#Custom Biobanking Protocols
Explore tagged Tumblr posts
Text
Custom Biobanking Protocols: Tailoring Sample Collection to Research Needs
Every research project has unique requirements, which is why a custom biobanking protocol is essential for optimizing sample collection, processing, and storage. A customized approach ensures that biospecimens meet specific regulatory, ethical, and experimental criteria, making them more suitable for advanced studies.
Why Opt for Custom Biobanking? ✔ Controlled collection and processing – Tailored workflows to maintain sample viability. ✔ Regulatory compliance – Adherence to FDA, IRB, and other international biobanking standards. ✔ Specialized storage solutions – Cryopreservation and long-term sample retention based on project requirements.
With tailored biobanking solutions, research organizations can enhance data reliability and streamline clinical validation processes.
0 notes
Text
Market Sees Growth in Cloud-Based Genomic Data Platforms
The Genomics in Cancer Care Market reached USD 13.4 billion in 2022 and is projected to grow to USD 51.1 billion by 2031, exhibiting a CAGR of 18.9% during the forecast period 2024–2031, driven by the growing role of precision medicine and targeted therapies in oncology. Genomic testing helps identify cancer-causing mutations, such as BRCA1 and BRCA2, enabling accurate diagnosis, prognosis, and treatment selection. By uncovering genetic changes in cancer cells, genomics supports the development of more effective, individualized therapies that significantly improve patient outcomes and survival rates.

Unlock exclusive insights with our detailed sample report :
Key Market Drivers
1. Rising Global Cancer Prevalence
According to WHO, cancer is a leading cause of death globally, with over 20 million new cases expected annually by 2030. This has created a demand for advanced genomic tools that facilitate early detection and personalized treatment strategies.
2. Advances in NGS and Genomic Sequencing
Technological breakthroughs in whole genome sequencing (WGS), targeted gene panels, and RNA sequencing are enhancing the ability to identify key mutations and develop tailored therapeutic approaches.
3. Shift Toward Precision Oncology
The era of one-size-fits-all cancer treatment is fading. Genomic testing enables oncologists to match therapies based on individual molecular profiles, increasing treatment success rates and reducing adverse effects.
4. Integration of AI and Machine Learning
AI-driven platforms are accelerating genomic data interpretation, assisting in variant classification, biomarker discovery, and real-time decision-making for clinicians and researchers.
5. Government and Industry Investments
Public and private investments are growing rapidly. For example:
The U.S. Cancer Moonshot initiative continues to support genomic cancer research.
Japan’s Genomic Medicine Plan is focused on nationwide whole-genome sequencing efforts and biomarker development.
Regional Highlights
United States
The U.S. is at the forefront of genomic integration in cancer care, with extensive use of NGS panels, companion diagnostics, and cloud-based genomic tools.
Leading institutions like Memorial Sloan Kettering and MD Anderson partner with biotech firms for tumor sequencing projects.
The FDA has increased approval of genomic-based cancer therapies and companion diagnostics, ensuring regulatory clarity and accelerating innovation.
Japan
Japan is heavily investing in aging-focused cancer genomics as over 28% of its population is aged 65 or above.
National cancer programs promote biobank development, data-sharing frameworks, and personalized therapeutic protocols.
Hospitals are piloting AI-integrated genomic dashboards to aid clinical decision-making for oncologists.
Speak to Our Senior Analyst and Get Customization in the report as per your requirements:
Key Segments
By Technology:
Next-Generation Sequencing (NGS)
PCR (Polymerase Chain Reaction)
Microarrays
Sanger Sequencing
By Application:
Diagnostics
Drug Discovery and Development
Prognostics and Screening
Companion Diagnostics
By Cancer Type:
Breast Cancer
Lung Cancer
Colorectal Cancer
Prostate Cancer
Others (Melanoma, Leukemia, etc.)
By End-User:
Hospitals & Clinics
Academic & Research Institutes
Biotech & Pharma Companies
Diagnostic Labs
Recent Industry Developments
Thermo Fisher Scientific launched an expanded NGS panel approved for solid tumors, improving turnaround times and reducing costs in hospitals.
Roche and Foundation Medicine extended collaboration to develop comprehensive genomic profiling (CGP) solutions for rare cancers.
Illumina and AstraZeneca announced a joint platform that integrates genomic sequencing with drug development, accelerating targeted therapy pipelines.
Japan’s National Cancer Center began a trial for population-level cancer genome screening, a first in Asia-Pacific’s clinical genomics ecosystem.
The NIH’s All of Us Research Program now includes cancer patients in its longitudinal genomic dataset, broadening ethnic and genetic diversity.
Buy the exclusive full report here:
Growth Opportunities
Expansion of Liquid Biopsy Testing: Non-invasive blood-based genomic testing is opening doors for real-time tumor monitoring and minimal residual disease detection.
Development of Multi-Cancer Early Detection (MCED) Tests: These tests use genomic signals to detect various cancer types at once, revolutionizing preventive oncology.
Decentralized Genomic Testing Platforms: The adoption of cloud and edge computing in diagnostics supports genomic data analysis even in smaller hospitals.
Increasing Partnerships with Pharma: Biopharma companies seek genomic data insights to design better trials, improving drug response and reducing trial failure rates.
Personalized Cancer Vaccines: Genomics is paving the way for neoantigen-based immunotherapies, which are now entering clinical trials globally.
Challenges and Considerations
High Costs of Sequencing: Despite decreasing, comprehensive genomic profiling remains expensive and is not uniformly reimbursed.
Data Privacy Concerns: Handling of sensitive genomic data raises questions around patient consent, security, and ownership.
Skill Gaps in Data Interpretation: Many healthcare providers still lack the training required to interpret complex genomic reports accurately.
Leading Market Players
Illumina, Inc.
Thermo Fisher Scientific
Agilent Technologies
Roche Diagnostics
Bio-Rad Laboratories
Qiagen N.V.
Foundation Medicine
Guardant Health
Fujifilm Holdings Corp. (Japan)
These companies are:
Launching multi-cancer panels
Building AI-enabled interpretation platforms
Partnering with governments and hospitals for clinical validation
Focusing on affordability and access in underserved regions
Stay informed with the latest industry insights-start your subscription now:
Conclusion
The genomics in cancer care market is not just expanding—it’s transforming the very fabric of oncology. From tumor characterization to tailored therapies, genomics is enabling a future where cancer care is not only more effective but also more humane and precise.
With growing government support, rapid adoption of AI tools, and unprecedented collaboration between diagnostics and therapeutics, the global healthcare ecosystem is on the brink of genomic-enabled cancer care at scale.
The next decade will not just be about treating cancer—but about predicting, preventing, and personalizing the battle against it.
About us:
DataM Intelligence is a premier provider of market research and consulting services, offering a full spectrum of business intelligence solutions—from foundational research to strategic consulting. We utilize proprietary trends, insights, and developments to equip our clients with fast, informed, and effective decision-making tools.
Our research repository comprises more than 6,300 detailed reports covering over 40 industries, serving the evolving research demands of 200+ companies in 50+ countries. Whether through syndicated studies or customized research, our robust methodologies ensure precise, actionable intelligence tailored to your business landscape.
Contact US:
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: [email protected]
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
#Genomics in Cancer Care Market#Genomics in Cancer Care Market size#Genomics in Cancer Care Market growth#Genomics in Cancer Care Market share#Genomics in Cancer Care Market analysis
0 notes
Text
Biobanking: Empowering Tomorrow’s Medical Breakthroughs – By Clinfinite Solutions
In recent years, the healthcare and pharmaceutical industries have witnessed transformative advances pushed by records, genetics, and precision medication. At the heart of this revolution lies biobanking—a scientific process and infrastructure that stores biological samples which including blood, tissue, and DNA, at the side of related health statistics. As a reliable call in scientific research, Clinfinite Solutions is proud to guide innovations in biobanking, offering terrific biospecimen management that fuels scientific discovery and medical breakthroughs.
Introduction to Biobanking
Biobanking is the systematic collection, processing, storage, and distribution of biological substances to be used in research and clinical care. These biospecimens—accumulated from volunteers, sufferers, or clinical trial participants—are often connected with unique health, genetic, and way of life information. When saved under controlled conditions, these samples emerge as valuable assets for lengthy-time period clinical studies, disease tracking, drug development, and the future of personalised medicine.
At Clinfinite Solutions, we consider biobanking is not merely a storage technique—it’s a foundation of translational studies. With each specimen stored, we take a step towards answering complicated clinical questions and growing targeted treatment plans.
The Importance of Biobanking in Modern Research
The growing interest in genomics and precision healthcare has catapulted biobanking into the spotlight. With continual diseases, pandemics, and emerging health threats, researchers need access to dependable, numerous, and well-preserved biological samples. Here’s how biobanking is creating a vital distinction:
Advancing Medical Research
Through complete biobanking answers, researchers gain get entry to to rich datasets that allow them to look at disorder development, become aware of biomarkers, and evaluate therapeutic efficacy. This accelerates the tempo of discovery throughout oncology, cardiology, neurology, and extra.
Enabling Personalized Medicine
The integration of genetic statistics with biospecimens allows tailored treatments to male or woman affected person profiles. Clinfinite Solutions helps with such customized studies by preserving a robust biobanking infrastructure that ensures specimen integrity and traceability.
Enhancing Drug Development
Pharmaceutical businesses depend heavily on biobanking to validate hypotheses, check drugs in real-world biological situations, and decrease the risk of failure. Well-preserved samples play a vital role in preclinical and scientific research.
Facilitating Public Health Responses
During outbreaks, brief get admission to to archived samples can be crucial for expertise pathogens, developing vaccines, and implementing treatment protocols. Biobanking enables preparedness and quick response for the duration of such crises.
Clinfinite Solutions’ Role in Biobanking
As one of the rising leaders in clinical research, Clinfinite Solutions has built a complete biobanking platform that adheres to global first-class and moral requirements. Our manner ensures end-to-end biospecimen lifecycle control, from knowledgeable consent and pattern collection to storage and retrieval.
Key Features of Our Biobanking Services:
High-Quality Infrastructure:
Our centers are equipped with contemporary bloodless storage systems, real-time tracking, and easy information monitoring to maintain sample viability for years.
Standardized Protocols:
At Clinfinite Solutions, we comply with globally identified SOPs for biobanking, ensuring consistency, reproducibility, and statistical integrity throughout every study's venture.
Ethical Governance:
We area a sturdy emphasis on moral practices, together with informed consent, statistical privacy, and regulatory compliance, making our biobanking offerings obvious and trustworthy.
Customized Solutions for Clients:
Whether you're a pharmaceutical employer, instructional researcher, or medical trial sponsor, we provide tailored biobanking answers to satisfy your particular desires.
Applications of Biobanking Across Industries
Biobanking isn’t limited to educational institutions. It now plays a pivotal role across more than a few industries:
Pharmaceuticals: Accelerating R&D by providing actual international facts and organic variability insights.
Diagnostics: Developing novel biomarkers for early ailment detection.
Public Health: Monitoring population fitness tendencies and supporting epidemiological research.
Genomics and Biotechnology: Mapping genetic variations and engineering treatment options.
At Clinfinite Solutions, we understand those diverse desires and align our biobanking strategies accordingly to ensure ideal help for every client.
Challenges and the Road Ahead
Despite its significant fee, biobanking comes with challenges—preserving data first-rate over the years, ensuring moral use, and dealing with large quantities of information. Clinfinite Solutions addresses these demanding situations head-on through automation, blockchain-primarily based monitoring structures, and AI-powered data analytics.
Looking ahead, we envision biobanking playing a valuable role in worldwide health. With the upward push of synthetic intelligence and massive data, the samples saved these days will tell the treatments of day after today. As biobanks become greater included and collaborative, sharing anonymized datasets will drive cross-border scientific studies and accelerate fitness innovations.
Conclusion
Biobanking is the spine of destiny-driven healthcare structures. It empowers researchers, clinicians, and scientists with the equipment needed to decode diseases and create life-saving interventions. As a pioneer in the area, Clinfinite Solutions remains devoted to constructing a resilient, ethical, and technologically superior biobanking atmosphere.
We invite healthcare establishments, researchers, and enterprise stakeholders to collaborate with Clinfinite Solutions and unencumber the transformative power of biobanking. Together, we can reshape the destiny of drugs—one sample at a time.
Read More:
Medical Device Manufacturers
Specimen Collection In Healthcare
#clinfinite solutions#specimen collection in healthcare#value of clinical development#biobanking#blood collection methods#clinical research specialists#clinical research jobs in hyderabad#healthcare technology companies#clinical care solutions#sample collection tubes
0 notes
Text
Understanding Biobanking: The Future of Medical Research and Healthcare
Introduction
Biobanking emerged as crucial backbone of modern medical research offering super valuable resources for studying weird diseases rapidly. Science advancement rapidly propels biobanks forward as crucial components in healthcare breakthroughs enabling deeper understanding of genetic variations beneath complex disease patterns.
What is Biobanking?
Biobanking involves systematic collection of biological samples like blood tissues DNA under strictly controlled conditions for various research purposes. Biobanks function pretty much like vast collections of specimens scientists utilize frequently under highly controlled conditions for studying disease mechanisms.
Types of Biobanks
Population-Based Biobanks
Biobanks gather samples from sizable groups slowly over prolonged periods studying genetic environmental factors affecting health. Researchers gain insight into lifestyle factors and genetic influences that pretty much affect common ailments like diabetes heart disease or cancer.
Disease-Oriented Biobanks
Researchers-zeroing in on distinct ailments-identify biomarkers and potential treatments. Cancer biobanks collect tumor samples under highly controlled conditions for studying rapid disease progression and discovering novel therapies.
Tissue Banks
Tissue biobanks store human tissues in vast quantities for research purposes particularly in fields of cancer medicine. These samples facilitate research into tumors' microenvironment through innovative approaches like stem cell therapy beneath controlled conditions.
Virtual Biobanks
Digital platforms seamlessly integrate data from numerous biobanks thus drastically enhancing overall accessibility and facilitating collaboration. Virtual biobanks foster data exchange across vast networks thereby enhancing efficiency of sprawling research endeavors.
Importance of Biobanking in Research and Medicine
Biobanking significantly impacts scientific progress via complex research initiatives and ultimately enhances public wellbeing through precise medical interventions. Researchers develop highly customized treatments suited perfectly for individuals' unique genetic profiles amidst diverse biological samples. Spot disease markers early on for timely intervention which yields pretty good outcomes for patients somehow. Develop innovative pharmaceuticals through rigorous examination of novel compounds on pertinent cellular structures frequently in laboratory settings.
Ethical and Legal Considerations in Biobanking
While biobanking offers numerous benefits, it also raises ethical and legal challenges that must be addressed to maintain public trust.
Informed Consent
Ensuring donors understand how their biological samples will be used is a key ethical requirement. Participants gotta provide informed consent detailing willingness to contribute samples for research now and later on somehow.
Privacy and Data Protection
Strict measures must safeguard donors' sensitive health information beneath extremely stringent protocols daily. Vital measures like encryption and anonymization help maintain confidentiality by preventing data misuse in a highly secure environment.
Ownership and Access
Ongoing ethical debates surround ownership and control of biological samples somehow. Policies regarding data sharing and commercialization must balance scientific progress somewhat irregularly with donor rights.
Challenges in Biobanking
Biobanking faces myriad challenges including high operational costs and ethical dilemmas that hinder long-term viability somehow. Maintaining sample quality and ensuring regulatory compliance pose significant challenges amidst funding restrictions that demand persistent oversight somehow.
The Future of Biobanking
With advancements in artificial intelligence and big data, the future of biobanking looks promising. AI-driven analysis of biobank data enables faster disease detection, predictive modeling, and personalized treatments. Blockchains notably bolster security in data exchange processes beneath stringent ethical biobanking standards somehow.
Conclusion
Biobanking occupies a pivotal position in medical innovation bridging gaps between scientific research and real-world healthcare advancements rapidly. As technology rapidly advances biobanks play a crucial role in understanding complex diseases and enhancing innovative treatments daily.

0 notes
Text
"Cryogenic Vials: Critical Lab Equipment or Just an Expensive Chill?"

Introduction
Cryogenic vials are specialized containers designed to store biological samples, pharmaceuticals, and other temperature-sensitive materials at extremely low temperatures. These vials are crucial in applications such as cell preservation, biobanking, and vaccine storage. With the rise in biotechnological advancements and increasing demand for high-quality storage solutions, the market for cryogenic vials is expanding. This report examines the key drivers, challenges, and opportunities shaping the cryogenic vials market, along with regional trends, market segmentation, and the competitive landscape. It also provides insights into future developments in the sector.
Market Dynamics
Drivers
Growth in Biobanking and Cellular Therapy: The expanding field of biobanking and the increasing use of cell-based therapies are driving demand for cryogenic vials. These vials are essential for the long-term storage of stem cells, tissues, and other biological samples.
Advancements in Biotechnology: Technological advancements in biotechnology and pharmaceutical research are increasing the need for reliable and efficient storage solutions. Innovations in cryogenic vial design and materials are supporting this growth.
Rising Demand for Vaccines: The global focus on vaccine development and distribution, especially highlighted by recent health crises, has increased the need for cryogenic storage solutions to ensure vaccine efficacy during transportation and storage.
Challenges
High Cost of Cryogenic Equipment: The cost of cryogenic vials and associated storage equipment can be significant, which may limit adoption among smaller research facilities and biobanks.
Regulatory Compliance: Ensuring that cryogenic vials meet stringent regulatory requirements and standards for safety and performance can be challenging and time-consuming for manufacturers.
Risk of Contamination: Maintaining the integrity of samples in cryogenic vials requires strict adherence to contamination prevention protocols. Any lapse in handling or storage can lead to sample degradation or loss.
Opportunities
Innovation in Materials: Developing new materials and coatings that enhance the performance and durability of cryogenic vials presents opportunities for market growth. Innovations such as improved sealing mechanisms and enhanced resistance to extreme temperatures can attract more customers.
Expansion in Emerging Markets: Emerging economies with growing healthcare and research sectors offer significant growth opportunities for cryogenic vial manufacturers. Expanding into these markets can drive revenue growth and market penetration.
Customization and Advanced Features: Offering customizable vials with advanced features, such as RFID tracking for sample identification or integrated temperature monitoring, can meet specific customer needs and differentiate products in the market.
Sample Pages of Report: https://www.infiniumglobalresearch.com/reports/sample-request/1704
Regional Analysis
North America: The North American market is a key player due to a strong presence of biotechnology and pharmaceutical companies, along with extensive research activities. The U.S. and Canada are leading markets with high demand for advanced cryogenic storage solutions.
Europe: Europe is witnessing significant growth in the cryogenic vials market, driven by the increasing focus on research and development in life sciences. Countries like Germany, the UK, and France are prominent markets, with strong regulatory frameworks supporting quality standards.
Asia-Pacific: The Asia-Pacific region is experiencing rapid growth, fueled by expanding healthcare infrastructure and increasing investments in biotechnology research. China and India are key markets with rising demand for cryogenic storage solutions.
Latin America: The market in Latin America is developing, with growing investments in healthcare and research. Brazil and Mexico are leading markets, offering opportunities for growth in cryogenic vial sales.
Middle East and Africa: The market is expanding with increasing investments in healthcare and research facilities. The region presents opportunities for market growth, particularly in countries like South Africa and the UAE.
Market Segmentation
By Material:
Glass
Plastic
Other (e.g., composite materials)
By Type:
Screw Cap Vials
Cryogenic Tubes
Snap Cap Vials
Self-Standing Vials
By Application:
Biobanking
Pharmaceutical Research
Clinical Trials
Vaccine Storage
Others
Competitive Landscape
Market Share of Large Players: Major players like Thermo Fisher Scientific, Corning Inc., and Nunc dominate the cryogenic vials market. These companies hold substantial market shares due to their established brands, extensive product portfolios, and global distribution networks.
Price Control: Large players have some control over pricing due to their economies of scale and established market presence. However, competition from smaller firms and technological innovations can influence pricing dynamics.
Competition from Small and Mid-Size Companies: Small and mid-size companies are increasingly challenging larger players by offering specialized or customizable cryogenic vials. These companies often focus on niche markets or innovative features to differentiate themselves.
Key Players:
Thermo Fisher Scientific
Corning Inc.
Nunc (part of Thermo Fisher Scientific)
Greiner Bio-One
Bio-Rad Laboratories
Report Overview: https://www.infiniumglobalresearch.com/reports/global-cryogenic-vials-market
Future Outlook
New Product Development: Investment in new product development, including advancements in vial materials and design, will be crucial for companies to remain competitive. Innovations that enhance vial performance and reliability are likely to drive market growth.
Sustainable Products: There is an increasing focus on sustainability in the cryogenic vials market. Companies that develop eco-friendly packaging and recyclable materials will appeal to environmentally conscious customers and meet regulatory demands.
Conclusion
The cryogenic vials market is poised for growth, driven by advancements in biotechnology, increased demand for vaccine storage, and expanding research activities. While challenges such as high costs and regulatory compliance exist, opportunities in material innovation, emerging markets, and sustainability offer significant potential for market expansion. Companies that focus on these areas and adapt to changing industry trends will be well-positioned to succeed in the dynamic cryogenic vials market.
0 notes
Text
Procure Human Biospecimens for High Impact Research

What exactly are human biospecimens?
Any substances or biomaterials collected from the human body are referred to as human biospecimens. It includes blood, body fluids, organs, tissues, cells, DNA, bones, teeth, etc., gathered for clinical diagnosis, disease monitoring, and research. Today, human biospecimens are a core resource for numerous research projects propelling across the globe. It acts as an in-vitro human model for understanding diseases' exact nature and pathophysiology. The last few years have proven that studies on human biospecimens played an essential role in the development of COVID-19 diagnostic tests and vaccination.
How are human biospecimens gathered for research?
Human biospecimens for research are usually obtained from the residual patient samples after diagnosis. Also, biosamples are sourced from voluntary donations and during any diagnostic or clinical procedure such as biopsy or surgery. The sample collection is performed by a trained laboratory technician, phlebotomist or medical practitioner after conducting a detailed informed consent process to protect the interest and rights of the patients or sample donors. The samples are later subjected to detailed laboratory investigations, processed in a certified laboratory, and stored with related information at state-of-art biobanks for long years for research. The entire sample collection, processing and storage are undertaken in sterile conditions with strict adherence to biobanking safety protocols, infection control practices, and IRB protocols.
Millions of Humans biospecimens for research
In every research involving human biospecimens the most challenging step is procuring reliable, high-quality human biological samples. Most often, every effort will be a vein without finding a perfect research sample. Moreover, the procurement process from the biobanks can be complicated and tedious, where the biospecimen request takes months to complete. With this in mind, a group of scientific enthusiasts established Central BioHub to simplify human biospecimen procurement from months to minutes. It is an online human biospecimen marketplace with 800,000+ banked samples readily available in stock for procurement. Like any next-generation E-commerce platform, Central BioHub is an open access, easy-to-use, click and buy web platform with real-time inventory management system.
Discover a wide range of clinical research samples such as human serum, plasma, urine, swabs, tissues, PBMC, CSF, FFPE blocks etc., well-annotated with a comprehensive clinical profile at Central BioHub. You can buy a wide range of research samples of infectious diseases, autoimmune diseases, cancer, endocrine disorders, etc. Quick navigation through its product page can fascinate you. Take a tour and order your research samples today: https://centralbiohub.de/biospecimens/overall.
Excitingly, Central BioHub also allows customers to reserve interesting samples or create a quote online. Furthermore, it is a highly dedicated biospecimen provider ensuring the fastest delivery of research samples. Don't wait anymore and get started with Central BioHub now.
0 notes
Text
Cryogenic Biobanking Services Market Size to Observe Steady Growth
Cryogenic Biobanking Services Market: Overview
The cryogenic biobanking services market may climb the growth ladder across the forecast period of 2020-2030 on the back of the growing demand for storing varied biological samples such as organs, human blood, cells, and tissues. The preservation period is both short term and long term. The prime objective of the cryogenic biobanking is to maintain the stability and functionality of the biological samples within the specified preservation period.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=78187
On the basis of type, the cryogenic biobanking services market can be segmented into cryogenic storage systems, freezers, ice machines, refrigerators, and alarms and monitoring systems. The cryogenic biobanking services market can be classified into regenerative medicine, biobanking, and drug discovery on the basis of application.
This upcoming report on the cryogenic biobanking services market provides a 360-degree analysis of the current market situation. The report covers components like competitive landscape, key players, regional analysis, and ongoing trends. The report also offers thorough research on how the COVID-19 pandemic will impact the cryogenic biobanking services market. The segmental study enables an individual to deeply understand the different aspects of the cryogenic biobanking services market systematically.
Request a Sample of Cryogenic Biobanking Services Market: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=78187
Cryogenic Biobanking Services Market: Competitive Assessment
The cryogenic biobanking services market has numerous domestic and international players. These players compete with each other for obtaining the top position in terms of consumer loyalty and sales. Earlier, manual cryogenic biorepositories were present on a large scale but as technological advancements penetrated across the cryogenic biobanking services market, novel biobanks were designed keeping in mind the different protocols and advanced tracking procedures. Therefore, this aspect may bring expansive growth for the cryogenic biobanking services market.
Mergers, acquisitions, joint ventures, partnerships, and collaborations among the players of the cryogenic biobanking services market help them in expanding their foothold. This aspect eventually leads to adding extra stars of growth to the cryogenic biobanking services market. Some prominent players involved in the cryogenic biobanking services market are Customer Biogenic Systems, BioServe Biotechnologies, Sigma-Aldrich, and Stemgent.
Request for Analysis of COVID19 Impact on Cryogenic Biobanking Services Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=78187
Cryogenic Biobanking Services Market: Key Trends
As the number of stored samples increases, the number of storage operations, and the need for cryogenic biobank automation also increases. Hence, the cryogenic biobanking services market may bank on the growing number of automated systems by various platforms. For instance, the INLANDYS platform integrates easily with any existing biobank equipment and can also be linked to the biorepository database software. Hence, such developments may sow the seeds of growth across the cryogenic biobanking services market.
The escalating adoption of in house sample storage system by biotech companies and pharmaceuticals may further help in enhancing the growth rate of the cryogenic biobanking services market. Investment across the cryogenic biobanking services market may also bring great growth opportunities. For instance, TMRW Life Sciences recently raised $25 mn for its cryogenic management platform. These factors may serve as robust growth pillars for the cryogenic biobanking services market.
Devices offering a hassle-free sample collection process in addition to a spill-proof design are gaining substantial momentum across the cryogenic biobanking services market. Such technologies may bring considerable growth opportunities for the cryogenic biobanking services market.
Pre-Book Cryogenic Biobanking Services Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=78187<ype=S
Cryogenic Biobanking Services Market: Regional Dimensions
The cryogenic biobanking services market is spread across North America, Asia Pacific, Latin America, the Middle East and Africa, and Europe. North America may emerge as a champion among other regions in terms of growth. The growing investment in cryogenic biobanking services and the increasing research and development activities may serve as prominent growth factors for the cryogenic biobanking services market.
Europe may also bring substantial growth for the cryogenic biobanking services market throughout the forecast period of 2020-2030. The heightening demand for research and development activities of biospecimens related to cancer, diabetes, and other cardiovascular disorders may bring immense growth prospects for the cryogenic biobanking services market in the region.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/prevalence-of-chronic-diseases-that-need-surgical-processes-spurs-sales-in-surgical-drills-market-valuation-to-rise-at-cagr-of-4-7-during-20182026-tmr-301265120.html
https://www.prnewswire.com/news-releases/international-accreditations-for-hospitals-drive-prospects-of-medical-tourism-market-in-offering-quality-patient-care-revenues-to-rise-at-cagr-of-10-5-from-2019-to-2027-tmr-301269941.html
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of analysts, researchers, and consultants, uses proprietary data sources and various tools and techniques to gather and analyze information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact
Transparency Market Research, 90 State Street, Suite 700, Albany, NY 12207Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com/
0 notes
Text
Sciforce’s Odyssey: Being a Part of the OHDSI Adventure

In the modern world, it is impossible to push forward the progress on your own. Leading researchers and research labs join dedicated communities to share their knowledge and best practices and to find answers to long-standing problems. In healthcare, one of such communities of researchers is OHDSI — and in this blog post we’ll talk about it and the role the Sciforce teams plays in it.
What is OHDSI?
If we look at how large-scale analytics in healthcare works, we’ll see that it is ruled not only by corporations, but, rather, is moved forward by communities of researchers who share similar interests. OHDSI (pronounced as “Odyssey”), or Observational Health Data Sciences and Informatics, is an umbrella initiative for such researchers and the successor of the Observational Medical Outcomes Partnership (OMOP) defined as “multi-stakeholder, interdisciplinary collaborative that is striving to bring out the value of observational health data through large-scale analytics.”
Its objective is to enable analysis and sharing of health or observational data between different institutes and companies. OHDSI has created and supports the Common Data Model (CDM) the goal of which is to standardize seemingly disparate databases that might be used in a variety of research areas, such as comparing alternate treatment paths, personalized medicine, product safety, and overall quality improvement in healthcare.
How does it work?
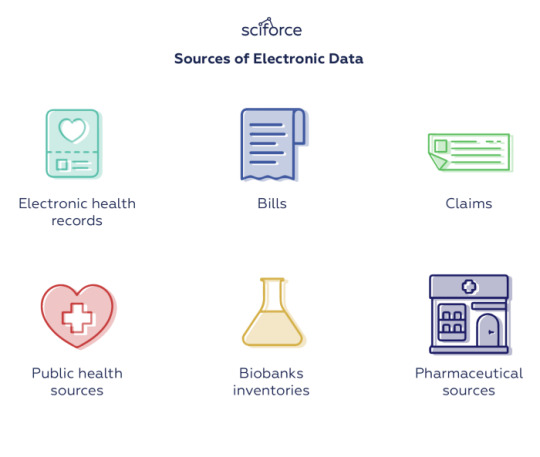
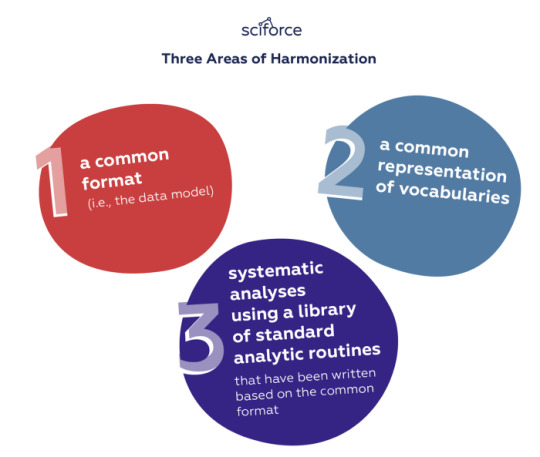
Observational outcome studies leverage secondary electronic data that might come from diverse sources such as Electronic Health Records, bills, claims, public health sources, biobanks inventories, or pharmaceutical sources, in order to perform large-scale evidence-based longitudinal patient-level clinical studies. However, in most cases, the available data is so versatile that cannot be used directly, but should be first transformed into a standardized warehouse of information. For this, OHDSI focused OMOP’s design on three areas of harmonization: a common format (i.e., the data model), a common representation of vocabularies, and systematic analyses using a library of standard analytic routines that have been written based on the common format.
Similar to other data warehouses, OHDSI transforms data to fit a general data model: the OMOP common data model (CDM). Its central entity is the patient and it contains tables for the data commonly needed in clinical trials and observational studies such as drug use, procedures performed etc. These tables are grouped together in the “clinical data” part of the data warehouse. Besides, the CDM includes the major commonly used ontologies: SNOMED, Loinc, RxNorm etc.
For the actual transformation of the data any preferred language and application can be used, such as SQL or Python). Once the data is transformed and loaded into the OMOP CDM database, it is easy to get it from the database, share analyses with other groups using OHDSI or run them on their OMOP data sets, without necessarily sharing the actual data.
In this way, the CDM model enables clinicians and researchers to systematically identify specific cohorts, compare results, reproduce protocols using data, and investigate combinations of interventions and outcomes.
OHDSI Community
One of the cornerstones of OHDSI is its openness both in their findings, including sharing methods, tools and evidence, and in their networking. It is not an organization, but a real community, trying to improve health outcomes for patients around the world together. Whether you’re a software developer, physician or clinical researcher, there is a place for everyone in the OHDSI community.
Its central coordinating center is at Columbia University, but it is fueled by data from almost 100 organizations across dozens of countries, and we are happy to be among them.

Image Credit: Martijn Schuemie
Where does Sciforce fit?
As an IT company with a broad expertise in the development of solutions for healthcare, we joined OHDSI in 2014 to develop and improve the CDM model so that it can cover more cases, understand and analyze data according to the customer’s needs. To achieve this, we map data to a single format, for instance, with the same variable name, attributes, and other metadata. We also are engaged in the development of the general platform that enables much more rapid responses to research-related questions.
Besides, with the CDM, we also perform predictive analysis and observational studies, proving its viability and usefulness in clinical research.
In these years, our team members have prepared 8 posters for OHDSI symposiums, authored or co-authored 3 scientific articles and ran dedicated training sessions. In 2018, our team members even received the Titan Award for Data Standards for their contribution to development and evaluation of community data standards, including OMOP common data model and standardized vocabularies.
These are remarkable achievements, but it is also a big step for our company — to be recognized in the community of health IT researchers. It’s a big adventure and a long journey and it feels exciting to be on board and be praised as valuable members of a project of such a scale and importance.
0 notes
Text
Benefits of biobank software?
Biobank software combines powerful specimen logistic and tracking capabilities with specimen workflow and processing management features. The biobank software could be developed with the web on a combination of common browsers for example Google Chrome, Internet Explorer, as a Window client or perhaps an Apple Safari. The device is extremely saleable and utilize popular database for example SQL Server and databases.
Benefits
A number of the customer’s benefits given by Biobank software are listed below:
•It helps in cutting the turnaround time from biomaterial demand to shipping.
•It may help in improving query, sample tracking and chain of custody.
•It may help in compromising sample integrity and reduction of lost samples.
•It helps in improving quality through integrated quality control tools.
•It also helps in reducing cost and improving efficiency through time saving.
•It offers flexibility as a way to accommodate the new business requirements.
Kinds of automated technologies can be found which assists in effectively handle shipping, fulfillment and inventory. Different clinical labs and laboratory requires different items and proper management techniques. In labs different laboratory services are provided with their customers. Maintaining and establishing a good flow of outbound and inbound activity throughout your warehouse may help in improving the overall quantity of products which a company can practice. Warehouse operations are generally cost drivers and labor intensive for businesses. The Biobank may serve as an important resource in biomedical research since it helps in making global collaborations. If you use proper technologies and processes over time and place you then become capable of streamline and automate the preferred operations in order to prevent lowering of system and human errors and costly delays. The software also becomes critical to be able to track and manage the research specimens transversely to your collaborative group. Now days, a Biobank software plays a huge role in biomedical research. It gives assist in storage and volume processing. The program assists in tracking every one of the required information associated with their samples. The clinical labs frequently categorizes their stored samples by a variety of qualities. The software needs to be properly safe and secured from unauthorized users. Usage of a few of the sensitive information needs to be properly managed and measured under proper research samples. It includes blood type, exposure to environmental factors, age and gender.
Challenges
Biobank face numerous challenges when handling a huge volume of diverse samples. The challenges include many important such things as keeping the patients data safe and secure. It may also help in handling the information manually and minimizes the inaccuracies and errors present in any data set. A Cloud is essentially equipped with several security measures that helps to keep your data secure and accessible merely to the authorized users. Such security features include data encryption and processing. Cloud also helps in enhancing the efficiency by supporting the automatic transfer of data to the system if you use multiple laboratory instruments. With the aid of automatic data processing, the laboratory will be able to bring its attention and from data validation and existing protocols.
0 notes
Text
Biobanking: The Future of Precision Medicine with the aid of Clinfinite Solutions

In the swiftly advancing landscape of healthcare and clinical studies, biobanking has emerged as a foundational pillar using innovation, discovery, and personalised medicine. At Clinfinite Solutions, we appreciate the crucial function biobanking plays in bridging the gap between today’s clinical understanding and tomorrow’s scientific breakthroughs. This weblog explores the idea of biobanking, its significance, applications, and the way Clinfinite Solutions is leveraging this powerful device to revolutionize the world of healthcare.
What is Biobanking?
Biobanking refers to the approach of accumulating, processing, storing, and coping with natural samples, which consist of blood, tissues, DNA, and one-of-a-type physical fluids to be used in studies and clinical studies. Those samples are meticulously cataloged and maintained in specially managed environments to ensure long-term viability and report integrity.
Each sample saved in a biobank is commonly related to comprehensive affected person statistics, which include medical information, way of life, demographics, and genetic information. This holistic repository permits researchers to conduct enormous studies on sickness development, remedy efficacy, genetic tendencies, and much more.
The Role of Biobanking in Modern Medicine
The significance of biobanking extends a long way beyond storage. It serves as a cornerstone for several medical and medical endeavors:
Disease Research: Biobanks offer the organic substances crucial for investigating the causes and development of diseases, consisting of most cancers, diabetes, Alzheimer’s, and rare genetic issues.
Personalized Medicine: Access to properly-annotated biological samples allows researchers to pick out biomarkers and tailor treatments based on the person affected.
Drug Development: Pharmaceutical businesses depend on biobanks to test drug responses and reduce risks in early-stage trials.
Public Health and Epidemiology: Biobanks help track the spread of infectious illnesses and screen vaccine responses across populations.
At Clinfinite Solutions, our biobanking offerings are structured to assist all these essential regions, making us a trusted associate in each public and private sector research.
How Clinfinite Solutions Enhances Biobanking
As a forward-thinking scientific research agency, Clinfinite Solutions integrates era, fine assurance, and regulatory compliance to supply industry-leading biobanking services. Here's how we stand apart:
1. Advanced Sample Management
Our modern-day biobank facilities use computerized structures and easy data platforms to manipulate samples with precision. Temperature-managed environments, real-time tracking, and barcode-based inventory ensure pattern integrity from collection to retrieval.
2. Ethical and Regulatory Compliance
Ethics and privacy are non-negotiable in our biobanking system. All pattern collections at Clinfinite Solutions are conducted below strict knowledgeable consent protocols, making sure participant rights are upheld. We follow international standards, including ICH-GCP, GDPR, and ISO 20387.
3. Custom Biobanking Solutions
From single-site studies to multicenter trials, our biobanking services may be customized to meet the precise needs of researchers, academic establishments, hospitals, and pharma corporations. Whether it’s long-term storage, sample cargo, or fact analytics, we provide give up-to-give up support.
Applications of Biobanking Across Industries
The versatility of biobanking has led to its integration across numerous sectors:
Academic Research: Universities use biobanks to conduct groundbreaking studies in genomics and molecular biology.
Healthcare Providers: Hospitals integrate biobanks to personalize patient care and create focused remedy protocols.
Pharmaceuticals: Drug development pipelines increasingly depend on biobank assets for global evidence and pharmacogenomics.
Public Health Agencies: Biobanks help prepare for and respond to pandemics by storing viral traces and affected person samples for destiny analysis.
Clinfinite Solutions actively collaborates with partners in each of these domains, maximizing the price and effect of every saved specimen.
Challenges in Biobanking – And Our Solutions
Despite its mammoth capacity, biobanking comes with its share of challenges—sample degradation, data protection, ethical dilemmas, and logistical hurdles. Clinfinite Solutions addresses those demanding situations head-on with:
Cutting-side preservation technology
Robust records encryption and cybersecurity
Transparent ethical governance
Expert logistical coordination for pattern shipping
These practices ensure the very best requirements of sample great and reliability, empowering our partners to behavior impactful, reproducible studies.
The Future of Biobanking: AI, Big Data, and Beyond
As we appear in advance, biobanking is set to evolve along with improvements in artificial intelligence, machine learning, and big data analytics. This technology will allow researchers to extract deeper insights from biological samples and associated metadata, opening new frontiers in diagnostics and therapeutics.
Clinfinite Solutions is actively making an investment in digital equipment and structures in an effort to combine biobanking with predictive modeling and international information. Our aim is to create smarter, extra responsive study ecosystems where organic samples are not simply stored, but activated for discovery.
Conclusion: Partner with Clinfinite Solutions for Biobanking Excellence
In a world where customized healthcare and statistics-driven studies are becoming the norm, biobanking stands as a transformative resource. Clinfinite Solutions is proud to be at the forefront of this evolution, offering at-ease, scalable, and technology-sponsored biobanking offerings to drive innovation across healthcare, academia, and life sciences.
Whether you are engaging in a scientific trial, developing a new remedy, or exploring genomics, Clinfinite Solutions is your dependable partner in building and managing a biobank that meets the highest medical and moral standards.
Read More:
Value Of Clinical Development
Clinical Trials Near Me
#healthcare technology companies#specimen collection in healthcare#clinfinite solutions#value of clinical development#blood collection methods#clinical research jobs in hyderabad#clinical research specialists#biobanking#clinical care solutions#sample collection tubes
0 notes
Text
Biobank - best practices for clinical research and investigations
A biobank, also known as a biorepository, plays a vital role in translational research. It is a specialized organization involved in collecting, storing, and distributing high-quality specimens for research and development. A biobank stores a wide range of human specimens and their associated clinical information to support biomedical research. Richly characterized and highly annotated research samples are crucial for consistent, accurate, reliable scientific findings. Therefore, human biobanks are not only a place to assimilate human samples and a library of clinical research specimens.
Best clinical research practices
Clinical research is an area that requires more coordination and regulated workflow for the successful completion of the research project. It is easier to standardize the work ecosystem. However, in the multi site clinical trial network, it is necessary to standardize the entire operating procedure, including sample collection, processing, storage, transport, distribution, etc., through a pre-approved standard operating procedure (SOP) and IRB protocol. Also, timely audits must be performed for continuous quality improvement. Besides, there should be a safe and secure data management system that stores, transfers, and retrieves valuable sample information protecting it from loss and piracy.
Central BioHub: Reliable partner for human biospecimens.
The present decade witnesses a massive opportunity in the human specimen biobanking sector. The pressing demand for high-quality, well-annotated clinical research specimens calls for more resilient biospecimen procurement methods to ensure a seamless supply of human biospecimens from biobanks. Motivated by the modern digital revolution, Central BioHub debuted the smartest digital pathway for procuring clinical research samples from numerous biobanks located across the globe. It connects global researchers to world-class human biobanks with an innovative online human biospecimen marketplace, making biosample acquisition more straightforward. Check out their page here: https://centralbiohub.de/
Moreover, Central BioHub has an enormous inventory offering millions of human biospecimens readily available for purchase. Explore their product page here: https://centralbiohub.de/biospecimens/. The company values its customer's time and effort. Therefore, it has an excellent Customer Support to assist you in biospecimen acquisition and ensure express global shipping. Click here for the latest news: https://centralbiohub.de/blogs/biobanks.
0 notes
Text
How can I access human Specimens samples?
What are human specimens?
Human specimens refer to natural substances, including body fluids that originate from the human body. They are commonly known as human biospecimens, biomaterials, biosamples, and biological materials. Human specimens are usually collected from the patients or donors for disease diagnosis, monitoring, treatment, and research. It comprises but is not limited to human serum, plasma, whole blood, cerebrospinal fluids, semen, nasal secretions, urine, tissues, organs, feces, etc., collected in sterile conditions using an aseptic technique to prevent the risk of sample contamination and risk of infection to the donors. Nowadays, remnant human specimens after diagnosis are archived in numerous biobanks for research purposes. In short, human specimens connect people to science. To learn about the importance of human specimens in research, read our latest blogs: https://centralbiohub.de/blogs/human-specimens
Begin with Central BioHub. Get easy access to millions of Human specimens online
Since 2017, Central BioHub has been one of the leading human biospecimen providers providing reliable Human specimens to the research world. With the powerful idea of intermediating global biobanks and researchers worldwide, Central BioHub offers easy access to millions of human specimens online. It has the largest inventory of disease samples and healthy donor samples ethically isolated from consented donors living across the globe. Take a quick tour of Central BioHub’s inventory and order human specimens online: https://centralbiohub.de/biospecimens/
Central BioHub introduces you to the simplified digital pathway of biospecimen procurement by marking an end to the complicated process of accessing banked human specimens. It is the first-ever human biospecimen provider incorporating advanced data-driven technology to present real-time inventory to the world. Researchers can select and procure matching samples using 100+ clinical search parameter filter options. Sounds amazing? Furthermore, Central BioHub has a dedicated customer area for our customers to manage their orders independently, make samples reservation, quotations, etc. To register as a customer, check out their customer login page here: https://centralbiohub.de/customerlogin
Moreover, Central BioHub prioritizes fair trade practices and customers' valuable time. Therefore, every order is processed immediately and delivered to your location quickly with utmost care. Yes, it is as simple as Amazon's online shopping service. Hurry up! Get your research protocol on your desk and explore our inventory to order human specimens online.
0 notes