#IoTHealth
Explore tagged Tumblr posts
Text
#DigitalHealthRevolution#HealthTechInnovations#InformaticsInAction#FutureOfHealthcare#TechTransformsHealth#PrecisionMedicine#GlobalHealthTech#InnovateWithInformatics#EthicalHealthTech#PatientCenteredCare#DataDrivenWellness#TechForHealthEquity#HealthcareEvolution#SmartHealthSolutions#ConnectedCareGlobally#EHRsEmpowerPatients#TelehealthAdvancements#AIinMedicine#BlockchainHealth#IoTHealth#DataSecurityMatters#DigitalHealthEthics#GlobalCollaborationInHealth#HealthInformaticsJourney#CrisisResponseTech
0 notes
Text
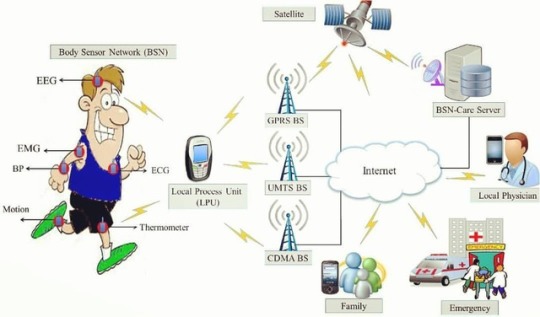
Transforming Healthcare: The Internet of Healthcare Things (IoHT) Revolution

IoHT, short for the Internet of Healthcare Things, is a network of interconnected computing devices utilized for storing and transmitting patient information. These devices include ingestible sensors that use the FHIR protocol to communicate data over the internet, ultimately creating a digital patient care record. To read full log visit: https://www.rangtech.com/blog/iot/transforming-healthcare-the-internet-of-healthcare-things-ioht-revolution
0 notes
Photo
IoT Health... . wwe.iothought.com . . #InternetOfThings #IoT #technology #hightech #futuretechnology #hightech #future #futuretech #whatisnext #connected #iotworld #iotlife #techlifestyle #techlife #smartproducts #health #iothealth #healthmonitor #healthtechnology #futurehealth #elderlycare #patientmonitor https://www.instagram.com/p/BwJ2kj1Byma/?utm_source=ig_tumblr_share&igshid=glsreq79on4q
#internetofthings#iot#technology#hightech#futuretechnology#future#futuretech#whatisnext#connected#iotworld#iotlife#techlifestyle#techlife#smartproducts#health#iothealth#healthmonitor#healthtechnology#futurehealth#elderlycare#patientmonitor
1 note
·
View note
Text
The Internet of Things via Medicines – FDA Approves Digital Pill
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.
The post The Internet of Things via Medicines – FDA Approves Digital Pill appeared first on HealthPopuli.com.
The Internet of Things via Medicines – FDA Approves Digital Pill posted first on http://ift.tt/2sNcj5z
0 notes
Text
The Internet of Things via Medicines – FDA Approves Digital Pill
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.
The post The Internet of Things via Medicines – FDA Approves Digital Pill appeared first on HealthPopuli.com.
The Internet of Things via Medicines – FDA Approves Digital Pill posted first on http://ift.tt/2sF7oEr
0 notes
Text
The Internet of Things via Medicines – FDA Approves Digital Pill
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.
The post The Internet of Things via Medicines – FDA Approves Digital Pill appeared first on HealthPopuli.com.
Article source:Health Populi
0 notes
Text
Transforming Healthcare: The Internet of Healthcare Things (IoHT) Revolution

The Internet of Healthcare Things (IoHT), also known as the Internet of Medical Things (IoMT), is reshaping the healthcare landscape. It involves a network of interconnected devices designed to store and transmit patient information securely, offering unprecedented opportunities for improving patient care, enhancing medical practices, and even transforming the health insurance industry.
In this comprehensive guide, we explore the diverse applications of IoHT, its advantages, challenges, and the latest trends shaping the healthcare sector.
What is IoHT?
IoHT, short for the Internet of Healthcare Things, is a network of interconnected computing devices utilized for storing and transmitting patient information. These devices include ingestible sensors that use the FHIR protocol to communicate data over the internet, ultimately creating a digital patient care record.
Applications of IoT in Healthcare:
For Patients: IoHT empowers patients with personalized care through wearable devices such as fitness bands and wireless monitoring equipment. These devices, including blood pressure and heart rate monitors, can send reminders, track vital signs, and provide continuous medical monitoring. This technology is particularly transformative for elderly patients and individuals living alone, offering a safety net for their health.
For Physicians: IoHT enables physicians to monitor patients more effectively by leveraging wearables and home monitoring devices. Physicians can track treatment adherence and assess the need for emergency care, leading to proactive healthcare interventions. Data from IoT devices enhances communication between medical professionals and patients, facilitating more tailored treatment plans and improved outcomes.
For Health Insurance Companies: Health insurers benefit from IoT-connected devices by using health monitoring data for underwriting and claims processing. This data aids in identifying suitable candidates for coverage and detecting fraudulent claims. IoT devices enhance transparency between insurers and clients, providing customers with insights into the decision-making process.
Types of IoMT Devices
IoMT devices come in various forms:
Wearables-on the Body: These include biosensors for real-time health monitoring. Some are even implanted under the skin for discreet, continuous monitoring. The key categories are consumer health wearables (e.g., fitness bands) and clinical-grade wearables (used under medical supervision).
In Hospitals and Clinics: Large equipment and smart devices are used for patient monitoring, supply management, and environmental control in healthcare facilities. Leading MedTech companies employ IoMT for diagnosing and maintaining medical equipment.
Community IoMT: IoMT extends to communities, enabling tracking of patients during travel and offering remote healthcare services in non-traditional settings, such as field hospitals and kiosks for medication distribution.
Advantages of IoT in Healthcare:
IoT in healthcare offers numerous benefits:
Enhanced Quality: IoHT improves the quality of care by enabling personalized treatment plans and data-driven decision-making, supported by technologies like cloud computing, big data, and IoT.
Improved Patient Experience: Real-time data from connected devices allows doctors to make more accurate diagnoses, resulting in individualized care and an enhanced patient experience.
Reduced Costs: Unnecessary doctor visits decrease, and homecare services improve, reducing healthcare costs. Combining automation with data-driven approaches can lower expenses significantly.
Challenges of IoMT Healthcare Networks:
IoMT also presents unique challenges:
Data Security: The exchange of vast amounts of medical data makes healthcare systems vulnerable to cyber threats. Real-time data monitoring, cyber threat analysis, and secure networking technologies are crucial to mitigate these risks.
Regulatory Compliance: Strict regulations govern the use and safeguarding of sensitive medical data, requiring healthcare organizations to adhere to guidelines like those issued by the FDA for medical device cybersecurity.
Technical Challenges: Ensuring secure communication among IoMT devices is essential. Outdated systems may struggle to adapt to evolving security guidelines and interoperability requirements.
Popular Trends in IoMT in 2022:
Several trends are shaping IoMT in 2022:
Nano-Enabled Medical Products: Nanotechnology-based medical devices, including medications and diagnostics, are gaining popularity for their potential in diagnosing and treating various health conditions.
Personalized Healthcare: Rising chronic conditions and health awareness are driving demand for personalized healthcare. Wearable devices and biosensors enable continuous monitoring and reduce the burden on healthcare institutions.
Connected Inhalers: IoT-connected inhalers help patients identify triggers for pulmonary problems and ensure correct usage, improving the management of respiratory conditions.
Market Outlook: The IoMT market is on a growth trajectory, with a compound annual growth rate (CAGR) of 19.9% from 2019 to 2025. In 2020, the market size was USD 219.5 billion, projected to reach USD 534.3 billion by 2025.
Conclusion
The Internet of Healthcare Things is revolutionizing healthcare by providing real-time data, personalized care, and cost-effective solutions. IoMT devices are reshaping patient care records, enhancing communication between patients and healthcare providers, and driving innovations in healthcare. As IoMT continues to evolve, it holds the promise of further improving the quality and accessibility of healthcare services.
About Rang Technologies
Rang Technologies, based in New Jersey, has dedicated over a decade to delivering innovative staffing solutions and the best talent to help businesses of all sizes unlock the full potential of the latest technologies and build high-performing teams to achieve their digital transformation goals.
0 notes
Text
The Internet of Things via Medicines – FDA Approves Digital Pill
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.
The post The Internet of Things via Medicines – FDA Approves Digital Pill appeared first on HealthPopuli.com.
The Internet of Things via Medicines – FDA Approves Digital Pill posted first on http://ift.tt/2sNcj5z
0 notes
Text
The Internet of Things via Medicines – FDA Approves Digital Pill
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.
The post The Internet of Things via Medicines – FDA Approves Digital Pill appeared first on HealthPopuli.com.
The Internet of Things via Medicines – FDA Approves Digital Pill posted first on http://ift.tt/2sNcj5z
0 notes