#FDA-Approved Drugs
Text
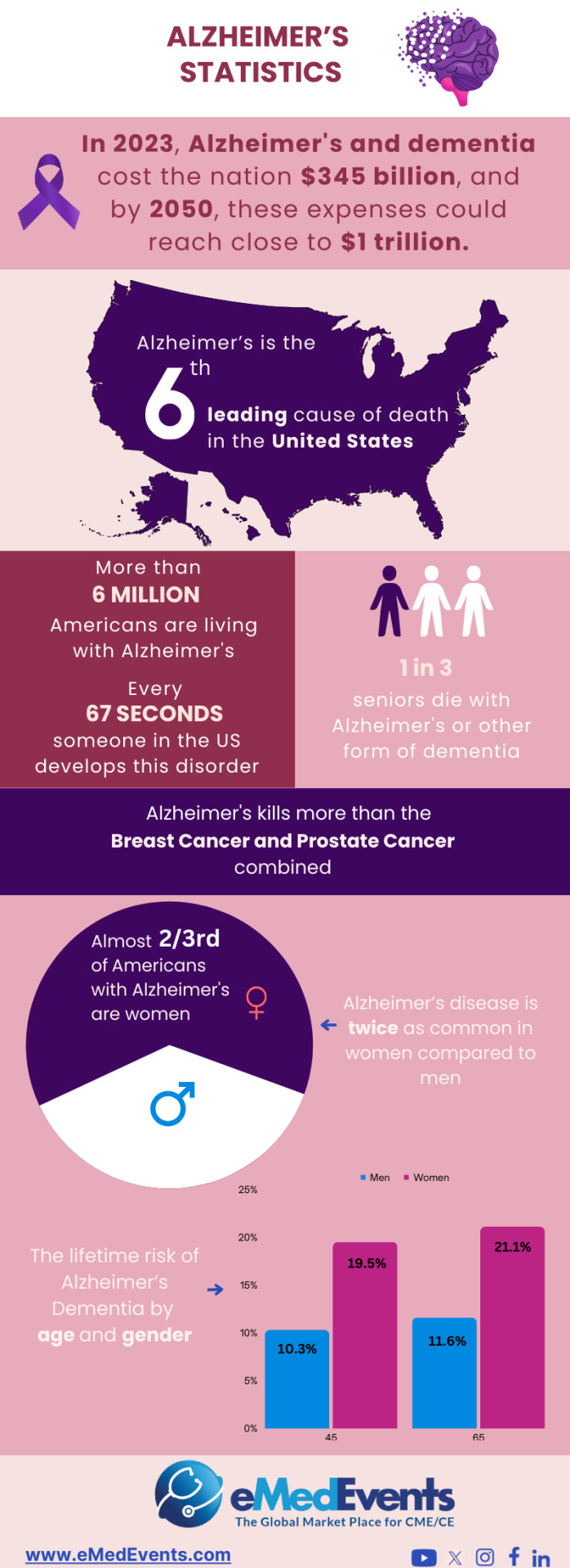
Recent Insights in Alzheimer Research |
Discover the latest insights into Alzheimers research, shedding light on advancements in diagnosis and treatment.
#Alzheimer's#dementia#x27;s disease#neurodegenerative disease#x27;s research insights#x27;s research#Neurological Biomarkers#Tau Protein#FDA-Approved Drugs#Nanotechnology#CME Programs#CME Conferences#CME Medical courses#CME credits#doctor conferences#medical CME#Primary Care Conference#Medical CME Online#Emergency Medicine Online CME#Medical Conference Website#Physician Conferences#Medical CME Courses#Doctors CME Conferences#CME training for Physicians#Medical Event Organizer#Organizing Medical Events#Medical Conferences#Medical Speakers#Healthcare Marketplace
0 notes
Text
living in the USA really just is reading a news headline like "BREAKING: FDA outlaws cancer-causing food coloring" and you click it and its "beta-dextraflubenrabinol #5 (shortened to beta DFR5), is commonly used in the manufacturing of paintballs. it has been around since 1943. today, it is present in 95% of sodas." and u just kinda go "wtf wasn't that already illegal?" and u look MORE into it on google and find some shit like "the FDA allows up to 12% toxicity in all products meant for consumption" and u just kinda stare into the camera like the office
#us american#usa news#united states#united states of america#usa#fda approved#us fda#fda#fda corruption#food and drug administration#roan.txt#fuck the usa
2 notes
·
View notes
Text
5 reasons that Choosing an online pharmacy may be right for you
Choosing an online pharmacy like DiRx can save you time, money, and hassle. With convenient home delivery, lower costs, no waiting in lines, and extended hours for customer care, it's a smart choice for many. Ensuring you select a trustworthy, FDA-approved provider guarantees the same safety and quality as your local pharmacy. If these benefits resonate with you, it might be time to consider ordering your prescriptions online.
#Online pharmacy USA#Buy prescription drugs online#FDA-approved online pharmacy#Affordable online pharmacy#Save money on prescriptions
2 notes
·
View notes
Text
5 reasons that Choosing an online pharmacy may be right for you
Choosing an online pharmacy like DiRx can save you time, money, and hassle. With convenient home delivery, lower costs, no waiting in lines, and extended hours for customer care, it's a smart choice for many. Ensuring you select a trustworthy, FDA-approved provider guarantees the same safety and quality as your local pharmacy. If these benefits resonate with you, it might be time to consider ordering your prescriptions online.
#Online pharmacy USA#Buy prescription drugs online#FDA-approved online pharmacy#Save money on prescriptions
2 notes
·
View notes
Text
.
#really struggling today#my vet suspects my cat has FIP and I’m crushed since that’s basically a death sentence and he only just turned 5 :(#I know GS-441524 is somewhat available in Canada now but since it’s not FDA approved it’s like 8k#what’s worse is my family and I have a 2 week vacation scheduled on May 11#so even though there’s this drug with a 90% success rate it’s just so incredibly expensive I doubt we could afford it#even if we did manage to get our hands on it we wouldn’t be able to administer it to him for those 2 weeks#and even though he’s doing somewhat ok at the moment who knows what his condition will be like during those 2 weeks :(#ultimately we’re trying to decide whether or not to put him down before our trip#like if he does have it and died alone and in pain while we were out of the country I would be crushed#but I’d also be crushed if we put him down when there’s the chance he doesn’t have it since FIP is so hard to diagnose#it’s the not knowing what’s going on that makes it worse#it’s so hard because he’s still so sweet and curious and has really been my rock since we got him I’m just absolutely beside myself#like the whole day yesterday he’s done nothing but cuddle me and my mom like he knows we’re upset but doesn’t know why#I just feel like I’m abandoning and failing him in his time of need#I desperately don’t want to go on this trip so I can spend more time with him and maybe scrounge up enough money to buy the drugs#and give him a fighting chance#but I can’t and I feel sick and trapped about it
3 notes
·
View notes
Text
Another WOTUS decision from an activist judge, and I just can't stress enough how much the U.S. is captured by an activist, right-wing judiciary. If the courts were ever neutral arbiters who's only existence was to settle disputes and occasionally interpret the law, that time is long past. Our government is, in theory, set up on a system of checks and balances. Except that there is no realistic check upon the court system. At least, there is no realistic check upon the court system if the federal government refuses to act (either by ignoring clearly biased rulings, removing activist judges from the bench, or packing the courts with more moderate jurists).
Unless the judiciary is meaningfully reigned in, judges will continue to take more and more power. If judges continue to overrule federal agencies - who are empowered to act by Congress and the President, and employ thousands of experts to make informed decisions - for no reason other than that particular judge disagrees personally with the agency's decision, then we're effectively letting judges run the government. It doesn't matter who the President is, or what Congress does, it matters what some Harvard grad who wears a polyester robe to work and hasn't every worked an honest day in their life is. And that's a fucking problem.
#woolly rambles#this applies to the recent decision about abortion medication too btw#the FDA approved that drug according to its expertise and authority well over two decades ago#if Chevron deference was protecting us as it was meant to then this should have been an open and shut case with a decision in favor of FDA#but activist judges don't care about precedent or established rule of law#they care about weilding as much fucking power as they can#and what is our recourse there? another fucking court? where there are no consequences when judges misapply or purposefully ignore the law?#they should be shot like dogs if you ask me
4 notes
·
View notes
Text
Guys with a special interest in drugs will spend half an hour researching a drug mentioned in one line in a show
#throttling rashid. intravenous levodopa isn’t even FDA APPROVED.#they make it sound like these are maybe weekly or monthly treatments that daniel’s getting#but it’s a daily drug!!!!#yeah yeah child vampire and what not. im focusing on the medical inaccuracies.#ray says stuff
7 notes
·
View notes
Text

Thirsting so hard for Sqwincher right now………………
#sqwincher#thirst#drink#swincher#beverage#drinking#food and beverage industry#food and drink#fda approved drug library#drinking liquid#liquid#juice#squinch#juicy#thisting#thirsting*#thirsting#goofy ahh#adam sandler
2 notes
·
View notes
Text
Discover Leading Pharmaceutical Solutions with Chemxpert Database
Looking for a medicine company near me or exploring the landscape of generic pharma companies? Chemxpert Database connects you with US pharmaceuticals, excelling in "pharmaceutical excipient expertise and pharma management. Whether you're interested in the top pharma companies in the world or niche market players, Chemxpert Database is your ultimate resource for insights and connections in the pharmaceutical industry. Uncover the leaders shaping the future of medicine.

#tablet formulation process#packaging for pharmaceutical products#FDA approved drugs#pharma exhibition#food and drug
1 note
·
View note
Text
Understanding the Regulatory Framework of FDA for Drug Approval

FDA Drug Approval, FDA drug approval process, FDA regulatory compliance, FDA drug approval process timelineThe Food and Drug Administration (FDA) of the US is the key regulatory body responsible for ensuring the safety and effectiveness of medications sold in the US. It defines approval for any product intended to alter the body’s structure or function and used for disease diagnosis, mitigation, treatment, or prevention as a medication.
Before a medicine is commercialized, the FDA must first approve it. In this process, the Center for Drug Evaluation and Research (CDER) plays a crucial role in ensuring that pharmaceuticals, both name brand and generic, function as intended and that the benefits to health outweigh any known risks.
The whole FDA drug approval process timeline, from comprehensive research development to final approval process, last from 12 to 15 years. For this, an independent group of physicians and scientists, who are experts in their respective fields, rigorously assesses each medication’s safety, effectiveness, and labeling under thorough examination. Their expertise and dedication ensure that only safe and effective medications are approved for public use.
Ensuring Safety within the FDA Drug Approval Process
A pharmaceutical business must fulfill the following five-step process in order to receive FDA clearance to commercialize a new prescription drug: FDA review, preclinical research, clinical research, post-market safety monitoring, and discovery/concept. To ensure the drug is safe and effective, the manufacturer must conduct a test in a lab before moving on to humans.
Before human trials, manufacturers must conduct preclinical research, synthesizing and screening drug candidates for toxicity in animals. An Investigational New Drug (IND) Application is then submitted, detailing the drug’s chemistry, production, and human testing schedule.
Human trials can only begin once the IND Application is approved by the FDA and the local institutional review board (IRB). Remarkably, only one in 1,000 laboratory-tested chemicals reaches human testing. The investigational medication will proceed through multiple stages of clinical trials and post-marketing approval if the FDA approves it and undergoes the following phases:
Phase 1: It primarily focuses on establishing a drug’s safety, and it involves about 20 to 80 healthy volunteers. In addition to safety, the drug’s metabolism and excretion are also reviewed and highlighted.
Phase 2: Phase 2 concentrates on the medication’s efficacy and also reviews side effects and safety in patients with a particular condition or disease, between 100-300 patient volunteers. This stage lasts for roughly two years.
Phase 3: If Phase 2 results indicate that the medication is effective, Phase 3 studies can start. In clinics and hospitals, several hundred to three thousand patients are usually monitored to closely assess effectiveness and detect additional side effects. This stage lasts, on average, three years. Fewer study participants may be found in very rare diseases. Patients of various kinds and ages are assessed. The manufacturer may consider experimenting with various dosages and the experimental medication in conjunction with other therapies.
Phase 4: The studies in phase 4 collect more data regarding a product’s effectiveness, safety, or best use following approval. Post-marketing research may involve patient groups using the medication in real-world situations. These investigations might reveal new applications, long-term efficacy, and previously unrecognized adverse effects.
Once a drug is approved in Phase 4, a post-marketing review takes place to ensure that the new medicine remains safe for public use. Pediatric studies or special safety studies may be completed during this time frame.
FDA Regulatory Compliance: Building Trust in Every Dose
FDA regulatory compliance in the pharmaceutical industry has evolved into an intricate and diverse matter. Pharmaceutical companies are subject to a plethora of regulations and standards enforced by the Food Drug Administration (FDA) in their efforts to guarantee the safety, effectiveness, and moral development of pharmaceutical products.
Amendments to FDA regulations have a major impact on the total expense of medication development. This includes the possible financial burdens and reputational concerns resulting from non-compliance, in addition to the direct costs related to meeting strict standards at various stages. Furthermore, there may be significant delays in medication development due to the complexity of integrating FDA regulatory compliance changes.
For instance, a major advancement in the treatment of Alzheimer’s disease, for which there has been little therapeutic success in recent decades, was made possible by a multinational biotechnology company’s Alzheimer’s medicine. Complicacies and inconsistencies along the drug’s approval journey attracted the attention of many parties.
Two significant Phase III clinical trials were carried out to assess the effectiveness of the medication. The primary endpoint of the second experiment was not met, despite the first trial demonstrating a decrease in cognitive decline. Divergent trial results placed the company under regulatory scrutiny, which led to lengthy negotiations with regulatory authorities and highlighted the difficulties in evaluating novel therapy methods in illnesses like Alzheimer’s disease, where it can be difficult to identify endpoints.
The trial data’s intricacies and the ensuing talks with the FDA resulted in delays in the drug approval process, which had an impact on when the product may potentially enter the market. In addition, the company’s stock price had considerable fluctuations during this time, which was indicative of the strain on finances and the unpredictability of the regulatory procedure during FDA approval for drugs.
Eventually, the medication was added to the FDA drug approval list in June 2021 under the accelerated FDA drug approval process.
Key Differences between NMPA and FDA Drug Approval Process
The US FDA and the National Medical Products Administration (NMPA) of China (formerly known as the China Food and Drug Administration, CFDA) are both critical regulatory agencies responsible for the oversight of drugs, medical devices, and other health products in their respective countries.
Thus, both the FDA regulatory compliance and NMPA play crucial roles in ensuring the safety, efficacy, and quality of drugs, medical devices, and other health products. While there are similarities in their regulatory frameworks and processes, there are also distinct differences shaped by regional legal requirements, market dynamics, and historical contexts.
Prioritizing Drug Safety: Closing Thoughts on FDA Approval Process
FDA approval for drugs is seen as a mark of credibility and trust. Patients and healthcare providers rely on its rigorous review process to ensure that drugs are safe and effective. This further boosts consumer confidence, as people are more likely to trust and use medications that have undergone stringent testing and review by a reputable regulatory body.
Thus, FDA drug approval is essential for entering the US market, which is one of the largest and most lucrative pharmaceutical markets in the world. Many insurance companies and government healthcare programs require FDA approval for drugs for reimbursement. Without the FDA drug approval, patients may encounter out-of-pocket expenses, limiting the drug’s market potential. Evidently, the FDA drug approval is recognized by regulatory bodies across the world and thus facilitates smooth approval in other countries thereby enhancing the global marketability of the drug.
#FDA Drug Approval#FDA drug approval process#FDA regulatory compliance#FDA drug approval process timeline
0 notes
Text
Navigating the world of medications can be overwhelming, especially when faced with the choice between generic and brand-name drugs. At our pharmacy in Boynton Beach, Florida, we aim to provide clarity and guidance to help you make informed decisions about your health.
0 notes
Text
FDA approves Eli Lilly's Alzheimer’s drug that slows memory decline
The Food and Drug Administration approved a new Alzheimer’s drug from Eli Lilly that has been shown in clinical trials to modestly slow a decline in memory and thinking abilities in people with the disease, the drugmaker said Tuesday.
The drug donanemab, which will be sold under the brand name Kisunla, is a monoclonal antibody infusion given every four weeks.
AD BY PURE ENCAPSULATIONS
Have you…
View On WordPress
0 notes
Text
What can we do about the soaring cost of prescription medicine?
Satish Srinivasan, CEO of DiRx, discusses the impact of soaring prescriptionmedicine costs and how DiRx is addressing this issue by offering affordablemedications directly from manufacturers and bypassing intermediaries. DiRx's modelhelps consumers save significantly on their prescriptions, making essential medicinesmore accessible to those in need.
#Affordable prescription medicine#Generic medication savings#Online#pharmacy New Jersey#Cost-effective prescriptions#Prescription cost reduction#Direct#pharmacy services#Low-cost medications USA#Prescription drug affordability#Skip the#middleman pharmacy#DiRx pharmacy savings#Prescription cost solutions#Discounted#generic drugs#Affordable healthcare solutions#Prescription medicine prices#generic pharmacy#Save on medications#Prescription savings plan#Online medication#savings#FDA-approved generics#DiRx online pharmacy
0 notes
Text
Choosing a trustworthy online pharmacy
Choosing a trustworthy online pharmacy is crucial for your health and safety. DiRx outlines five key factors: ensuring the pharmacy sells only FDA-approved medicines, is licensed and accredited, offers pharmacist support, monitors prescription interactions, and complies with privacy laws. By focusing on these aspects, you can confidently select an online pharmacy that prioritises your well-being.
#Trustworthy online pharmacy#FDA-approved medications#Accredited online pharmacy#Online pharmacysafety#Buy prescription drugs online
0 notes
Text
5 reasons that Choosing an online pharmacy may be right for you
Choosing an online pharmacy like DiRx can save you time, money, and hassle. With convenient home delivery, lower costs, no waiting in lines, and extended hours for customer care, it's a smart choice for many. Ensuring you select a trustworthy, FDA-approved provider guarantees the same safety and quality as your local pharmacy. If these benefits resonate with you, it might be time to consider ordering your prescriptions online.
#Online pharmacy USA#Buy prescription drugs online#FDA-approved online pharmacy#Affordable online pharmacy#Prescription delivery service
0 notes
Text
people still actually believe nic vapes caused popcorn lung HELP
#I love the idea that the FDA would approve a product that puts you at good risk to give you popcorn lung okay lmao#very “your kids are given drugs at halloween” core#I love people who read news headlines and take them as fact <3 yay!#personal diary#diary entry#online diary
0 notes