive Landscape:
The major players operating the endoscope reprocessing device market are STERIS Plc., Cilag GmbH International, Steelco S.p.A., Minntech Corp PENTAX Medical, Medivators Inc., Wassenburg Medical B.V., Soluscope, ENDO-TECHNIK W.Griesat GmbH, Johnson & Johnson and Getinge AB.
Key Market Drivers:
Rising prevalence of colorectal cancer and other endoscopy-related diseases are anticipated to augment the growth of the global endoscope reprocessing device market. For instance, as per the American Cancer Society, nearly 97,220 people are diagnosed with colorectal cancer and 43,030 rectal cancer by the end of 2018.
Furthermore, increasing cardiovascular diseases which are treated with using ERs is anticipated to boost the growth of the global endoscope reprocessing device market. For instance, as per OECD, 45,307 people have undergone the coronary heart bypass surgery/operation in Germany as of 2018.
Covid-19 Impact Analysis:
During the pandemic, along with the sterilization the ERs have also increased as the reprocessors have been a crucial part for the endoscopy surgeries which led to the growth of the global endoscope reprocessing device market.
Key Takeaways:
The global endoscope reprocessing device market is expected to exhibit a CAGR of XX % during the forecast period due to frequent approvals, quick launches of products. For instance, in April 2019, Advanced Sterilization Products gained US. FDA, launched for the ASP AEROFLEX automated endoscopes reprocessors.
Among Regions North America, Asia Pacific and Europe are anticipated to witness robust growth in the global endoscope reprocessing device market due to increasing prevalence of diseases, frequent approvals. As per the Globocan, 3,244 people suffer from stomach cancer in Canada and 5,139 people suffer from pancreatic cancer.
0 notes