#IRB Protocol Solutions
Explore tagged Tumblr posts
Text
Breakthrough Research: Clinical Trials In Las Vegas with Clinfinite Solutions

In the dynamic landscape of medical research, medical trials function as the cornerstone of innovation. These established studies deliver new therapies, medicines, and scientific gadgets to life by way of very well evaluating their safety and effectiveness. As the need for advanced healthcare answers grows, so does the significance of carrying out well-organized and moral medical trials. Among the rising hubs for research, Clinical Trials in Las Vegas are gaining considerable attention for their ability to boost healthcare progress. At Clinfinite Solutions, we are proud to contribute to this evolution via imparting complete trial aid and revolutionary research methodologies.
Why Las Vegas Is Becoming a Clinical Research Destination
Traditionally acknowledged for its amusement and tourism, Las Vegas is speedy carving out a niche as a key location for clinical studies and development. The city gives a unique mix of modern-day healthcare infrastructure, numerous demographics, and a growing interest in clinical discovery. The upward push of Clinical Trials in Las Vegas is not merely coincidental—it is a strategic flow that benefits both researchers and the general public.
Las Vegas hosts a diffusion of studies, establishments, and hospitals equipped with contemporary facilities, an experienced clinical group of workers, and a population willing to participate in trials. This mixture fosters an environment in which progressive treatments can be studied successfully and ethically. The range of participants additionally guarantees that clinical trials produce statistics reflective of real international populations, making consequences greater relevant to large affected person groups.
Clinfinite Solutions: Driving Innovation in Clinical Research
At Clinfinite Solutions, we are devoted to reworking healthcare through medical excellence. Our participation in Clinical Trials in Las Vegas is rooted in our notion that localized research, whilst finished to international standards, yields powerful results. We collaborate with hospitals, study institutions, and biopharmaceutical agencies to execute trials that meet regulatory expectations at the same time as retaining patient safety and well-being at the forefront.
Our services span from protocol development to regulatory submissions, patient recruitment, site tracking, information control, and the very last reporting. Whether it’s a Phase I safety have a have a look at or a multi-phase III efficacy trial, Clinfinite Solutions has the infrastructure and understanding to supply excellence at each stage.
Benefits of Participating in Clinical Trials
For individuals, taking part in Clinical Trials in Las Vegas gives several benefits. Participants may additionally gain early get entry to to breakthrough remedies and get hold of care from main medical professionals. Additionally, they make contributions to the development of drugs, assisting destiny sufferers get access to better remedies and treatment plans.
Healthcare providers and researchers additionally benefit extensively. Clinical trials offer valuable insights into disorder mechanisms and remedy responses. Furthermore, they open doorways to collaboration with pharmaceutical agencies, beautify the reputation of study institutions, and bring financial sources into the neighborhood healthcare environment.
Ensuring Ethical and Regulatory Compliance
Clinfinite Solutions guarantees that every medical trial meets the best ethical and regulatory requirements. We adhere to Good Clinical Practice (GCP) hints, keep transparency throughout the process, and prioritize knowledgeable consent and patient rights. All Clinical Trials In Las Vegas facilitated with the aid of us are reviewed by Institutional Review Boards (IRBs) to protect participant welfare.
Our group of regulatory experts in India and the U.S. Paintings in tandem to control documentation, approvals, and compliance, ensuring that trials aren't only a success but additionally legally and ethically sound. This robust method helps faster approvals and smoother operations, contributing to the success of every take a look at.
Supporting a Healthier Future
The contribution of Clinical Trials in Las Vegas to the global healthcare landscape can not be overstated. These trials are not just medical research; they may be the bridge between innovation and patient care. With the proper infrastructure, ethical oversight, and medical rigor, Las Vegas is rising as a crucial participant in this worldwide attempt.
At Clinfinite Solutions, we take satisfaction in our function as a catalyst for development. We believe in the transformative strength of studies and are devoted to improving lives through science. As we enlarge our footprint in Las Vegas and beyond, we invite stakeholders from all sectors—sufferers, providers, and partners—to sign up for us in shaping the destiny of healthcare.
Conclusion
The future of medication lies in evidence-based research, and Clinical Trials In Las Vegas are poised to steer this modification. Through the dedication and understanding of Clinfinite Solutions, these trials are carried out with integrity, precision, and care. From early-phase improvement to post-marketplace studies, our holistic method ensures that each trial contributes meaningfully to clinical understanding and affected person fitness.
Whether you are a sponsor in search of a dependable companion, a healthcare expert interested in research collaboration, or a patient seeking to make a contribution to clinical advancement, Clinfinite Solutions is here to guide you. Together, we are able to liberate progressive solutions and build a healthier tomorrow—one medical trial at a time.
Read More;
Biomedical Device Companies
Clinical Research Specialists
#clinfinite solutions#specimen collection in healthcare#biobanking#healthcare technology companies#clinical research specialists#blood collection methods#value of clinical development#clinical care solutions#sample collection tubes#clinical research jobs in hyderabad
0 notes
Text
How Audio Transcription Strengthens Academic Research
Making recorded data clearer, searchable, and more usable in every stage of research.
In academic research, spoken content is everywhere, interviews, lectures, focus groups, field notes. These recordings often capture some of the most insightful and nuanced material, yet they’re also the hardest to work with in their raw form.
Audio transcription offers a practical solution. It transforms hours of spoken data into text you can analyze, reference, and share—making your research process smoother, more accurate, and ultimately more impactful.
Let’s explore how transcription helps at every step of the academic journey.
1. Turning Audio into Searchable, Structured Data
When you're dealing with multiple interviews or hours of recorded material, it becomes nearly impossible to find key moments quickly. Transcripts give structure to your data. You can scan, annotate, and tag themes without constantly replaying audio files.
For qualitative researchers, transcription becomes the foundation for coding and thematic analysis. For students, it makes referencing sources during writing or presentations much easier. In short, it turns scattered recordings into an organized, usable asset.
2. Supporting Deeper and More Accurate Analysis
Listening to audio repeatedly not only eats up time but also increases the risk of overlooking details. A professionally transcribed document gives you the ability to read carefully, cross-reference quotes, and engage more deeply with your content.
Written transcripts also support collaborative research. Team members can read and interpret the same material without waiting for audio playback—helping everyone stay aligned and efficient.
3. Preserving Accuracy and Context
While automated transcription tools are convenient, they often miss important cues, especially when dealing with heavy accents, domain-specific language, or overlapping speakers.
In academic work, these inaccuracies can compromise your results. A slight misinterpretation of a word or an incorrectly attributed quote can change the meaning entirely. Human transcription offers far greater reliability, especially in research where context matters.
4. Enhancing Accessibility and Inclusivity
Transcripts aren’t just for the researcher—they benefit others who engage with your work. Students with hearing impairments, collaborators from non-native English backgrounds, or peer reviewers can access your material more easily through text.
This level of accessibility supports a more inclusive academic environment and also aligns with many institutions’ requirements for accessible content in publications and presentations.
5. Meeting Ethical and Privacy Standards
Academic research often involves sensitive material—interviews with vulnerable populations, confidential project data, or unpublished findings. It’s critical that this information is handled responsibly.
While some AI tools store uploaded data or use it to train their models, professional academic transcription services typically operate under strict non-disclosure agreements. For researchers working under IRB protocols or institutional guidelines, this level of confidentiality is non-negotiable.
6. Tailoring Output to Academic Needs
Beyond just accuracy, human transcription services can offer custom formatting, speaker labels, timestamps, or APA/MLA style formatting, based on your project’s requirements. This saves time and ensures your transcripts are ready for citation, publishing, or submission.
Quick Comparison: AI vs. Human Transcription
Here’s a side-by-side look at why researchers often opt for human transcription when quality matters:
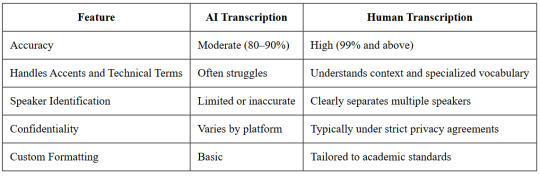
Final Thought
Transcription might not seem like the most glamorous part of research—but it’s one of the most transformative. It bridges the gap between raw, unstructured audio and usable, insightful content.
If your goal is to save time, improve accuracy, and ensure the quality of your findings, transcription isn’t just a helpful tool—it’s a strategic advantage. Partnering with experienced professionals who understand the academic context can make all the difference in how effectively your work moves forward.
0 notes
Text
What Skills Do You Gain from Pursuing a Career in Clinical Research?
One area of the pharmaceutical and healthcare industries that is expanding quickly is clinical research. The need for qualified experts in clinical research has grown dramatically as more novel therapies and drugs are created. What skills do you get by pursuing a career in clinical research? Is a question you may have if you're thinking about taking clinical research courses?
Let’s explore the core competencies and transferable skills that make clinical research a dynamic and rewarding career path.
1. Analytical and Critical Thinking Skills
Analytical thinking is one of the fundamental abilities you acquire in clinical research. To ascertain the efficacy and safety of novel medications or therapies, clinical trials entail gathering, evaluating, and analysing enormous data sets. You will get knowledge of how to: • accurately interpret clinical data; • Use statistical software to analyse outcomes; Analyse possible risks and rewards; find patterns and trends in clinical outcomes; these abilities contribute to the validity, dependability, and action ability of study findings.
2. Attention to Detail
A high degree of precision is required for clinical research. Your attention to detail is essential whether you're organizing case report forms, recording patient replies, or making sure ethical compliance is maintained. Clinical research courses help you to: • Excellent documentation abilities • the practice of cross-checking and error-checking • Adherence to legal requirements; • Regularity in data collection since a single mistake in paperwork can affect the entire trial, accuracy is one of the most highly regarded qualities in this field.
3. Ethical and Regulatory Understanding
In clinical research, adhering to Good Clinical Practice (GCP) and ethical standards is non-negotiable. You’ll gain a thorough understanding of:
Informed consent procedures
Patient rights and confidentiality
Institutional Review Board (IRB) processes
International guidelines like ICH-GCP, FDA, and EMA standards
These skills ensure that you can conduct trials ethically and legally across diverse settings.
4. Communication and Interpersonal Skills
Clinical research is a collaborative field. Whether you’re coordinating with doctors, interacting with participants, or reporting to sponsors, strong communication is vital. During your training, you’ll build:
Verbal and written communication skills
Report writing and documentation expertise
Conflict resolution and patient counselling abilities
Team collaboration strategies
Clear communication improves study outcomes and participant trust.
5. Project Management and Organizational Skills
Managing a clinical trial requires juggling multiple responsibilities, from budgeting and resource allocation to timeline tracking. Clinical research courses equip you with:
Time management strategies
Budget and resource planning
Task delegation and workflow coordination
Monitoring and reporting progress
These skills are especially beneficial if you pursue roles like Clinical Research Associate (CRA) or Project Manager.
6. Technical Proficiency
Modern clinical research relies heavily on technology. You’ll gain hands-on experience with tools and platforms used for:
Electronic Data Capture (EDC)
Clinical Trial Management Systems (CTMS)
Statistical software like SPSS or SAS
Remote monitoring tools
This technical foundation enhances your efficiency and employability.
7. Problem-Solving and Decision-Making Abilities
Unexpected issues often arise in clinical trials—participant dropouts, protocol deviations, or data inconsistencies. Clinical research training helps you:
Identify problems quickly
Develop logical solutions
Make informed decisions under pressure
Adapt to changes and stay compliant
These capabilities are crucial in dynamic and high-stakes environments.
8. Career-Ready Professionalism
Beyond technical skills, clinical research courses also nurture professional behaviour. You learn to:
Maintain confidentiality
Act responsibly and ethically
Work independently and as part of a team
Uphold a high standard of integrity
These soft skills are key to building long-term success and credibility in the field.
Conclusion
What abilities can a job in clinical research provide, then? The answer is: a broad range of interpersonal, analytical, ethical, and technical abilities that set you up for success in one of the most important sectors of healthcare innovation. Clinical research courses provide a strong basis for a rewarding career if you have a strong interest in science, patient care, and advancing medical knowledge. Regardless of your professional goals—clinical research coordinator, CRA, data manager, or regulatory specialist—the abilities you gain will provide access to a wide range of public and private sector employment options.
0 notes
Text
Custom Biobanking Protocols: Tailoring Sample Collection to Research Needs
Every research project has unique requirements, which is why a custom biobanking protocol is essential for optimizing sample collection, processing, and storage. A customized approach ensures that biospecimens meet specific regulatory, ethical, and experimental criteria, making them more suitable for advanced studies.
Why Opt for Custom Biobanking? ✔ Controlled collection and processing – Tailored workflows to maintain sample viability. ✔ Regulatory compliance – Adherence to FDA, IRB, and other international biobanking standards. ✔ Specialized storage solutions – Cryopreservation and long-term sample retention based on project requirements.
With tailored biobanking solutions, research organizations can enhance data reliability and streamline clinical validation processes.
0 notes
Text
Clinical Trials Organizations From Research to Reality
Introduction: Understanding Clinical Trials Organization
A Clinical Trials Organization plays a crucial role in the drug development process, ensuring that new treatments are thoroughly tested for safety and efficacy before they reach the market. These organizations facilitate clinical trials, which are essential in determining whether a new drug, medical device, or treatment method is safe and effective for human use. The success of new therapies largely depends on how efficiently a Clinical Trials Organization manages the research process.
What is a Clinical Trials Organization?
A Clinical Trials Organization is a specialized entity responsible for planning, conducting, and overseeing clinical trials. It ensures compliance with regulatory guidelines and ethical standards while managing the entire trial process. These organizations work closely with pharmaceutical companies, government agencies, and healthcare institutions to develop new medical solutions.
Key Components of a Clinical Trials Organization
A Clinical Trials Organization consists of several essential stakeholders who collaborate to conduct successful trials:
Sponsors – Pharmaceutical companies, biotech firms, and research institutions often act as sponsors, providing financial support and initiating clinical trials.
Contract Research Organizations (CROs) – CROs assist in managing clinical trials by offering expertise in regulatory compliance, patient recruitment, and data analysis.
Institutional Review Boards (IRBs) – IRBs review and approve trial protocols to ensure they meet ethical and safety standards, prioritizing patient welfare.
Clinical Research Sites – Hospitals, universities, and research centers serve as locations where trials are conducted, and participants receive treatments.
Phases of Clinical Trials in a Clinical Trials Organization
Clinical trials are conducted in multiple phases to ensure thorough testing of new treatments:
Phase 1 – This phase primarily assesses the safety and dosage of a new drug by testing it on a small group of healthy volunteers.
Phase 2 – A larger group of patients receives the treatment to evaluate its effectiveness and identify potential side effects.
Phase 3 – Conducted on a large scale, this phase compares the new treatment against existing standard treatments to confirm its efficacy.
Phase 4 – After the drug receives regulatory approval, this phase involves post-market surveillance to monitor long-term effects and ensure continued safety.
Challenges Faced by Clinical Trials Organizations
Despite advancements in medical research, Clinical Trials Organizations encounter various challenges, including regulatory hurdles, complex approval processes, and the high costs associated with trials. Patient recruitment is another significant challenge, as finding willing and eligible participants can delay the trial process. Additionally, ensuring data integrity and managing potential biases in clinical studies remains a critical concern.
The Role of Technology in Modern Clinical Trials Organizations
Technology is revolutionizing how Clinical Trials Organizations operate. Artificial intelligence (AI) enhances data analysis and patient monitoring, while blockchain ensures secure and transparent record-keeping. Decentralized clinical trials, where participants can engage remotely through digital tools, are becoming more popular, reducing logistical barriers and improving patient engagement.
Best Practices for an Efficient Clinical Trials Organization
To ensure successful clinical trials, organizations must adopt best practices such as:
Regulatory Compliance – Adhering to FDA, EMA, and other regulatory standards is essential to gaining drug approval.
Patient-Centric Approach – Improving patient engagement through digital tools and communication enhances trial participation rates.
Data Integrity & Transparency – Maintaining accurate records and ensuring open communication fosters trust and credibility in the trial process.
Conclusion: The Future of Clinical Trials Organizations
The future of Clinical Trials Organizations lies in embracing technological advancements, enhancing patient engagement, and streamlining regulatory procedures. As clinical research continues to evolve, adopting innovative approaches will help accelerate drug development and improve healthcare outcomes worldwide.
0 notes
Text
Empowering Startups: Equipment Financing Solutions in Texas, Hawaii, and NYC

Understanding Clinical Trial Registries are at the heart of medical innovation, offering a systematic way to test new treatments, therapies, and drugs for safety and efficacy. In Canada, clinical trial registries and proper management of trials are essential to ensuring transparency, safety, and ethical standards. This article explores the importance of clinical trial registries, the role of investigational drug studies, and managing adverse events in clinical research.
What Are Clinical Trial Registries?
A clinical trial registry is a publicly accessible database where information about ongoing and completed clinical trials is recorded. These registries serve as a valuable resource for researchers, healthcare professionals, and the general public. In Canada, the Health Canada Clinical Trial Registry Database (HCCTD) is a key registry that tracks trials approved by Health Canada.
Registries ensure transparency in clinical research by documenting the purpose, methodology, and outcomes of trials. This helps prevent duplication, promotes accountability, and allows participants and stakeholders to make informed decisions. Additionally, the International Clinical Trials Registry Platform (ICTRP), managed by the World Health Organization (WHO), provides a global framework for harmonizing clinical trial registries, including those in Canada.
The Importance of Investigational Drug Studies
Investigational drug studies focus on testing new drugs or combinations of drugs to evaluate their safety and efficacy. These studies are conducted in phases:
Phase 1: Focuses on safety and dosage, typically involving a small number of healthy volunteers.
Phase 2: Tests the drug's efficacy on a larger group with the target condition while continuing to assess safety.
Phase 3: Involves even larger populations to confirm efficacy, monitor side effects, and compare the drug to existing treatments.
Phase 4: Conducted after market approval to gather additional data on long-term safety and effectiveness.
Canada follows strict guidelines under the Food and Drugs Act and associated regulations to ensure that investigational drugs undergo rigorous testing before approval. Ethics committees and institutional review boards (IRBs) oversee these studies to protect the rights and safety of participants.
Managing Adverse Events in Clinical Trials
Managing clinical trial adverse events Adverse events (AEs) are unintended effects that occur during clinical trials, ranging from mild side effects to serious medical complications. Proper management of AEs is critical to ensuring participant safety and maintaining the integrity of the trial.
Researchers in Canada are required to report serious adverse events (SAEs) to Health Canada and the trial's ethics board within a stipulated timeframe. Managing AEs involves:
Monitoring and documentation: Accurate recording of AEs to assess their frequency and severity.
Risk mitigation: Adjusting dosages, altering protocols, or pausing trials to address safety concerns.
Participant communication: Ensuring participants are informed about potential risks and updates during the trial.
Conclusion
Clinical trial registries, investigational drug studies, and adverse event management form the backbone of ethical and effective medical research. By leveraging tools like the Health Canada Clinical Trials Database and adhering to stringent safety protocols, Canada continues to lead in advancing healthcare innovation. For researchers, participants, and healthcare providers, Understanding investigational drug studies these processes is essential to fostering trust and progress in clinical research.
1 note
·
View note
Text
Role of Clinical Research Services in Advancing Healthcare
In the dynamic landscape of healthcare, clinical research plays a pivotal role in driving innovation, improving patient outcomes, and advancing medical knowledge. Clinical research services encompass a wide range of activities aimed at evaluating the safety, efficacy, and effectiveness of medical interventions, from pharmaceutical drugs to medical devices and beyond. Through rigorous scientific inquiry and collaboration between researchers, clinicians, and industry partners, clinical research services contribute to the development of new therapies, treatments, and healthcare solutions. Let's delve into the world of clinical research services and explore their profound impact on the future of healthcare.
Comprehensive Study Design and Protocol Development
At the heart of clinical research services lies the meticulous design and development of study protocols that guide the conduct of clinical trials and research studies. Experienced research teams work closely with sponsors, investigators, and regulatory agencies to design studies that adhere to ethical principles, regulatory requirements, and scientific standards. This involves defining study objectives, selecting appropriate study populations, determining study endpoints, and establishing methodologies for data collection and analysis.
By employing robust study design and protocol development methodologies, clinical research services ensure that research studies are conducted with scientific rigor and integrity. This helps to minimize bias, confounding variables, and other sources of error, leading to reliable and credible study results that inform clinical practice and regulatory decision-making.
Patient Recruitment and Enrollment
Patient recruitment and enrollment are critical aspects of clinical research services that involve identifying and enrolling eligible participants in research studies. Research teams employ various strategies to recruit participants, including advertising, outreach efforts, and collaboration with healthcare providers and patient advocacy groups. Additionally, electronic health record systems and patient registries may be utilized to identify potential participants who meet study criteria.
Efficient and timely patient recruitment is essential for the successful completion of clinical trials and research studies. By engaging with diverse patient populations and communities, clinical research services strive to ensure adequate representation and participation in research studies, ultimately enhancing the generalizability and applicability of study findings to real-world clinical settings.
Regulatory Compliance and Ethical Oversight
Ensuring regulatory compliance and ethical oversight is paramount in clinical research services to protect the rights, safety, and well-being of research participants. Research teams work closely with regulatory agencies, institutional review boards (IRBs), and ethics committees to obtain approvals and oversee the conduct of research studies in accordance with applicable regulations and guidelines.
This involves submitting study protocols, informed consent forms, and other study-related documents for review and approval, as well as addressing any regulatory or ethical concerns that may arise during the course of the study. By adhering to rigorous regulatory and ethical standards, clinical research services uphold the highest standards of integrity and accountability in the conduct of clinical research.
Data Collection, Monitoring, and Analysis
Data collection, monitoring, and analysis are essential components of clinical research services that involve gathering, recording, and analyzing data generated during the course of research studies. Research teams utilize electronic data capture systems, case report forms, and other data collection tools to collect study data in a standardized and systematic manner.
Furthermore, clinical research services employ rigorous monitoring and quality assurance measures to ensure the accuracy, completeness, and integrity of study data. This may include on-site monitoring visits, source data verification, and data validation checks to identify and address any discrepancies or deviations from the study protocol.
Once data collection is complete, research teams conduct statistical analysis and interpretation of study findings to assess the safety, efficacy, and effectiveness of the investigational intervention. This involves employing advanced statistical methods and techniques to analyze study endpoints, identify trends, and draw meaningful conclusions from the data.
Dissemination of Study Results and Knowledge Translation
The dissemination of study results and knowledge translation are essential components of clinical research services that involve sharing research findings with the broader scientific community, healthcare providers, policymakers, and the public. Research teams publish study results in peer-reviewed journals, present findings at scientific conferences, and communicate results through various channels to facilitate knowledge dissemination and translation into clinical practice.
Furthermore, clinical research services play a vital role in promoting evidence-based medicine and informing healthcare decision-making by providing stakeholders with timely access to accurate and reliable research evidence. This helps to bridge the gap between research and practice, ultimately improving patient care and outcomes.
Conclusion
In conclusion, clinical research services are essential drivers of innovation and progress in healthcare, facilitating the development and evaluation of new therapies, treatments, and healthcare solutions. Through comprehensive study design, patient recruitment, regulatory compliance, data collection and analysis, and dissemination of study results, clinical research services contribute to advancing medical knowledge, improving patient outcomes, and shaping the future of healthcare. By embracing the principles of scientific inquiry, collaboration, and ethical conduct, clinical research services play a vital role in promoting evidence-based medicine and ultimately enhancing the quality of care for patients worldwide.
0 notes
Text
Proxies and Academic Research: Gathering Data for Studies

Introduction
In the realm of academic research, data collection is a crucial step in conducting meaningful studies. However, researchers often encounter challenges when gathering data due to various restrictions, such as geographical limitations and access restrictions imposed by websites or online platforms. Proxies offer a valuable solution to overcome these barriers by providing researchers with the ability to gather data from diverse sources while maintaining privacy and complying with ethical considerations. In this article, we will explore the role of proxies in academic research and how they can assist researchers in collecting data for their studies. We will discuss the benefits of using proxies, considerations for ethical data collection, and best practices for incorporating proxies into academic research workflows.
Understanding Proxies in Academic Research
Proxy Basics: A proxy acts as an intermediary between a researcher’s computer and the internet. It allows researchers to route their internet traffic through a server located in a different geographical location, enabling them to access websites and online platforms that may have restrictions based on the researcher’s location.
Benefits of Proxies in Academic Research: Proxies offer several advantages for data collection in academic research. They provide researchers with the ability to access geographically restricted content, gather data from websites that implement access restrictions, and maintain anonymity during data collection.
Ethical Considerations for Data Collection
Respect Terms of Service: When using proxies for data collection, it is essential to respect the terms of service of the websites or platforms being accessed. Researchers should review and comply with the terms, ensuring that their data collection activities align with the guidelines set by the website or platform.
Privacy and Anonymity: Researchers must prioritize privacy and anonymity when using proxies. Data collection activities should not infringe upon the privacy of individuals or violate any ethical guidelines. Proper anonymization techniques should be employed to protect the identities of individuals or entities involved in the collected data.
Institutional Review Board (IRB) Approval: If the research involves human subjects or sensitive data, researchers should seek approval from their institution’s IRB or ethics committee. The IRB can assess the ethical considerations of the study, including data collection methods involving proxies, and provide guidance on the proper implementation of research protocols.
Best Practices for Incorporating Proxies in Academic Research
Proxy Selection: Choose reputable proxy providers that offer reliable and secure services. Consider factors such as server locations, connection speeds, and the provider’s privacy policy. Opt for proxies that offer a wide range of geographical locations to access diverse data sources.
Data Collection Planning: Develop a comprehensive data collection plan that outlines the objectives, methodologies, and specific data sources. Consider the limitations and potential biases associated with proxy-based data collection and devise strategies to address them.
Proxy Configuration: Configure the proxy settings on the researcher’s computer or network to route traffic through the selected proxy server. Ensure that the configuration is properly implemented and tested to verify that the desired websites or platforms can be accessed successfully.
Web Scraping and Crawling: Use web scraping and crawling techniques to automate data collection from websites and online platforms. Employ tools or programming libraries specifically designed for web scraping, ensuring compliance with the terms of service and ethical considerations of the targeted websites.
Data Storage and Security: Maintain proper data security measures throughout the data collection process. Store collected data securely and implement necessary encryption and access controls to protect the integrity and confidentiality of the data.
Data Cleaning and Validation: Thoroughly clean and validate the collected data to ensure its accuracy and reliability. Remove any duplicates, outliers, or irrelevant information that may affect the integrity of the research findings.
Ethical Data Handling: Adhere to ethical guidelines when handling collected data. Protect the privacy and confidentiality of individuals or entities involved in the data, and anonymize any personally identifiable information in accordance with ethical considerations and applicable regulations.
Collaboration and Knowledge Sharing: Engage in collaboration and knowledge sharing within the academic community when using proxies for data collection. Share insights, experiences, and best practices with fellow researchers to promote ethical and responsible data collection practices.
Conclusion
Proxies play a vital role in academic research by enabling researchers to overcome geographical and access restrictions when collecting data. By using proxies, researchers can access diverse data sources, gather information from websites with access limitations, and maintain privacy and anonymity during the data collection process. However, it is essential to adhere to ethical considerations, respect terms of service, and obtain necessary approvals from institutional review boards when using proxies for data collection. By incorporating proxies into academic research workflows and following best practices, researchers can enhance their ability to collect comprehensive and meaningful data, contributing to the advancement of knowledge in their respective fields.
1 note
·
View note
Text
Unlocking Innovation: Clinical Trials in Las Vegas by Clinfinite Solutions
The healthcare and pharmaceutical industries depend closely on medical research to make bigger, secure, and effective remedies. Across America, principal cities have emerged as study hubs, with Las Vegas step by step gaining prominence in this area. Known for more than its amusement and tourism, Las Vegas is now setting up a strong presence in the medical studies landscape. Clinical trials in Las Vegas are getting increasingly important, imparting sufferers early get right of entry to to cutting-edge healing procedures even as advancing technological know-how and medicine.
Why Las Vegas Is Becoming a Clinical Research Hub
Las Vegas offers numerous strategic benefits that make it perfect for conducting scientific trials. With a developing populace, diverse demographics, and more than a few healthcare establishments, the town presents a fertile ground for medical researchers. The affected character population proper right here consists of humans from numerous ethnic, socioeconomic, and age groups, providing rich and inclusive records for trial consequences.
Additionally, Las Vegas boasts a properly developed infrastructure, making it clean for patients to travel to trial sites. This convenience often improves patient compliance and retention rates. These elements blended are drawing the eye of study sponsors, CROs (Contract Research Organizations), and pharmaceutical companies seeking to behavior Clinical trials in Las Vegas.
Types of Clinical Trials in Las Vegas
Las Vegas is host to a broad spectrum of clinical trials, from early-segment exploratory research to late-phase confirmatory trials. The metropolis sees trials in therapeutic regions along with:
Oncology
Cardiology
Neurology
Infectious sicknesses
Dermatology
Mental health
Both authority-sponsored and private-region trials are energetic in the location, presenting nearby citizens several possibilities to participate in studies which can at once impact their fitness or that of future generations.
Benefits for Participants
Patients and volunteers who take part in Clinical trials in Las Vegas experience numerous benefits. These consist of:
Access to New Treatments: Many contributors advantage early get admission to to novel treatment options not yet available to the general public.
Comprehensive Care: Clinical trial participants often obtain loose scientific tests, lab checks, and health monitoring throughout the study duration.
Contributing to Science: Taking part in a medical trial is a way to contribute to medical advancement and help others with similar situations.
Most importantly, Las Vegas trial facilities ensure that participant safety and ethics are prioritized, adhering to strict regulatory guidelines.
Role of Research Institutions and CROs
The metropolis’s developing ecosystem of research establishments, hospitals, and CROs performs a crucial role in handling and executing medical trials. Organizations just like the University of Nevada, Las Vegas (UNLV), nearby hospitals, and dedicated scientific studies facilities collaborate to provide first rate have a look at talents. These establishments paintings under the supervision of Institutional Review Boards (IRBs) and observe the requirements set with the aid of the FDA and ICH-GCP (Good Clinical Practice).
Moreover, Las Vegas-based CROs streamline the trial method for sponsors, supplying cease-to-quit services like protocol design, affected person recruitment, regulatory submissions, records control, and reporting. This permits pharmaceutical and biotech agencies to behavior green and reliable medical trials in Las Vegas.
Opportunities for Medical Professionals
The scientific research discipline in Las Vegas additionally creates numerous job possibilities for healthcare experts. Physicians, nurses, lab technicians, and data analysts are finding new career paths in scientific studies. These roles no longer best provide professional growth, but also contribute to life-saving innovations.
Clinfinite Solutions, as an example, has been actively taking part with trial sponsors and investigators to provide tailored scientific solutions. With a commitment to transparency, performance, and patient welfare, the agency performs an essential role in making Clinical trials in Las Vegas successful and impactful.
Overcoming Challenges
Like any clinical studies hub, Las Vegas faces challenges, consisting of patient recruitment, public recognition, and regulatory complexities. However, neighborhood researchers are growing to the event via community outreach, education campaigns, and digital recruitment systems. These tasks are assisting in bridging the gap between studies and the general population.
Additionally, technology is transforming the manner trials are carried out. From electronic facts capture to digital trials, Las Vegas study centers are adopting modern equipment to enhance observation efficiency and participant experience.
Future of Clinical Trials in Las Vegas
The destiny looks promising for Las Vegas as a hub for medical studies. With improved funding in healthcare infrastructure and developing collaboration among academia, organisations, and authorities, the metropolis is poised to increase its abilities even further. Efforts are also underway to contain customized medicinal tablets, genomics, and AI-driven diagnostics into the clinical trial framework.
As attention grows and more citizens bear in mind collaborating in studies, the metropolis will continue to provide a robust platform for groundbreaking research. This progress will no longer simply benefit local groups, but also make contributions to global health.
Conclusion
In precis, Clinical trials in Las Vegas are reshaping the healthcare landscape of the place. They offer vital possibilities for sufferers, experts, and pharmaceutical innovators alike. With strong institutional resources, affected folks, and a willingness to moral studies practices, Las Vegas is turning into a key participant in advancing the scientific era.
Whether you're an affected person searching for new remedy alternatives, a healthcare provider, or a research sponsor, Las Vegas offers a dynamic environment for medical trials. The metropolis’s evolution into a scientific research hub is a testimony to its readiness to aid the destiny of drugs, one examine at a time.
Read More:
Clinical Care Solutions
Blood Collection Storage
#clinfinite solutions#healthcare technology companies#specimen collection in healthcare#biobanking#blood collection methods#value of clinical development#sample collection tubes#clinical research specialists#clinical care solutions#clinical research jobs in hyderabad
0 notes
Photo

Rating: 5
Genre: nonfiction, sociology
"One of the most important things to say about the gender data gap is that it is not generally malicious, or even deliberate. Quite the opposite. It is simply the product of a way of thinking that has been around for millennia and is therefore a kind of not thinking. A double not thinking, even: men go without saying, and women don't get said at all. Because when we say human, on the whole, we mean man."
Coming into this book, I was afraid that it would mostly be generalizations with a few random statistics thrown in here and there, but I was pleasantly amazed.
I was also angry, and rightfully so given the myriad of evidence that so many of today's male-oriented problems are killing women, and the solutions given are equally male-oriented that they make the problems worse. For example, more women than men died in the 2004 tsunami in Indonesia. Why? Women are more likely than men to care for children and the elderly. They are more likely to be in remote locations for work where warning signals are unreachable. They are less likely to be equipped with the ability to swim, and even should they be able to make it out alive, men are more likely to undertake a crime of opportunity-- rape, assault, murder. Urban planning is often made by men without the intention of obscuring women, and this is seen even on a smaller scale. It took a Google executive to become pregnant for her to suggest implementing closer parking spots for pregnant workers. It took an individual in a position of power to experience it in order to suggest and make receptable change (if at all).
What angered me even more was the aspect of public transport. Women are more likely to use public transport than men. If there is a car in a household, it is implicit that the man will use the car and the woman the bus. Yet, public transport systems were designed by men without the input of women. In Southern America, bus stops placed even two blocks closer to a "safer" neighborhood can be the difference between life and death. Women are more afraid of the to and fro than their actual workplace, as gang violence often leads to kidnapping and rape. On the topic of transportation, women are exponentially more likely to "trip-chain" than their male counterparts, expected to plan sub-trips involving driving home from work, dropping a child off at an extracurricular activity, going grocery shopping, picking up their child from said activity, and then returning home. Men are not expected to do the same. Work, social, and home lives are so male-oriented that the female perspective goes overlooked.
And research? The fact that women are commonly excluded from clinical trials yet given the same medicinal protocols is staggering. Biological men don't get pregnant, they don't have fluctuating hormones, they thus don't exhibit the same symptomatology or physiological ramifications as women might have. Yet, the decision to increase female participation in research remains unprioritized, and this finding might be attributed to the fact that most individuals on IRB panels are men. The ability to recognize such a discrepancy is amiss. The facts and data are already here, but the catalyst to change the norm is up in the air.
Empowering women and engaging female leadership starts with this change, and after reading this book, it is impossible not to see every little nuance all around us. Criado-Perez has truly gone above and beyond in her research and what could have been a didactic-heavy book was incredibly captivating to the lay-reader.
#carolina criado perez#criado perez#invisible women#nonfiction#sociology#book review#goodreads#data science#feminism#bias#gender
16 notes
·
View notes
Text
SCRO Stem Cell Research Oversight Protocol Software
Key Solutions offers SCRO stem cell research oversight protocol software which is responsible for developing guidelines on issues like donors, registries etc.
Key Solutions offers research administration and compliance software which are integrated with modules like iacuc and irb.
The SCRO module ensures that any stem cells research conducted in an organization is in compliance with the organization’s regulatory policies by streamlining the approval workflow between the investigators, the coordinators, and the review committee.
1 note
·
View note
Text
What to Look for When Partnering with a CRO for CNS Clinical Trials
When your organization develops a drug that affects the central nervous system, early clinical development can be complex, and requires teams with extensive clinical research expertise. As a result, many organizations partner with a contract research organization that has the necessary experience to conduct their CNS trials during the early stages of the drug development process. Here are a few factors to keep in mind when your organization is searching for the right CRO partner for your CNS trials.

Experience Handling Regulations Regulations for CNS drugs can be intricately detailed. You want to find a CRO with experience in adaptive trial designs often associated with CNS clinical trials and therapies, as well as bioanalysis needed for on-time regulatory submissions. The CRO team should also have experience working with Institutional Review Boards. Familiarity with IRBs means your protocol will be submission-ready while minimizing the potential for making amendments later, thus shortening your study’s start-up time. Specialized Recruiting Strategies Your chosen CRO should have experience recruiting potential participants for CNS studies. These can be different from typical first in human clinical trials, which may have broader needs. Experience recruiting for both types of studies is ideal. Having an in-house, full-time recruitment team means the CRO does not need to relay your requirements and preferences to outside organizations. Instead, the CRO can start creating efficient recruitment strategies through the most effective channels to meet any of your target milestones. Plus, access to a curated database of potential participants can help drastically reduce recruiting time. Sterile Drug Preparation Facilities Find a CRO with clean rooms for sterile drug preparation and staff experience in extemporaneous and intravenous preparation, including biologics. You will need staff with experience in preparing and dosing studies, particularly in the method your specific drug will be administered. This could be oral, sublingual, intranasal, or parenteral routes. Make sure the pharmacies are secured, such as with electronic fobs, facial recognition, or video monitoring. A Team of Dedicated Research Physicians and Analysts Having a team of dedicated research physicians that are deeply involved in all aspects of your CNS clinical trials can help ensure the proper technical and medical procedures are completed. Keeping lines of communication open is vital. A full-service CRO can easily provide the physicians with the information they need from previous phases, helping information flow seamlessly. The physicians should be familiar with how CNS clinical trials differ from typical clinical trials. Driving Simulations and Cognitive Testing Finally, having qualified resources available to do additional tests, such as cognitive tests or driving simulations studies is a great benefit. The driving simulation, for example, can help provide accurate information on driving performance data that compares to over-the-road testing but in far less time. It also costs less and doesn’t risk any potential property damage or injuries to the participant. These types of studies can highlight the effects of age, trauma, drowsiness, neurologic disease, CSN stimulants, and CNS depressants. About Altasciences As a mid-sized contract research organization and drug development solution, Altasciences offers partners more than 25 years of research experience for preclinical studies and clinical trials. As an integrated CRO, CDMO pharma services are just one of many that Altasciences offers, with an innovative approach both biotechnology and pharmaceutical companies rely on. Clients also gain the Altasciences teams’ expertise in a wide variety of study types and therapeutic indications, including vast experience in first-in-human clinical trials, CNS clinical trials, and ethnobridging. Altasciences provides partners with access to dedicated Phase 1 clinical trial units across North America, both in the USA and Canada. Partners can utilize Altasciences’ resources, including access to over 580 beds, an experienced, highly trained staff, and a recruiting database of more than 400,000 potential participants. Partner with this trusted CRO/CDMO for all your early clinical development needs. Partner with Altasciences for your early clinical development and CNS clinical trials at https://www.altasciences.com/ Original Source: https://bit.ly/3Mg4ZZM
0 notes
Text
Lessons from the COVID-19 swab crisis -- LiveScience.Tech

A major crisis that accompanied the rise of the pandemic was lack of availability of the nasopharyngeal swab — necessary for testing for COVID-19, which in turn, was necessary to get a grip on the pandemic. An account of how one group addressed that crisis is published this week Journal of Clinical Microbiology, a journal of the American Society for Microbiology.
“We met the challenge by creating all-new swabs, which were ready and clinically tested in just three weeks,” said Ramy Arnaout, M.D., D.Phil., Associate Professor of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, and Associate Director of the Clinical Microbiology Laboratories, Beth Israel Deaconess Medical Center (BIDMC).
“Handling crises successfully requires a different set of skills than the everyday,” said Dr. Arnaout. “Competition and secrecy are out. Cooperation and openness are in. Resolving the swab crisis was a case study in these and other valuable lessons.”
As the first wave of COVID-19 broke out across the United States, BIDMC, which had the largest in-house COVID-19 testing center in Boston, found themselves with only a week’s supply of swabs. “More manufacturing was the only lasting solution,” Dr. Arnaout said. He and his colleagues began reverse engineering swabs, to determine if they could make them from scratch.
Swabs must be engineered to be neither too stiff, nor too flexible, and must be individually packaged and sterile. BIDMC needed around ten thousand a week; the country needed roughly ten million.
The first week, group members floated, shot down, resurrected, and repurposed various ideas, said Dr. Arnaout. Ultimately, the team saw two options: to find a scalable means to assemble swabs, or else to “find a way to make a stripped-down swab in a single go, without the need for assembly.” 3D printing had “advantages in speed of development and in the variety of structures it can make.”
Dr. Arnaout had previously demonstrated that open and collaborative crowdsourcing is a viable route to solving complex computational problems, specifically his work in computational immunology. He put this lesson to work in the COVID crisis.
“We navigated our… networks, letting manufacturers know about the swab crisis and what we needed from them to solve it,” said Arnaout. “We set up a free publicly viewable knowledge base online in the form of a GitHub repository — a type of website usually used by software engineers to collaborate on coding projects — to share everything we knew with everyone who might want to know it. This was critical for lowering the activation energy for anyone who wanted to join the effort… By the end of the first week, prototypes were rolling in.”
Over the course of the second week, the team tested more than 150 prototypes. “We were giving manufacturers feedback and suggestions one day and receiving new prototypes the next,” Dr. Arnaout said. “We put our protocols and results online.”
The team spoke often with BIDMC’s Institutional Review Board, “whose help and quick feedback were indispensable for cutting through red tape,” Dr. Arnaout said. “We put our IRB-approved protocol online as well.” BIDMC’s technology ventures office assured the team that the evaluation and feedback they were providing to manufacturers would not constitute intellectual property, thereby avoiding any haggling over ownership, which could have wasted precious time.”
By the fourth week, the team had validated four prototypes for clinical use. By summer’s end, “millions of the swabs our coalition helped design, vet, and mass-produce had been sold and used for COVID-19 testing” across the United States and in Europe, Dr. Arnaout wrote.
That experience suggested five lessons:
Define the mission — “a simple, clear, and concrete unifying goal for the entire team,” said Dr. Arnaout.
Establish norms for behavior. At BIDMC, that took place “mostly via conversations, repetition of the main message and personal example,” said Dr. Arnaout. “Conversations often began or ended with an explicit acknowledgment of the temptation to go it alone… and a reminder that we were not going to give in to such temptation.”
Leverage expertise. “At BIDMC, the clinical trials office handled paperwork that the investigators would have handled themselves under normal circumstances.”
Practice open and clear communication, “by eliminating the friction of gatekeeping access to information,” said Dr. Arnaout.
Stay positive.
“Perhaps we all can take the opportunity afforded by this trying time to improve how we meet our everyday challenges,” said Dr. Arnaout. “By doing so, we might find ourselves further along, more capable, and better prepared for when the next crisis inevitably hits.”
New post published on: https://livescience.tech/2021/08/19/lessons-from-the-covid-19-swab-crisis-livescience-tech/
0 notes
Text
Overcome Challenges in Cancer Research & Improve Decision-Making Using a Cloud-Based Informatics Solution

The fight against cancer has remained a great challenge for patients, their families and physicians as well as oncology researchers due to the dynamic and versatile nature of the disease. To date, there are over 100 different types of cancer that have been identified and they affect different cells in the body. They include carcinomas, sarcomas, leukemias, myelomas, melanomas, and lymphomas among others. Consequently, oncology researchers are constantly dealing with an avalanche of data and metadata (e.g., omics data or radiology data), which require robust infrastructure to collect, manage and interpret. It becomes even more difficult when these data sets have to be shared across different teams while maintaining compliance and other industry standards.
Why is Cancer Research Important?
Cancer research involves the discovery of cancer biology and genetic diversity and translating this knowledge to improve cancer therapy. Often, this involves the use and reuse of data from disparate sources (databases and samples). It may be necessary to get this data on one streamlined platform to allow oncology researchers faster access to the data and also facilitate collaboration, compliance, and maximize the value of research. This helps shorten the time to diagnosis and treatment and expedite the entry of drugs into the market.
Cancer treatment, from diagnosis to treatment, is always a race against time.
Cancer research sites and institutions often have limited access to research staff, facilities, and study participants or samples. Therefore, it is necessary to form networks where data, knowledge and samples can be shared collaboratively which is often a massive undertaking that requires advanced technological solutions. Multiple technical interfaces (interoperability) have to come in place since the data is usually found in varying documentation systems and at different sites. Data privacy, in this case, can be easily compromised if adequate measures are not put in place. Hence, appropriate technological solutions for pseudonymization have to be created and integrated into existing workflows to support compliance as well as yield acceptable and reproducible research results. Modern IT solutions can be used to help cancer research laboratories to maximize the value of cancer research.
As much as IT solutions will not necessarily cure patients; advances in medical IT research will go a long way in accelerating cancer research and facilitate timely treatments and consequently improve patient outcomes. With all forms of cancer, time is always of the essence.
How can a LIMS Help with Cancer Research?
The prototypical nature of cancer research requires the processing and integration of datasets in a robust technological environment. An appropriate LIMS for Cancer Research should support the whole process from tissue and sample collection and annotation to data analysis and reporting. A LIMS helps to collate data from multiple sources and studies without data redundancy, providing researchers access to high-quality data for fast decision-making. A LIMS for cancer research also helps in automating and monitoring complex workflows and flags steps that deviate from defined SOPs.
A LIMS for cancer research should be flexible, adaptive, and compliant. It needs to be able to manage data flow across different disciplines and allow the researchers to easily share and receive data, metadata, and results with other researchers in real time.
Integrating a LIMS with the different instruments such as DNA sequencers, visual tools, and other software, such as EMR, EHR, pathology systems, statistical tools, will provide the oncologist with a holistic view of the cancer patient in a real-life situation.
Ensuring Compliance in Cancer Research
Compliance is a top priority for every cancer laboratory. The Health Insurance Portability and Accountability Act (HIPAA) regulates the use and sharing of protected health information such as data related to the operation of cancer registries and registry-supported cancer research.
The lab’s LIMS should support the HL7 standards for data exchange in the cancer research industry. It also helps with consent management and meeting HIPAA requirements for safeguarding patient privacy & IRB protocols for tissue acquisition and data sharing.
Conclusion
With the complexity of cancer research and treatment, researchers can easily get overwhelmed by the multiform data that they are required to process. Deciphering the significance or irrelevance of data and metadata and drawing conclusions from the generated data sets requires the support of an intelligent and automated LIMS software. Such a software offers broader insights that can easily be translated into therapy at a faster turnaround time.
A cloud-based LIMS that manages large datasets and facilitates data access, visualization, analysis, and transmission can be integrated with other systems to facilitate informed decision-making. A cloud solution, besides storing data, may be able to execute algorithms that can be used by other researchers to gain insights from data without needing deep technical knowledge. This data can be easily accessed from different sources and locations at any given time, thereby overcoming the time constraints which is a major challenge in cancer research. This, hopefully, will speed up the decision-making process, improve patient outcomes, and clinical care of patients.
Originally published at https://cloudlims.com.
0 notes
Text
JOIN WITH US FOR the Webinar - "How to Make Efficient FDA-Compliant Clinical Protocol Amendments"
Live Webinar: "How to Make Efficient FDA-Compliant Clinical Protocol Amendments" When: Tuesday Jul 6, 2021 - 01:00 PM EST Duration: 60 Minutes.
Click here to Register Webinar

On an average a clinical protocol gets amended anywhere from 3-6 times after it has been finalized. Most protocol amendments happen for ongoing studies and require re-consenting subjects, and numerous operational and regulatory updates including FDA review and IRB approval prior to implementing an amended clinical protocol. Protocol amendments are not only operationally burdensome; they could also impact the scientific validity of the study, if not appropriately designed or implemented. This webinar, presented by a leading clinical protocol expert will discuss FDA expectations from protocol amendment process, and best practices for avoiding errors and accidents. Areas Covered in the Session: Five common reasons for amending a clinical protocol When a protocol should not be amended FDA expectations from protocol amendment process Operational aspects of protocol amendment Statistical issues that must be considered when amending protocols Differences between protocols for drugs, biologics and medical devices Best practices for training and monitoring clinical sites during and after protocol amendment Common FDA audit findings for improperly amended protocols and potential solutions
0 notes
Text
Global Clinical Trials Market– Industry Trends and Forecast to 2025
Market Definition: Global Clinical Trials Market
Clinical trials can be termed as research studies that are performed on human to gain specific information about biomedical interventions such as vaccines, treatments, drugs in order to generate a safe data. According to United States Food and Drug (FDA) form 1572, protocol and amendments for some information is must for clinical trials such as inform consent, other written information for participants, recruitment advertisement, financial disclosure form (FDF), master clinical trial agreement (MCTA), institutional review board (IRB) approval, medical licensure, training records, laboratory accreditation, visit monitor reports, miscellaneous document, signature sheet and documentation of investigational drug destruction

Market Analysis: Global Clinical Trials Market
The Global Clinical Trials Market is expected to reach USD 1.76 billion by 2025, from USD 1.04 billion in 2017 growing at a CAGR of 6.7% during the forecast period of 2018 to 2025. The upcoming market report contains data for historic year 2016, the base year of calculation is 2017 and the forecast period is 2018 to 2025..
Major Market Competitors/Players: Global Clinical Trials Market
Some of the major players operating in the global Clinical trials market are Clinipace Worldwide, LabCorp, Eli Lilly and Company, ICON Plc., Novo Nordisk, Parexel, Pfizer Inc., Pharmaceutical Product Development, LLC, IQVIA, Roche Holding, Ranbaxy Laboratories, Ltd., Sanofi Aventis A.S. and Roche Group., Aaipharma Services Corp, Accell Clinical Research LLC, Aptiv Solutions, Chiltern International Limited , Congenix, Covance Inc.
READ MORE
CONTACT US:
Customer Support 24*7
USA Number – +1 888 387 2818 (Toll – Free)
Europe Number – +44 208 089 1725
Asia Pacific Number – +81 34 520 8959
Skype.Address: databridgemarketresearch
Website :https://databridgemarketresearch.com
0 notes