#PPD = project planning and design
Text
next ARE scheduled october 20th.... this exam is harder and 1 hour longer....... wish me luck lads 🫡🫡🫡🫡🫡🫡🫡🫡
#i did PA so now i'm attempting PDD#and the. going back and doing PPD after#hoping that i can do pdd and ppd within 2 weeks of each other ah#ITS SO SCARY GUYS#these are the exams which test me on how to make a building that won't kill you#so it matters LOL#fyi PA = programming and analysis#PDD = project development and documentation#PPD = project planning and design#ME = anxious
1 note
·
View note
Text
SAP PPDS Online Training: Elevate Your Planning & Scheduling Skills
At Best Online Career, we offer comprehensive SAP PPDS Online Training designed to help professionals master Production Planning and Detailed Scheduling (PPDS) in SAP S/4HANA. This advanced module ensures efficient production planning and helps optimize manufacturing processes, making it a critical skill for industries looking to stay competitive.
Why Choose SAP PPDS?
SAP PPDS is an integral part of SAP S/4HANA and is widely used by organizations to improve production efficiency and meet customer demands promptly. Learning PPDS enables you to:
Perform advanced scheduling and planning activities.
Balance resource load effectively.
Manage complex manufacturing scenarios with ease.
Optimize production orders and capacities.
Benefits of SAP PPDS Online Training
Our SAP PPDS Online Training at Best Online Career is tailored to provide hands-on experience, enabling learners to understand core concepts and apply them in real-world scenarios. Key benefits include:
Flexible Learning: Access SAP PPDS training at your convenience with live sessions and recorded materials.
Expert Trainers: Learn from certified SAP professionals with years of experience in SAP PPDS and production planning.
Real-Time Projects: Work on real-time projects and case studies that mirror industry challenges in production scheduling.
Certification Preparation: Our course is aligned with SAP’s certification guidelines, helping you to achieve your SAP PPDS certification and advance your career.
Key Features of Our SAP PPDS Course
In-depth coverage of SAP PPDS module functionalities.
Training in SAP S/4HANA PPDS integration with other modules such as SAP MM and SAP SD.
Hands-on exercises in production planning, demand management, and supply network planning.
Guidance on SAP PPDS configuration and customization.
Who Should Attend?
SAP consultants looking to expand their skillset in production planning.
Supply chain and production professionals aiming to optimize manufacturing operations.
IT professionals are interested in SAP’s advanced planning tools.
Enroll in SAP PPDS Online Training Today!
Boost your career with our industry-recognized SAP PPDS Online Training. Whether you're an experienced professional or just starting your SAP journey, Best Online Career provides the resources and support you need to succeed.
Explore our course offerings and take the next step in mastering production planning and scheduling with SAP PPDS. Visit www.bestonlinecareer.com and enroll today! Contact Us - 9146038100 / Email - [email protected]
0 notes
Text
SAP S4 HANA PP

SAP S4 HANA PP
SAP S/4HANA PP: Streamlining Production Processes in the Digital Age
SAP S/4HANA is a revolutionary ERP (Enterprise Resource Planning) suite designed to help businesses thrive in a rapidly changing, data-driven environment. Among its powerful modules, SAP S/4HANA PP (Production Planning) stands out as a cornerstone of modern manufacturing operations.
What is SAP S/4HANA PP?
SAP S/4HANA PP is a comprehensive suite of tools within the SAP S/4HANA ecosystem that addresses the intricate demands of production planning and execution. It provides integrated functions for managing:
Master Data: Material masters, bills of materials (BOMs), routings, work centers, and more.
Demand Planning: Sales forecasts, independent requirements, and planning strategies.
Material Requirements Planning (MRP): Calculates the needs for dependent materials.
Capacity Planning: Optimizes resource utilization and schedules production.
Shop Floor Control: Manages production orders, execution tracking, and confirmation.
Why SAP S/4HANA PP?
SAP S/4HANA PP offers compelling advantages over traditional PP solutions in several ways:
Real-Time Speed & Insight: Built on the in-memory HANA database, S/4HANA PP allows lightning-fast calculations, simulations, and ‘what-if’ scenarios. This empowers faster decision-making and plan adjustments.
Advanced Planning & Optimization (PPDS): For more complex scenarios, S/4HANA PPDS incorporates finite scheduling, constraint-based planning, and advanced algorithms to optimize resource usage and create highly detailed schedules.
Embedded Analytics: Gain real-time insights into production processes, identify bottlenecks, and visualize KPIs for continuous improvement.
Internet of Things (IoT) Integration: Connect to shop floor sensors and machines to monitor performance and trigger real-time adjustments in production plans.
Fiori User Interface: S/4HANA PP delivers a modern, intuitive interface making it easier to learn and adopt by users.
Key Functionalities of SAP S/4 HANA PP
Demand Management:
Sales and Operations Planning (S&OP): Collaborate on long-term plans
Demand-Driven Replenishment (DDR): Buffers for flexible replenishment.
Predictive MRP: Leverages forecasts and trends to anticipate needs
Supply Planning:
MRP Live: Run MRP dynamically based on up-to-date changes.
Multi-level Production Planning: Accounts for dependent demand across complex BOM structures.
Long-Term Planning (LTP): Projecting long-range materials needs
Detailed Scheduling (PPDS):
Production Planning & Detailed Scheduling: Handles complex scheduling requirements.
Optimizer: Creates the most efficient production sequences accounting for constraints.
Manufacturing Execution
Production Orders: Manage the entire production order lifecycle.
Kanban Control: Replenishment using visual cues.
Shop Floor Reporting and Confirmation: Track production progress.
Embarking on S/4HANA PP
If you’re considering implementing or upgrading to SAP S/4HANA PP, here are some things to keep in mind:
Assess Readiness: Evaluate your current production processes and technology to identify gaps.
Choose the Path: Opt for new implementations, system conversions, or landscape transformations based on your current systems.
Partner Wisely: Select an experienced SAP implementation partner.
Change Management: Training and communication are vital.
The Future of Production Planning
SAP S/4HANA PP positions businesses to embrace Industry 4.0 principles, characterized by automation, smart factories, and data-driven agility. By leveraging the power of this module, organizations can unlock the efficiency and responsiveness needed to stay competitive in the ever-evolving manufacturing landscape.
youtube
You can find more information about SAP PP in this SAP PP Link
Conclusion:
Unogeeks is the No.1 IT Training Institute for SAP PP Training. Anyone Disagree? Please drop in a comment
You can check out our other latest blogs on SAP PP here – SAP PP Blogs
You can check out our Best In Class SAP PP Details here – SAP PP Training
Follow & Connect with us:
———————————-
For Training inquiries:
Call/Whatsapp: +91 73960 33555
Mail us at: [email protected]
Our Website ➜ https://unogeeks.com
Follow us:
Instagram: https://www.instagram.com/unogeeks
Facebook:https://www.facebook.com/UnogeeksSoftwareTrainingInstitute
Twitter: https://twitter.com/unogeek
#Unogeeks #training #Unogeekstraining
0 notes
Text
UAL Awarding Body
Candidate Name
Candidate Number
Pathway
Project Title
Unit 13- FMP Proposal –Insert title here
Your Project Proposal must provide an outline brief of the project. This brief will normally be of your own choosing. Where your tutor sets the brief, or where you are working on a team/ group brief (with other Learners), please make sure that you cover how you will respond individually to the brief and what your contribution to the work of the group will be.
Prepare your proposal carefully using the guidance in each section below. The guidance should be seen as a prompt and not be regarded as being prescriptive. The four sections carry an approximate number of words and these exclude the Appendix which is where you should include your bibliography, timescale and action plan
Your Project Proposal must be approximately 500 words long (450-550)
SECTION 1 Rationale (approx. 150 words)
What is your chosen pathway? What do you know now that you didn’t before? What are the characteristics you feel most affinity with (your strengths & preferences)? What has been your strongest work? Which has been your favourite project? Which designers/ artists influence you most?
SECTION 2 Project Concept (approx. 250 words)
What could you work towards producing and what could be your possible end point? Explain how this relates to your work and ideas produced so far and how it extends your knowledge, understanding and creative ability.
What are the influences, starting points and contextual references and how they are relevant to your ideas? Indicate the subject areas you intend to research and the likely sources of information including any exhibitions, museums or specific locations you plan to visit.
SECTION 3 Evaluation (approx. 100 words)
How will you critically review and analyse your work and determine if it is successful? How will you identify directions for ongoing development? How often will you review your performance against the 7x PPD grade descriptors? Do you have a method to record the critical response to your ideas? How do you propose to assess the success of your project and what will be your methods of evaluation?
Proposed Research Sources and Bibliography (Harvard Format)
You MUST keep a detailed log of ALL research sources used in the FMP as assessment evidence and because you are exhibiting your work to the general public. You must do this using the Harvard referencing system (we will show you how to do this) but for now keep a list of all books, magazines, films, websites and journals. YOU MUST USE RESEARCH FROM AT LEAST 3 OF THESE SOURCES.
http://www.harvardgenerator.com
https://www.sciencebuddies.org/science-fair-projects/science-fair/writing-a-bibliography-examples-of-apa-mla-styles
3 notes
·
View notes
Text
Nreal unveils lightweight Nreal Air AR glasses for entertainment
The Transform Technology Summits start October 13th with Low-Code/No Code: Enabling Enterprise Agility. Register now!
Nreal is one of the few companies still standing from the early wave of augmented reality glasses makers. And today it is unveiling the second-generation Nreal Air glasses with big improvements.
The goal of the Beijing-based company is to keep moving fast on the leading edge of the technology and create multiple generations of products so that it can be ready with the right one when the AR market takes off. The company is pitching the device as a “portable movie theater.”
The newest glasses weigh just 2.7 ounces (77 grams), and they’re smaller and more compact than the Nreal Light glasses (100 grams) that the company launched in 2019, said Nreal CEO Chi Xu in an interview with GamesBeat.
Xu said that advancements in AR technology have accelerated exponentially since Nreal Light was first unveiled. And that makes smaller and more compact augmented reality technology available. The company has also designed the new glasses for specific applications, such as streaming TV shows and playing mobile games.
Webinar
Three top investment pros open up about what it takes to get your video game funded.
Watch On Demand


Above: Nreal Air looks like a pair of sunglasses.
Image Credit: Nreal
“We got the Nreal light into hands in 2019, and it was like a concise version of the HoloLens,” Xu said. “We thought it would be great for productivity, but it turned out most of the usage was entertainment and media consumption.”
The aim is to create an immersive experience in a form factor indistinguishable from daily worn sunglasses. Xu didn’t disclose the exact tech details, but he said the Nreal Air boasts the best display on the market available for any AR device, as it is capable of projecting up to a (simulated) 201-inch virtual display, when viewed at a distance of six meters, which is well suited for watching multimedia content.
The glasses will still be tethered to your smartphone, just like the Nreal Light. But the glasses are about a third lighter and are more comfortable to wear than many previous AR glasses, Xu said.
The glasses have an adjustable three-step rake system, which enables users to adjust the viewing angle by tilting the lens, and elastic temples that tightly hug the head and won’t slip. Nreal Air’s design has an outward-facing camera to focus on the theater experience. At 46 degrees, the glasses have a wider field of view than the previous glasses.
Rivals include the Microsoft HoloLens, the Magic Leap One, and whatever mysterious object Apple is working on but never revealing.
Impressive specs


Above: Side view of the Nreal Air.
Image Credit: Nreal
The device has a micro-OLED display. Nreal Air’s display has a high density of colors, with as many as 49 pixels per degree (PPD). This ensures that fine details are clearly visible and enhances the realism of the content, Xu said. And it has a refresh rate of up to 90Hz, and the features don’t drain the phone’s battery.
“We put a lot of resources into image quality and color,” Xu said.
It is also compatible with Apple iOS devices, a rarity in that it will support both iPhones and iPads, and will also be compatible with most Android devices.
Xu found that 78% of users in South Korea used Nreal Light to watch streaming content. Nreal will first launch Nreal Air in December 2021 in three Asian markets: Japan, China, and South Korea in partnership with leading carriers. Nreal Air’s roll out to other markets will continue through to 2022. Pricing will be determined by local carrier partners but will retail at a fraction of the price of Nreal Light.
The company is planning to take the Nreal Light product into enterprises, while the Nreal Air will focus on consumers. The Nreal Light Developer Kit is available for order here.
As one of the survivors, Nreal has been able to raise more than $185 million to date. The first glasses debuted in South Korea, Japan, and Europe. Xu said the company has more than 250 employees. Xu said he believes the headquarters in China is an advantage.
“We are closer to the supply chain and we can move faster,” he said. “We go early and we go first. We can get the early market share.”
GamesBeat
GamesBeat's creed when covering the game industry is "where passion meets business." What does this mean? We want to tell you how the news matters to you -- not just as a decision-maker at a game studio, but also as a fan of games. Whether you read our articles, listen to our podcasts, or watch our videos, GamesBeat will help you learn about the industry and enjoy engaging with it. How will you do that? Membership includes access to:
Newsletters, such as DeanBeat
The wonderful, educational, and fun speakers at our events
Networking opportunities
Special members-only interviews, chats, and "open office" events with GamesBeat staff
Chatting with community members, GamesBeat staff, and other guests in our Discord
And maybe even a fun prize or two
Introductions to like-minded parties
Become a member
0 notes
Text
From trucks to trails, Philly’s new $181 million bond will pay for improvements citywide
A new dock for dragon boats, HVAC systems for the city’s homeless shelters, and a cap for I-195 along Penn’s Landing are among the capital projects Mayor Jim Kenney will fund with the $181 million municipal bond Philadelphia voters approved on Tuesday.
The bond dollars constitute a relatively small slice of the $4.7 billion capital budget for next year. But the bond proceeds will help the city leverage private, state and federal money sources, which often require local matching funds — combined, those outside sources make up 35 percent of the city’s FY 2019 capital budget.
The credit line increase comes at a time when the city is working to realize a number of ambitious public works projects. Many of them will share in the $174 million that the city will ultimately put in the bank, after paying fees tied to the bond’s issuance.
Here's what Philadelphia voters bought on Tuesday
The Philadelphia Museum of Art officially broke ground on its Frank Gehry-designed, $196 million overhaul last year. The museum is city-owned, and so the city is chipping in a little: $5 million of the bond proceeds will go toward some of the infrastructure upgrades needed to make way for the ambitious reimagining of one of the city’s most iconic buildings.
On the other side of the Schuylkill River, another beloved cultural institution, the Philadelphia Zoo, will get $1.5 million for renovations to some of the city-owned buildings there.
The Commerce Department will oversee $1.5 million earmarked for improvements to commercial corridors throughout the city. The money will go toward upgrading sidewalks, lighting, landscaping and parking, in an effort to support existing local storefront businesses and inspire new ones to open.
The Philadelphia Industrial Development Corporation will get $3 million from the bond to boost its revolving fund for acquiring and investing in industrially zoned sites throughout the city, which it then sells off to private companies.
One of the city’s largest long-term capital projects is the plan to cap I-95 along Penn’s Landing with an 8-acre park. Kenney committed $90 million in city funds to the project in 2017, PennDOT pledged $100 million, and the William Penn and Knight Foundations have chipped in the rest of the $225 million cost.
This coming year, $500,000 of the bond will go toward preliminary design and permitting stages of the cap project. Another $7.5 million will be spent on other improvements to Penn’s Landing and the Central Delaware Waterfront. And $500,000 is pegged for the North Delaware River Waterfront.
The city’s other river will also get a splash of the bond monies: $2 million is pledged for the Schuylkill River Trail’s ongoing expansion projects.
The city’s other ambitious capital project, Rebuild, got off to a slow start, as opponents fought a long legal battle over the constitutionality of the sweetened beverage tax funding most of it. With that fight now behind the city, work can begin in earnest. But the start is also a bit modest: $1 million of the bond is pegged for the Free Library’s Rebuild budget, and another $7 million will go toward Department of Parks and Recreation-led projects.
Parks and Recreation will see other projects funded by the bond, too, with $1 million going toward an ongoing project to maintain and rebuild the retaining wall along the Schuylkill River. The Horticultural Center will get $200,000 for building upgrades. Design work on improving the East Park Canoe House will get a $400,000 infusion, and another $250,000 will go toward a new dock for dragon boats. The Mann Center is also slated to get $1 million for various improvements.
The Philadelphia Fire Department will get $2 million for ongoing and new upgrades at its stations across the city, including the city’s oldest, the 1891-built Engine 37 in Chestnut Hill.
The city also plans to spend $19.1 million of the bond on some new vehicles. The Fire Department will get the bulk, $10 million, and the Streets Department will get $7.6 million, leaving $1.5 million for cars and trucks used by other city departments.
The city’s homeless shelters will spend $1.2 million from the bond proceeds to replace heating and cooling systems.
A large chunk of the bond will be spent on updating the city’s old technology and computer systems: $15.2 million for hardware, and $6.7 million for software, including upgrades to the city’s accounting systems and the Streets Department’s right-of-way management system. That system tracks and coordinates the dozens of street closures for construction, parties, parades and other events that happen every day.
The Police Department will get money to support its move to 400 N. Broad St. The former Inquirer building will also house the 6th and 9th police districts, the medical examiner’s office and the city’s 911 call center. Along with upgrades to the 2nd, 15th and 22nd police districts, the PPD is getting $21.1 million in budget year 2019.
In addition to that, $5 million will go toward “substantial improvement to meet modern operational and safety requirements,” at the PPD’s firearms training facility. A $6.2 million extension of the Baxter Trail running behind it has been mostly closed because of the danger of ricocheting bullets, despite improvements at the time of the trail’s construction to keep shots within the gun range.
The Streets Department will heavily leverage the $1.5 million share of the bond pegged for Vision Zero traffic safety improvements. Streets officials expect it to yield $3 million in additional federal funds and another $3 million from the state.
The city will also allocate $500,000 for its ongoing replacement of city’s streetlights with brighter, but less energy-intensive LEDS.
Streets will also see $4.7 million used to rehabilitate and stabilize bridges. Another $23.5 million is earmarked for street repaving and the installation of curb ramps mandated by the Americans with Disabilities Act.
Philadelphia will also chip in $5 million for SEPTA-led construction projects across the city, which are estimated to total $227 million in the coming fiscal year.
Another $200,000 will go toward expanding Indego.
Looking beyond the new bond, the largest chunk of the city’s capital budget comes from “self-sustaining” sources. For instance, the Philadelphia Water Department reinvests utility bill payments into replacing old pipes, and Philadelphia International Airport uses the fees airlines pay to upgrade terminals and expand runways. So, while the airport is one of the largest line items on the budget — $600 million in capital spending is earmarked for the coming fiscal year — none of the recently approved municipal bond will go toward it.

Source: http://planphilly.com/articles/2018/11/09/from-trucks-to-trails-philly-s-new-181-million-bond-will-pay-for-improvements-citywide
0 notes
Text
Biographical Data
The intern was Jose Manuel J. Ambita and have so many things to do but neither lazy nor Hardworking but diligent when it comes to work, he have a family with stable income and have a family that so supportive to him but no achievement in school. Elementary and High School graduate.
Acknowledgement
Thank you for the people who contributed to the internship because of them we have now work experience and for the people in the department that given us some work and experience that are unforgettable.
Introduction
An internship is a period of work experience offered by an organization for a limited period of time. Once confined to medical graduates, the term is now used for a wide range of placements in businesses, non-profit organizations and government agencies. They are typically undertaken by students and graduates looking to gain relevant skills and experience in a particular field. Employers benefit from these placements because they often recruit employees from their best interns, who have known capabilities, thus saving time and money in the long run. Internships are usually arranged by third-party organizations which recruit interns on behalf of industry groups. Rules vary from country to country about when interns should be regarded as employees. The system can be open to exploitation by unscrupulous employers.
Internships for professional careers are similar in some ways, but not as rigorous as apprenticeships for professions, trade, and vocational jobs. The lack of standardization and oversight leaves the term "internship" open to broad interpretation. Interns may be high school students, college and university students, or post-graduate adults. These positions may be paid or unpaid and are temporary.
Typically, an internship consists of an exchange of services for experience between the intern and the organization. Internships are used to determine if the intern still has an interest in that field after the real-life experience. In addition, an internship can be used to create a professional network that can assist with letters of recommendation or lead to future employment opportunities. The benefit of bringing an intern into full-time employment is that they are already familiar with the company, their position, and they typically need little to no training. Internships provide current college students the ability to participate in a field of their choice to receive hands on learning about a particular future career, preparing them for full-time work following graduation.
Internship was a way to carefully understand the student of how the job it works in their place, to experience the difficulties of having a job, to train your-self as how the job works, and in order to gain work experience as a student.
Internship was designed as guide for you on how you will interact with other people on the job, how you will manage your own work using your own strategy, and how you will manage your time that are consumed by doing some work, this will be the stepping stone for you when you got a job in the future.
The students was assigned to their designated work place and have their schedule that given by the coordinator to them.
Linkage Institution

General Mariano Alvarez, the newest town of Cavite is formerly a part of the Municipality of Carmona. This new Municipality was named after General Mariano Alvarez, one of the foremost sons of Cavite, in the town of Noveleta during the revolution. It is previously called Carmona Resettlement Project. This project started in March 1968 because of the need to clear Quezon Memorial Park in Diliman, Quezon City of squatter’s shanties and other illegal constructions situated in it. The People’s Home site and Housing Corporation (PHHC) prepared the basic development concepts with the National Planning Commission (NPC) and the Department of Health (DOH). The group prescribed a minimum size of 12 x 12 or 144 square meters per relocated family.
VISION
A Municipality of diverse but unified, empowered and God-Loving People, enjoying the blessings of progressive economy with balance environment, under a firm and transparent leadership, recognizes as a commercial and agro-industrial haven in CALABARZON.
MISSION
To serve, sustain, protect and provide GMAians a safe drug free community, effective programs on health, environment, education, housing, employment, agriculture, commerce and industry through skills, development trainings, infrastructure and strict implementation of laws.
PERFORMANCE PLEDGE
Giving you effective plans and Programs that promotes social health, environment, education, housing, employment, agriculture, commerce and industry for the benefit of GMAnians;
Maintaining that all frontline service providers are courteous, approachable, polite and happy in serving each client;
Achieving the highest service performance and client satisfaction in delivery of frontline services offered by line and staff offices;
CORE VALUES STATEMENT
"Competence, Professionalism, Diversity towards Service Excellence"
MANAGEMENT ORGANIZATIONAL STRUCTURE, FUNCTIONS, RESPONSIBILITIES

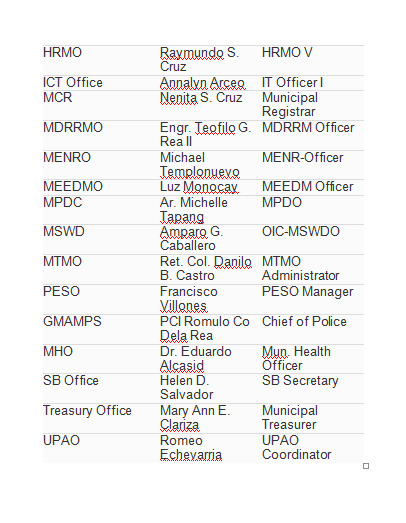
Social Services
Timely Registration of Civil Registry Documents (Birth/ Marriage/ Death)
Delayed Registration of Civil Registry Documents (Birth/ Marriage/ Death)
Issuance of Certified True Copies of Civil Registry Documents (Birth, Marriage, and Death)
Request for Authenticated Civil Registry Documents (BREQS)
Application for Legitimation
Applicaiton of Out-Of-Town Registration of Birth
Application for use of Surname of the Father / Affidavit to use surname of the Father (AUSF)
Application for Adoption / Registration of Annulment of Marriage
Request for Indorsement of Civil Registry Documents
Issuance of Certification (No Record/ On Process)
Request for Correction of Clerical Error/ Change of Firstname / Change of Firstname (RA 9048 & RA 10172)
Registration of Certificate of Foundling
Application for Marriage License
Endorsement of Supplemental Report
Burial Contract
Mayors Permit
Financial Assistance
PhilHealth Endorsement
Other Endorsement
Solemnization of Civil Wedding
Special Mayors Permit
Public Employment
Dog Registration and Vaccination
Vegetable Seeds Dispersal
Swine Dispersal
E-governance
Business Permit
Certificate of No Business
Business Closure
Real Property Certificate
Cedula
Official Receipt
Real Property Tax
Tax Clearance
Traceback Real Property
Simple Transfer RP
Issuance of Building Permit
Electrical Permit Private and Public
Environmental Permit
Health Services
Out-Patient Consultation
Prenatal Checkup
Postnatal Checkup
Woman who about to give birth
Family Planning
TB DOTS Clinic
Direct Sputum Smear Microscopy
PPD Skin Testing
Animal Bite Treatment Center
Expanded Program on Immunization
Issuance of Health Card / Sanitary Permit
Laboratory Services
Medical Certificate / Medicolegal Certificate
Dental Services
0 notes
Text
FDA panel backs Sage’s novel antidepressant but turns down Alkermes’
Sage’s Zulresso (brexanolone) is on course for approval for postnatal depression in the US after a positive vote from an FDA advisory committee at its meeting last week, but the same panel had bad news for Alkermes’ two-drug therapy for major depressive disorder.
The committee voted 17-1 on Friday that the benefit-risk profile for Zulresso (brexanolone) supported approval of the drug as an injectable therapy for post-partum depression (PPD) – provided it is delivered on an inpatient basis, by qualified staff, in a clinic licensed to prescribe the drug.
If the FDA follows its panel’s advice, Zulresso will become the first drug specifically approved for PPD, which is estimated to affect around 400,000 women every year in the US. The regulator is due to deliver a decision by 19 December, setting up a launch in the new year for what could be an important new women’s health drug.
The panel unanimously voted that Zulresso was effective in PPD and given that the drug represents a whole new class of antidepressant, much of the discussion centred on labelling, how to make sure the drug was used appropriately, and patient monitoring.
Brexanolone is a GABAA receptor modulator given as a 60-hour infusion, and has been shown in trials to start working within days of dosing, sometimes as quickly as 14 hours later, while traditional antidepressants can take weeks to exert their effects.
In Europe, Sage has said it hopes to have feedback from the EMA before the end of the year which will “guide our future plans in the EU as well as the timing of the build of our EU footprint,” according to recent comments by the company’s chief business officer Michael Cloonan.
The verdict puts Sage on the brink of commercialising its first product, a big achievement for the company which was hit hard by the failure of brexanolone as a therapy for super-refractory status epilepticus (SRSE) – a severe type of epilepsy – last year.
Meanwhile, the company is also planning to extend the use of brexanolone into the depressive phase of bipolar disorder, with phase II trials at the planning stage and due to start before year-end.
As Sage’s follow-up drug SAGE-217 is also a GABAA receptor modulator – in trials for major depressive disorder (MDD) – analysts at Stifel said the outcome also reduces the risk in that project, which is in phase III testing.
The verdict has also reignited speculation that a larger company may decide the time is right to buy into Sage’s pipeline. The company featured high on a list of potential takeover targets in the US biotech sector published earlier this year.
Alkermes setback
Alkermes’ bid to get its new antidepressant ALKS 5461 approved in the US took a big knock a day earlier when the advisory committee voted 21-2 that the drug’s benefit-risk profile is not adequate to support its approval for treatment-resistant MDD.
The once daily drug – due for an FDA verdict by 31 January – combines buprenorphine, a mixed mu opioid receptor agonist/kappa opioid receptor antagonist already used to treat pain and opioid addiction, with an opioid antagonist called samidorphan. Buprenorphine is thought to have an antidepressant effect via the kappa receptor, but its stimulation of the mu receptor means it has abuse potential which Alkermes says can be overcome by giving it alongside samidorphan.
Alkermes was already labouring under the weight of one failed trial out of three submitted in support of the drug, and the panel was critical of the design deployed in trials, specifically a format known as sequential parallel comparison design (SPCD) that is designed to correct the placebo effect which can be a big factor in depression studies.
SPCD – which involves re-randomising placebo non-responders to either the drug or placebo again – has never been used in an FDA submission before, and the agency’s reviewers were sceptical about its benefit in briefing documents filed ahead of the meeting. On the day, that scepticism was shared by the panel, which also had concerns about a decision to change the endpoint in one study mid-way through.
The committee voted 20-3 that the company had not provided sufficient evidence to support the efficacy of ALKS 5461, but concluded it was safe – just – by a margin of 13 to 10.
Jefferies analysts said the vote resulted from “poor choice in statistical methods and an unsupported change in clinical endpoints,” adding that the big question now is whether an ongoing phase IIIb trial (study 217) – not due to read out until August 2021 – will be able to meet the regulator’s concerns.
The post FDA panel backs Sage’s novel antidepressant but turns down Alkermes’ appeared first on Pharmaphorum.
from Pharmaphorum https://pharmaphorum.com/news/fda-panel-backs-sages-novel-antidepressant-but-turns-down-alkermes/
0 notes
Text
7 years
When I first got sick, I posted about it on facebook. That’s how I know for sure it was 7 years ago. We (myself, my husband, my doctor) thought it was a simple case of anemia, easy enough to fix and the fatigue would go away. I had been sick with a stomach bug in March, and after I recovered I had started going to the gym. Only I couldn’t work out, every time I went I felt worse and was able to do less before I felt like I’d pass out. I started feeling dizzy and exhausted at home. I would space out. I had trouble staying focused. I had trouble carrying the kids. I felt weak, almost like I had the flu, but without any other symptoms - no headache, no fever etc.
At the time, my daughter was 3.5 and my son was on his way to 2. It was normal for me to feel tired (the girl wasn’t sleeping through the night consistently yet). The fatigue wasn’t a big deal, it was the dizzyness and weakness that drove me crazy. I posted on facebook about it as a way to explain why I was changing plans, why I needed to carpool, why I was having trouble keeping on top of the housework.
At the time, I was taking antidepressants because I had gone through PPD and wasn’t ready to try going off them yet, just in case. At the time, I was attributing the pelvic pain to SPD. At the time, I really thought all I needed was some iron pills and I’d be fixed.
Except I wasn’t. My iron went up, but my symptoms got worse. I had other tests, and then more tests, and then more again. I saw a different doctor, and had even more tests. I went on a new antidepressant, then weaned off them completely. There were theories and more tests and still I was sleeping 18 hrs a day and hardly functional when I was awake.
And then in November, for some reason, things got better. I was given a 4 day run of steroids to see how I reacted, and most of my symptoms went away. The dizzyness and weakness were gone, the fatigue wasn’t nearly as strong, and over time it got better. I got stronger. My doctor told me he wasn’t sure what it was, if it came back it was likely autoimmune, if it didn’t, it must have been viral.
I went back to the gym, I got a job. I told everyone I was recovered. I had been sick but now I wasn’t. I had gastro issues, I still had pelvic pain, but I was better, I wasn’t sick anymore. I managed the gastro problems through diet, I used light exercise to help deal with the pelvic pain (which I still assumed was from the SPD, even years later). I went on with life. Things were really good for a while. I kept getting blood draws every 6 months or so to check on the anemia - my levels were still generally low even though I was feeling so much better.
It would come back in spells and I’d blame it on being stressed or not sleeping well, or on eating poorly and dealing with the consequences. The pelvic pain was getting worse and that was my main concern - 3 years ago I was wondering if maybe it was cancer - either my uterus or ovaries - because it would have explained all my symptoms, including the fatigue. I wasn’t working again, and sometimes the pain was so bad I couldn’t function. More doctors appointments, more tests, more waiting, a lot of fighting with my doctor because he wasn’t taking me seriously. I thought the fatigue was a result of the pain and the meds. I thought if I could get the pain under control I’d be well again.
Last November I finally got my GP to send me to a gynecologist for the pelvic pain. It took her about 10 minutes to diagnose me with endometriosis and discuss treatment options. About 3 weeks into treatment and things were much better. I could walk, sleep, move, function, focus on a conversation and not be distracted by pain. Life was good again.
But the fatigue is still there, and now there was nothing to blame it on. Now there’s a new doctor, more tests, more waiting. I’m not going out so much in the evenings, I avoid driving, I schedule naps into my day. I adjust my schedule and my expectations and my plans. Just like 7 years ago. I miss things - events, conversations, laughter, moments with friends and family.
I don’t post on facebook anymore about being sick. A few close friends know, but a lot of people don’t. They only see me on good days, or just after a nap, or for short enough periods of time that I can fake it. Those that do know don’t really get it - they joke about how nice it would be to get a scheduled nap time every day, make helpful comments about this supplement or that exercise. I feel isolated. I feel like I’m failing. My kids are now 10.5 and almost 9. They know on bad days we can’t do some things - friends can’t come over, there can’t be too much noise, that I’m trying to listen and I don’t mean to space out.
I knit and I spin and I weave because it gives me something to focus on, something to do, something that makes me feel productive without taking too much out of me. It gives me a way to feel like I’m still doing something, but on my worst days I can’t even manage that. I love teaching knitting classes, but it’s getting harder and harder because classes are in the evenings. I love designing, but lately keeping track of numbers and ideas has been really tough. I haven’t even dressed my loom since my last project, because it’s an all day job and I don’t have the energy to spare.
I don’t post about my illness on facebook anymore, because I don’t want people to think I’m just looking for attention. I know there are lots of people sicker than I am, who have lived with it for longer, who have lost more and suffered more than I have. I don’t talk about it outside of a few people because I don’t want to have to defend myself, because I know I don’t look sick. I don’t share with people because sometimes I really want to be able to pretend I’m normal, even though at the same time i really wish more people understood. The fatigue is isolating, but I’ve also isolated myself.
This started because of a facebook memory. I wish I could post this there - that I could be honest and open about how things are going, but I can’t. I don’t feel safe making it public, so I’ll just keep it here where people understand, finish my coffee and try to get some chores done before naptime.
#this is my life#chronic illness#spoonie#spoonie struggles#chronic pain#chronic fatigue#cfs/me#endometriosis#visanne#treatment#medical stuff#Longer than I expected#sorry for the rant#doctors#can't put this on facebook#so tired
2 notes
·
View notes
Text
Tasks
Please factor the following into your day/ weekend action plan:
-Please send me your wall art composition drawing asap before 12:15 pm.
-Contextualise your response/ progress to the GLEAM project brief. Explain the point of your outcome, identify the audience, how you hope they respond, your responsibility to them, the client and the population of the city as an artist or designer.
-Review your attention to the 7 x PPD- what needs improving and what are you doing well.
-Explain & evaluate what you are experimenting with new and reasons for using these materials.
-Give examples of how research has influenced your ideas/ outcomes.
-Make a statement in your journal about what you have learned about your pathway since the summer.
-Make an addition to your pathway resource about the characteristics/ expectations of your pathway and what to expect as an art student.
2 notes
·
View notes
Text
Contract Research Organization Services Market - Industry Size, Growth, Trends, and Analysis, 2018-2026
The contract research organization services are the organizations that provide support to the biopharmaceutical or biotechnological industries and academic institutes in the form of outsourced pharmaceutical research services. The CROs work for both drugs and medical devices and also range from large, international full service organizations to small niche specialty groups to fulfill the clients’ requirement. Initially, the pharmaceutical companies used to carry their own discovery work, along with every other elements to get the drug or medical device in the market. But nowadays, any research work required by biotechnology or pharmaceutical companies from designing assay to planning and running the clinical trials are outsourced from CROs. Outsourcing or partnering with a CROs by the biotechnological companies provide a strategic benefit to the manufacturers, such as cost and time saving in the development and approval process of new drug or therapeutic device, which is expected to increase the demand for CROs.
Download PDF Brochure @ https://www.coherentmarketinsights.com/insight/request-pdf/1592
Contract Research Organization Services Market Dynamics
Increasing number of Food and Drug administration (FDA) approvals are supporting the growth of biopharma industry, which in turn is fuelling growth of the CROs market. According to article published in Nature, the U.S. FDA approved 46 novel drugs for various indication by the agency’s Center for Drug Evaluation and Research (CDER) in 2017 and 22 drugs in 2016. As the drug companies and few research institutes face challenges in marketing their product due to limited resources and huge capital investment, which decreases the affordability of errors in research work. Therefore CROs play an important role in reducing the cost of research and help in successful clinical development program, which is the most important step of drug development process. As developing a safe and efficacious biological product for human is demonstrated through clinical trials.
Contract Research Organization Services Market - Regional Insights
North America market accounted for the largest share in the contract research organization services market, followed by Europe in 2016. This is attributed to increasing number of biotechnology-based companies with rising demand for CROs outsourcing services. Furthermore, increasing number of clinical trials and robust pipeline of Novartis, AstraZeneca, Merck and Pfizer, Inc. in the North America region are the factors responsible for growth of market in North America region over the forecast period.
In Asia Pacific region, the countries such as China and India particularly have increasing number of clinical trials for many application areas. Furthermore, due to large patient population, these countries provides a huge opportunity to the manufactures to market their drugs in Asia Pacific region, which is considered as major driver for rapid growth of CRO services. Besides, in some cases, the multinational companies are not allowed to conduct first-in-human Phase I studies in India and China where CROs plays a vital role to conduct such projects under such circumstances.
Request TOC of the Report @ https://www.coherentmarketinsights.com/ongoing-insight/toc/1592
Contract Research Organization Services Market - Competitive Analysis
The Contract research organization players are involved in acquisition to expand the geographical presence of the CROs. For instance, in April 2018, Frontage Laboratories, Inc. an early-stage contract research organization located in China and the U.S. acquired Concord Biosciences, a preclinical organization based in Cleveland, OH aiming the company’s goal to build a global CRO with integrated services. Furthermore, in May 2018, RxCelerate acquired Total Scientific, a CRO focused in bioanalysis, protein assays and gene-phenotype association.
Key players operating in the global contract research organization services market include IQVIA, LabCorp, PAREXEL, ICON plc, PPD, PRA Health Sciences, Syneos Health, Charles River, Wuxi PharmaTech, Medpace Holdings, SGS, Envigo, and MPI Research.
Contract Research Organization Services Market - Taxonomy:
By Type: Clinical Research Services, Early Phase Development Services, Laboratory Services, Consulting Services,. By Therapeutic Application: Oncology, Infectious Diseases, Central Nervous System (CNS) Disorders, Immunological Disorders, Cardiovascular Diseases, Respiratory Disorders, Diabetes, Other Therapeutic Areas,. By End User: Pharmaceuticals & Biopharmaceutical Companies, Medical Device Companies, Academic Institutes,.
Click To Continue Reading On Contract Research Organization Services Market
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
Visit our news Website: https://www.coherenttimes.org
#Contract Research Organization Services Market#Contract Research Organization Size#Contract Research Organization Growth
0 notes
Text
Rant about parenting from someone with OCD... (mainly in favor of it)
Hi, I’m a young woman with OCD and anxiety. Knowing this, I’m sure you can take a guess how much I appreciate the recent self-depreciating or self-inflating mindsetw about parenting, coupled with the pretentious child-free people who think parents are absolute idiots: the only two sides of the spectrum that have ever chosen to speak up and put their thoughts in writing.
I think, regardless of all the media I’ve consumed in my life by growing up in the digital age, that the public commentary on parenting as of late has left more scars- more fear, guilt, and pangs of emptiness and cluelessness- than anything else. The commentary on choosing whether to have a family has been overwhelmingly toxic, negative, insulting, and nosy from every side of the discussion. I’ve never been more convinced that people really can’t mind their own business around here.
Where are the people saying shit will be okay? Nowhere, because things that aren’t extraordinarily wonderful or horrible just do not make the news. All I see is “my friends don’t visit me anymore and judge me for not buying designer baby shoes.” Are these actually real people? Who the fuck buys designer baby shoes?! Do you have to be a demigod to realize how fast babies grow?
I absolutely fucking hate it. Here is something in my life that I do not know if I want, but think I do, that is being absolutely smeared as a life-ending experience for all these weird as fuck reasons. Telling me it’s the “worst job I’ll ever love” and bullshit Nanny Diaries phrases like that does not help me, mind you.
I really, really wish I could talk casually about what it’s like to be a parent. Because I think I might be a good parent to one child. Not perfect, but good. I was raised well (I respected my parents’ style), and I watched my little cousins get raised well to the point of inspiration. Kids have always liked and respected me even when I resented them. I don’t want a child for selfish reasons, or to project myself onto them, or to fill a void, or to save a relationship, or for “fun.” I want to answer their questions, teach them how to be a respectful and imaginative person, rediscover the world with them, watch them grow, let them learn, and show them that there are a million different ways you can be happy in life. Is that enough to be a good parent? Will that desire alone rob me of being an individual? I just don’t see how that could be possible. I know it will be hard. I know I won’t sleep at first, I know I won’t be able to “go out” (WHERE were all these people allegedly “going” all the time before a kid, anyway? Peru??? I really want to know this), I know they’ll make me pissed at their helplessness (lots of things do). I know not to spoil, I know to instill lessons of managing money and helping around the house, I know how to be patient with emotions, I hate going to restaurants and movies and all that anyway (I hear “you’ll have no free time” and I think, I won’t be able to watch videos on my computer while making noodles???) so no loss there... I’m a homebody in general. I only want to travel to Japan and plan on doing so before having a family anyway. Does that help? Is that enough? I genuinely want to know if the fact that I don’t think I can even relate to any of the common personal “struggles” in the first place is a good sign. I’m worried about a horrible OCD spike and PPD. Very, very worried. But not worried enough to let that win.
I hate this because OCD makes every risk seem like the end of the line as it is, and you’re expected to jump right into the frightening and unknown in order to overcome that. But with all these fucking messages of absolute regret and bitterness over children stemming from these apparently high-energy people with extravagant lives that went downhill, which even I- a person who feels rather indifferent to children when I once disliked them- find disgusting, it really does make me wonder whether parenthood would be an irreversible mistake. I’m not sure how to weigh my options, and “You won’t know if you’re ready until you try” might be good advice for regular people, but it’s the opposite of helpful for me.
I truly don’t know what to think. I just want to be free of trying to understand everything one day. I’m quickly learning that I’ll never, ever know everything. I really want a nap.
Edit for minirants.
- Wear your baby. Deadlift your baby. who gives a fuck
- babies poop. and u have diarrhea especially after dairy if you’re lactose intolerant so don’t think you’re some kinda deity. buy flushable moist wipes. you’re welcome
- NO ALLOWANCES UNLESS THEY DO BASIC CHORES. if they want $20 item, they save up
- say thank you to the lady for handing you the cookie
- they’re gonna ask questions all the fucking time. so do i. baby doesn’t know shit but I don’t either. maybe we’ll both learn something
- of course you’re gonna have an awful time if you’re expecting to be perfect?? is that RLY what all these people are doing? also dont buy expensive clothes and shit. Babies don’t care, people who do care don’t matter. TJ maxx
- It’s really gonna help to be creative with your kid, build them stuff that helps build their imagination. thats the one thing that always, always stuck with me and never failed
- People who judge the way you parent are actual assholes. your mom in law isn’t the parent, your coworker isn’t, your friend from college isn’t. unless you’re being a straight up shit person, who cares what they think about your diaper genie or breastfeeding decisions. call them out on this and don’t worry
- MY MOM ALWAYS SAID THAT NOT EvERYTHING IS FUN AND GAMES. GUESS WHAT. YES IT IS. JUST PRETEND THAT BASIC SHIT IS AN ADVENTURE
- toys are cool and fun. no regret spending dollars on toys in this household.
- clean your FCKING space. this is not a parents-only task
- your child does not have to be a sports/dance genius or spelling bee champion. literally nobody cares
- you still love your partner. you want to kill them sometimes because they didn’t do the dishes like you asked when it was the ONLY THING YOU ASKED THEM TO DO, STEVEN. u still love them. lower ur voice, get a babysitter and go get ice cream together or something.
- the stars still shine, you’re still you. life is fucking weird. do whatever
2 notes
·
View notes
Text
Healthcare Contract Research Organization Market poised for growth over the coming years by 2027
Healthcare Contract Research Organization market report is the major research for those who look for an entire analysis of markets. The report covers all information on the Global and regional markets including old and future trends for market demand, size, trading, supply, competitors, and prices as well as Global predominant vendors’ information. We have provided CAGR, value, volume, sales, production, revenue, and other estimations for the global as well as regional markets. The market is designed to serve as a ready-to-use guide for developing accurate pandemic management programs allowing market players to successfully emerge from the crisis and retract numerous gains and profits. The players included in this report are chosen in terms of their product portfolio, market share, brand value, and the well-being of the organizations. Our report based on current situations across the globe.
You can get a sample copy of the report here @ https://www.datalabforecast.com/request-sample/197651-healthcare-contract-research-organization-market
**Note: Don’t miss the trading opportunities on Healthcare Contract Research Organization Market. Talk to our analyst and gain key industry insights that will help your business grow as you create sample reports.
Note- This report sample includes:
• Brief Introduction to the research report
• Table of Contents (Scope covered as a part of the study)
• Research framework (Structure of The Report)
• Top players in the market
• The research methodology adopted by Data Lab Forecast
North America is expected to hold dominant position in the global Healthcare Contract Research Organization market, owing to increasing collaboration activities by key players over the forecast period.
Healthcare Contract Research Organization Market: Dynamics
Based on the current scenario, the industry has a fairly positive impact on the Healthcare Contract Research Organization Market, owing to increasing use and adoption of Healthcare Contract Research Organization during COVID-19. The spread of COVID-19 has forced the industry to drive both a stronger online presence and discover new ways to provide analysis. Hence, end users are adopting market to overcome business challenges. This is increasing spending on Healthcare Contract Research Organization across the globe.
The research study offers a substantial knowledge platform for entrants and investors as well as veteran companies, manufacturers functioning in the global Healthcare Contract Research Organization market. The report includes CAGR, market shares, sales, gross margin, value, volume, and other vital market figures that give an exact picture of the growth of the global Healthcare Contract Research Organization market. We have also focused on SWOT, PESTLE, and Porter’s Five Forces analyses of the global Healthcare Contract Research Organization market.
We are currently offering Quarter-end Discount to all our high potential clients and would really like you to avail the benefits and leverage your analysis based on our report.
Avail 30-50% Discount on various license type on immediate purchase (Use Corporate email ID to Get Higher Priority) @ https://www.datalabforecast.com/request-discount/197651-healthcare-contract-research-organization-market
Healthcare Contract Research Organization Market
Thinking One Step Ahead
In today’s competitive world you need to think one step ahead to pursue your competitors, our research offers reviews about key players, major collaborations, union & acquisitions along with trending innovation and business policies to present a better understanding to drive the business in the correct direction.
Healthcare Contract Research Organization Market: Impact of COVID-19
The Coronavirus (COVID-19) pandemic has affected every aspect of life worldwide. The report considers the impact of COVID-19 on market growth. The study provides full coverage of the impact of the COVID-19 pandemic on the Healthcare Contract Research Organization market and its key segments. Furthermore, it covers the present and future impact of the pandemic and offers a post-COVID-19 scenario to provide a deeper understanding of the dynamic changes in trends and market scenarios.
Healthcare Contract Research Organization Market: Key Players
The major market players that are operating in the Healthcare Contract Research Organization market are Quintiles, ICON, LabCorp (Covance), Parexel, PPD, inVentiv Health, Charles River Laboratories, Medidata Solutions, IQVIA
Detailed Segmentation:
Global Healthcare Contract Research Organization Market, By Product Type:
⇛ Drug Discovery, Pre-Clinical, Clinical.
Global Healthcare Contract Research Organization Market, By End User:
⇛ Pharmaceutical Industry, Biotechnology, Medical Device Industry.
Do You Have Any Query or Specific Requirement? Drop Your Query Here @ https://www.datalabforecast.com/request-enquiry/197651-healthcare-contract-research-organization-market
The Healthcare Contract Research Organization Market report incorporates the detailed analysis of the leading organizations and their thought process and what are the methodologies they are adopting to maintain their brand image in this market. The report aides the new bees to understand the level of competition that they need to fight for to strengthen their roots in this competitive market.
Healthcare Contract Research Organization Market: Prominent Regions
• Asia-Pacific (Vietnam, China, Malaysia, Japan, Philippines, Korea, Thailand, India, Indonesia, and Australia)
• Europe (Turkey, Germany, Russia UK, Italy, France, etc.)
• North America (United States, Mexico, and Canada.)
• South America (Brazil etc.)
• The Middle East and Africa (GCC Countries and Egypt.)
What benefits does DLF research studies provide?
1. Supporting company financial and cash flow planning
2. Latest industry influencing trends and development scenario
3. To resize powerful market opportunities
4. A key decision in planning and to further expand market share
5. Identify Key Business Segments, Market proposition & Gap Analysis
6. Assisting in allocating marketing investments
Buy Now this Premium Report to Grow your Business @ https://www.datalabforecast.com/buy-now/?id=197651-healthcare-contract-research-organization-market&license_type=su
In conclusion, the Healthcare Contract Research Organization Market report is a genuine source for accessing the research data which is projected to exponentially grow your business. The report provides information such as economic scenarios, benefits, limits, trends, market growth rates, and figures. SWOT analysis and Porters Five analysis is also incorporated in the report.
About Us
Transforming Information into Insights
We pride ourselves in being a niche market intelligence and strategic consulting and reporting firm driven towards resulting in a powerful impact on businesses across the globe. Our accuracy estimation and forecasting models have earned recognition across majority of the business forum.
We source online reports from some of the best publishers and keep updating our collection to offer you direct online access to the world’s most comprehensive and recent database with skilled perceptions on global industries, products, establishments and trends. We at ‘Data Lab Forecast’, wish to assist our clients to strategize and formulate business policies, and achieve formidable growth in their respective market domain. Data Lab Forecast is a one-stop solution provider right from data collection, outsourcing of data, to investment advice, business modelling, and strategic planning. The company reinforces client’s insight on factors such as strategies, future estimations, growth or fall forecasting, opportunity analysis, and consumer surveys, among others.
Contact:
Henry K
Data Lab Forecast
86 Van Wagenen Avenue, Jersey,
New Jersey 07306, United States
Phone: +1 917-725-5253
Email: [email protected]
Website: https://www.datalabforecast.com/
Follow Us on: LinkedIN | Twitter |
0 notes
Link
As the United States accelerates the search for a coronavirus vaccine, tensions have erupted between government scientists and Moderna, one of the leading developers, Reuters has learned.
The federal government is supporting Moderna’s vaccine project with nearly half a billion dollars and has chosen it as one of the first to enter large-scale human trials.
But the company – which has never produced an approved vaccine or run a large trial – has squabbled with government scientists over the process, delayed delivering trial protocols and resisted experts’ advice on how to run the study, according to three sources familiar with the vaccine project. The sources said those tensions, which have not been previously reported, have contributed to a delay of more than two weeks in launching the trial of the Moderna’s vaccine candidate, now expected in late July.
Moderna “could be on schedule if they were more cooperative,” one of the sources told Reuters.
Some of the disagreements have stoked concerns over the young biotech firm’s relative inexperience and what the sources described as its lack of staff and expertise to oversee the most critical phase of human trials. The U.S. government is not facing similar problems with established drugmakers, such as AstraZeneca Plc and Johnson & Johnson, working on other leading vaccine candidates, the sources said.
Moderna and other vaccine developers are working with the U.S. National Institutes of Health (NIH) and the Food and Drug Administration (FDA), along with networks of immunologists and other vaccine experts tasked by the NIH to help oversee trial design.
Moderna denied any missteps on its part but acknowledged “differences of opinion” with experts involved in the unprecedented effort to deliver on the Trump administration’s pledge to find a vaccine within months. It typically takes about a decade to develop a vaccine – and many efforts fail to produce one at all. Moderna said it has an experienced team that includes people who have run multiple large-scale trials.
“It has not been smooth or easy,” said Moderna spokesman Ray Jordan. “No one has ever done anything like this before – not Moderna, not the NIH, and not any of the other companies.”
In one disagreement, Moderna executives resisted experts’ insistence on close monitoring of trial participants who might contract COVID-19 for changes in oxygen levels that could signal dangerous complications. While other drugmakers complied, Moderna questioned the recommendation as a “hassle” that slowed development, one of the sources told Reuters. Jordan said the company preferred to defer all decisions about monitoring to patients’ physicians but that the company ultimately agreed to some monitoring.
Despite a bumpy process, Moderna remains ahead of other firms in the race for a vaccine, according to statements from the government and the companies. The firm, founded a decade ago, has outpaced much larger companies despite the steeper challenges Moderna faces in scaling up staff and capacity to create a vaccine at breakneck speed. AstraZeneca and Johnson and Johnson are also steaming toward their own large-scale trials, but they are behind Moderna in the United States.
Stephen Thomas, a vaccine developer who is chief of infectious diseases at SUNY Upstate Medical University, said vaccine development can spark such disagreements even without the pressures of an out-of-control pandemic.
“Those tensions, in and of themselves, don’t indicate that Moderna is incapable of doing it,” Thomas said.
In a statement to Reuters, the U.S. Health and Human Services Department (HHS) said the government’s collaboration with Moderna, as with all organizations in the project, has been “extremely cooperative.” The agency said Moderna’s vaccine candidate is the most advanced and has shown excellent performance in early trials.
HHS declined to respond to further questions. NIH and the FDA declined to comment.
‘WARP SPEED’
The Trump administration’s “Operation Warp Speed” vaccine program is run by HHS in partnership with other agencies. It is led by Moncef Slaoui, a former GlaxoSmithKline executive who more recently served on Moderna’s board of directors. He stepped down in May to run the government’s COVID-19 vaccine project.
Moderna’s vaccine technology uses genetic material called messenger RNA to instruct human cells to produce coronavirus proteins that prompt an immune response. The firm was picked early by NIH because of its technology’s potential to accelerate development. Moderna developed a vaccine candidate in about two months, making it the first to move to early human testing in a small U.S. trial of healthy volunteers in March.
The NIH had hoped to launch Moderna’s large-scale trial by July 10 but the disputes with the company caused the delay, the sources said. Medical news site STAT first reported the trial delay on Thursday.
The company attributed the delay to the need to accommodate last-minute compromises with the NIH and to allow the government to coordinate trials with multiple drugmakers. Moderna’s Jordan called its interactions with government experts a healthy scientific debate. “There have, of course, been differences of opinion, but we believe there has always been good intent,” he said.
Moderna and other vaccine developers have been given significant control and responsibility over the large-scale, so-called Phase 3 trials by HHS. But Moderna has been less forthcoming than other drugmakers about its plans, the sources said.
Moderna outsourced the handling of data collection to the contract research firm PPD Inc. At one meeting set up with the leading companies and government officials, Moderna did not allow PPD to share details of the trial plans, as other companies had done, the sources said. PPD did not respond to request for comment.
Moderna disputed that it withheld information, calling the complaint a misunderstanding about the company’s presentation at the meeting, which was not as detailed as others.
Moderna delayed submitting its clinical trial protocols by several weeks, the sources said. The protocols lay out goals and detailed procedures for researchers to manage the trial safely. While Moderna maintained it made the call to delay the trial launch, the sources said Moderna lacked enough staff to complete the protocols on time.
Moderna also initially sought a lower threshold for proving whether its vaccine worked than what was ultimately set by the FDA, one of the sources said. The company says it has aligned with the FDA’s guidance after discussions with the agency.
One of the sources said such disputes and delays speak to a larger problem in the government’s interactions with Moderna. “They try to test every boundary,” the person said.
The post COVID-19 vaccine: Tensions erupt between US scientists and Moderna appeared first on ARY NEWS.
https://ift.tt/36T6vfX
0 notes
Text
Time Plan (25th February - 20th May)
Week 1 - 25.2.2020
Review the year so far, evaluate which units you thought were successful, identify which projects you would like to develop further. Write this evaluation for your blog.
Week 2 - 4.3.2020
Research 3 ideas / themes for investigation in the FMP, presented informally to 4 students and 1 staff. Review feedback and post on blog to see what can be improved.
Week 3 - 11.3.2020
A well written Project Proposal on UAL pro forma. Include rationale, project proposal and evaluation (500 words).
Week 4 - 18.3.2020
Comprehensive primary and secondary research (approx. 20 A4 sides (pass), 30 (merit) or 40 (Distinction) with detailed analysis that clearly shows links to ideas and defines the starting point in sketchbook, journal and blog.
Week 5 - 25.3.2020
Complete experiments - including possibilities of video, virtual reality, painting, digitally drawn art, pencil sketch, graphic design, 3D design. A bibliography detailing research sources used to date in the Harvard format.
Week 6 - 1.4.2020
A self assessment/ review of the first stage and your performance against the 7 x PPD categories.
Week 7 - 8.4.2020
Extensive experimental and developmental practical work that explores a wide range of possibilities in response to your intentions with detailed evaluations and links to your research sources in sketchbook, journal AND blog. A clearly defined statement of intent describing what your final outcome will be
Week 8 + 9 - EASTER - KEEP UPDATED WITH ALL WORK, ADD ANY EXTRA DETAIL AND COMPLETE FULLY STAGE 1 + 2 + 3.
Week 10 - 29.4.2020
A self assessment/ review of stage 2 and 3 and your performance so far against the 7 x PPD categories.
Week 11 - 6.5.2020
A final outcome presented in exhibition with detailed evaluation against original proposal which may require refining/ finishing by 5th June.
Week 12 - 13.5.2020
A self assessment/ review of stage 4 and your performance against the 7 x PPD categories, A well populated blog recording significant stages of your project progress with outcomes.
Week 13 - 20.5.2020 - FINAL PRESENTATION OUTCOME
Week 14- MAY HALF-TERM
Final assessment and overall grade of presented outcome and all developmental work in sketch book, journal, blog, experiments/ samples/design sheets etc. against project proposal. Self assessment / review of the stage 5 and your performance against the 7 x PPD categories.
Week 15- HAND IN SKETCHBOOK / JOURNAL / BLOG FOR ASSESSMENT
0 notes
Text
Contract Research Organization Services Market Size, Share, Industry Insights, Trends, Outlook, and Analysis, 2020-2027
The contract research organization services are the organizations that provide support to the biopharmaceutical or biotechnological industries and academic institutes in the form of outsourced pharmaceutical research services. The CROs work for both drugs and medical devices and also range from large, international full service organizations to small niche specialty groups to fulfill the clients’ requirement. Initially, the pharmaceutical companies used to carry their own discovery work, along with every other elements to get the drug or medical device in the market. But nowadays, any research work required by biotechnology or pharmaceutical companies from designing assay to planning and running the clinical trials are outsourced from CROs. Outsourcing or partnering with a CROs by the biotechnological companies provide a strategic benefit to the manufacturers, such as cost and time saving in the development and approval process of new drug or therapeutic device, which is expected to increase the demand for CROs.
Furthermore, increasing demand for effective biotherapies and increasing competition between manufacturers to discover new drugs are expected to foster growth of CROs market. Increasing need for product development is leading to high demand for experience and high skilled professionals to implement and conduct the biotechnological research and clinical trials, which can be provided by CROs.
Ask for the Sample of the Study:
https://www.coherentmarketinsights.com/insight/request-sample/1592
Increasing number of Food and Drug administration (FDA) approvals are supporting the growth of biopharma industry, which in turn is fuelling growth of the CROs market. According to article published in Nature, the U.S. FDA approved 46 novel drugs for various indication by the agency’s Center for Drug Evaluation and Research (CDER) in 2017 and 22 drugs in 2016. As the drug companies and few research institutes face challenges in marketing their product due to limited resources and huge capital investment, which decreases the affordability of errors in research work. Therefore CROs play an important role in reducing the cost of research and help in successful clinical development program, which is the most important step of drug development process. As developing a safe and efficacious biological product for human is demonstrated through clinical trials.
Furthermore, stringent regulatory policies for drug development make process more complex, as it required more resources to develop new drugs, devices, and biologics. These process requires expertise in broad scientific disciplines of preclinical, clinical, ancillary clinical in chemistry, packaging, manufacturing, project management, and regulatory affairs which are provided by the CRO’s, considered as a major reason for drug companies to outsource clinical trials to CROs. Besides, the pharmaceuticals & biopharmaceutical companies are collaborating to undergo clinical trials with CROs to undergo the drug development process.
Get Free PDF Brochure of Research Report: https://www.coherentmarketinsights.com/insight/request-pdf/1592
For instance, in May 2018, Ritter Pharmaceuticals, Inc. a developer of novel therapeutic products signed an agreement with the clinical research organization (CRO) Medpace to conduct the first of two pivotal Phase 3 clinical trials for RP-G28 in patients with lactose intolerance (LI).
North America market accounted for the largest share in the contract research organization services market, followed by Europe in 2016. This is attributed to increasing number of biotechnology-based companies with rising demand for CROs outsourcing services. Furthermore, increasing number of clinical trials and robust pipeline of Novartis, AstraZeneca, Merck and Pfizer, Inc. in the North America region are the factors responsible for growth of market in North America region over the forecast period.
In Asia Pacific region, the countries such as China and India particularly have increasing number of clinical trials for many application areas. Furthermore, due to large patient population, these countries provides a huge opportunity to the manufactures to market their drugs in Asia Pacific region, which is considered as major driver for rapid growth of CRO services. Besides, in some cases, the multinational companies are not allowed to conduct first-in-human Phase I studies in India and China where CROs plays a vital role to conduct such projects under such circumstances.
Furthermore, ongoing adoption of international standards and patent protection laws are been improved over the years particularly in India, China, and Japan. For instance, International Conference on Harmonization (ICH) Guidance document on Good Clinical Practice (GCP) followed by U.S. and Europe is been now adopted in India, Japan, and China. Hence the entrance of CROs in Asia Pacific region helps to provide clinical, regulatory infrastructure and practices in this region.
The Contract research organization players are involved in acquisition to expand the geographical presence of the CROs. For instance, in April 2018, Frontage Laboratories, Inc. an early-stage contract research organization located in China and the U.S. acquired Concord Biosciences, a preclinical organization based in Cleveland, OH aiming the company’s goal to build a global CRO with integrated services. Furthermore, in May 2018, RxCelerate acquired Total Scientific, a CRO focused in bioanalysis, protein assays and gene-phenotype association.
Key players operating in the global contract research organization services market include IQVIA, LabCorp, PAREXEL, ICON plc, PPD, PRA Health Sciences, Syneos Health, Charles River, Wuxi PharmaTech, Medpace Holdings, SGS, Envigo, and MPI Research.
Browse the Full Report at
https://www.coherentmarketinsights.com/ongoing-insight/contract-research-organization-services-market-1592
About us:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Name: Mr. Shah
Phone: US +12067016702 / UK +4402081334027
Email: [email protected]
Visit our Blog: https://hospitalhealthcareblog.wordpress.com/
0 notes