#MaterialsPosts
Explore tagged Tumblr posts
Text




Glasses: Flint Glass
As with many other "types" of glasses, flint glass does not have a set composition, and the name has shifted in meaning over the years. The original flint glasses contained flint, hence the name, however it wasn't long before the term flint glass was used to refer to glass that had relatively high amounts of lead present. Lead is known to have a high refractive index, allowing for brilliant glass pieces when cut. As such, flint glass was often decorative, though also practical in the form of dishware, or used for its optical properties. Flint glass was typically clear, but colors could also be introduced on rare occasions.
Historic flint glass was primarily popular in the United States and United Kingdom in the 1800s. In more modern times, flint glass as a term is used to describe optical glasses regardless of their composition. Some still contain lead, while in others the lead has been replaced by materials such as titanium or zirconium dioxide. Modern day lead glass is more often known as simply that, or as crystal.
Sources/Further Reading: (Images source - Incollect) (Wikipedia) (Pattern Glass) (RP Photonics)
18 notes
·
View notes
Photo




Minerals: Veatchite
With the chemical formula of Sr2B11O16(OH)5·H2O, veatchite is classified as a borate mineral. The first known discovery of this mineral was in 1938, in a borax mine, and it was named for John Veatch, “the first person to detect boron in the mineral waters of California”.
Veatchite crystalizes in the monoclinic crystal system and has a Mohs hardness of ~2, thereby making it a relatively soft mineral. It is often transparent or translucent, and typically is only found in white and colorless varieties. A relatively rare mineral, veatchite has no known commercial uses.
For more in depth information about veatchite, check out what mindat.org, webmineral.com, handbookofmineralogy.org, and mineralatlas.eu have to say about it.
Sources/Further Reading: ( 1 - image 1 ) ( 2 - image 2 and 3 )
Image 4.
12 notes
·
View notes
Photo




Minerals: Xenotime
A rare earth phosphate mineral, the primary composition of xenotime is YPO4, though it does form a solid solution with the mineral chernovite-(Y) and may therefore include arsenic impurities. As with many other rare earth minerals, the yttrium is also often substituted with other rare earth elements, including dysprosium, erbium, terbium and ytterbium. Uranium can be found in xenotime as well, so caution should be taken with the mineral as radioactive crystals can be found, though it is typically mild.
Xenotime crystalizes with a tetragonal crystal structure and is typically brownish, yellow, or red in color. On the Mohs hardness scale it has a value of 4.5. Considered a minor accessory mineral, xenotime does not have any large scale applications, but has been processed for its yttrium content when mined in conjunction with other minerals. Ornamental applications also exist.
For more in depth information about xenotime, check out what mindat.org, webmineral.com, minerals.net, handbookofmineralogy.org, and mineralatlas.eu have to say about it.
Sources/Further Reading: (1 - image 1) (2 - image 2) (3 - image 3) (4 - image 4)
18 notes
·
View notes
Photo




Minerals: Natrolite
A mineral in the zeolite group, natrolite is primarily sodium and aluminum silicate with the formula Na2Al2Si3O10·2H2O. Its name comes from the Greek word for soda, in reference to its sodium content, and was named in 1803.
Natrolite is relatively brittle, with an acicular crystal habit, usually forming thin needles, and has no widespread commercial uses beyond its status among collectors. It crystalizes with an orthorhombic crystal structure and falls on the Mohs hardness scale somewhere between 5-6. Natrolite’s color is typically white or colorless, but it can be pink, red, yellow, or other rare colors on occasion.
For more in depth information about natrolite, check out what mindat.org, webmineral.com, minerals.net, handbookofmineralogy.org, and mineralatlas.eu have to say about it.
Sources/Further Reading: (1 - images 1/4) (2 - image 2) (3 - image 3) ( 4 ) ( 5 )
9 notes
·
View notes
Text

Glasses: Chemically Strengthened Glass
Typical soda-lime-silica glasses can be strengthened in a method known as chemical strengthening (also known as chemical tempering or ion exchange strengthening). Submersing the glass in a solution containing potassium (typically potassium nitrate) allows for the sodium ions near the surface of the glass to exchange places with potassium ions from the solution. Because the potassium ions are larger than the sodium ions, this leads to a compressive layer at the surface of the glass that is stronger than the glass was prior to the reaction. Gorilla Glass is one example of a trademarked chemically strengthened glass.
This technique was first discovered in the 1960s and is not limited to sodium-potassium exchanges, though those are most common. The goal is simply to exchange smaller ions with larger ions. Because glass is strong but brittle, the technique only needs to be applied to the surface - to prevent the formation of flaws from which failure would follow from - to strengthen the entire glass, however it is typically limited to thinner sheets. Thermal tempering methods, which are more common, are limited when it comes to thin materials, so the technique filled in a niche for thin glass applications. Early applications of the technology included aircraft windshields; today chemically strengthened glass is used in smart phones and EpiPens, among other applications.
Sources/Further Reading: (Image source - Cat-i Glass) (Corning) (ACS) (MSE Student) (Wikipedia)
26 notes
·
View notes
Text



Alloys: Britannia Metal
Britannia metal is the name given to a specific pewter alloy first produced in the late 1700s. Pewter itself was historically a tin alloy, often with lead and sometimes with copper, though modern variants of pewter avoid lead and often use antimony instead. Britannia metal, also known as Vickers white metal, is approximately 92% tin, 6% antimony, and 2% copper and was first created by James Vickers.
The alloy is relatively soft, with a melting point of 255°C, and is usually used for decorative purposes thanks to its silvery appearance. It is also commonly used as the base metal for electroplating because of its smooth surfaces. When produced in bulk, Britannia metal is typically cold worked and spun, rather than being cast.
Sources/Further Reading: (Image 1 - Assignment Point) (Images 2 and 3 - Silver collection) (Wikipedia) (The Pewter Society)
11 notes
·
View notes
Text


Alloys: Gum Metal
A beta-titanium alloy, gum metal typically consists of just over 20% niobium, with small amounts of tantalum, zirconium, and oxygen, though compositions can also include hafnium and vanadium. These alloys are also known as TNTZ (for titanium, niobium, tantalum, and zirconium). Gum metal was first described in the early 2000s and is known for its unique elastic properties, including superelasticity. Given its status as a 'newer' alloy, applications are rare and the best processing routes for the metal are still being researched.
Sources/Further Reading: (Images source - 2023 article) (UC Berkeley) (Wikipedia)
15 notes
·
View notes
Text

Fiber Cement
A composite material used primarily in construction and architecture, fiber cement (or fibre cement) is usually made from water, Portland cement, fly ash or sand, and cellulose (wood). Considered to be a durable, strong material, thanks in part to the fiber reinforcement, it is used as siding and roofing for homes. Because some varieties contain silica sand, care must be taken when cutting the composite as silica dust is a respiratory hazard.
Sources/Further Reading: (Image source - This Old House) (Lowes) (JamesHardie) (Wikipedia)
25 notes
·
View notes
Text

Thoriated Glass
A now defunct glass used for optical lenses, thoriated glass is a composition of glass with up to nearly 30%, by weight, of thorium dioxide. Composition varied beyond that, but typically included boron, lanthanum, barium, and/or calcium in the glass. The composition was chosen because of the resulting refractive index; a higher refractive index typically means lenses can be made thinner, as the glass itself doesn't need to bend as much. Thorium, specifically, was chosen to maintain a high refractive index while lowering dispersion.
Because, however, thorium (and thorium dioxide) is a radioactive substance, these lenses eventually fell out of use. The first patents appeared around 1940, with the first commercial applications found in the 1950s, with production ending by the 1980s.
Sources/Further Reading: (Image source - ORAU) (Lens Legend) (Wikipedia)
22 notes
·
View notes
Text

Semiconductors: Copper Zinc Tin Sulfide (CZTS)
A quaternary compound, Cu2ZnSnS4 is most commonly known as copper zinc tin sulfide, or simply CZTS. Today, it is most of interest for thin film solar cells, such as the one depicted in the image above. First created in the late 1960s, the compound's photovoltaic properties started to generate interest (and further research) in the 1980s and 90s. It has a reported band gap of 1.4–1.5 eV and is said to take the form of greenish-black crystals in bulk.
Beyond it's properties, CZTS is of interest because of how it's elemental composition differs from other common solar cell materials: all four elements in the compound are considered common, relatively inexpensive, safe raw materials. Whereas other solar cells may use expensive indium or tellurium, or toxic cadmium, CZTS cells promise lower costs and higher safety.
Challenges with CZTS include the formation of secondary phases, such as CuS or ZnS, which have been shown to have detrimental results on the desirable solar cell properties. These phases can be difficult to detect, though there are processes that can remove certain impurities. Elements and compounds like Zn and SnS are also relatively volatile, which can lead to processing challenges.
Sources/Further Reading: (Image source - 2024 article) (2015 Book chapter) (Stanford University) (AZoM) (Wikipedia)
#Materials Science#Science#Semiconductors#MaterialsPosts#Copper#Zinc#Tin#Sulfides#Solar power#MyMSEPost
5 notes
·
View notes
Text

Alloys: Cerrosafe
A fusible alloy, Cerrosafe has the general composition of 42.5% bismuth, 37.7% lead, 11.3% tin, and 8.5% cadmium. These four elements are the same as in Wood's metal, but whereas Wood's metal is a eutectic alloy, Cerrosafe's composition is non-eutectic (it has lower amounts of bismuth, tin, and cadmium, and a higher lead content). Cerrosafe's main usage derives from its coefficient of thermal expansion; the alloy contracts on solidification, allowing easily removal from casting molds, before expanding at a well known rate. It's melting point is around 74°C (165°F).
Because of the presence of both lead and cadmium, Cerrosafe is not a prevalent alloy choice. Past applications have included toy soldier castings, gun barrels, to create mold/casting references and prototypes, and dental crowns.
Sources/Further Reading: (Wikipedia) (Chemistry Learner) (CS Alloys) (1944 article)
Image source.
Previous materials post: Wood's metal
11 notes
·
View notes
Photo




Minerals: Orthoclase
A potassium feldspar mineral, orthoclase has the chemical formula KAlSi3O8 and is also known as K-feldspar or K-spar. It is a common mineral found in most granites and other igneous rocks. Because of this it had several commercial uses, including in tiles and dinnerware among other ceramics. As a raw material it can be used in the production of porcelain and glass.
Often translucent, orthoclase can range in color from white to brown to yellow to pink, among other varieties. It has a Mohs hardness of 6, and is actually one of the minerals used to define the Mohs scale. Orthoclase can be found across the globe and has also been found on the Moon and Mars. It crystalizes in the monoclinic system.
For more in depth information about orthoclase, check out what mindat.org, webmineral.com, minerals.net, and mineralatlas.eu have to say about it.
Sources/Further Reading: ( 1 - image 4 ) ( 2 - image 2 ) ( 3 - image 3 ) ( 4 )
Image 1.
13 notes
·
View notes
Text

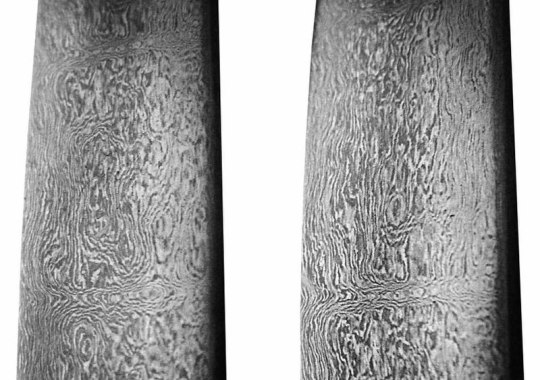

Alloys: Wootz Steel
A high-carbon crucible steel, the history of wootz steel dates back to over 2,000 years ago in the south of India. Though the exact timeline of this alloys invention is unknown, historical records suggest it existed as early as 300-500 BCE. The word wootz is an anglicized version of ukku or urukku, what the steel was known as by the locals in the Dravidian languages.
Wootz steel is characterized by a banded microstructure. Higher carbon varieties contain carbides in a martensitic or pearlitic structure, while lower carbon varieties contain ferrite and pearlite bands (because of its age, there is no set composition for wootz steel). Distinct patterns known as wave, ladder, and rose patterns can be created based on processing. Damascus steel, meanwhile, is merely wootz steel that has been forged into sword blades, and acquired its name from wootz steel that was imported to the city of Damascus before being further processed.
India maintained secrecy around the production of wootz steel for centuries, and thus managed to maintain their status as the primary producer until the 19th century, when Europeans began to produce it as well. The crucible process used to create it involves heating the iron for several days in a clay crucible before slow cooling.
Sources/Further Reading: (Images 1 and 3 - Ancient Origins) (Image 2 - Wikipedia) (ThoughtCo.) (Noblie) (Indian Institute of Science) (Matthew Forde Military Antiques) (Ancient Science) (National Institute of Advanced Studies, Bangalore)
23 notes
·
View notes
Text



Semiconductors: Cadmium sulfide
An inorganic compound that exists naturally in two forms, cadmium sulfide is used primarily as a pigment but holds increasing interest in semiconducting applications. Cadmium sulfide, chemical formula CdS, is a direct band gap semiconductor that exhibits electroluminescence and piezoelectronic properties. When doped it exhibits cathodoluminescence and can be used as a phosphor, and has been used in photovoltaics. Recent research has focused on cadmium sulfide nanoparticles, including quantum dots. However, applications of this compound must maintain awareness of cadmium's toxicity.
Sources/Further Reading: (Images source - Wikipedia) (LibreTexts) (2023 article) (PubChem)
#Materials Science#Science#Cadmium sulfide#Cadmium#Sulfides#Semiconductors#Nanotechnology#MyMSEPost#MaterialsPosts
5 notes
·
View notes
Text


Semiconductors: Zinc oxide
An inorganic compound used in a wide variety of applications, zinc oxide (ZnO) also finds applications as a semiconductor. Pure ZnO is a white powder, though the naturally occurring mineral (zincite) often contains impurities that have it varying in color from yellow to red. It has two main crystal structures, hexagonal and cubic, and in addition to being a semiconductor is also piezoelectric and thermochromic.
As a semiconductor, ZnO is considered a wide band gap semiconductor (typically n-type) with a value of around 3.3-3.4eV at room temperature. As such, it is often used in higher temperature and higher power applications. Tuning of ZnO's bandgap is usually done by alloying the materials with magnesium or cadmium oxides. Dopant atoms typically include group III elements (such as aluminum) to substitute with zinc or group VII elements (such as chlorine) to substitute with oxygen. Doping with transition metal or rare earth elements can provide interesting optical properties as compared to pure ZnO and as such applications also often include optoelectronics.
One advantage ZnO has over other some other semiconducting materials (such as gallium nitride) is that it can be grown in bulk as single crystals. However, ZnO typically displays n-type superconductivity and doping to produce a p-type semiconductor has proven to be very challenging, which limits the current applications of the material. Primarily, as a semiconductor, it is used in LEDs and lasers as well as transparent contacts.
Sources/Further Reading: (Images source - Wikipedia) (2022 article) (2014 article) (2009 article)
16 notes
·
View notes
Text




Glasses: Colored glass
When many people think of glass, they tend to think of the transparent, clear panes that make up most windows, or the screens on their mobile devices, but glass, by definition, isn't necessarily clear or transparent. Examples of glasses that aren't, which I've posted about before, include metallic glass and goldstone, among others. For the purposes of this post, therefore, I'm defining 'colored glass' to be any ordinary soda-lime glass that has been colored by the addition of a coloring agent (this blog has posted about a few of these glasses as well; see Cranberry Glass and Cobalt Glass, as examples).
Soda-lime glass, in its purest form, is colorless, but in practicality can often have a green tint due to iron impurities. This is one of the main techniques to add color to glass: in the form of metal ions, often as sulfides or oxides. Different ions result in different colors, as they each absorb different wavelengths of light, including copper creating turquoise, manganese resulting in a purple color, and nickel, which can produce a range of colors from blue to purple to black.
Other methods of creating color in glass include striking glasses (including cranberry glass and photochromic lenses) and adding inclusions (such as milk glass). Other forms of colored glass don't necessarily involve additives, but coatings. Iridescent glass can be produced this way, as well as dichroic glasses.
Sources/Further reading: ( Wikipedia - images 1 and 4 ) ( colored glass in design ) ( commercial colored glass supplier ) ( glassblowing website )
Image 3.
20 notes
·
View notes