#Plexiform Neurofibroma
Text
Neurofibromatosis Treatment Drugs Industry Growth

Neurofibromatosis (NF) is a genetic disorder that causes tumors to form on nerve tissues. There are two main types - NF1 and NF2. NF1, also known as von Recklinghausen disease, is the more common form affecting around 1 in 3000 people. The main symptoms include light brown spots on the skin, tumors on or under the skin (neurofibromas), and Lisch nodules on the iris. NF2 is rarer and causes bilateral vestibular schwannomas (tumors on the eighth cranial nerve), which can lead to hearing loss and balance problems if not treated. Other features may include meningiomas (tumors of the meninges) and ependymomas (tumors of the central nervous system).
Medical Management of NF1
For NF1, the treatment approach depends on the symptoms. If neurofibromas are small and cause no problems, only monitoring is needed. However, larger or painful neurofibromas may require surgery to remove them. Magnetic resonance imaging (MRI) scans are useful to monitor the growth of tumors. Children with NF1 are closely followed to watch for the development of optic pathway gliomas, which can affect vision if not treated. Medical therapy focuses on managing complications like high blood pressure, learning disabilities, and bone abnormalities.
Targeted Neurofibromatosis Treatment Drugs Therapies
In recent years, research has led to the development of targeted drug therapies that interfere with molecular pathways driving tumor growth in NF. One such pathway involves the RAS family of oncogenes, which are mutated in a high percentage of NF1 tumors. Selumetinib (Koselugo) is a MEK inhibitor drug approved by the FDA to treat inoperable plexiform neurofibromas in patients with NF1. By blocking the MEK protein, it helps control tumor growth. Another RAS pathway drug, sotorasib (Lumakras), showed efficacy against KRAS G12C mutant solid tumors in a clinical trial and may offer an option for NF patients with specific mutations. Several other MEK and RAF inhibitors are under investigation for NF.
Medical Management of NF2
For NF2, treatment goals are to halt tumor growth and preserve hearing and neurological function as long as possible. Surgery continues to play a major role by removing tumors causing symptoms. Stereotactic radiosurgery uses focused beams of radiation to control residual or growing tumors without the risks of open surgery. Monitoring with serial MRIs helps determine when intervention is needed. The multikinase inhibitor sorafenib was shown to slow tumor growth in an NF2 clinical trial and represents a potential medical option. However, effective drug therapies are still quite limited for systemic treatment of NF2.
Research Directions
Ongoing research aims to discover new drugs that more specifically target signaling pathways driving NF tumor formation and growth. Candidate pathways include PI3K-AKT-mTOR, Hedgehog, Notch, Wnt, and Hippo signaling. Therapies modulating these cascades are in preclinical testing. Immunotherapies are another area of investigation since NF tumors can express tumor antigens that may stimulate anti-tumor immune responses. Combining targeted drugs with immunotherapy is a strategy to make treatments more effective. Advances in gene therapy also offer hope that someday, mutations causing NF could be directly corrected. Progress is being made, but more work is still required to develop curative options for these currently incurable genetic tumor predisposition syndromes.
#Neurofibromatosis Treatment Drugs Analysis#Neurofibromatosis Treatment Drugs Demand#Neurofibromatosis Treatment Drugs Trend
0 notes
Text
Short Description on Plexiform Neurofibroma
Short Description on Plexiform Neurofibroma
Plexiform neurofibroma is a rare type of tumor that affects the peripheral nerves. It is a benign tumor that can occur anywhere in the body, but it is most commonly found in the head, neck, and extremities. Plexiform neurofibroma is often associated with neurofibromatosis type 1 (NF1), a genetic disorder that affects the development and growth of nerve…
View On WordPress
0 notes
Photo

“Why do we need Disability Pride?”
A comic I made for Disability Pride. Under the keep reading is an in-depth image description of the comic.
[description one: a seven-panel comic titled ‘Why do we need Disability Pride?’ where two ignorant able-bodied people have their misconceptions about disability challenged. at the end of the comic, they are involved in the community.
[description two: A text at the beginning of the comic reads, “Why do we need Disability Pride?” Beneath that is a balanced scale with two people sitting on either side. On one side is Ekam, a Sikh man wearing a turban, button-up, and khakis. Above it reads, “Ekam is uncomfortable around disabled people.” On the other side of the scale is Bailey, a fat white woman wearing a long-sleeved shirt, tights, and a carrier purse which are all purple. Above her it reads, “Bailey thinks every disabled person is an inspiration.” To either side of them are panels providing examples of how their ableism manifests.
To the left of Ekam is a panel of him speaking to a coworker, Alazaye, a fat Black woman with half-up hair buns in a wheelchair wearing a tank top and jean shorts. Ekam looks unsettled, but polite. In a speech bubble above him he says, “So.. uh,... hey..” Imposed over-top of his dialogue is Alazaye’s thought bubble that reads, “he’s only awkward around me..” while looking away, frustrated.
To the right of Bailey is a panel of her speaking to a stranger, Caden, a white man with facial and limb differences due to burns using arm crutches. He is wearing a t-shirt and long-sleeved shirt with jeans. Bailey is touching his shoulder comfortingly while saying, “it’s so BRAVE of you to be OUTSIDE!” Caden is looking at her directly while scowling, but not speaking.
In the next panel is a landscape of a Disability Pride parade. Two people in mobility scooters are leading the parade while carrying a banner of zigzag Disability Pride Flag. On the left side of the banner is a fat East Asian woman wearing a blouse with her hair up. On the right is a dark-skinned Black woman with locs, self-harm scars, and Plexiform neurofibromas on her face wearing a bucket hat and t-shirt. Around them are other disabled people and allies carrying signs or celebrating in the street. Above the panel it reads, “Disability Prides in America have been held since 1990 when the ADA was signed.” Below it reads, “The work of disabled activists during the month encourages others to break down bias they may have towards those with disabilities.”
In the next panel Ekam approaches a group of people within the parade, smiling and waving nervously. In the foreground of the illustration is Ruben, a South Asian enby waving to Ekam with one of their prosthetic arms. Caden from before is standing beside Ekam wearing a sunhat to protect his eyes. Below the panel it reads, “Be brave and reach out.”
In the next panel Bailey listens to a white redheaded woman wearing goth clothes in a wheelchair speak while Ernie, a chubby Black man with a high-top fade wearing an autistic infinity t-shirt and jeans, is prompting Bailey to listen to the other. Above the panel it reads, “Really listen to what we have to say.”
In the final panel, Ekam and Bailey are engaging with and listening to their disabled friends at the parade. Ekam is telling a joke to Ruben in the left corner of the panel, Caden is listening to Ernie infodump about his special interest in the middle, and to the right Alazaye is joined hands with Bailey while looking at each other romantically. Above the group it reads, “You’ll begin to see what we’ve been trying to show you.” Below the group it reads, “WE ARE DISABLED!” Then outside the panel it reads in italics, “NOT DISPOSABLE.”
Thank you for reading. Happy Disability Pride!
#disability#disability pride#image transcribed#disabled rights#mobility devices#disability pride month#face difference#cw self harm scars#burn survivor#mobility scooter#autism#disabled not disposable#wheelchair user#tw self harm scars#plexiform neurofibromas#physically disabled#accessibility#image described#comic#my art#daly art
4K notes
·
View notes
Text
About Neurofibromitosis
Hi. I’m Pepper. I’m a 20-something and I have a genetic disorder called Nf1, or Neurofibromitosis type 1. What the hell is neurofibromitosis? I’ll tell you! There are 3 different types, but I will be covering Nf1 only.
So, cells naturally like to divide and replicate, right? That’s what they do. 6th grade biology 101. But obviously, cells dividing and replicating everywhere unmitigated would be a bad thing, because that’s how you get tumors, cancers, etc.
So. Your body has a gene, called the Nf1 gene, that produces a chemical called neurofibromin. Neurofibromin is a tumor-inhibitor. So your body basically has a natural gene that says “no tumors”.
My body does’t have that gene! So my body says “yes tumors”!!! Which means that my body spontaneously grows thousands of tiny tumors wherever and whenever it wants. For this reason I joke that Nf1 is Yes Tumors Disease.
Some of these tumors are about the size of a pea or a pinhead and some are only a little bigger than this period . They might resemble a skin tag or a pimple. Those are called cutaneous neurofibromas, and they grow anywhere on the surface of the skin. They do not go away once they’re there, and there aren’t a lot of options to have them removed. But some of them are more of what you might think of as a traditional tumor - fleshy, large masses that grow and become deeply enmeshed in the nerves, and can become cancerous. These are called plexiform tumors and they can grow anywhere, including the face. I have three of them; I had surgeries to remove two of them that were not successful, and the third isn’t at the size or rate of growth where I’d be considering surgery yet, especially given that it’s on my neck and it would be a complicated surgery. Brain tumors are common with Nf1 too; I myself have a stable brain tumor, an optic glioma, so I need to get annual or biannual MRIs for... the rest of my life, essentially, to make sure that tumor doesn’t grow and new ones don’t appear.
Tumor growth is related to hormones, and one may go through periods of their life where no tumors grow, followed by periods of their life where tumors grow extremely frequently. I didn’t get a single neurofibroma until I was 22, but now that I’m in my mid-20s I have to check my body nearly daily to keep track of when and where new ones are showing up.
Nf1 affects every organ and system in the body and comes with a host of other symptoms, including bone deformities, learning disabilities, and problems with vision. It is the most common genetic disorder in humans, affecting about 1 in 2500 people. It’s autosomnal dominant, meaning a patient with Nf1 has a 50% chance of passing the condition onto their child, if their partner does not have Nf1. If their partner also has Nf1, it’s a 100% chance. For this reason, I have made the personal decision not to carry a child.
One of the main ways that Nf1 affects me (other than the tumors, duh, which absolutely suck) is scoliosis. I developed severe scoliosis at the age of 6 and needed to have back surgery at Johns Hopkins by the age of 8, to fuse my spine with metal rods and screws so it wouldn’t continue to curve. I then had surgery to fuse additional vertebrae at age 12, and a surgery at 22 to replace hardware that had cracked. I’m fused T2-T12, all but one of my thoracic vertabrae, so half of my spine does not bend. I physically cannot slouch. I set off metal detectors everywhere and I always get patted down by TSA. I have ramrod straight posture with no effort put in, which is sometimes a plus! Spinal fusions are common in Nf1 patients and the more vertebrae are fused, the less mobility you have. I am lucky that I am still able to twist and bend, though I can’t touch my toes and I’m supposed to squat instead of bending over.
Here’s a Johns Hopkins page where you can learn more :) Though please ask me any questions if you’re curious! I love getting to talk to people about my disorder and I don’t find questions offensive. Nf1 is a fascinating condition. And if you have nf1, please reach out to me! I’ve never had a friend with my disorder and it’s an unimaginably lonely feeling
32 notes
·
View notes
Text
Lupine Publishers | Micro-Environmental Systems and Endothelial Cells in Cooperative Tumorigenesis Account for Potential Malignant Transformation in Neurofibromatosis Type 1 Patients
Open Access Journal of Oncology and Medicine (OAJOM)

Lupine Publishers Open Access Journal of Oncology and Medicine (OAJOM)
Abstract
Overall tumorigenesis in neurofibromatosis type 1 patients constitutes a series of specific targeting events with a central role enacted by proliferation of fibroblasts and endothelial cells in overproduction of growth factors and cytokines such as transforming growth factor-beta and CXCL12 cytokine. The plexiform neurofibroma well-illustrates dimensions of such cooperative participation within operative fields of the initial Schwann cell proliferation leading in a significant number of patients to malignant transformation of the peripheral nerve sheath tumors. Inclusive directions in operative targeting of Schwann cells or astrocytes are staged performance in the transformation of hyperproliferative induction and constitute further evolutionarily defined incorporation of such systems as endothelial cells. Hyperproliferative cell subsets are initial and also consequential target formulation of potential malignant states as induced in malignant peripheral nerve sheath tumors.
Introduction
Neurofibromatosis type 1 (NF1) is a neurogenetic disorder and involves both heterozygous and homozygous absence/reduction of neurofibromin that acts normally as a tumor suppressor. There is a need to assess predisposing genetic factors and loss of heterozygosity causing emergence of aggressive neoplasms in patients with NF1 [1]. The two hit hypothesis helps account for the emergence of Schwann cell-based proliferations and for neurofibromas and plexiform neurofibromas. Gherkin may act on tumorigenesis of cutaneous neurofibromas via growth hormone secretagogue receptor [2]. It is important to consider the neurofibroma that is based on micro-environmental potentiation of tumor generation in patients that develop malignant nerve sheath tumors and astrocytomas in patients with NF1 +/- genotype; this occurs in a manner that involves growth factor overactivity and mast cell and endothelial overactivity within a milieu that dysfunctionally stimulates tumorigenesis. Reactive oxygen species overproduction lead to epithelial-mesenchymal transit in patients with neurofibromin deficiency and plays a crucial role in NF1 tumor growth [3]. RAS activation alone is not sufficient for malignant transformation of peripheral nerve sheath tumors; signal transduction may potentially help identify therapies for this neoplasm type [4].
Neurofibromin
The dynamics of neurofibromin as a cytoplasmic protein involve the regulation of K-Ras, and the PI3K/Akt pathways; absence of neurofibromin leads to overactivation of these pathways in various ways in inducing tumorigenesis in such lesions as optic tract pilocytic astrocytomas, brain stem astrocytomas and also other CNS astrocytomas in terms of progression of these lesions. The cell of origin determines the temporal course of neurofibromatosis-1 low-grade glioma formation [5]. The micro-environment of plexiform neurofibromas of peripheral nerves and of nerve plexi include a 10% risk of malignant change with subsequent aggressive clinical behavior in the affected patients. Over expression of cellular retinoid acid binding protein 2 is reported in several cancer types, including malignant peripheral nerve sheath tumors (MPNSTs) [6].
Related Tumor Predispositions
The neurofibromin insufficiency status in Schwann cells and fibroblasts allows for enhanced participation of immune system component cells such as microglia as evidenced in optic pathway low-grade astrocytomas. Telomere erosion is described in many tumor types and may potentially drive genomic instability and clonal progression in NF1-associated MPNSTs [7]. Tumor dimensions include proliferation of astrocytic cells in optic pathways, and of various subtypes of stromal cells such as fibroblasts and mast cells in the peripheral nervous system. It is significant to consider particularly the micro-environmental active participation in the genesis of the most common tumor type in Neurofibromatosis type 1 patient, that is the neurofibroma, which invokes proliferation of fibroblasts and endothelial cells. The congenital plexiform neurofibroma is in fact a hypervascular lesion that transgresses tissue margins and induces a significant risk for malignant transformation. NF1 loss is the primary driver of tumorigenesis in neurofibromatosis type 1-related plexiform neurofibroma [8]. It is further to such considerations that important cooperative intervention in malignant transformation of plexiform neurofibromas invokes multi-type cells in inducing proliferation of an integral Schwann cell-fibroblastic twin population in enhancing potential malignant transformation of the peripheral nerve sheath. A therapeutic window for neuroprotective intervention exists as detected by optical coherence tomography in mice with optic glioma, and particularly as an accurate biomarker of retinal ganglion cell apoptosis [9]. The heterozygous absence of one neurofibromin allele in mice results in plexiform neurofibromas and low-grade optic pathway astrocytomas. Mast cells appear to play a causal role in neurofibroma formation and also in microglia in optic pathway glioma evolution [10]. Such implications of the micro-enviromental factors includes a distinctive cooperative participation that carries implications for significant enhancement of cell proliferation and of such cytokines such as transforming growth factor and CXCL12 in formulating malignant transformation in such tumors. The methylemetetrahydrofolate reductase 1298 and 677 gene polymorphisms are related to optic glioma and hamartoma risk in NF1 patients through effects on DNA synthesis and methylation [11].
Convergent Targeting
The related tuberous sclerosis complex is analogous to neurofibromatosis type 1 as a neurogenetic disorder associated with increased risk for astrocytomas in the form of subependymal giant cell astrocytomas. A convergent targeting of systems of cell proliferation include in particular cyclic AMP and Ras in a manner that includes dimensions of micro-environmental conditioning. Mutations of the NF 1 gene are frequent in many cancer types in patients without NF1 and this is suggestive of a more general role for the NF1 gene in oncogenesis. In melanoma NF1 mutations potentially drive tumorigensis and promote drug resistance [12]. Inclusive dynamics allow for permissive tumorigenesis in a manner that includes the incorporation of malignant transformation within confines of a Schwann cell-fibroblast-endothelial cell system in the case of malignant peripheral nerve sheath tumors. Astrocytes and microglia are analogous counterparts in the induction of CNS astrocytomas. Such considerations are inclusive phenomena of multi-component induction of potential malignancy that recharacterizes conditioning of the micro-environment of proliferative states preceding tumorigenesis. Interaction between neoplastic Schwann cells and their surrounding neural microenvironment has important implications for early cellular events promoting tumorigenesis in neurofibroma development [13].
Performance Dynamics
Performance dynamics of tumors in neurofibromatosis type 1 may potentially modify the biologic significance of a two-hit hypothesis in a manner that implicates micro-environmental conditioning of the resultant cell hyperplasias and proliferations in such lesions as peripheral nerve sheath tumors and astrocytomas. NF1 provides unique vantage points to examine co-contributions of molecular, cellular, and tissue processes in tumor biology [14]. Such proposed dimensions invoke in particular an over-activation in production and action of growth factors that provoke selective malignant transformation of hyper-proliferative lesions composed of Schwann cells and astrocytes in the peripheral and central nervous systems respectively. Plasma soluble levels of transforming growth factor-beta and interleukin-6 are increased in NF1 patients and a shift towards an anti0inflammatory profile has been reported in cells expressing cytokines [15].
Hyperproliferation
The hyperproliferative states affecting Schwann cells and astrocytes invoke also fibroblast and microglial cell proliferations in a manner transforming tumorigenesis. Such facilitation to tumorigenesis invokes dimensions of transformation as well seen in plexiform neurofibromas that may undergo malignant transformation in a significant number of affected individuals. Such considerations are selective targeting of specific cell subpopulations in a manner that allows permissive transformation. Insertional mutagenesis identifies a STAT3/Arid1b/beta-catenin pathway that drives neurofibroma initiation in the context of Nf1 loss [16]. Mast cells and fibroblasts may potentially incorporate endothelial cells that may participate as central dysregulatory dimensions in plexiform neurofibroma tumorigenesis. The provocations for malignant transformation further cooperate in systems of derivative consequence as hypervascular lesions that subsequently lead to potential malignant cells in individual patients. Cross species comparative oncogenomic may identify driver mutations in mouse cancer models and allow validation in human tumors [17].
Concluding Remarks
Propositional implications in tumorigenesis include the multi-component participation of Schwann cells on the one hand and of fibroblasts, mast cells, endothelial cells and also of microglia in an inductive process that includes specific pathways of malignant transformation. Endothelial cell proliferation is related to substantial participation in modes related to key-events of increased proliferation of Schwann cells and astrocytes in initial stages of lesion infliction. Inclusive phenomena have thus become systems of consequence in affecting such specific cell proliferative states. Such events occur within the added dimensions of directed targeting of multiple-agent micro environmental modeling of the initial proliferation of the Schwann cells or astrocytes. A pivotal series of roles played by fibroblasts, endothelial cells, mast cells and of microglia and astrocytes appears a dynamic milieu within added consequences of malignant transformation of both Schwann cells and astrocytes that progress as cooperative systems of tumorigenesis.
For more Lupine Publishers Open Access Journals Please visit our website: h
http://lupinepublishers.us/
For more Journal of Oncology and Medicine Impact Factor articles Please Click Here:
https://lupinepublishers.com/cancer-journal/index.php
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
6 notes
·
View notes
Video
instagram
✅Follow ➡️ @my_name_is_dentist Plexiform Neurofibroma : Complex Reconstructive Craniofacial Surgery being planned next month. History : 2 prior surgeries www.facesurgeon.in www.drsunilrichardson.com +91943182860 _______________________________ FOLLOW : 📷 Instagram/dentistryzone 👤 Facebook/dentistry.zone 💎 Twitter/dentistryzone1 👻 Dentistryzone ▶️ YouTube/DentistryZone ⚫️ Tumblr/dentistryzone1 _______________________________ Tag your friends 👇 #dentistryzone #neurofibromatosis #neurofibromatose #neuroma #face #craniofacial #facialnerve #omfs #plasticsurgery #plasticsurgeon #surgery #surgeon #cirugia #medical #medic #medico #medicalstudent #medicalstudents #medstudent #medstundets #dental #dentalstudents #reconstructivesurgery #dentalstudent #medicine #drsunilrichardson #muscat #oman #kerala #tamilandu https://www.instagram.com/p/Btb_KT1IftI/?utm_source=ig_tumblr_share&igshid=1ehsnsv8haalu
#dentistryzone#neurofibromatosis#neurofibromatose#neuroma#face#craniofacial#facialnerve#omfs#plasticsurgery#plasticsurgeon#surgery#surgeon#cirugia#medical#medic#medico#medicalstudent#medicalstudents#medstudent#medstundets#dental#dentalstudents#reconstructivesurgery#dentalstudent#medicine#drsunilrichardson#muscat#oman#kerala#tamilandu
2 notes
·
View notes
Text
Selumetinib
In this article, we will discuss Selumetinib (Dosage Overview). So, let’s get started.
IndicationsSelumetinib is indicated for the treatment of pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN).DosageRecommended DosageThe recommended dosage of Selumetinib is 25 mg/m² orally twice daily (approximately…
View On WordPress
0 notes
Text
I've been sad about my body recently. I have a large Plexiform Neurofibroma on my chest and it's heavily disfigured me. I try and ignore it but idk it's just making me self conscious latley. I bind my chest anyway when I go out so that kinda hides it. However I still see it and feel it other times. It makes me feel ugly. Living with Nf1 is tough.
1 note
·
View note
Text
Best Neurologist in Pitampura - What is Neurofibromatosis Type 1
Dr Shailesh Jain - Neurofibromatosis type 1 is characterized by changes in skin color and also the development of tumors along the veins within the skin, brain, and other parts of the body. The signs and symptoms of this condition are widely different among affected people.
Neurofibromatosis type 1 also called as Recklinghausen disease or von Recklinghausen disease or von Recklinghausen’s phakomatosis or von Recklinghausen’s neurofibromatosis or neurofibroma (multiple) or peripheral neurofibromatosis.
At birth or early childhood, affected individuals may have relatively large, benign tumors that consist of bundles of nerves and other tissue (plexiform neurofibromas). Individuals with Neurofibromatosis type 1 may also develop benign nodules on the colored regions of the eyes (Lisch nodules), or tumors in the nerves of the visual pathway (optic pathway gliomas) by Best doctor for brain stroke in Shalimar Bagh.
According to Dr Shailesh Jain, Neurofibromatosis type 1 could also be characterized by unusually large head size (macrocephaly) and comparatively small height. Additional abnormalities may be present, like episodes of uncontrolled electrical activity within the brain (seizures), Learning disabilities, and lack of attention; Speech difficulties; Abnormally increased activity (hyperactivity), And skeletal malfunctions, including progressive curvature of the spine (scoliosis), bending of the lower legs (pseudoarthrosis) and improper development of some bones.
Symptoms of Neurofibromatosis type 1
Flat, brown spots (cafe au lait) on the skin -These harmless spots are common in many folks. Having more than six cafe au lait spots suggests Neurofibromatosis type 1. they're usually present at birth or appear during the primary years of life. After childhood, new spots stop appearing.
Freckling in the armpits or groin area - Freckling usually appears by ages 3 to 5 Freckles are smaller than cafe-au-lait spots and tend to occur in clusters in skin folds.
Tiny bumps on the eye's iris (lynch nodules) - These harmless nodules aren’t easily seen and don't affect vision.
Bone deformity - Abnormal bone development and reduction in bone mineral density can lead to bone deformities such as curved spine (scoliosis) or tilted lower leg.
Tumor on the optic nerve (optic glioma) - These tumors usually appear at the age of 3, rarely in childhood and adolescence, and almost never in adults.
Larger than average head size - Children with Neurofibromatosis type 1 are larger than the average head size due to increased brain volume.
short stature - Children who have Neurofibromatosis type 1 often have lower-than-average height.
Causes of Neurofibromatosis type 1
The Neurofibromatosis type 1(NF1) gene on chromosome 17 produces a protein called neurofibromin that regulates the growth of your cells. Mutation of this gene causes neurofibromin loss and uncontrolled cell growth.
NF1 complications
Neurological problems - Thinking and learning difficulties are the most common neurological problems associated with NF1. Unusual complications include epilepsy and the creation of excess fluid in the brain.
Vision problems - Sometimes a tumor develops on the optic nerve, which can affect vision.
Heart problems - People who have NF1 have an increased risk of hypertension and blood vessel abnormalities may develop.
Trouble breathing - Rarely, plexiform neurofibromas can exert pressure on the airway.
About Dr. Shailesh Jain, Neurosurgeon, Neurologist
Dr. Shailesh Jain is the Best neurologist in pitampura and Shalimarbagh has been performing with excellent results for the last 16 years. He has vast experience in this field. Dr. Shailesh Jain runs his Arihant Neurospin Clinic in Pitampura and Max Superspeciality Hospital, Shalimar Bagh. Dr Shailesh Jain is Principal Consultant Neurosurgery and Neurointervention at Max Hospital Shalimar Bagh and runs his own Arihant Neurospine clinic in Pitampura and Shalimar Bagh.
#Dr shailesh jain AIIMS Neurosurgeon#Best doctor for brain stroke in pitampura#Best neurologist in pitampura#Top Neurosurgeon in pitampura#Best doctor for back pain in pitampura#Best doctor for headache in pitampura#Best doctor for migraine in pitampura#Best doctor for fits in pitampura#Best doctor for brain hemorrhage in pitampura#Best doctor for depression in pitampura#Best doctor for Stroke Intervention in pitampura#best doctor for head and spine injury in pitampura#Best doctor for epilepsy in pitampura
0 notes
Text
CHMP backs Roche’s Enspryng for rare nerve disease NMOSD

Roche’s Enspryng has been recommended for approval in the EU for treating neuromyelitis optica spectrum disorder (NMOSD), extending the treatment options for people with the life-threatening rare disease.
The CHMP has backed the drug in patients aged 12 or more with NMOSD that tests positive for aquaporin-4 (AQP4) antibodies, a biomarker seen in around 80% of NMOSD cases. Enspryng was approved for the same indication in the US last year.
NMOSD is a lifelong and debilitating autoimmune disorder of the central nervous system, often misdiagnosed as multiple sclerosis, that causes damages to the optic nerves and spinal cord, leading to blindness, muscle weakness and paralysis. It affects roughly one to two people per 100,000 in the EU.
IL-6 inhibitor Enspryng isn’t the first drug to treat NMOSD to get a green light from the CHMP, as Alexion’s complement C5 blocker Soliris (eculizumab) has been approved for the disease in the EU since 2019. However, according to Roche is the only therapy that can be used in adolescents over 12 as well as adults. Alexion has phase 3 trials on the go in children and adolescents with NMOSD.
The Swiss pharma group also says its product is the only subcutaneous treatment option for NMOSD that can be administered at home every four weeks, after an initial three injections given two weeks apart. Soliris is given by intravenous infusion every two weeks, after an initial period of weekly dosing.
Another NMOSD therapy, Horizon Pharma/Viela Bio’s Uplizna (inebilizumab), has been approved in the US but isn’t yet available in Europe. It too is administered by intravenous infusion.
Meanwhile, Alexion’s follow-up to Soliris – Ultomiris (ravulizumab) – is also in late-stage clinical development as an infusion for NMOSD, although the drugmaker is also working on a subcutaneous formulation of the antibody. Also back in early-stage development is an oral therapy based on cladribine developed by Swiss biotech Chord Therapeutics.
Enspryng’s approval based on two clinical trials which showed that the rate of relapse with Enspryng was a quarter of that in a placebo group, both given on top of other immunosuppressive drugs.
Roche’s drug was one of several new medicines recommended for approval at the CHMP’s April meeting.
Regeneron’s first-in-class ANGPTL3 inhibitor Evkeeza (evinacumab) got a positive opinion for homozygous familial hypercholesterolemia (HoFH), a genetic form of high cholesterol, in patients aged 12 and over, and the committee also backed AstraZeneca’s MEK 1/2 inhibitor Koselugo (selumetinib) for children with neurofibromatosis type 1 (NF1) plexiform neurofibromas (PN).
Leo Pharma’s IL-13 inhibitor Adtralza (tralokinumab) got the go-ahead as a treatment for adults with moderate to severe atopic dermatitis, as did Celgene’s Onureg (azacitidine) as a maintenance therapy for patients with acute myeloid leukaemia (AML).
Other highlights included an extension of the Indications for AbbVie’s Venclyxto (venetoclax) as a first-line treatment – in combination with azacytidine or decitabine – in newly-diagnosed AML patients ineligible for intensive chemotherapy.
AZ also got the go-ahead for Tagrisso (osimertinib) for the adjuvant treatment after surgery of patients with early-stage EGFR-positive non-small cell lung cancer (NSCLC).
The post CHMP backs Roche’s Enspryng for rare nerve disease NMOSD appeared first on .
from https://pharmaphorum.com/news/chmp-backs-roches-enspryng-for-rare-nerve-disease-nmosd/
0 notes
Text
NEUROFIBROMATOSIS TREATMENT DRUGS MARKET ANALYSIS
Neurofibromatosis Treatment Drugs Market, by Disease Type (Neurofibromatosis 1 (NF1) and Neurofibromatosis 2 (NF2), and Schwannomatosis), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Press Release : Neurofibromatosis Treatment Drugs Market
Request Sample Download Pdf
There are three types of Neurofibromatosis; Neurofibromatosis type 1, Neurofibromatosis type 2, and Schwannomatosis. Neurofibromatosis type (NF) 1 is a common monogenic disorder of neurocutaneous tissue growth that arises secondary to mutations in the tumor suppressor gene NF1. It features an autosomal dominant pattern of inheritance. Individuals with NF1 typically have an increased predisposition to a variety of benign and malignant tumors and develop neurofibromas, and axillary and inguinal freckling. Neurofibromatosis 2 (NF2) is less common than NF1. Common signs and symptoms of NF 2 include benign, slow-growing tumors affecting the cranial, spinal, and peripheral nerves, as well as the meninges. KOSELUGO- selumetinib is the only drug available for the treatment of Neurofibromatosis Type-1. Moreover, some drugs such as bevacizumab are also used as off-label for the treatment of Neurofibromatosis
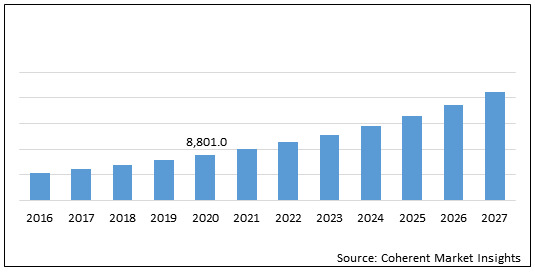
The global neurofibromatosis treatment drugs market is estimated to be valued at US$ 8,801.0 million in 2020 and is expected to exhibit a CAGR of 13.40% during the forecast period (2020-2027).
Figure 1. Global Neurofibromatosis Treatment Drugs Market Value (US$ Mn)
Global Neurofibromatosis Treatment Drugs Market: Drivers
Increasing focus of major players on research and development of novel therapies for treating neurofibromatosis. Moreover, clinical studies are well supported by regulatory authorities which is expected to drive the growth of the global neurofibromatosis treatment drugs market. For instance, in October 2019, SpringWorks Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing life-changing medicines for patients with severe rare diseases and cancer, initiated the Phase 2b ReNeu clinical trial to evaluate the mirdametinib (formerly PD-0325901), an oral, small molecule designed to inhibit MEK1 and MEK2, in children and adult patients with neurofibromatosis type 1 (NF1)-associated plexiform neurofibromas (NF1-PN). In July 2019, the European Commission granted Orphan Drug Designation for SpringWorks Therapeutics’ mirdametinib (formerly PD-0325901), an oral, small molecule inhibitor of MEK1 and MEK2, for the treatment of neurofibromatosis type 1 (NF1).
Global Neurofibromatosis Treatment Drugs Market – Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID 19) pandemic and lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Private healthcare is one such sector, which has been majorly impacted by the COVID-19 pandemic.
The lockdown in various countries due to the pandemic has placed an economic burden on the private healthcare sector.
Moreover, the coronavirus pandemic has hampered the development, production, and supply of healthcare products (medical devices and medicinal products) and affected growth of healthcare businesses of various companies across the globe.
The lockdowns have led to closure of industrial establishments, except manufacturing of essential commodities, and disrupted supply chains.
The COVID-19 pandemic has affected the economy in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; and 3) by its financial impact on firms and financial markets
As a result, the impact of coronavirus (COVID-19) pandemic is also expected to limit growth of the global neurofibromatosis treatment drugs market during the forecast period.
Figure 2. Global Neurofibromatosis Treatment Drugs Market Value (US$ Mn), by Region, 2020
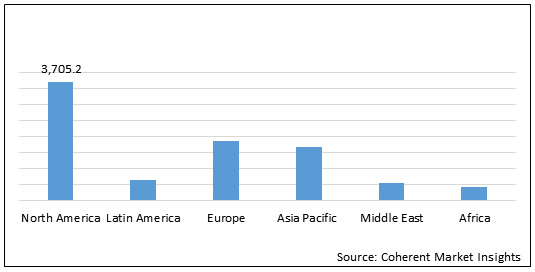
North America neurofibromatosis treatment drugs market is expected to show significant growth during the forecast period owing to presence of key players and research institutes who are involved in clinical trial programmes. For instance, on November 28, 2017, the University of Alabama at Birmingham initiated phase 2 clinical study on Binimetinib in children and adults with nf1 plexiform neurofibromas. On February 18, 2020, the University of Alabama at Birmingham initiated phase 2 clinical trial of crizotinib for children and adults with neurofibromatosis type 2 and progressive vestibular.
Key Players
Major Player operating in the global neurofibromatosis treatment drugs market is AstraZeneca Plc.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
0 notes
Text
Neurofibromatosis Treatment Drugs Market Forecast - 2027
To Gain More Insights into the Neurofibromatosis Treatment Drugs Market, Browse Summary of the Research Report –
Neurofibromatosis is a genetic disorder of the nervous system. It mainly affects growth of nerve cells. It is characterized by the growth of tumors on nerves. Neurofibromatosis is considered as a hereditary disease which mainly occurs in children or it can happen due to mutation (change) of genes. Usually the Neurofibromatosis tumors are benign, but sometimes they can become cancerous. There are three types of neurofibromatosis: Type 1 (NF1), Type 2 (NF2), and Schwannomatosis. All three types show varied symptoms.
https://www.coherentmarketinsights.com/market-insight/neurofibromatosis-treatment-drugs-market-4188
There are three types of Neurofibromatosis; Neurofibromatosis type 1, Neurofibromatosis type 2, and Schwannomatosis. Neurofibromatosis type (NF) 1 is a common monogenic disorder of neurocutaneous tissue growth that arises secondary to mutations in the tumor suppressor gene NF1. It features an autosomal dominant pattern of inheritance. Individuals with NF1 typically have an increased predisposition to a variety of benign and malignant tumors and develop neurofibromas, and axillary and inguinal freckling. Neurofibromatosis 2 (NF2) is less common than NF1. Common signs and symptoms of NF 2 include benign, slow-growing tumors affecting the cranial, spinal, and peripheral nerves, as well as the meninges. KOSELUGO- selumetinib is the only drug available for the treatment of Neurofibromatosis Type-1. Moreover, some drugs such as bevacizumab are also used as off-label for the treatment of Neurofibromatosis
Increasing focus of major players on research and development of novel therapies for treating neurofibromatosis. Moreover, clinical studies are well supported by regulatory authorities which is expected to drive the growth of the global neurofibromatosis treatment drugs market. For instance, in October 2019, SpringWorks Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing life-changing medicines for patients with severe rare diseases and cancer, initiated the Phase 2b ReNeu clinical trial to evaluate the mirdametinib (formerly PD-0325901), an oral, small molecule designed to inhibit MEK1 and MEK2, in children and adult patients with neurofibromatosis type 1 (NF1)-associated plexiform neurofibromas (NF1-PN). In July 2019, the European Commission granted Orphan Drug Designation for SpringWorks Therapeutics’ mirdametinib (formerly PD-0325901), an oral, small molecule inhibitor of MEK1 and MEK2, for the treatment of neurofibromatosis type 1 (NF1).
“We Do Offer Sample of Neurofibromatosis Treatment Drugs Market Report. Kindly go through the follow information in order to access sample copy.”
https://www.coherentmarketinsights.com/insight/request-sample/4188
Table of Contents
Research Objectives and Assumptions
Research Objectives
Assumptions
Abbreviations
Market Purview
Report Description
Market Definition and Scope
Executive Summary
Market Snippet, By Drug Type
Market Snippet, By Distribution Channel
Market Snippet, By Region
Coherent Opportunity Map (COM)
Market Dynamics, Regulations, and Trends Analysis
Market Dynamics
Drivers
Restraints
Market Opportunities
Impact Analysis
Market Trends
Regulatory Scenario
Reimbursement Scenario
Epidemiology
PEST Analysis
Recent Product Launch
Merger and Acquisition Scenario
Top players in the market
Major Player operating in the global neurofibromatosis treatment drugs market is AstraZeneca Plc.
Research methodology adopted by Coherent Market Insights
Coherent Market Insights followsa comprehensive research methodology focused on providing the most precise market analysis. The company leverages a data triangulation model which helps company to gauge the market dynamics and provide accurate estimates. Key components of the research methodologies followed for all our market reports include:
Primary Research (Trade Surveys and Experts Interviews)
Desk Research
Proprietor Data Analytics Model
In addition to this, Coherent Market Insights has access to a wide range of the regional and global reputed paid data bases, which helps the company to figure out the regional and global market trends and dynamics. The company analyses the industry from the 360 Degree Perspective i.e. from the Supply Side and Demand Side which enables us to provide granular details of the entire ecosystem for each study. Finally, a Top-Down approach and Bottom-Up approach is followed to arrive at ultimate research findings.
Request A Sample Copy Neurofibromatosis Treatment Drugs Market Report Click here:
https://www.coherentmarketinsights.com/insight/request-sample/4188
Get PDF Research Report Brochure @
https://www.coherentmarketinsights.com/insight/request-pdf/4188
Buy Now this Premium Report to Grow your Business @
https://www.coherentmarketinsights.com/insight/buy-now/4188
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
Contact Us:
Name: Mr. Raj Shah
Email: [email protected]
Phone: US +12067016702
Country: United States
Visit our Blog: https://hospitalhealthcareblog.wordpress.com/
0 notes
Text
Lupine Publishers | Micro-Environmental Systems and Endothelial Cells in Cooperative Tumorigenesis Account for Potential Malignant Transformation in Neurofibromatosis Type 1 Patients

Lupine Publishers | Open Access Journal of Oncology and Medicine (OAJOM)
Abstract
Overall tumorigenesis in neurofibromatosis type 1 patients constitutes a series of specific targeting events with a central role enacted by proliferation of fibroblasts and endothelial cells in overproduction of growth factors and cytokines such as transforming growth factor-beta and CXCL12 cytokine. The plexiform neurofibroma well-illustrates dimensions of such cooperative participation within operative fields of the initial Schwann cell proliferation leading in a significant number of patients to malignant transformation of the peripheral nerve sheath tumors. Inclusive directions in operative targeting of Schwann cells or astrocytes are staged performance in the transformation of hyperproliferative induction and constitute further evolutionarily defined incorporation of such systems as endothelial cells. Hyperproliferative cell subsets are initial and also consequential target formulation of potential malignant states as induced in malignant peripheral nerve sheath tumors.
Introduction
Neurofibromatosis type 1 (NF1) is a neurogenetic disorder and involves both heterozygous and homozygous absence/reduction of neurofibromin that acts normally as a tumor suppressor. There is a need to assess predisposing genetic factors and loss of heterozygosity causing emergence of aggressive neoplasms in patients with NF1 [1]. The two hit hypothesis helps account for the emergence of Schwann cell-based proliferations and for neurofibromas and plexiform neurofibromas. Gherkin may act on tumorigenesis of cutaneous neurofibromas via growth hormone secretagogue receptor [2]. It is important to consider the neurofibroma that is based on micro-environmental potentiation of tumor generation in patients that develop malignant nerve sheath tumors and astrocytomas in patients with NF1 +/- genotype; this occurs in a manner that involves growth factor overactivity and mast cell and endothelial overactivity within a milieu that dysfunctionally stimulates tumorigenesis. Reactive oxygen species overproduction lead to epithelial-mesenchymal transit in patients with neurofibromin deficiency and plays a crucial role in NF1 tumor growth [3]. RAS activation alone is not sufficient for malignant transformation of peripheral nerve sheath tumors; signal transduction may potentially help identify therapies for this neoplasm type [4].
Neurofibromin
The dynamics of neurofibromin as a cytoplasmic protein involve the regulation of K-Ras, and the PI3K/Akt pathways; absence of neurofibromin leads to overactivation of these pathways in various ways in inducing tumorigenesis in such lesions as optic tract pilocytic astrocytomas, brain stem astrocytomas and also other CNS astrocytomas in terms of progression of these lesions. The cell of origin determines the temporal course of neurofibromatosis-1 low-grade glioma formation [5]. The micro-environment of plexiform neurofibromas of peripheral nerves and of nerve plexi include a 10% risk of malignant change with subsequent aggressive clinical behavior in the affected patients. Over expression of cellular retinoid acid binding protein 2 is reported in several cancer types, including malignant peripheral nerve sheath tumors (MPNSTs) [6].
Related Tumor Predispositions
The neurofibromin insufficiency status in Schwann cells and fibroblasts allows for enhanced participation of immune system component cells such as microglia as evidenced in optic pathway low-grade astrocytomas. Telomere erosion is described in many tumor types and may potentially drive genomic instability and clonal progression in NF1-associated MPNSTs [7]. Tumor dimensions include proliferation of astrocytic cells in optic pathways, and of various subtypes of stromal cells such as fibroblasts and mast cells in the peripheral nervous system. It is significant to consider particularly the micro-environmental active participation in the genesis of the most common tumor type in Neurofibromatosis type 1 patient, that is the neurofibroma, which invokes proliferation of fibroblasts and endothelial cells. The congenital plexiform neurofibroma is in fact a hypervascular lesion that transgresses tissue margins and induces a significant risk for malignant transformation. NF1 loss is the primary driver of tumorigenesis in neurofibromatosis type 1-related plexiform neurofibroma [8]. It is further to such considerations that important cooperative intervention in malignant transformation of plexiform neurofibromas invokes multi-type cells in inducing proliferation of an integral Schwann cell-fibroblastic twin population in enhancing potential malignant transformation of the peripheral nerve sheath. A therapeutic window for neuroprotective intervention exists as detected by optical coherence tomography in mice with optic glioma, and particularly as an accurate biomarker of retinal ganglion cell apoptosis [9]. The heterozygous absence of one neurofibromin allele in mice results in plexiform neurofibromas and low-grade optic pathway astrocytomas. Mast cells appear to play a causal role in neurofibroma formation and also in microglia in optic pathway glioma evolution [10]. Such implications of the micro-enviromental factors includes a distinctive cooperative participation that carries implications for significant enhancement of cell proliferation and of such cytokines such as transforming growth factor and CXCL12 in formulating malignant transformation in such tumors. The methylemetetrahydrofolate reductase 1298 and 677 gene polymorphisms are related to optic glioma and hamartoma risk in NF1 patients through effects on DNA synthesis and methylation [11].
Convergent Targeting
The related tuberous sclerosis complex is analogous to neurofibromatosis type 1 as a neurogenetic disorder associated with increased risk for astrocytomas in the form of subependymal giant cell astrocytomas. A convergent targeting of systems of cell proliferation include in particular cyclic AMP and Ras in a manner that includes dimensions of micro-environmental conditioning. Mutations of the NF 1 gene are frequent in many cancer types in patients without NF1 and this is suggestive of a more general role for the NF1 gene in oncogenesis. In melanoma NF1 mutations potentially drive tumorigensis and promote drug resistance [12]. Inclusive dynamics allow for permissive tumorigenesis in a manner that includes the incorporation of malignant transformation within confines of a Schwann cell-fibroblast-endothelial cell system in the case of malignant peripheral nerve sheath tumors. Astrocytes and microglia are analogous counterparts in the induction of CNS astrocytomas. Such considerations are inclusive phenomena of multi-component induction of potential malignancy that recharacterizes conditioning of the micro-environment of proliferative states preceding tumorigenesis. Interaction between neoplastic Schwann cells and their surrounding neural microenvironment has important implications for early cellular events promoting tumorigenesis in neurofibroma development [13].
Performance Dynamics
Performance dynamics of tumors in neurofibromatosis type 1 may potentially modify the biologic significance of a two-hit hypothesis in a manner that implicates micro-environmental conditioning of the resultant cell hyperplasias and proliferations in such lesions as peripheral nerve sheath tumors and astrocytomas. NF1 provides unique vantage points to examine co-contributions of molecular, cellular, and tissue processes in tumor biology [14]. Such proposed dimensions invoke in particular an over-activation in production and action of growth factors that provoke selective malignant transformation of hyper-proliferative lesions composed of Schwann cells and astrocytes in the peripheral and central nervous systems respectively. Plasma soluble levels of transforming growth factor-beta and interleukin-6 are increased in NF1 patients and a shift towards an anti0inflammatory profile has been reported in cells expressing cytokines [15].
Hyperproliferation
The hyperproliferative states affecting Schwann cells and astrocytes invoke also fibroblast and microglial cell proliferations in a manner transforming tumorigenesis. Such facilitation to tumorigenesis invokes dimensions of transformation as well seen in plexiform neurofibromas that may undergo malignant transformation in a significant number of affected individuals. Such considerations are selective targeting of specific cell subpopulations in a manner that allows permissive transformation. Insertional mutagenesis identifies a STAT3/Arid1b/beta-catenin pathway that drives neurofibroma initiation in the context of Nf1 loss [16]. Mast cells and fibroblasts may potentially incorporate endothelial cells that may participate as central dysregulatory dimensions in plexiform neurofibroma tumorigenesis. The provocations for malignant transformation further cooperate in systems of derivative consequence as hypervascular lesions that subsequently lead to potential malignant cells in individual patients. Cross species comparative oncogenomic may identify driver mutations in mouse cancer models and allow validation in human tumors [17].
Concluding Remarks
Propositional implications in tumorigenesis include the multi-component participation of Schwann cells on the one hand and of fibroblasts, mast cells, endothelial cells and also of microglia in an inductive process that includes specific pathways of malignant transformation. Endothelial cell proliferation is related to substantial participation in modes related to key-events of increased proliferation of Schwann cells and astrocytes in initial stages of lesion infliction. Inclusive phenomena have thus become systems of consequence in affecting such specific cell proliferative states. Such events occur within the added dimensions of directed targeting of multiple-agent micro environmental modeling of the initial proliferation of the Schwann cells or astrocytes. A pivotal series of roles played by fibroblasts, endothelial cells, mast cells and of microglia and astrocytes appears a dynamic milieu within added consequences of malignant transformation of both Schwann cells and astrocytes that progress as cooperative systems of tumorigenesis.
For more Lupine Publishers Open Access Journals Please visit our website: h
http://lupinepublishers.us/
For more Journal of Oncology and Medicine Impact Factor articles Please Click Here:
https://lupinepublishers.com/cancer-journal/index.php
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
#lupine publishers#lung cancer#Lupinepublishers#lupine publisher#Cancer#Oncology and Medicine (OAJOM)
0 notes
Text
FDA Approves Koselugo for Pediatric Neurofibromatosis Type 1

TUESDAY, April 14, 2020 — Koselugo (selumetinib) has received the first approval for treatment of neurofibromatosis type 1 (NF1) in children ages 2 years and older, the U.S. Food and Drug Administration announced Friday.
Approved specifically for patients with symptomatic, inoperable plexiform neurofibromas (PNs), Koselugo, a kinase inhibitor, works by blocking a key enzyme to help stop the…
View On WordPress
0 notes
Text
AstraZeneca, Merck cue up FDA verdict on selumetinib next year

After failing to make the grade in multiple cancer types, AstraZeneca and Merck & Co/MSD’s selumetinib could get approval in the US for a rare paediatric disease next year.
The MEK 1/2 inhibitor has been submitted to the FDA for children with neurofibromatosis type 1 (NF1), an incurable genetic condition that affects one in 3,000-4,000 newborns worldwide, and been granted a priority review by the agency.
If approved, selumetinib would be the first drug available to treat NF1 with symptomatic, inoperable plexiform neurofibromas – benign tumours of the peripheral nerves that can occur anywhere in the body, can cause pain, motor dysfunction and disfigurement, and in some cases can become malignant.
The FDA is due to rule on the marketing application for selumetinib as a twice-daily, oral monotherapy for NF1 in the second quarter of 2020.
NF1 is usually characterised by lumps under the skin and pigmentation, with plexiform neurofibromas occurring in around 25% of cases. Some patients also experience other complications such as learning difficulties, visual impairment, twisting and curvature of the spine, high blood pressure, and epilepsy.
Selumetinib was once tipped as a major new cancer prospect for AZ and Merck, but was all-but written off after it failed trials as a single-agent therapy in various solid tumours including thyroid cancer, lung cancer, and uveal melanoma.
It’s been reborn as a potential NF1 treatment thanks to the 50-patient phase 2 SPRINT trial – sponsored by the US National Cancer Institute (NCI) – which found an objective response rate of 66% in children with NF1 and symptomatic, inoperable plexiform neurofibromas. A response in the trial was defined as a 20% of better reduction in tumour volume.
On the strength of those results selumetinib was granted breakthrough status by the FDA in April, having already been awarded orphan drug status in 2018.
Selumetinib also remains in development by AZ and Merck as a component of combination treatment regimens for various cancer types.
The drug is being tested alongside EGFR inhibitor Tagrisso (osimertinib) in non-small cell lung cancer, for example, as well as in combination with PARP inhibitor Lynparza (olaparib) in ovarian cancers and with PD-L1 inhibitor Imfinzi (durvalumab) and experimental CTLA4 inhibitor tremelimumab in NSCLC.
The post AstraZeneca, Merck cue up FDA verdict on selumetinib next year appeared first on .
from https://pharmaphorum.com/news/astrazeneca-merck-cue-up-fda-verdict-on-selumetinib-next-year/
0 notes