#Medical Device Reprocessing Market Trends
Explore tagged Tumblr posts
Text
#Medical Device Reprocessing Market#Medical Device Reprocessing Market Share#Medical Device Reprocessing Market Size#Medical Device Reprocessing Market Trends
0 notes
Text
#Reprocessed Medical Devices Market#Reprocessed Medical Devices Market Trends#Reprocessed Medical Devices Market Growth#Reprocessed Medical Devices Market Industry#Reprocessed Medical Devices Market Research#Reprocessed Medical Devices Market Report
0 notes
Text
0 notes
Text
Automated Endoscope Reprocessing Market Overview: Global Industry Snapshot
The Automated Endoscope Reprocessing Market has emerged as a pivotal segment within the broader landscape of medical device disinfection and sterilization. As minimally invasive surgeries (MIS) continue to gain traction, the demand for high-level disinfection of endoscopic instruments has become increasingly critical. Automated endoscope reprocessors (AERs) have thus transformed from optional support systems into indispensable components of modern healthcare infrastructure.

What is Automated Endoscope Reprocessing?
Automated Endoscope Reprocessing refers to the use of specialized machines to clean, disinfect, and dry endoscopes after clinical use. The process ensures a standardized, consistent, and safe method of preparing endoscopes for reuse. AERs reduce the risks associated with human error in manual cleaning and provide traceable documentation to meet stringent regulatory compliance standards.
Market Size and Dynamics
The global Automated Endoscope Reprocessing Market has experienced consistent growth over the past decade. This growth is driven by a combination of rising surgical procedures, hospital-acquired infection (HAI) prevention protocols, and global health initiatives pushing for sterilization automation in medical settings. The market is projected to continue expanding from 2025 to 2032 due to:
Increasing geriatric population and chronic illnesses requiring endoscopy
Surge in demand for MIS procedures
Regulatory enforcement for infection control
Rising investment in hospital infrastructure
As of 2024, the market valuation is estimated to be in the range of USD 1.5 to 2 billion, with robust CAGR expectations of around 8–10% through the forecast period.
Key Market Segments
The Automated Endoscope Reprocessing Market can be segmented based on product types, end users, and regions:
Product Types: Single basin, double basin, and advanced multi-cycle units
End Users: Hospitals, ambulatory surgical centers (ASCs), specialty clinics
Geography: North America leads due to strict regulations and advanced hospital facilities, followed by Europe and Asia-Pacific, where rapid infrastructure growth is boosting demand.
Drivers of Market Growth
Several factors fuel the adoption of automated endoscope reprocessors:
Stringent Regulatory Requirements: Regulatory bodies like the U.S. FDA, CDC, and European CE authorities mandate validated cleaning protocols.
HAI Prevention and Patient Safety: AERs reduce the risk of cross-contamination and infection outbreaks.
Growing Demand for Reusable Endoscopes: Single-use scopes are costlier in the long term; thus, proper reprocessing becomes essential.
Operational Efficiency: Automated systems reduce manpower needs and provide traceable reports for compliance audits.
Challenges in the Market
Despite its rapid growth, the Automated Endoscope Reprocessing Market faces several obstacles:
High Initial Investment: The cost of acquiring and maintaining AER systems may be prohibitive for small healthcare providers.
Complexity in Operation and Maintenance: Technical training and ongoing support are critical to ensure safe and correct use.
Infrastructure Gaps in Low-Income Regions: Limited access to utilities and space can hinder adoption in rural or underdeveloped areas.
Competitive Landscape
Major players in the Automated Endoscope Reprocessing Market include:
Olympus Corporation
Getinge AB
Cantel Medical (Steris)
Ecolab Inc.
Advanced Sterilization Products (ASP)
These companies are investing in technological innovation, AI integration, and ergonomically designed units to offer better cleaning efficiency, traceability, and operator convenience.
Global Trends and Future Outlook
Global trends in the Automated Endoscope Reprocessing Market include the adoption of smart reprocessors with integrated software systems for performance monitoring, IoT-based devices for maintenance alerts, and energy-efficient designs to align with green hospital initiatives.
Moreover, the COVID-19 pandemic has led to heightened awareness of sterilization and disinfection in healthcare settings. The resulting surge in sterilization compliance continues to positively influence market growth even in the post-pandemic era.
Conclusion
The Automated Endoscope Reprocessing Market represents a critical aspect of infection control within the healthcare system. With rising emphasis on patient safety, regulatory compliance, and healthcare automation, the market is poised for significant expansion in the coming years. As technology evolves and cost-effectiveness improves, adoption is expected to widen globally—particularly in emerging healthcare markets seeking to enhance their clinical hygiene standards.
0 notes
Text
Trocars Market : Size, Trends, and Growth Analysis 2032
The Trocars Market was valued at US$ 820.90 million in 2024 and is projected to grow at a CAGR of 5.90% from 2025 to 2032. This growth is propelled by the global shift toward minimally invasive surgeries (MIS), which offer significant benefits such as faster recovery times, reduced patient trauma, and lower hospital costs. Trocars — key components in laparoscopic, thoracoscopic, and endoscopic procedures — play a vital role in enabling this transformation in surgical care.
What Are Trocars?
Trocars are specialized surgical instruments that serve as gateways into body cavities during minimally invasive procedures. A typical trocar assembly includes three parts:
Cannula: The hollow tube inserted into the body, allowing access for instruments.
Seal: Maintains insufflation pressure and prevents fluid or gas leakage.
Obturator: A pointed or blunt-tipped device used to puncture tissue and facilitate cannula insertion.
Trocars are designed to safely introduce surgical tools like cameras, scissors, graspers, and energy devices into the operative field without requiring large incisions, supporting enhanced surgical precision and patient outcomes.
Key Market Drivers
1. Rising Adoption of Minimally Invasive Surgery (MIS)
The growing preference for MIS in treating gastrointestinal, gynecological, urological, and cardiovascular conditions is a key growth factor. These procedures use fewer and smaller incisions, leading to shorter hospital stays and lower risk of complications — advantages that have made them a gold standard in many surgical disciplines.
2. Technological Advancements in Trocar Design
The market has seen continuous innovation, including bladeless and optical trocars that reduce insertion-related injuries. Newer models offer integrated valves, ergonomic handles, and anti-leakage seals, improving surgeon control and patient safety.
3. Increased Surgical Volumes Worldwide
An aging population, rising prevalence of chronic conditions like obesity and cancer, and the growing availability of healthcare services are leading to higher surgical volumes — particularly in Asia-Pacific and Latin America. This directly boosts the demand for reliable and efficient trocar systems.
4. Surge in Outpatient and Ambulatory Surgeries
With healthcare systems encouraging cost-effective treatments, many laparoscopic procedures are shifting to outpatient settings. Trocars enable these surgeries to be performed efficiently and safely with minimal infrastructure.
5. Surgeon Training and MIS Education
Medical education has increasingly integrated laparoscopic techniques into curricula, and professional bodies are encouraging adoption. The resulting increase in trained surgeons is expanding the global MIS footprint.
Product Types and Features
Bladed vs. Bladeless Trocars: Bladed trocars are sharp-tipped and used for easier penetration, while bladeless (dilating tip) trocars minimize tissue trauma and bleeding.
Optical Trocars: Equipped with a clear pathway for endoscope insertion, these allow real-time visualization during entry, reducing the risk of accidental injury.
Disposable vs. Reusable: Disposable trocars ensure sterility and eliminate reprocessing costs. Reusable trocars, while more expensive upfront, are preferred in cost-sensitive facilities.
Insufflation Trocars: These allow the introduction of CO₂ gas to expand the operative field, especially in laparoscopic procedures.
Radially Expanding Trocars: Designed to stretch tissue instead of cutting it, reducing the incidence of hernia formation at the insertion site.
Key Application Areas
General Surgery: Includes appendectomy, hernia repair, and gallbladder removal. These procedures often rely on multiple trocar ports for effective access.
Gynecology: Laparoscopic hysterectomy, ovarian cyst removal, and endometriosis treatment rely heavily on trocars.
Urology: Trocars are essential in prostatectomy and nephrectomy surgeries.
Bariatric Surgery: In weight-loss procedures like sleeve gastrectomy, multiple access points created by trocars facilitate organ resection and stapling.
Pediatric Surgery: Smaller trocar sizes are tailored for child patients, ensuring minimal impact and faster recovery.
Regional Insights
North America: Leads the market due to the high volume of minimally invasive procedures, advanced healthcare systems, and early adoption of innovative surgical technologies.
Europe: Follows closely with strong demand driven by government-funded healthcare, supportive regulatory policies, and the presence of leading manufacturers.
Asia-Pacific: The fastest-growing region, attributed to rising medical tourism, healthcare infrastructure development, and increasing awareness about MIS benefits in countries like China, India, and Japan.
Latin America and Middle East & Africa: These are emerging markets with increasing surgical intervention rates, though limited by affordability and access to advanced medical devices in certain regions.
Competitive Landscape
Global trocar manufacturers are focusing on R&D, product innovation, and strategic partnerships to expand market reach. Key players include:
Medtronic A leader in MIS equipment, Medtronic offers a wide portfolio of bladed, bladeless, and optical trocars with advanced sealing technology for enhanced surgical outcomes.
Johnson & Johnson (Ethicon Division) Offers innovative trocar systems under the Endopath brand, known for ergonomic designs, secure seals, and intuitive insertion mechanisms.
B. Braun Melsungen AG Provides high-precision trocar systems that integrate seamlessly with B. Braun's comprehensive laparoscopic surgery suite.
Teleflex Incorporated Offers disposable trocar products that ensure sterility and efficiency in high-volume surgical centers.
CONMED Corporation Supplies a range of access devices, including optical and radially expanding trocars, emphasizing surgeon comfort and patient safety.
The Cooper Companies, Inc. Through its medical device subsidiary, CooperSurgical, offers gynecology-focused trocar systems designed for precision and safety.
GENICON, INC. Specializes in cost-effective and intuitive laparoscopic access systems that cater to both developed and emerging markets.
LaproSurge and Purple Surgical UK-based manufacturers known for customizable, single-use trocar systems with competitive pricing.
Applied Medical Resources Corporation A well-known brand for its Kii® trocar line, combining high flow, low profile, and safe entry systems.
Trocar Site Closure Systems Focuses on post-procedure closure solutions that complement trocar usage and reduce postoperative complications such as hernias.
Browse more Report:
Translation Management Systems Market
Smart Implants Market
Small Molecule Sterile Injectable Drugs Market
Respiratory Syncytial Virus Therapeutics Market
Pulmonary Fibrosis Biomarkers Market
0 notes
Text
Titanium Recycling Market Growth Analysis, Market Dynamics 2025
The global Titanium Recycling market was valued at US$ 998.43 million in 2023 and is anticipated to reach US$ 1,812.07 million by 2030, witnessing a CAGR of 9.10% during the forecast period 2024-2030.
Get more reports of this sample : https://www.intelmarketresearch.com/download-free-sample/489/titanium-recycling-market
Titanium recycling is the process of recovering and reprocessing titanium and titanium-based alloys from scrap or used products for use in new applications.
Titanium is a valuable and widely used metal in a variety of industries, including aerospace, automotive, and medical devices, but it can be difficult and expensive to extract from natural sources. As a result, there is a growing interest in recycling titanium and titanium-based alloys to reduce the environmental impact and cost of production.
The major global companies of Titanium Recycling include TIMET, Kymera International, Metraco NV, EcoTitanium (Aubert & Duval), Monico Alloys, Baoji Titanium, Mega Metals, United Alloys and Metals, and Globe Metal, etc. In 2023, the world's top three vendors accounted for approximately 12% of the revenue.
This report aims to provide a comprehensive presentation of the global market for Titanium Recycling, with both quantitative and qualitative analysis, to help readers develop business/growth strategies, assess the market competitive situation, analyze their position in the current marketplace, and make informed business decisions regarding Titanium Recycling.
The Titanium Recycling market size, estimations, and forecasts are provided in terms of and revenue ($ millions), considering 2023 as the base year, with history and forecast data for the period from 2019 to 2030. This report segments the global Titanium Recycling market comprehensively. Regional market sizes, concerning products by Type, by Application, and by players, are also provided.
For a more in-depth understanding of the market, the report provides profiles of the competitive landscape, key competitors, and their respective market ranks. The report also discusses technological trends and new product developments.
The report will help the Titanium Recycling companies, new entrants, and industry chain related companies in this market with information on the revenues for the overall market and the sub-segments across the different segments, by company, by Type, by Application, and by regions.
Market Segmentation
By Company
TIMET
Kymera International
Metraco NV
EcoTitanium (Aubert & Duval)
Monico Alloys
Baoji Titanium
Mega Metals
United Alloys and Metals
Globe Metal
Grandis Titanium
Goldman Titanium
Hanwa
Toho Titanium
OSAKA Titanium
Kobe Steel
Dong-A Special Metal
Hansco
Posco
Western Metal Materials
Pangang Group Titanium Metal Materials
Qinghai Supower Tianium
Segment by Type
Titanium Solids
Titanium Turnings
Segment by Application
Titanium Ingot
Steel Industry
Others
By Region
North America
United States
Canada
Asia-Pacific
China
Japan
South Korea
Southeast Asia
India
Australia
Rest of Asia
Europe
Germany
France
U.K.
Italy
Russia
Rest of Europe
Latin America
Mexico
Brazil
Rest of Latin America
Middle East & Africa
Turkey
Saudi Arabia
UAE
Rest of MEA
Drivers
The titanium recycling market is driven by increasing demand across aerospace, automotive, and medical industries due to titanium's lightweight and corrosion-resistant properties. Recycling titanium is essential to meet supply challenges and environmental mandates, as primary titanium extraction is energy-intensive. The growing adoption of sustainable manufacturing practices further bolsters market growth. North America and Europe dominate the market due to robust aerospace industries and stringent environmental regulations.
Restraints
Despite its growth potential, the market faces challenges such as high costs associated with titanium scrap processing and limited technological advancements in some regions. The industry also grapples with supply chain issues, particularly in collecting and sorting high-quality scrap.
Opportunities
Emerging economies in Asia-Pacific present lucrative opportunities due to expanding manufacturing bases and increasing awareness about sustainable practices. Innovations in recycling technologies, such as more efficient separation and refinement processes, are anticipated to enhance market dynamics.
Get more reports of this sample : https://www.intelmarketresearch.com/download-free-sample/489/titanium-recycling-market
0 notes
Text
Single-Use Bronchoscopes Market Emerging Trends Shaping Future Healthcare Practices
The single-use bronchoscopes market is experiencing a remarkable transformation, driven by technological advancements, increasing infection control awareness, and the growing demand for cost-effective, disposable medical devices. Unlike traditional reusable bronchoscopes, single-use bronchoscopes offer convenience, reduced contamination risk, and operational efficiency, making them a preferred choice in both developed and emerging healthcare settings. As the healthcare industry continues to emphasize patient safety and improved procedural outcomes, several emerging trends are influencing the trajectory of this rapidly evolving market.

Increasing Preference for Infection Control and Patient Safety
One of the most significant trends shaping the single-use bronchoscopes market is the heightened emphasis on infection prevention. Healthcare-associated infections (HAIs) have long been a concern in hospital environments, particularly in intensive care units where bronchoscopic procedures are common. Single-use bronchoscopes, by design, eliminate the risk of cross-contamination between patients. This feature is especially crucial during outbreaks of communicable diseases like COVID-19, where reusable medical equipment posed a higher risk of viral transmission.
In response, hospitals are increasingly replacing traditional reusable bronchoscopes with disposable alternatives. This shift is not only improving safety standards but also reducing the time and resources required for cleaning and sterilization.
Integration of High-Resolution Imaging and Advanced Technology
Technological innovation is playing a pivotal role in shaping the future of single-use bronchoscopes. Manufacturers are integrating high-definition (HD) imaging systems and enhanced light sources into these devices, providing clearer visuals and improved diagnostic accuracy during procedures. These advancements help clinicians perform complex procedures with greater precision and confidence.
Moreover, the incorporation of CMOS camera technology and real-time video capabilities in single-use bronchoscopes is setting new benchmarks in respiratory diagnostics. These features are no longer confined to high-end reusable scopes and are increasingly being adopted in disposable models, thereby elevating the standard of care without compromising sterility.
Rising Adoption in Ambulatory and Emergency Care Settings
The portability and ease of use of single-use bronchoscopes have expanded their utility beyond hospitals to include ambulatory surgical centers (ASCs), emergency departments, and field hospitals. In such settings, time-sensitive care delivery and lack of sterilization infrastructure make single-use devices particularly advantageous.
Emergency care professionals prefer these bronchoscopes for rapid intubation and airway management, especially in critical cases where time is a constraint. Additionally, these scopes reduce the need for reprocessing delays and equipment failures, ensuring uninterrupted patient care in fast-paced environments.
Cost-Effectiveness Driving Adoption in Resource-Limited Settings
Initially, cost concerns limited the widespread adoption of single-use bronchoscopes. However, a growing body of evidence now supports their long-term cost efficiency. When factoring in reprocessing costs, staff labor, maintenance, and potential liabilities related to infections, single-use bronchoscopes often present a more economical solution.
Healthcare facilities in resource-constrained regions are increasingly recognizing these benefits. The absence of sterilization infrastructure in many rural and remote areas further reinforces the need for disposable medical devices. As a result, single-use bronchoscopes are becoming a viable and scalable option in emerging markets.
Growing Demand for Customization and Specialty Applications
Another notable trend is the customization of single-use bronchoscopes to suit various medical specialties. While initially developed for pulmonology and intensive care applications, manufacturers are now designing models tailored to otolaryngology, anesthesiology, and thoracic surgery.
These specialized bronchoscopes offer enhanced flexibility, specific diameters, and features like steerable tips and suction channels, catering to niche procedural needs. As the demand for customized tools increases, the market is witnessing a surge in product innovation to meet the evolving expectations of healthcare professionals.
Expansion of Strategic Partnerships and Global Market Reach
Market players are actively pursuing strategic partnerships, mergers, and acquisitions to enhance product portfolios and expand geographic reach. Collaborations between device manufacturers and healthcare providers are accelerating the development and deployment of advanced single-use bronchoscopes across regions.
Additionally, increasing regulatory approvals across North America, Europe, and Asia-Pacific are enabling manufacturers to penetrate new markets. Government initiatives promoting infection control measures and improving healthcare infrastructure are also boosting adoption rates, especially in countries with developing healthcare systems.
Conclusion
The single-use bronchoscopes market is poised for sustained growth, driven by critical trends such as infection control prioritization, technological enhancements, and expanding applications in diverse care settings. As the healthcare industry continues to navigate challenges related to safety, cost, and efficiency, single-use bronchoscopes are emerging as a reliable, scalable solution. Manufacturers that invest in innovation, customization, and strategic expansion are well-positioned to capitalize on the growing demand and shape the future of bronchoscopy.
0 notes
Text
0 notes
Text
Clean & Critical: Endoscope Reprocessing Market Reaches $4.8B Goal 🔁🧼

As hospitals and clinics strive to reduce the risk of healthcare-associated infections (HAIs), the need for reliable endoscope reprocessing systems — ranging from automated reprocessors and disinfectants to drying cabinets and tracking solutions — has intensified. The goal is clear: prevent contamination, ensure compliance, and boost patient trust in clinical environments.
Market Dynamics
The surge in demand for endoscope reprocessing is being powered by several intertwined dynamics. First, the increasing prevalence of chronic gastrointestinal disorders and cancers is fueling endoscopic procedure volumes. In parallel, global infection control standards are becoming more rigorous, compelling healthcare providers to invest in high-quality reprocessing solutions.
Click to Request a Sample of this Report for Additional Market Insights: https://www.globalinsightservices.com/request-sample/?id=GIS10277
Among the product categories, automated endoscope reprocessors (AERs) are leading the charge due to their efficiency and consistency, helping reduce human error. The trend toward automation is transforming the market, ensuring standardization and compliance across facilities. Complementary technologies like UV-C disinfection and hydrogen peroxide vaporization are also gaining traction.
On the flip side, challenges like high equipment costs, skilled labor shortages, and regulatory complexities pose hurdles. Additionally, the rise of single-use endoscopes could potentially disrupt the market by reducing the need for reprocessing altogether. Nevertheless, the drive toward sustainable and eco-friendly solutions presents fresh opportunities, with companies innovating to meet environmental and economic goals.
Key Players Analysis
The competitive landscape is vibrant, with leading firms like Olympus Corporation, Steris PLC, and Cantel Medical Corporation dominating the market. These companies continue to invest in advanced technologies, R&D, and strategic partnerships to stay ahead. Innovations in endoscope tracking, energy-efficient devices, and smart integration with hospital systems have set them apart.
In addition to established giants, emerging players such as Medi Clean Innovations, Scope Care Systems, and Endo Pure Solutions are making waves with cost-effective, specialized, and eco-conscious offerings. This diversity of players contributes to a dynamic marketplace brimming with innovation and adaptability.
Regional Analysis
North America leads the global market, underpinned by robust healthcare infrastructure, high awareness levels, and strict regulatory oversight. The United States is a standout performer, investing heavily in infection control technologies.
Europe follows closely, with countries like Germany and the UK playing key roles due to their emphasis on healthcare quality and technological innovation. The continent benefits from harmonized standards that support consistent market growth.
Browse Full Report : https://www.globalinsightservices.com/reports/endoscope-reprocessing-market/
Meanwhile, Asia-Pacific is emerging as a powerhouse. Nations like China and India are investing in healthcare modernization, driven by a growing middle class and medical tourism. With improved accessibility and infrastructure, this region is likely to witness exponential growth.
Latin America and the Middle East & Africa show moderate but steady development. While challenges remain in terms of economic and logistical constraints, the growing awareness of infection risks is pushing healthcare providers to upgrade their reprocessing practices.
Recent News & Developments
The past year has seen a flurry of activity in the endoscope reprocessing market. Pricing dynamics are shifting, influenced by the integration of smart features, energy-efficient systems, and compliance-focused design. Costs for AERs now range from $10,000 to $50,000, depending on their capabilities and sophistication.
Regulatory frameworks from agencies like the FDA and EU MDR have become more stringent, demanding robust compliance and spurring innovation in sterilization protocols. Key players are aligning with these regulations by enhancing automation, traceability, and sustainability in their products.
Additionally, strategic mergers and acquisitions are reshaping the landscape. Companies are joining forces to expand global footprints, strengthen product portfolios, and explore untapped regional markets. This consolidation trend is expected to intensify as the market matures.
Scope of the Report
This report offers an in-depth exploration of the endoscope reprocessing market, analyzing trends across product types, technologies, services, applications, and end-users. It maps out the historical journey and provides accurate forecasts from 2025 to 2034, highlighting growth drivers, restraints, competitive strategies, and technological innovations.
The report evaluates key development strategies, including product launches, partnerships, and acquisitions. It provides actionable insights into market segmentation, regional performance, and regulatory landscapes. Stakeholders can leverage this information to navigate challenges, identify growth hotspots, and capitalize on emerging trends.
Whether you’re a manufacturer, healthcare provider, or investor, this comprehensive outlook equips you with the knowledge to make informed decisions in an increasingly complex and competitive market.
Discover Additional Market Insights from Global Insight Services:
Outpatient Oncology Infusion Market : https://www.globalinsightservices.com/reports/outpatient-oncology-infusion-market/
Neurorehabilitation Devices Market : https://www.globalinsightservices.com/reports/neurorehabilitation-devices-market/
Aquaculture Vaccines Market : https://www.globalinsightservices.com/reports/aquaculture-vaccines-market/
Bioanalytical Testing Services Market : https://www.globalinsightservices.com/reports/bioanalytical-testing-services-market/
Biodegradable Implant Devices Market : https://www.globalinsightservices.com/reports/biodegradable-implant-devices-market/
#endoscopereprocessing #healthtech #infectioncontrol #patientcare #medicaldevices #hospitaltech #disinfectiontech #aers #uvdisinfection #hydrogenperoxide #healthcareinfrastructure #automatedreprocessing #cleaningprotocols #gastrotech #pulmonologycare #urologytech #medicalstandards #hospitalequipment #diagnostictech #surgicalsafety #globalhealth #medinnovation #sterileprocessing #regulatorycompliance #healthtechinnovation #medicaltourism #asiahealthcare #northamericahealth #europehealthcare #latamhealth #middleeasthealthcare #reprocessingtech #singleusevsreusable #biomedicaldevices #greenhealthcare #sustainablehospitals #ecofriendlytech #medtechstartups #infectionprevention #endoscopytech #futureofhealthcare
About Us:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC 16192, Coastal Highway, Lewes DE 19958 E-mail: [email protected] Phone: +1–833–761–1700 Website: https://www.globalinsightservices.com/
0 notes
Text
Medical Device Reprocessing Market: Market Trends and Competitive Analysis 2024-2032

The Medical Device Reprocessing Market was estimated at USD 2.69 billion in 2023 and is projected to reach USD 9.63 billion by 2032, growing at a compound annual growth rate (CAGR) of 15.29% during the forecast period of 2024-2032. Get Free Sample Report @ https://www.snsinsider.com/sample-request/3485 Regional Analysis North America: Dominates the market due to advanced healthcare infrastructure and stringent regulatory standards promoting reprocessing practices. Europe: Exhibits significant growth driven by cost-containment measures in healthcare and increasing adoption of reprocessed devices. Asia-Pacific: Anticipated to witness rapid expansion owing to rising healthcare expenditures, growing awareness about reprocessing benefits, and the presence of a large patient pool. Market Segmentation By Type: Reprocessing Support & Services Reprocessed Medical Devices By Device Category: Critical Devices Semi-Critical Devices Non-Critical Devices By Application: Surgical Instruments Endoscopy Equipment Respiratory Care Devices Others Key Players The major key players are Stryker, Innovative Health, NEScientific, Inc., Medline Industries, LP, Arjo, Vanguard AG, Cardinal Health, SureTek Medical, Soma Tech Intl, Johnson & Johnson MedTech and other players. Key Points Reusable medical devices, such as surgical forceps and endoscopes, are essential in healthcare for their cost-effectiveness and utility across multiple patients. Proper reprocessing of these devices is crucial to eliminate contaminants and prevent infections, thereby ensuring patient safety. The increasing prevalence of chronic diseases, like asthma and COPD, underscores the importance of reprocessed surgical instruments in managing healthcare costs and sustainability efforts. Adherence to industry standards and best practices in medical device reprocessing significantly reduces infection risks and enhances patient outcomes. Supply chain challenges, including material shortages and manufacturing complexities, can impact the availability and cost of reprocessed devices. Future Scope The future of the medical device reprocessing market appears promising, with technological advancements enhancing the efficiency and safety of reprocessing methods. Innovations in sterilization technologies and the development of more durable medical devices suitable for multiple reprocessing cycles are expected to drive market growth. Additionally, increasing environmental concerns and the push for sustainable healthcare practices will likely encourage the adoption of reprocessed devices, further propelling the market forward. Conclusion The medical device reprocessing market is set for substantial growth, driven by the need for cost-effective healthcare solutions, environmental sustainability, and stringent infection control measures. As healthcare systems worldwide strive to balance quality patient care with economic and environmental considerations, the role of medical device reprocessing becomes increasingly vital. Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Other Related Reports: Fertility Services Market Medical Power Supply Market Post Traumatic Stress Disorder Treatment Market MRI Guided Neurosurgical Ablation Market
#Medical Device Reprocessing Market#Medical Device Reprocessing Market Share#Medical Device Reprocessing Market Trends#Medical Device Reprocessing Market Size
0 notes
Text
The Clean Sweep: A Report on the Japan Medical Device Cleaning Market
Japan Medical Device Cleaning Market Growth & Trends
The Japan Medical Device Cleaning Market size is anticipated to reach USD 1,169.49 million by 2030 and is projected to grow at a CAGR of 10.83% over the forecast period, according to a new report by Grand View Research, Inc. This growth can be attributed to the increasing competition among the market players and the growing efforts to reduce hospital-acquired infections.
Several studies are being published focusing on the increasing number of infections acquired in hospitals and the measures that can prevent them. For instance, a study published by the National Library of Medicine in May 2020 found that nosocomial infections, also known as healthcare-acquired infections, are a significant burden on hospitalized patients in Japan. This study analyzed the Japanese claims database and found that out of 73,962,409 inpatients registered in the database, 9.7% had community-acquired infections (CAI), and 4.7% had nosocomial infections (NI). As a result, the growing burden of hospital-acquired infections is expected to increase the demand for medical device cleaning products in Japan.
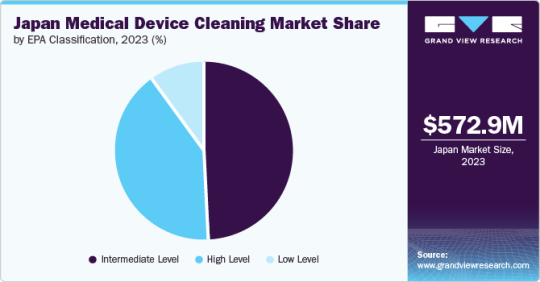
Moreover, the growing use of single-use devices and the rising manufacturing of medical devices are anticipated to propel the Japanese medical device cleaning market. Industry stakeholders are focusing on increasing the development of medical devices in the country. For instance, in May 2023, Terumo Corporation, a medical device company, invested around USD 360 million to construct a new manufacturing facility for the Medical Care Solutions Company in Japan.
In addition, in January 2022, Kaneka Corporation invested around USD 69 million to build a new medical device plant in the Tomatoh Industrial Area in the northern area of Japan. Companies are expanding their manufacturing facilities. These expanding manufacturing facilities will require medical device cleaning solutions for sterilization, reprocessing, and cleaning in the future. Thus, the rise in investments in medical device manufacturing and development in Japan is projected to increase the demand for medical device cleaning products in the coming years.
Curious about the Japan Medical Device Cleaning Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Japan Medical Device Cleaning Market Report Highlights
Based on device type, the semi-critical segment dominated the market in 2023 and accounted for 46.02% of revenue share. However, the critical segment is anticipated to grow fastest over the forecast period due to the increasing infection control awareness and growing aging population.
Based on technique, the disinfection segment dominated the market in 2023 and accounted for 49.53% of the revenue share. However, the sterilization segment is anticipated to grow fastest from 2024 to 2030. Advancements in sterilization technologies are expected to boost segment growth in the coming years.
Based on EPA classification, the intermediate-level segment dominated the Japan market and accounted for the largest revenue share, 48.84%, in 2023. In contrast, the high-level segment is expected to grow fastest, with the fastest CAGR over the forecast period.
Japan Medical Device Cleaning Market Segmentation
Grand View Research has segmented the Japan Medical device cleaning market based on the device type, technique, and EPA classification:
Japan Medical Device Cleaning Device Type Outlook (Revenue, USD Million, 2018 - 2030)
Non-Critical
Semi-Critical
Critical
Japan Medical Device Cleaning Technique Outlook (Revenue, USD Million, 2018 - 2030)
Cleaning
Detergents
Buffers
Chelators
Enzymes
Others
Disinfection
Chemical
Alcohol
Chlorine & Chorine Compounds
Aldehydes
Others
Metal
Ultraviolet
Others
Sterilization
Heat Sterilization
Ethylene Dioxide (ETO) Sterilization
Radiation Sterilization
Japan Medical Device Cleaning EPA Classification Outlook (Revenue, USD Million, 2018 - 2030)
High Level
Intermediate Level
Low Level
Download your FREE sample PDF copy of the Japan Medical Device Cleaning Market today and explore key data and trends.
0 notes
Text
Single-Use Bronchoscopes Market Drivers: Increasing Focus on Infection Control and Patient Safety in Healthcare Settings.
The global healthcare industry has seen remarkable advancements over the years, one of which is the growing demand for single-use medical devices. The Single-Use Bronchoscopes Market, particularly the single-use variant, has witnessed a significant rise due to various factors. These instruments, designed for visualizing and diagnosing respiratory system conditions, have become indispensable in critical care and diagnostic procedures. The single-use bronchoscope market, driven by innovations in medical technology and patient safety concerns, is poised for significant growth.

Increasing Focus on Patient Safety and Infection Control
A major driver for the single-use bronchoscope market is the heightened focus on patient safety and infection control. Traditional reusable bronchoscopes, despite their robust functionality, carry the risk of cross-contamination between patients if not properly sterilized. In healthcare settings, especially in hospitals, this can result in the transmission of infections, some of which may be severe or even fatal. Single-use bronchoscopes mitigate this risk by being disposed of after each use, ensuring that each patient receives a sterile device, thereby reducing the potential for healthcare-associated infections (HAIs). The increasing awareness about infection prevention is leading to more healthcare providers opting for single-use bronchoscopes, thus driving market growth.
Advancements in Technology
The development of new technologies and improved materials has made single-use bronchoscopes more effective and efficient. Manufacturers are continuously working to enhance the image quality, ease of use, and flexibility of these devices. For example, innovations in fiber optics, miniaturization, and digital imaging have enhanced the diagnostic capabilities of single-use bronchoscopes. These advances make it easier for healthcare providers to conduct accurate and efficient bronchoscopies, which is particularly crucial in emergencies and high-risk patients. As technology improves, single-use bronchoscopes are becoming increasingly sophisticated and reliable, further fueling their adoption.
Cost-Effectiveness and Economic Efficiency
Another driving factor is the cost-effectiveness associated with single-use bronchoscopes. While reusable bronchoscopes might seem cost-effective initially, they require significant investment in cleaning, sterilization, and maintenance. Moreover, there is always the risk of improper sterilization, which could lead to contamination. Single-use bronchoscopes, on the other hand, are more economical in terms of overall lifecycle costs. Hospitals and clinics save on maintenance, sterilization services, and the manpower required to handle reusable devices. The ability to dispose of a bronchoscope after use also eliminates the need for costly reprocessing, making it a more attractive option for facilities with budget constraints or those seeking efficiency in their operations.
Rising Demand for Minimally Invasive Procedures
The growing preference for minimally invasive procedures is also a key driver for the single-use bronchoscope market. Bronchoscopies are typically performed for diagnosing lung diseases, airway disorders, and infections. Traditional methods may require invasive procedures with longer recovery times and higher risk of complications. However, with the advent of single-use bronchoscopes, these procedures are less invasive and can be performed with greater precision. Patients experience less discomfort and a shorter recovery time, contributing to their preference for such procedures. This growing trend for minimally invasive diagnostic and therapeutic options is driving the demand for single-use bronchoscopes.
Regulatory Approvals and Clinical Guidelines
Government regulations and clinical guidelines also play a crucial role in the growth of the single-use bronchoscope market. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved single-use bronchoscopes, reinforcing their safety and effectiveness in medical procedures. As these devices continue to meet high standards of regulatory approval, healthcare providers are more likely to integrate them into their practices. Clinical guidelines further endorse the use of single-use devices to reduce infection risks and improve patient outcomes. These endorsements help boost confidence in single-use bronchoscopes, driving market growth.
Conclusion
In conclusion, the single-use bronchoscope market is being driven by multiple factors such as the increasing demand for infection control, technological advancements, cost-effectiveness, and the preference for minimally invasive procedures. As the healthcare industry continues to evolve and patient safety becomes a priority, the adoption of single-use bronchoscopes will only continue to rise. Furthermore, with regulatory approvals and technological innovations, the future of single-use bronchoscopes looks promising, offering enhanced diagnostic capabilities and improved patient outcomes.
0 notes
Text
Automated Endoscope Reprocessing Market: Trends and Growth Opportunities
Introduction
The automated endoscope reprocessing market has been experiencing significant growth in recent years, driven by increasing concerns about infection control, advancements in reprocessing technologies, and the rising number of endoscopic procedures worldwide. The market is expected to witness steady expansion as healthcare facilities prioritize efficiency, safety, and regulatory compliance in endoscope reprocessing.

Market Trends
1. Rising Demand for Infection Control Solutions
Hospital-acquired infections (HAIs) have become a critical issue, leading to stringent sterilization guidelines. Automated endoscope reprocessors (AERs) ensure thorough and standardized cleaning, minimizing contamination risks. The shift from manual to automated endoscope reprocessing is accelerating due to growing awareness and compliance requirements.
2. Technological Advancements in AERs
Manufacturers are integrating smart technologies, such as automated tracking systems and real-time monitoring, to enhance efficiency and traceability in endoscope reprocessing. Innovations in chemical disinfectants and high-level disinfection (HLD) techniques are further improving the reliability of reprocessing systems.
3. Stringent Regulatory Standards
Regulatory bodies, including the FDA and CDC, have established strict guidelines for reprocessing reusable medical devices. Compliance with these standards is fueling the adoption of automated endoscope reprocessors, as healthcare providers seek to meet safety and hygiene protocols.
Growth Opportunities
1. Expansion in Emerging Markets
Developing regions, such as Asia-Pacific and Latin America, present lucrative growth opportunities due to increasing healthcare infrastructure investments. The rising prevalence of gastrointestinal and respiratory diseases is boosting the demand for endoscopic procedures, further driving the need for efficient reprocessing solutions.
2. Adoption of Single-Use Endoscopes
While the focus remains on automated endoscope reprocessing, the industry is also witnessing a surge in single-use endoscopes to reduce cross-contamination risks. This shift could lead to hybrid market trends where both automated reprocessing and disposable options co-exist.
3. Integration of Artificial Intelligence (AI) and IoT
The incorporation of AI-powered monitoring systems and IoT-enabled devices in automated reprocessors is revolutionizing infection control. Predictive maintenance, data analytics, and cloud-based reporting systems enhance efficiency and reduce operational costs, making AERs more attractive to healthcare providers.
Conclusion
The automated endoscope reprocessing market is poised for substantial growth, fueled by stringent regulatory mandates, technological advancements, and the need for effective infection control solutions. Companies investing in innovation and expanding into emerging regions will likely gain a competitive edge in this evolving landscape. As healthcare systems continue to prioritize patient safety, the demand for efficient, automated reprocessing solutions will remain strong.
0 notes
Text
Ortho Phthalaldehyde Market Size, Share, and Competitive Landscape
Rising Demand for High-Efficiency Disinfectants Drives Growth in the Ortho Phthalaldehyde Market

The Ortho Phthalaldehyde Market size was valued at USD 5.83 billion in 2023. It is expected to grow to USD 9.25 billion by 2032 and grow at a CAGR of 5.26% over the forecast period of 2024-2032.
The Ortho Phthalaldehyde (OPA) Market is driven by increasing demand for high-performance disinfectants in healthcare, pharmaceuticals, and industrial applications. OPA is widely recognized for its superior antimicrobial properties, making it a preferred alternative to traditional disinfectants like glutaraldehyde. The rising need for effective sterilization solutions, coupled with stringent hygiene regulations in hospitals and medical facilities, is fueling the market expansion. Additionally, its applications in chemical synthesis and water treatment contribute to the growing global demand.
Key Players in the Market
The Ortho Phthalaldehyde market features a competitive landscape, with key industry players focusing on product innovation, regulatory compliance, and sustainability. Leading companies in the sector include:
AK Scientific Inc.
Alfa Aesar
MP Biomedicals
DPX Fine Chemicals
Virox
Thermo Fisher Scientific Inc.
Parchem Fine & Specialty Chemicals
TCI America
Merck Millipore Corporation
Sigma-Aldrich Co. LLC
These companies are investing in advanced formulations and expanding their production capacities to cater to the growing demand for OPA-based disinfectants.
Future Scope and Emerging Trends
The future of the Ortho Phthalaldehyde Market looks promising as healthcare facilities worldwide prioritize infection control and patient safety. The shift toward non-toxic and biodegradable disinfectants is driving research into environmentally friendly OPA formulations. Moreover, the rising adoption of automated disinfection systems in hospitals and laboratories is increasing the use of OPA-based solutions. Additionally, innovations in high-purity OPA for pharmaceutical and biotechnology applications are expanding its market potential. With growing concerns over hospital-acquired infections (HAIs), the demand for OPA in sterilization and medical device reprocessing is expected to surge.
Key Market Points:
✅ Rising Healthcare Demand: Increased usage of OPA-based disinfectants in medical facilities. ✅ Superior Antimicrobial Properties: Preferred over glutaraldehyde for sterilization due to enhanced safety and efficacy. ✅ Growth in Water Treatment Applications: Expanding use in industrial and municipal water treatment processes. ✅ Regulatory Compliance: Companies focusing on meeting stringent safety and environmental standards. ✅ Advancements in Chemical Research: Ongoing R&D to develop safer and more sustainable OPA formulations.
Conclusion
The Ortho Phthalaldehyde Market is set for continued growth, driven by increasing hygiene awareness, advancements in disinfection technologies, and expanding industrial applications. As industries and healthcare providers seek safer and more effective sterilization solutions, OPA is emerging as a key component in infection control. Companies investing in sustainable production methods and innovative applications will be well-positioned to capitalize on this growing market.
Read Full Report: https://www.snsinsider.com/reports/ortho-phthalaldehyde-market-3861
Contact Us:
Jagney Dave — Vice President of Client Engagement
Phone: +1–315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Ortho Phthalaldehyde Market#Ortho Phthalaldehyde Market Size#Ortho Phthalaldehyde Market Share#Ortho Phthalaldehyde Market Report#Ortho Phthalaldehyde Market Forecast
0 notes
Text
Disposable Laparoscopic Surgical Trocars: A Market Analysis

The worldwide market for disposable laparoscopic surgical trocars is showing high growth rates due to growing use of minimally invasive procedures. This article offers a critical examination of the market, key drivers, issues, trends, and competitive position.
Market Overview
Laparoscopic surgery, also a minimally invasive surgical method, has grown popular as a result of the advantages it poses to open surgery, including small cuts, minimal pain, shorter recovery periods, and fewer infection risks. Trocars are tools needed for the conduct of laparoscopic operations that are designed to provide openings by which medical tools and cameras may be directed into the body of the patient.
Single-use trocars are used once and do not require reprocessing and sterilization, which minimizes cross-contamination and surgical site infection risks. They are convenient and efficient in the workflow of surgeries and are the preferred option for healthcare professionals.
The Disposable Laparoscopic Surgical Trocar Market will register a CAGR of 4.20% during the period from 2024 to 2031 and will have a market size grow from US$ XX million in 2024 to US$ XX Million in 2031.
Market Drivers
A number of drivers are fueling the growth of the disposable laparoscopic surgical trocar market:
Increased incidence of chronic illnesses: The rising prevalence of chronic illness, like cancer, cardiovascular illness, and gastrointestinal disease, is propelling demand for surgical intervention, including laparoscopic procedures.Increased utilization of minimally invasive surgery: The advantages of laparoscopic surgery compared to open surgery are driving adoption of the technology among different surgical disciplines, thereby enhancing demand for trocars.
Technological innovation: Ongoing innovation in trocar technology, including the evolution of bladeless trocars, optical trocars, and safety-enhanced trocars, is driving the market.
Growing healthcare spending: Growth in healthcare spending in developed and emerging economies is fueling market growth.
Older population: The aging world population is at higher risk for chronic conditions necessitating surgical treatment, further pushing demand for trocars.
Market Challenges
In spite of the expansion prospects, the market is confronted with some challenges:
Expensive disposable trocars: Disposable trocars tend to be pricier than reusable ones, and this may limit their adoption in some healthcare institutions, especially where costs are sensitive.
Environmental issues: The disposal of single-use trocars generates medical waste and creates environmental issues, necessitating sustainable options.
Stringent regulatory needs: The medical device market is governed by stringent regulatory needs, which could be challenging for manufacturers in terms of product development and approvals.
Market Trends
The disposable laparoscopic surgical trocar market is experiencing some major trends:
Bladeless trocar development: Bladeless trocars are becoming increasingly popular because they are able to reduce tissue trauma and the risk of complications during insertion.
Emphasis on safety features: Companies are emphasizing the creation of trocars with improved safety features like obturator locking and anti-rotation designs to avoid unintentional injuries.
Increased demand for optical trocars: Optical trocars enable real-time visualization upon insertion, enhancing accuracy and minimizing damage to underlying tissues.
Growing use of robotic surgery: The growth in robotic surgery is opening up new opportunities for trocar manufacturers, as the procedures are performed using specialized trocars.
Competitive Landscape
Applied Medical
Optcla
Genicon
Conmed
Specath
Victor Medical
J and J
B.Braun
Medtronic
Market Outlook
The market for disposable laparoscopic surgical trocar is anticipated to sustain its growth trend in the future, due to the above-discussed factors. The growing demand for minimally invasive surgery, improvement in technology, and increase in healthcare spending are likely to drive the market growth. However, manufacturers must deal with the issues of cost and environment in order to foster sustainable growth.
Conclusion
The market for disposable laparoscopic surgical trocars is a dynamic and developing market with high growth opportunities. There is growing demand for minimally invasive surgery, in combination with technology advancements, which is leading the market. Challenges are present, but the outlook for the market is good, and companies that can provide innovative and cost-efficient solutions are expected to thrive within this competitive market.
About Us-
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
0 notes
Text
Disposable Endoscopes Market: Emerging Trends Shaping Future Medical Practices
The disposable endoscopes market is rapidly gaining traction within the medical device industry, driven by the increasing demand for cost-effective, safe, and infection-free diagnostic and surgical procedures. Unlike traditional reusable endoscopes, disposable variants offer single-use functionality, eliminating the risk of cross-contamination and reducing the need for complex reprocessing. This market has witnessed significant evolution due to technological innovations, rising healthcare awareness, and growing regulatory support, positioning itself as a transformative force in modern medical diagnostics and treatment.

Market Overview
Disposable endoscopes are being widely adopted across various medical specialties including urology, pulmonology, gastroenterology, and otolaryngology. With healthcare providers under constant pressure to enhance patient safety and streamline operational efficiency, single-use devices have emerged as a practical solution. The demand is particularly high in outpatient settings, emergency rooms, and intensive care units, where rapid turnaround and infection prevention are top priorities.
The global market size for disposable endoscopes has been growing steadily and is expected to continue on an upward trajectory. This growth is largely attributed to the increasing prevalence of hospital-acquired infections, the rising geriatric population, and a broader shift toward value-based healthcare models.
Key Emerging Trends
1. Integration of Smart Technologies
One of the most influential trends in the disposable endoscopes market is the integration of smart and digital technologies. Many new devices now feature advanced imaging capabilities such as high-definition visualization, real-time data transmission, and AI-assisted diagnostics. These innovations are enhancing clinical outcomes by improving diagnostic accuracy and procedure efficiency.
2. Expansion into New Specialties
Initially concentrated in urology and bronchoscopy, disposable endoscopes are now finding applications in other fields such as gastrointestinal endoscopy and ENT (ear, nose, and throat) diagnostics. As manufacturers refine designs to suit the unique needs of each specialty, the scope of application is expanding, bringing with it a broader customer base.
3. Regulatory Momentum and Guidelines
Regulatory agencies around the world are increasingly acknowledging the safety advantages of disposable medical devices. Guidelines promoting single-use endoscopes to reduce infection risks have bolstered their adoption. This regulatory backing has encouraged hospitals to invest more in these devices, especially in high-risk procedures and vulnerable patient populations.
4. Cost-Benefit Analysis Favoring Single-Use
While disposable endoscopes might seem costlier per unit compared to reusable ones, they eliminate hidden costs associated with cleaning, maintenance, repair, and sterilization. Studies and internal hospital audits have begun to reveal the long-term economic advantages of adopting single-use endoscopes, especially when factoring in litigation risks and costs of treating infections.
5. Sustainability Concerns and Eco-Friendly Initiatives
A growing concern with disposable medical devices is environmental sustainability. However, recent advancements have seen the development of recyclable materials and eco-friendly disposal solutions. Companies are increasingly focusing on designing biodegradable or low-impact devices to align with healthcare institutions' environmental goals.
Competitive Landscape
The disposable endoscopes market is highly competitive, with both established medical device giants and innovative startups vying for market share. Leading players are investing heavily in R&D to improve device performance, miniaturize components, and enhance user ergonomics. Strategic partnerships, mergers, and acquisitions are also common, as companies seek to broaden their product portfolios and gain competitive advantages.
Startups are contributing significantly by offering niche solutions with specialized functionalities, catering to underserved segments of the market. This has resulted in a dynamic ecosystem that fosters rapid innovation and responsiveness to clinical needs.
Regional Insights
North America currently holds a significant share of the global disposable endoscopes market, driven by stringent infection control protocols and advanced healthcare infrastructure. Europe follows closely, supported by favorable regulatory frameworks and rising healthcare expenditures. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare access, a growing middle-class population, and expanding hospital networks in countries like China and India.
Future Outlook
The future of the disposable endoscopes market looks promising, with continuous improvements in materials science, imaging technology, and AI integration likely to revolutionize diagnostic and surgical procedures. As healthcare systems worldwide strive for improved outcomes, patient safety, and operational efficiency, the demand for single-use endoscopic devices is expected to rise further.
In the coming years, expect to see greater standardization, cost reduction through mass production, and deeper penetration into low- and middle-income countries. These trends, combined with a proactive regulatory environment and shifting clinical preferences, position the disposable endoscopes market as a pivotal component of the evolving global healthcare landscape.
0 notes