#Polymerase Chain Reaction (PCR) Market
Text
Digital Polymerase Chain Reaction (PCR) Market Report, Outlook and Forecast 2024-2032

The Reports and Insights, a leading market research company, has recently releases report titled “Digital Polymerase Chain Reaction (PCR) Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032.” The study provides a detailed analysis of the industry, including the global Digital Polymerase Chain Reaction (PCR) Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Digital Polymerase Chain Reaction (PCR) Market?
The digital polymerase chain reaction (PCR) market size reached US$ 533.3 Million in 2023. Looking forward, Reports and Insights expects the market to reach US$ 1,074.5 Million by 2032, exhibiting a growth rate (CAGR) of 8.1% during 2024-2032.
What are Digital Polymerase Chain Reaction (PCR)?
Digital Polymerase Chain Reaction (PCR) is a molecular biology method that offers highly sensitive quantification and amplification of specific DNA sequences. Unlike conventional PCR, which provides qualitative DNA analysis, digital PCR allows for precise quantification of target DNA molecules in a sample. This technique involves dividing a sample into numerous individual reactions, each containing either a single DNA molecule or none. By counting the number of positive reactions, digital PCR can accurately determine the absolute quantity of the target DNA, making it useful for applications like gene expression analysis, copy number variation analysis, and detecting rare mutations.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/1707
What are the growth prospects and trends in the Digital Polymerase Chain Reaction (PCR) industry?
The digital Polymerase Chain Reaction (PCR) market growth is driven by various factors. The market for digital Polymerase Chain Reaction (PCR) is experiencing significant expansion, primarily due to its rising adoption in molecular diagnostics, research, and forensic applications. Digital PCR's benefits include heightened sensitivity, precision, and the ability to accurately quantify nucleic acids, making it crucial for applications demanding precise DNA quantification like cancer research and infectious disease diagnostics. Moreover, technological advancements such as droplet digital PCR and chip-based systems are driving market growth by enhancing efficiency and scalability. Hence, all these factors contribute to digital Polymerase Chain Reaction (PCR) market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Product Type:
Instruments
Consumables
By Technology:
Droplet Digital PCR (ddPCR)
BEAMing Digital PCR
Others
By Application:
Clinical Diagnostics
Research
Forensic Analysis
Others
By End-Use:
Hospitals and Diagnostic Centers
Research Institutes
Pharmaceutical and Biotechnology Companies
Others
Segmentation By Region:
North America:
United States
Canada
Asia Pacific:
China
India
Japan
South Korea
Australia & New Zealand
Association of Southeast Asian Nations (ASEAN)
Rest of Asia Pacific
Europe:
Germany
The U.K.
France
Spain
Italy
Russia
Poland
BENELUX (Belgium, the Netherlands, Luxembourg)
NORDIC (Norway, Sweden, Finland, Denmark)
Rest of Europe
Latin America:
Brazil
Mexico
Argentina
Rest of Latin America
The Middle East & Africa:
Saudi Arabia
United Arab Emirates
South Africa
Egypt
Israel
Rest of MEA (Middle East & Africa)
Who are the key players operating in the industry?
The report covers the major market players including:
Bio-Rad Laboratories, Inc.
Thermo Fisher Scientific Inc.
QIAGEN N.V.
Fluidigm Corporation
Merck KGaA
Agilent Technologies, Inc.
Illumina, Inc.
Takara Bio Inc.
Becton, Dickinson and Company
Eppendorf AG
BioFire Diagnostics, LLC
Jena Bioscience GmbH
RainDance Technologies (Acquired by Bio-Rad)
Quantabio (Acquired by Agilent Technologies)
Analytik Jena AG
View Full Report: https://www.reportsandinsights.com/report/Digital Polymerase Chain Reaction (PCR)-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd.
1820 Avenue M, Brooklyn, NY, 11230, United States
Contact No: +1-(347)-748-1518
Email: [email protected]
Website: https://www.reportsandinsights.com/
Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/
Follow us on twitter: https://twitter.com/ReportsandInsi1
#Digital Polymerase Chain Reaction (PCR) Market share#Digital Polymerase Chain Reaction (PCR) Market size#Digital Polymerase Chain Reaction (PCR) Market trends
0 notes
Text
The PCR (Polymerase Chain Reaction) Detection Systems market refers to the market for the devices and reagents used to conduct PCR, which is a technique used in molecular biology to amplify DNA sequences.
0 notes
Text
Testing conducted by the Food and Drug Administration on pasteurized commercially purchased milk has found genetic evidence of the H5N1 bird flu virus, the agency confirmed Tuesday. But the testing, done by polymerase chain reaction, or PCR, cannot distinguish between live virus or fragments of viruses that could have been killed by the pasteurization process.
The agency said it has been trying to see if it could grow virus from milk found to contain evidence of H5N1, which is the gold standard test to see if there is viable virus in a product. The lengthy statement the agency released does not explicitly say FDA laboratories were unable to find live virus in the milk samples, but it does state that its belief that commercial, pasteurized milk is safe to consume has not been altered by these findings.
“To date, we have seen nothing that would change our assessment that the commercial milk supply is safe,” the statement said.
The document was long on assurances but short on details of what has been undertaken or found. It does not specify how many commercial samples were taken or in how many markets, nor does it indicate what percentage of the samples were PCR-positive for H5N1. The statement did not indicate if the testing suggested the amounts of viral genetic material in the milk were low or high.
Furthermore, the statement did not reveal if the milk products were purchased in parts of the country where outbreaks have occurred, or in areas where cows haven’t been seen to have been infected.
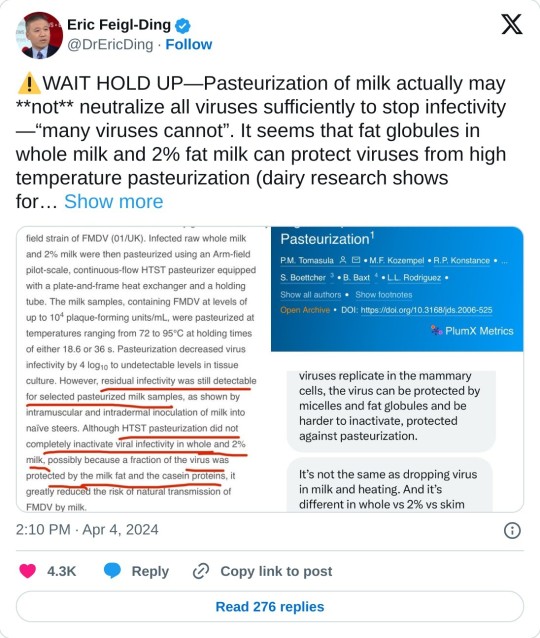
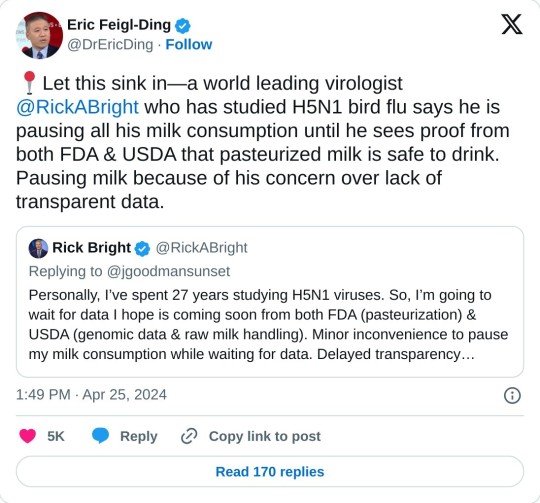
I put this in the tags but I guess it is worth saying here as well: I am not trying to panic anyone, but this definitely seems like something you should know if you are in the US. The milk supply is not definitely unsafe, but they don't know it is safe yet either, so make your own choices based on that.
#public health#bird flu#H5N1#psa#not trying to panic anyone but you should definitely know this if you are in the US#and at the very least avoid raw milk and raw milk products#apologies for the twitter links#AFAIK this is a US-only issue for now
285 notes
·
View notes
Text
Home PCR Tests: A Closer Look at the PCR Test At Home Dubai Option
The COVID-19 pandemic sparked major growth in the development and usage of diagnostic and antibody tests that patients can self-administer from home. Home PCR tests in particular enable private, convenient detection of active coronavirus infections. For those wondering whether accurate PCR Test At Home Dubai kits are available, exploring the leading options provides helpful guidance.

How Do Home PCR Tests for COVID-19 Work?
The PCR (polymerase chain reaction) technique is the gold standard for directly detecting the presence of the COVID-19 virus from respiratory samples. Home PCR test kits allow patients to collect their own nasal or saliva samples and perform the PCR assay without visiting a clinic.
PCR tests work by identifying the specific genetic material of the COVID-19 virus. Users collect a sample, mix it with chemical reagents, and insert the solution into the test kit for analysis. Results are displayed indicating whether viral genetic material was detected based on any color change reaction on the test strips.
Kits include step-by-step instructions to ensure patients perform the easy, quick tests properly using non-invasive nasal swabs or saliva collection. Many provide results within 10-30 minutes.
Here is a video from MedCram Youtube Channel about At Home Rapid COVID 19 Tests and False Positives (Coronavirus Antigen Tests). Watch the video
youtube
Benefits of At-Home PCR Testing
Here are some of the major advantages of having access to accurate home PCR tests for COVID-19:
Convenience: Test from the privacy of your residence without traveling to clinics.
Speed: Get results rapidly within minutes rather than waiting days for lab tests.
Self-Administered: Users can collect their own sample comfortably rather than relying on technicians.
Affordability: Individual kits are very competitively priced.
Detection Reliability: PCR technology directly identifies viral presence with high accuracy.
Ease of Use: Tests have simple, straightforward instructions for patients of all ages.
Infection Verification: Confirms active infections unlike antibody tests.
Having the option to privately, quickly, and accurately test for possible COVID-19 infections at home provides significant peace of mind during the pandemic.
How Reliable Are Home PCR Tests?
Many people reasonably wonder whether DIY home PCR test kits can match the reliability of lab-based PCR tests. The good news is that leading home PCR kits on the market have very high accuracy.
Most kits have published sensitivity and specificity above 90% when compared to lab PCR tests. High quality home tests analyze samples using comparable PCR methodology and match labs in detecting positives and negatives.
Furthermore, unlike Rapid PCR Test At Home kits some vendors offer, full home PCR tests analyze the sample through many amplification cycles to maximize accuracy. With good sampling collection, top home PCR kits offer laboratory-grade results conveniently at home.
Leading Home PCR Test Kit Options
For those exploring PCR Test At Home Dubai choices, here are some of the top-rated home PCR kits to consider:
Cue Health PCR Test: Cue offers an FDA-authorized home PCR test delivering highly accurate results in 20 minutes with nasal swab samples.
Lucira Check It PCR Test: This is a single-use PCR kit with 98% validated accuracy that provides molecular-level detection from nasal samples in 30 minutes or less.
Ellume COVID-19 Home Test: This over-the-counter home kit uses a mid-turbinate nasal sample and provides an amplified PCR digital reading of positive or negative in 15 minutes on a connected analyzer.
Pixel by LabCorp PCR Test: Pixel is a monitored at-home nasal PCR test analyzed through LabCorp with over 98% accuracy returning results within 1-2 days.
Doximity's Covid-19 PCR Test: Doximity partners with qualified labs for monitored video-observed PCR testing with 97%+ accuracy and results in 24 hours.
All these options allow for convenient, accurate at-home COVID-19 testing using PCR with trusted partners. Kits can be purchased online and shipped directly to your home in Dubai.
When Are Home PCR Tests Recommended?
The CDC recommends utilizing home PCR tests in situations such as:
If you have any symptoms of COVID-19. Home testing allows quick confirmation.
After exposure events to quickly check for possible infection.
Before visiting individuals at higher risk for severe illness.
Before travel or group events for added assurance.
For frequent screening in schools or workplaces.
Even fully vaccinated individuals should test if they experience COVID-like symptoms or have a known exposure. Home PCR tests make quick detection fast and easy.
Home PCR Tests Offer Accuracy and Convenience
High quality Home PCR Tests have become an important tool in the fight against COVID by making reliable diagnostic testing accessible outside of clinics. There are excellent PCR Test At Home Dubai options available matching the standards of lab PCR sensitivity and specificity. Home PCR kits allow people to conveniently and confidently check themselves for possible COVID-19 infections from the privacy of home. As the technology continues advancing, home collection PCR will likely take on an increasingly vital role supporting public health and safety.
2 notes
·
View notes
Text
Latin America Molecular Diagnostics Market: Analyzing Growth Drivers and Challenges
Meticulous Research®, a leading market research firm, has published a pivotal report titled, “Latin America Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software, Services), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology), End User - Forecast to 2031.”
According to this comprehensive report, the Latin America molecular diagnostics market is expected to reach an impressive valuation of $2.50 billion by 2031, exhibiting a robust compound annual growth rate (CAGR) of 6.3% from 2024 to 2031. This growth trajectory is largely fueled by several key factors: the rising geriatric population, the escalating prevalence of both communicable and non-communicable diseases, significant advancements in molecular diagnostics technologies, and increased healthcare expenditures.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759?utm_source=article&utm_medium=social&utm_campaign=product&utm_content=24-09-2024
Factors Driving Market Growth
Aging Population: The demographic shift towards an aging population in Latin America has led to a heightened demand for effective diagnostic solutions. As people age, the risk of developing chronic diseases such as cancer and cardiovascular disorders increases, driving the need for advanced diagnostic tools.
Prevalence of Diseases: The region is witnessing an increase in both communicable diseases, such as HIV and influenza, and non-communicable diseases, including various cancers. This surge necessitates the adoption of precise and rapid diagnostic methods, propelling the molecular diagnostics market.
Technological Advancements: Continuous innovations in molecular diagnostic technologies, including Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), and microarrays, enhance the accuracy and speed of disease detection. These advancements are crucial for timely diagnosis and treatment, thereby increasing the uptake of molecular diagnostic tests.
Healthcare Expenditure: Rising healthcare expenditures in Latin America signify a growing commitment to improving healthcare systems. Governments and private entities are investing more in healthcare infrastructure, which includes the adoption of advanced diagnostic technologies.
Emerging Opportunities: The increasing focus on companion diagnostics and the rising trend of direct-to-consumer (DTC) testing further open up avenues for growth in the market. Emerging economies in Latin America are gradually embracing these trends, enhancing access to molecular diagnostics.
Market Challenges
While the market presents numerous opportunities, it also faces challenges that could impede growth:
Shortage of Skilled Professionals: A significant challenge is the shortage of trained professionals in molecular diagnostics. The complexity of the technologies involved necessitates a workforce with specialized skills, which is currently lacking in many parts of the region.
Regulatory Hurdles: The regulatory environment surrounding molecular diagnostics can be cumbersome. Variations in regulations across countries can lead to delays in product approval and market entry, which can be a significant barrier for companies looking to expand in this sector.
Cost Constraints: The high cost associated with molecular diagnostic tests can restrict their accessibility, particularly in low- and middle-income countries. Cost-effective solutions and increased funding for diagnostics are essential to overcome this barrier.
Segmentation Analysis
The Latin America molecular diagnostics market is segmented into various categories:
Offering:
Kits & Reagents: This segment is anticipated to dominate the market in 2024, primarily due to the wide range of diagnostic reagents available and the growing awareness regarding the importance of early disease diagnosis.
Instruments: Instruments used in molecular diagnostics, such as PCR machines and sequencers, also play a vital role in the market, offering precision and reliability in testing.
Test Type:
Laboratory Tests: The laboratory test segment is expected to capture the largest market share in 2024. The abundance of laboratory tests available in healthcare facilities and their reliability make them the preferred choice for diagnostics.
Point-of-Care (POC) Tests: POC testing is gaining traction due to its ability to provide rapid results. This segment is likely to witness significant growth, especially in emergency care and rural settings.
Technology:
Polymerase Chain Reaction (PCR): This technology is projected to lead the market due to its versatility and efficiency in detecting various pathogens and genetic disorders. PCR's ability to provide accurate results rapidly is invaluable in clinical settings.
Next-Generation Sequencing (NGS): NGS is also emerging as a powerful tool for molecular diagnostics, particularly in oncology and genetic testing. Its capability to analyze multiple genes simultaneously is driving its adoption.
Application:
Infectious Diseases: This application segment is expected to dominate the market in 2024. The increasing incidence of infectious diseases, coupled with the funding for developing new diagnostic tools, is enhancing the market's growth.
Oncology: The demand for early cancer detection through molecular diagnostics is also increasing, with significant investments being made in developing targeted therapies and companion diagnostics.
End User:
Hospitals & Clinics: This segment is expected to account for the largest market share in 2024 due to the increasing number of hospitalizations requiring molecular diagnostics. The proliferation of hospitals and clinics in emerging economies, such as Brazil, Chile, and Colombia, is a driving factor.
Diagnostic Laboratories: These facilities are critical in the molecular diagnostics landscape, providing specialized services and driving technological advancements.
Key Players in the Market
Several key players are shaping the landscape of the Latin America molecular diagnostics market, including:
Bio-Manguinhos (Brazil)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Hologic, Inc. (U.S.)
Illumina, Inc. (U.S.)
OmicronLab (Mexico)
QIAGEN N.V. (Netherlands)
Danaher Corporation (U.S.)
Abbott Laboratories (U.S.)
Agilent Technologies, Inc. (U.S.)
These organizations are actively engaged in research and development, aiming to introduce innovative products and solutions that cater to the evolving needs of healthcare providers.
Future Outlook
The future of the Latin America molecular diagnostics market appears promising. The integration of advanced technologies, coupled with a focus on patient-centric care, is likely to drive growth. As healthcare systems continue to evolve and adapt, the demand for molecular diagnostics will increase, paving the way for more innovative solutions.
Stakeholders in the market must navigate challenges such as regulatory barriers and workforce shortages. Strategic partnerships, investments in training programs, and collaborations with local governments can help mitigate these challenges.
Moreover, as consumer awareness grows regarding the importance of early detection and personalized medicine, the acceptance and utilization of molecular diagnostics are expected to rise. The increasing role of telehealth and remote monitoring can also enhance access to molecular diagnostic tests, particularly in underserved areas.
Conclusion
In conclusion, the Latin America molecular diagnostics market is poised for substantial growth driven by various factors, including technological advancements, increasing disease prevalence, and rising healthcare expenditures. While challenges remain, the opportunities for innovation and expansion are significant. With a focus on overcoming obstacles and embracing new trends, the future of molecular diagnostics in Latin America looks bright.
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes
Text
Automated Liquid Handling Technologies Market 2024-2032 Analysed by Business Growth, Development Factors, Applications, and Future Prospects
The global automated liquid handling technologies market is poised for substantial expansion, with the market valued at USD 2.11 billion in 2023. It is projected to grow at a compound annual growth rate (CAGR) of 11.18% during the forecast period of 2024 to 2032, reaching a market size of USD 5.24 billion by 2032. This surge is driven by the increasing demand for accuracy, efficiency, and automation in laboratory processes across various industries, particularly in pharmaceuticals, biotechnology, and clinical research.
Automated liquid handling (ALH) systems are advanced robotic tools designed to perform precise liquid transfers in laboratories, significantly reducing human error and increasing throughput. These technologies are instrumental in streamlining workflows for applications such as drug discovery, genomic research, clinical diagnostics, and high-throughput screening.
Key Market Drivers
Increased Focus on Laboratory Efficiency and Accuracy: As research processes become more complex and data-driven, there is a growing need for laboratories to improve efficiency while maintaining high accuracy. Automated liquid handling systems enable researchers to perform complex liquid handling tasks faster and more accurately than manual methods, thus reducing variability and improving reproducibility in experiments. This, in turn, accelerates scientific discoveries and product development timelines, making automation an attractive investment for laboratories worldwide.
Rising Demand in Pharmaceutical and Biotechnology Sectors: The pharmaceutical and biotechnology industries are experiencing a surge in demand for automated liquid handling systems due to their role in accelerating drug discovery, compound screening, and genomic studies. These industries rely on high-throughput automation to manage large sample volumes, particularly in the early stages of drug development. Automated systems allow pharmaceutical companies to process large datasets quickly, leading to faster drug candidate identification and reducing time to market.
Growth in Genomics and Proteomics Research: The expanding field of genomics and proteomics is another significant growth driver for the automated liquid handling market. As researchers explore the human genome and proteome, automated systems are becoming essential for handling complex workflows involving high sample volumes. Automated liquid handlers are used in next-generation sequencing (NGS), polymerase chain reaction (PCR), and other molecular biology techniques, helping labs to achieve higher throughput and accuracy.
Advancements in AI and Robotics: The integration of artificial intelligence (AI) and advanced robotics into automated liquid handling systems is further propelling market growth. AI-enhanced systems offer real-time monitoring, optimization of protocols, and predictive maintenance, which significantly enhance productivity and minimize downtime. The incorporation of machine learning algorithms allows these systems to adapt to complex workflows, making them indispensable in research-intensive sectors.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4485
Challenges and Opportunities
While the automated liquid handling technologies market holds immense growth potential, certain challenges may impede its expansion. The high initial cost of automated systems can be a barrier for small and mid-sized laboratories. Additionally, the need for specialized training to operate and maintain these systems may limit their adoption in less experienced laboratories.
However, these challenges are being addressed as market players increasingly offer more cost-effective and user-friendly solutions. Many companies are investing in modular systems that can be tailored to specific needs, reducing upfront costs and allowing for gradual upgrades. Furthermore, the integration of cloud-based systems and remote monitoring tools enables laboratories to enhance their capabilities without significant infrastructure investments.
Regional Insights
North America currently dominates the automated liquid handling technologies market, largely due to the presence of leading pharmaceutical and biotechnology companies, as well as advanced research infrastructure. The region benefits from substantial R&D investments and early adoption of innovative laboratory automation technologies. Europe follows closely, driven by robust healthcare and life sciences sectors.
The Asia-Pacific region is expected to witness the highest growth during the forecast period, as countries like China, India, and Japan invest heavily in healthcare infrastructure, biopharmaceutical research, and laboratory automation. The increasing prevalence of diseases, growing demand for personalized medicine, and rising investments in biotechnology are major factors driving market growth in this region.
Future Outlook
As the demand for precision, reproducibility, and efficiency in laboratory workflows continues to grow, the automated liquid handling technologies market is set to witness significant advancements. From drug discovery to clinical diagnostics, the need for high-throughput, automated solutions is accelerating across industries. With a projected CAGR of 11.18% from 2024 to 2032, the market will see ongoing innovation, including AI-driven automation, modular designs, and increased integration with laboratory information management systems (LIMS).
In conclusion, the global automated liquid handling technologies market is positioned for rapid expansion, with its size expected to grow from USD 2.11 billion in 2023 to USD 5.24 billion by 2032. The continued evolution of laboratory automation technologies will shape the future of scientific research, diagnostics, and drug development, driving efficiencies and innovation across the life sciences sector.
Other Trending Reports
Pancreatic Cancer Treatment Market Size
gRNA Market Size
Patient Portal Market Size
Continuous Glucose Monitoring Market Size
0 notes
Text
How Pork Testing Labs in Dubai Support Halal Compliance for Local Food Producers | +971 554747210
In Dubai's multicultural and diverse culinary landscape, maintaining halal standards is not just a requirement—it's a commitment to consumer safety and cultural respect. With a significant portion of the population adhering to halal dietary laws, local food producers must ensure that their products meet stringent halal regulations. Pork testing labs play a pivotal role in this process, providing essential services to help food producers maintain compliance, ensure quality, and build consumer trust.
In this blog, we will explore how pork testing lab in Dubai support halal compliance for local food producers and the vital impact they have on the food industry.
Understanding Halal Compliance in Dubai
Halal compliance refers to adhering to Islamic dietary laws, which dictate what foods can be consumed and how they should be prepared. For food producers in Dubai, this means ensuring that their products are free from pork and any pork-derived ingredients. The consequences of non-compliance can be severe, including loss of business, fines, and damage to reputation.
Given the cultural significance of halal in the UAE, pork testing labs have emerged as critical partners for local food producers. They provide the necessary testing and certification to help businesses navigate the complexities of halal compliance.
1. Accurate Testing for Pork Contamination
One of the primary functions of pork testing labs is to conduct accurate and reliable tests for pork contamination. These labs utilize advanced methodologies to detect the presence of pork or its derivatives in food products.
DNA Testing: Techniques such as Polymerase Chain Reaction (PCR) allow labs to identify even trace amounts of pork DNA in various food items. This level of sensitivity is crucial for ensuring that products meet halal standards.
Protein Testing: Enzyme-Linked Immunosorbent Assay (ELISA) is another method employed to detect specific pork proteins. This helps food producers verify that their products are free from non-halal ingredients.
By providing precise testing services, pork testing labs empower local food producers to ensure that their offerings are completely compliant with halal regulations.
2. Building Trust with Consumers
Consumer trust is vital for any food business, especially in a market where halal compliance is a significant concern. Partnering with accredited pork testing labs can enhance a food producer's credibility and foster trust among consumers.
Transparency: Accredited labs offer comprehensive testing reports that detail the methodologies used and results obtained. This transparency reassures consumers that the products they are purchasing are safe and halal-compliant.
Quality Assurance: Regular testing not only ensures halal compliance but also promotes overall product quality. When consumers know a brand is committed to rigorous testing, they are more likely to choose that brand over competitors.
Building a reputation for reliability and quality can lead to increased sales and customer loyalty, which are essential for long-term success in the food industry.
3. Supporting Regulatory Compliance
Dubai has specific regulations governing food safety and halal compliance, which local food producers must follow. Pork testing labs help businesses navigate these regulations effectively.
Guidance on Standards: Accredited labs are well-versed in local regulations and can provide food producers with guidance on best practices for ensuring compliance with halal standards.
Documentation for Inspections: Maintaining proper documentation is crucial during regulatory inspections. Regular testing generates records that can serve as proof of compliance with halal standards, helping businesses avoid penalties.
By supporting regulatory compliance, pork testing labs enable food producers to focus on what they do best—creating quality food products.
4. Risk Management and Contamination Prevention
The risk of pork contamination can arise at various points in the food supply chain, from sourcing raw ingredients to food preparation. Pork testing labs play a crucial role in identifying and mitigating these risks.
Cross-Contamination Detection: Through comprehensive testing of both raw materials and finished products, pork testing labs can detect potential sources of contamination. This enables food producers to implement corrective measures promptly.
Ongoing Monitoring: Many pork testing labs offer ongoing testing services to ensure that products remain compliant throughout the production process. This proactive approach helps identify any issues before they escalate.
Effective risk management is essential for maintaining halal compliance and protecting consumer health, making pork testing labs invaluable partners for local food producers.
5. Enhancing Operational Efficiency
Investing in pork testing lab services can lead to greater operational efficiency for food producers. Regular testing can streamline processes and improve overall productivity.
Preventive Measures: By identifying contamination risks early, food producers can take preventive actions, minimizing waste and production delays.
Quality Control: Continuous testing supports quality control measures, helping producers maintain consistent standards in their products.
Enhanced operational efficiency not only saves time and resources but also contributes to a more sustainable business model.
6. Educating Food Producers
Pork testing labs also serve as valuable educational resources for food producers. Many labs offer training sessions and workshops focused on halal compliance and food safety.
Understanding Best Practices: These training programs can help food producers understand the importance of halal compliance and the best practices for maintaining it in their operations.
Staff Training: By equipping staff with knowledge about contamination risks and prevention strategies, food producers can foster a culture of safety and compliance within their organizations.
Education is key to long-term success in the food industry, and pork testing labs play a vital role in empowering local food producers.
7. Facilitating Export Opportunities
For local food producers looking to expand their markets beyond Dubai, halal compliance is a critical factor in securing export opportunities. Many international markets require strict adherence to halal standards, and partnering with accredited pork testing labs can facilitate this process.
Certification for Exports: Accredited labs can provide the necessary certifications that confirm a product's halal status, making it easier for producers to enter new markets.
Meeting Global Standards: By adhering to internationally recognized testing standards, local food producers can enhance their competitiveness in the global market.
Access to export opportunities can significantly boost a food producer’s growth potential, making the services of pork testing labs even more valuable.
Conclusion
Pork testing labs in Dubai are essential partners for local food producers striving to maintain halal compliance. From providing accurate testing for pork contamination to supporting regulatory compliance and building consumer trust, these labs play a crucial role in ensuring food safety in the local market.
As the demand for halal products continues to grow, the importance of reliable testing and certification will only increase. By investing in the services of accredited pork testing labs, local food producers can enhance their reputation, streamline operations, and ultimately succeed in delivering safe, high-quality halal products to consumers in Dubai and beyond. In a landscape where food safety is non-negotiable, the partnership with pork testing labs is a commitment to excellence and consumer health.
0 notes
Text
Blood Group Typing Market Analysis: New Opportunities in the Healthcare Sector

The Blood Group Typing Market is expected to grow at a rapid pace over the coming years. In 2023, the market was valued at USD 1.9 billion, and by 2030, it's projected to surpass USD 3.3 billion, growing at a compound annual growth rate (CAGR) of 8.2%. This surge in market value highlights the increasing demand for blood typing in various healthcare sectors.
What is Blood Group Typing?
Blood group typing is the process of determining an individual's blood group, typically classified into A, B, AB, or O types. This crucial medical procedure ensures compatibility for blood transfusions, organ transplants, and managing pregnancies where blood group incompatibility may lead to complications. It also plays an important role in research, diagnostics, and therapeutics.
Why is the Blood Group Typing Market Growing?
Several factors contribute to the rapid growth of the blood group typing market:
Rising Demand for Blood Transfusions: The growing number of surgeries and trauma cases has driven the need for safe and compatible blood transfusions.
Advancements in Technology: With the advent of advanced testing technologies, blood typing has become quicker and more accurate.
Increasing Prevalence of Chronic Diseases: As chronic diseases like cancer and cardiovascular conditions become more common, the need for blood transfusions, organ transplants, and diagnostic tests rises.
Government Initiatives: Government health agencies across the globe are launching campaigns to improve healthcare infrastructure, which includes better access to blood banks and safe transfusion practices.
Access Full Report @ https://intentmarketresearch.com/latest-reports/blood-group-typing-market-3123.html
Key Segments of the Blood Group Typing Market
1. Based on Products
Consumables: Reagents, anti-sera, and red blood cells that are essential for blood typing procedures.
Instruments: Machines used in hospitals and laboratories for blood typing tests.
Services: Outsourcing services for hospitals and laboratories to perform blood group testing.
2. Based on Test Type
Antibody Screening: Detects antibodies in the blood that may cause immune reactions.
HLA Typing: Human leukocyte antigen typing is often used in organ transplantation to ensure compatibility.
ABO Blood Group Typing: The most common test, determining whether a person has blood type A, B, AB, or O.
Crossmatching: Ensures compatibility between donor and recipient blood before a transfusion.
3. Based on Technique
PCR-Based Testing: Polymerase chain reaction (PCR) testing is becoming popular due to its accuracy in detecting rare blood types.
Microarray Testing: A cutting-edge technique that offers detailed insights into blood group antigens.
Gel Agglutination: A traditional method where blood cells clump together to reveal their type.
4. Based on End-User
Hospitals and Clinics: The largest users of blood typing services, especially in emergency care, surgeries, and transfusions.
Blood Banks: Critical for maintaining safe blood supplies for hospitals and clinics.
Diagnostic Laboratories: Play a key role in providing accurate blood typing for various medical needs.
Research Institutes: Blood typing is essential in biomedical research and the development of new medical treatments.
Geographical Insights into the Blood Group Typing Market
North America
North America dominates the global blood group typing market, thanks to its advanced healthcare infrastructure and high demand for blood transfusions. The U.S. leads the region with significant investments in healthcare research and technological advancements.
Europe
Europe holds the second-largest market share, driven by increased demand for organ transplants and blood donations. Countries like Germany, the UK, and France are key players in the region.
Asia-Pacific
Asia-Pacific is the fastest-growing market, with a rapidly improving healthcare infrastructure, especially in countries like China, India, and Japan. Increased government initiatives and rising awareness of safe blood transfusion practices are fueling growth in this region.
Latin America and the Middle East
These regions are experiencing moderate growth, with improving healthcare systems and increased focus on safe transfusion practices. Brazil, Saudi Arabia, and the UAE are emerging as key markets.
Download Sample Report @ https://intentmarketresearch.com/request-sample/blood-group-typing-market-3123.html
Technological Advancements in Blood Group Typing
Technological innovations have dramatically improved the accuracy and speed of blood group typing. Some of the most noteworthy advancements include:
Automation: Automated blood typing machines reduce human error and streamline the testing process.
DNA-Based Testing: Genetic testing methods like PCR and next-generation sequencing (NGS) have made it easier to identify rare blood types.
Artificial Intelligence: AI-driven platforms can now analyze blood typing data more efficiently, predicting rare blood types and crossmatching results with greater precision.
Challenges in the Blood Group Typing Market
Despite its rapid growth, the blood group typing market faces several challenges:
High Cost of Advanced Technologies: While innovative testing methods improve accuracy, they also raise costs, limiting access in some regions.
Limited Awareness in Low-Income Countries: In developing countries, there is still a lack of awareness regarding the importance of safe blood transfusions.
Regulatory Hurdles: Compliance with government regulations varies by region, sometimes slowing the approval process for new technologies.
Key Players in the Blood Group Typing Market
Several companies are leading the charge in the global blood group typing market:
Bio-Rad Laboratories: Specializing in diagnostics and testing solutions.
Grifols: A global leader in plasma-derived medicines and transfusion diagnostics.
Ortho Clinical Diagnostics: A top player in immunohematology and blood typing products.
Immucor: Focuses on improving transfusion and transplantation diagnostics.
Future Outlook of the Blood Group Typing Market
The future of the blood group typing market looks promising, with continued growth expected through 2030. Some key trends include:
Increased Investment in R&D: Research into advanced blood typing methods will likely continue, focusing on automation, accuracy, and cost reduction.
Emerging Markets: Countries with developing healthcare systems are investing in better blood typing technologies, creating new growth opportunities.
Personalized Medicine: The rise of personalized treatments will likely drive demand for more precise blood typing methods to ensure compatibility in therapies like immunotherapy and gene editing.
Conclusion
The global blood group typing market is on a path of exponential growth, driven by technological advancements, rising demand for blood transfusions, and government initiatives. With the market projected to surpass USD 3.3 billion by 2030, the future looks bright for companies and healthcare providers in this field. As the industry continues to innovate, the accuracy and accessibility of blood group typing will only improve, saving more lives and ensuring safer medical procedures.
FAQs
What is the CAGR of the blood group typing market from 2024 to 2030?
The market is expected to grow at a CAGR of 8.2% during this period.
What is the importance of blood group typing?
Blood group typing ensures compatibility for blood transfusions, organ transplants, and other medical procedures, reducing the risk of complications.
Which regions are leading the blood group typing market?
North America holds the largest market share, followed by Europe and the rapidly growing Asia-Pacific region.
What are the major technologies used in blood group typing?
Key technologies include PCR-based testing, microarray testing, and gel agglutination methods.
What are the major challenges facing the blood group typing market?
Challenges include the high cost of advanced technologies, limited awareness in developing regions, and regulatory hurdles.
About Us
Intent Market Research (IMR) is dedicated to delivering distinctive market insights, focusing on the sustainable and inclusive growth of our clients. We provide in-depth market research reports and consulting services, empowering businesses to make informed, data-driven decisions.
Our market intelligence reports are grounded in factual and relevant insights across various industries, including chemicals & materials, healthcare, food & beverage, automotive & transportation, energy & power, packaging, industrial equipment, building & construction, aerospace & defense, and semiconductor & electronics, among others.
We adopt a highly collaborative approach, partnering closely with clients to drive transformative changes that benefit all stakeholders. With a strong commitment to innovation, we aim to help businesses expand, build sustainable advantages, and create meaningful, positive impacts.
Contact Us
US: +1 463-583-2713
0 notes
Text
Point of Care Diagnostics: Revolutionizing Healthcare with Real-Time Testing
The Advent of Quick and Accurate Medical Testing
Point of Care Diagnostics have emerged as a groundbreaking development in the medical field by enabling accurate testing to be done quickly and conveniently. Traditional diagnostic methods usually require samples to be sent to a centralized laboratory for analysis, which can delay vital treatment decisions by several days. However, point-of-care tests provide results within minutes using portable devices, bringing testing closer to the patient. This revolutionary approach is transforming healthcare delivery.
Rapid Testing for Better Patient Outcomes
By facilitating timely diagnosis, point-of-care testing leads to better patient outcomes. Speedy detection of conditions like infections or chronic diseases allows doctors to prescribe appropriate treatment without delay. For example, point-of-care tests are commonly used in emergency rooms to quickly identify heart attacks, strokes or life-threatening infections. Getting fast diagnostic results is crucial for such medical emergencies as it ensures patients receive the right therapy as soon as possible. The timely administration of antibiotics, anti-clotting medications or other critical treatments improves survival rates and recovery.
Patient Comfort and Convenience
Besides clinical benefits, Point of Care Diagnostics enhance patient comfort and convenience. People no longer have to wait anxiously for days to learn about their health while potentially worsening conditions go untreated. With devices that analyze samples on-site, patients get actionable results during the same clinical visit when treatment decisions are made. This spares them follow-up trips to the doctor or lab and unnecessary stress. Home testing using self-administered point-of-care kits even allows monitoring health remotely while maintaining independence. Finger-prick blood samples or urine specimens are all that's needed, eliminating difficulties obtaining specimens.
More Efficient Use of Resources
Speedy diagnostic testing optimizes use of limited healthcare resources. Quick turnaround times avoid unnecessary reliance on expensive treatments initiated just to address uncertainty in diagnoses. Point-of-care devices reduce laboratory workloads too by decentralizing testing. Moreover, decentralized testing is vital for resource-constrained settings like rural areas, refugee camps or developing countries where access to centralized labs is limited. Portable devices overcome infrastructure barriers and enable basic medical services even in remote areas. This promotes healthcare equity globally.
A Proliferation of Diagnostic Platforms
Rapid technological progress has enabled the development of varied point-of-care testing systems. Examples include paper microfluidic devices, electrochemical sensors, molecular diagnostics platforms and portable ultrasound machines integrated with imaging analysis software. Immunology-based tests detecting proteins or antibodies through lateral flow or microarray methods are commonly used for conditions like infections and cardiac markers. Molecular diagnostic platforms employ techniques like polymerase chain reaction (PCR) for swift nucleic acid amplification and analysis of viruses or genetic markers. Newer technologies like CRISPR gene editing also hold promise as a basis for point-of-care genetic testing. With ongoing research, the types of conditions examinable at the point of care continue expanding in scope and complexity.
Get more insights on Point Of Care Diagnostics
Also read related article on Gastroesophageal Reflux Disease Treatment Devices Market
For Deeper Insights, Find the Report in the Language that You want
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)

#Point Of Care Diagnostics#Poc Testing#Rapid Diagnostics#Bedside Testing#Portable Diagnostics#PointOfCare Testing#Poc Devices#NearPatient Testing#Decentralized Diagnostics
0 notes
Text
Digital PCR Market — Forecast(2024–2030)
Digital PCR Market Overview
Request Sample
Report Coverage
The report: “Digital PCR Market Forecast (2024–2030)”, by Industry ARC, covers an in-depth analysis of the following segments of the Digital PCR Market.
By Product: Consumables & Reagents and Software & Services
By Technology Type: Droplet Digital PCR, Chip Based Digital PCR, and Beaming Digital PCR
By Indication: Infectious Disease, Oncology, Genetic Disorders, and Others
By Application: Research, Clinical Diagnostics, Forensics, and Others
By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (Middle East, and Africa).
Inquiry Before Buying
Key Takeaways
North America dominated the Digital PCR Market in 2020 owing to the increasing demand for rapid diagnostic tests and high diagnosis rates for infectious disease. The Digital PCR Market scope for different regions will be provided in the final report.
Technological advancements in digital PCR and growing adoption of digital PCR over real time PCR are likely to aid the market growth of the Digital PCR Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Digital PCR Market report.
High cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR is poised to create hurdles for the Digital PCR Market.
Digital PCR Market Segment Analysis — By Technology Type
Droplet Digital PCR held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR of 9.7% during the forecast period 2024–2030. This is attributed to the technological advances along with various new product launches. Droplet digital PCR is based on water oil emulsion droplet technology and for the amplification of the template molecules in each individual droplet. It also uses workflows and reagents for most standard probe based assays. Droplet digital PCR also measure the copy number variation by partitioning a PCR reaction into nanoliter droplets. The cross-contamination drawback of droplet digital PCR is increasing the demand of chip based digital PCR. Droplet digital PCR are estimated to register the highest CAGR over the period 2024–2030.
Schedule a Call
Digital PCR Market Segment Analysis — By Indication
Infectious disease held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR 8.6% during the forecast period 2021–2026. This is attributed to the advantages of the droplet digital PCR of infectious diseases such as bacterial, viral, and parasitic indications. Digital PCR provides more accurate, sensitive, and reproductive detection of pathogens according to the National Center for Biotechnology and it is better than real time polymerase chain reaction that are used for clinical diagnostics. The demand of oncology is increasing owing to the growing prevalence of the condition and introduction of new product launches. Oncology are estimated to register the highest CAGR over the period 2024–2030.
Digital PCR Market Segment Analysis — By Geography
North America dominated the Digital PCR Market with a major share of 37.6% in 2020. This is attributed to the high prevalence & diagnosis rates for infectious disease and high awareness among patient population towards new diagnostic options. Availability of digital PCR devices, rising incidences of various types of cancer and metabolic diseases requiring advanced diagnosis and therapeutics is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2024–2030 owing to the growing patient awareness regarding advanced digital polymerase chain reaction devices. Developing Healthcare infrastructure is also increasing the growth of the market in this region.
Buy Now
Digital PCR Market Drivers
Technological Advancements in Digital PCR
Technological advancements in digital PCR is increasing the growth of the Digital PCR Market. This is attributed to the growing demand for innovative devices and increasing research & development activities. Digital PCR is used to quantify and amplify nuclei acid. The introduction of various technologically advanced devices such as droplet digital PCR, chip based, and beam digital PCR is offering great benefits to the market. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Growing Adoption of Digital PCR over Realtime PCR
Growing adoption of digital PCR over Realtime PCR is increasing the growth of the Digital PCR Market. This is attributed to the fact that digital PCR helps to deliver a compete measure to target nucleic acid molecules that is achieved from real time PCR. DNA quantification allows for reproducibility, precision, and sensitive that enables the researches to quantify smaller differences and measure minor variants very precisely. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Digital PCR Market Challenges
High Cost of Digital PCR Devices and Reimbursement Issues Along with the Technical Limitations of PCR
Some of the factors that are set to impede the growth of the Digital PCR Market are high cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR. The adoption of digital polymerase chain reaction techniques is limited owing to the lack of awareness about the digital PCR and the use of its advanced types.
Digital PCR Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Digital PCR Market. In 2020, the Digital PCR Market share is consolidated by the top ten players present in the market. Digital PCR Market, top 10 companies are Thermo Fisher Scientific Inc., BioMerieux SA, Stilla Technologies, Merck KgaA, Combinati Inc., and Bio-Rad Laboratories among others.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Bronchiolitis Market: Overview, Causes, Symptoms, Diagnosis, and Treatment
Bronchiolitis is a common respiratory condition primarily affecting infants and young children, characterized by inflammation of the small airways in the lungs (bronchioles). This condition often leads to significant respiratory distress and is a major cause of hospitalization in infants during the winter months. This article provides a comprehensive overview of bronchiolitis, including its causes, symptoms, diagnostic methods, and treatment options.
Bronchiolitis is an acute viral infection that causes inflammation and swelling of the bronchioles, the smallest air passages in the lungs. The condition is most prevalent in children under two years of age and typically occurs in the winter and early spring. Bronchiolitis is usually caused by respiratory syncytial virus (RSV), but other viruses can also be involved. The severity of bronchiolitis can range from mild to severe, with some cases requiring hospitalization.
Causes of Bronchiolitis
The primary cause of bronchiolitis is viral infection, with several key viruses implicated:
1. Respiratory Syncytial Virus (RSV): RSV is the most common cause of bronchiolitis, accounting for the majority of cases. It is highly contagious and spreads through respiratory droplets.
2. Rhinoviruses: These viruses, commonly associated with the common cold, can also cause bronchiolitis, especially in combination with other pathogens.
3. Parainfluenza Viruses: These viruses can contribute to respiratory infections in children, including bronchiolitis.
4. Adenoviruses: Adenoviruses can also cause bronchiolitis, although they are less common than RSV.
5. Human Metapneumovirus (hMPV): This virus can cause respiratory infections similar to those caused by RSV and is increasingly recognized as a cause of bronchiolitis.
Signs and Symptoms
The symptoms of bronchiolitis typically develop within a few days after exposure to a virus and may include:
1. Cough: A persistent cough is a common symptom of bronchiolitis, often accompanied by wheezing and a rattling sound in the chest.
2. Wheezing: The inflammation of the bronchioles causes narrowing of the airways, leading to a wheezing sound during breathing.
3. Respiratory Distress: Infants may show signs of difficulty breathing, including rapid or shallow breathing, nasal flaring, and use of accessory muscles to breathe.
4. Fever: Mild to moderate fever may accompany bronchiolitis, although high fever is less common.
5. Runny or Stuffy Nose: Initial symptoms often include a runny or congested nose, which may progress to more severe respiratory symptoms.
6. Decreased Appetite: Infants with bronchiolitis may have a reduced appetite and difficulty feeding due to respiratory distress.
7. Cyanosis: In severe cases, the skin or lips may turn bluish, indicating inadequate oxygenation and requiring urgent medical attention.
Diagnosis of Bronchiolitis
Diagnosing bronchiolitis involves a combination of clinical evaluation and diagnostic tests:
1. Medical History and Physical Examination: A healthcare provider will assess the patient's medical history and perform a physical examination. Key signs include respiratory distress, wheezing, and abnormal lung sounds.
2. Chest X-Ray: While not always necessary, a chest X-ray can help rule out other conditions such as pneumonia or congenital heart defects. It may show hyperinflation of the lungs or other signs consistent with bronchiolitis.
3. Viral Testing: Diagnostic tests, such as polymerase chain reaction (PCR) or immunofluorescence assays, can identify the specific virus causing the infection. RSV is often confirmed using these methods.
4. Blood Tests: Blood tests may be performed to assess the severity of the infection, check for signs of dehydration, or rule out other conditions.
5. Oxygen Saturation Monitoring: Pulse oximetry is used to measure blood oxygen levels, helping to assess the severity of respiratory distress.
Treatment of Bronchiolitis
Treatment for bronchiolitis focuses on supportive care and alleviating symptoms, as there is no specific antiviral treatment for the condition. Management strategies include:
1. Supportive Care:
- Hydration: Ensuring adequate fluid intake is crucial to prevent dehydration, especially if the child is experiencing reduced appetite or difficulty feeding.
- Nasal Saline Drops: Saline nasal drops or sprays can help relieve nasal congestion and improve breathing.
- Humidified Air: Using a cool-mist humidifier can help ease respiratory symptoms by keeping the airways moist.
2. Medications:
- Bronchodilators: Medications such as albuterol may be used to relax the airways and ease wheezing, although their effectiveness in bronchiolitis is debated.
- Corticosteroids: These are generally not recommended for routine use in bronchiolitis, but they may be prescribed in severe cases or when there is evidence of underlying asthma.
3. Oxygen Therapy: For infants with low oxygen levels, supplemental oxygen may be administered to ensure adequate oxygenation.
4. Hospitalization: In severe cases, hospitalization may be required to provide intensive monitoring and supportive care, including intravenous fluids, respiratory support, and close observation.
5. Preventive Measures:
- RSV Prophylaxis: In high-risk infants, such as those with congenital heart disease or premature birth, a medication called palivizumab may be given as a preventive measure during RSV season.
Bronchiolitis Market Insights
The market for bronchiolitis treatments reflects the ongoing need for effective management strategies and innovations in respiratory care:
- Market Size and Growth: The global market for bronchiolitis treatments is expanding, driven by the prevalence of respiratory infections in children and the development of new therapeutic and diagnostic solutions. Key segments include pharmaceuticals, diagnostic tools, and supportive care products.
- Key Players: Leading companies involved in the bronchiolitis market include:
- GlaxoSmithKline: Known for its respiratory therapies and involvement in the development of treatments for respiratory conditions in children.
- Roche Holdings: Engaged in diagnostics and research related to respiratory infections, including bronchiolitis.
- AstraZeneca: Focuses on respiratory medicines and has a presence in the market for managing respiratory infections.
- Novartis Pharmaceuticals: Contributes to research and development in respiratory and infectious disease therapies.
- Research and Development: Ongoing research aims to improve understanding of bronchiolitis, develop new antiviral agents, and enhance supportive care strategies. Innovations in diagnostics and treatments are expected to contribute to better management and outcomes for affected children.
Bronchiolitis is a common and often challenging respiratory condition that affects infants and young children. Understanding its causes, symptoms, and treatment options is crucial for effective management and improved outcomes. With ongoing research and advancements in the field, there is hope for more effective therapies and better preventive measures. The growing market for bronchiolitis treatments highlights the importance of continued innovation and support for managing this prevalent and impactful condition.
Download sample report @ https://www.delveinsight.com/sample-request/bronchiolitis-market
0 notes
Text
Companion Diagnostics Market - Forecast(2024 - 2030)
Companion Diagnostic Market Overview:
The Harvard University, while addressing the risk associated with reactions of new drugs prescription, has stated some overwhelming facts. According to their findings, even properly prescribed drugs causes about 1.9 million hospitalizations a year and about 128,000 people die from drugs prescribed to them.[1] Such epidemic condition is being tailgated by the need of safe and effective and specific use of the drug. Owing to such demands, companion diagnostic drug market is poised for exponential growth. Companion diagnostics is an in-vitro diagnostic tool that assists physicians in optimizing treatment decisions for their patients and is crucial for myriad of cancer and other therapies. Riding on the back of economic burden of global healthcare and with abundant potential to restrict the liability, companion diagnostic market size is estimated to be $2,950 million as of 2018.
Inquiry Before Buying
Companion Diagnostic Market Outlook:
Companion diagnostic (CDx) is a diagnostic test used as an associate to a therapeutic drug to regulate its applicability to an individual person. It involves multiple monitoring methods including immunohistochemistry (IHC), polymerase chain Reaction (PCR), in-situ hybridization (ISH), real-time PCR (RT-PCR), and gene sequencing. The companion diagnostics uses technologies such as molecular biology technique, drug and diagnostic technology, and oncology therapy for the treatment of colorectal cancer, breast cancer, and other chronic diseases.
An acute analysis of the region-wise companion diagnostic market share concluded with reckoning North America as the most lucrative market for CDx. This region with cutting-edge healthcare technology in the United States and Canada generates 41% of the global companion diagnostic market demand for alarming need for cancer diagnosis and treatment. According to the American Cancer Society, prostate cancer is the most common cancer among males (19%), followed by lung (14%) and colorectal (9%) cancers and among females, breast (30%), lung (12%), and colorectal (8%) cancers are the most common. Increasing instances of cancer along with neurological disorders, infectious diseases, Hepatitis A is augmenting the North American companion diagnostic market.
Companion diagnostic market with abundant potential as an effective tool for personalized medicine has found a tremendous scope of application in pharmaceuticals, laboratories, research institutes and hospitals. Hospital as an end-user industry is the leading segment progressing with an application CAGR of 12.5% going through 2025. Hospitalized patients account for a total of 2.74 million serious adverse drug reactions. Each CDx test is specifically designed to be corresponding with an exact drug. Such tests can also save significant hospital expenditure by targeting specific patients with the most effective therapy.
Request Sample
Companion Diagnostic Market Trends and Growth Drivers:
· The necessity for personalized therapeutics for the cumulative geriatrics population and the increasing figure of diagnostics centers in both the developed and developing economies is predominant to determine profits in the global companion diagnostics market.
· FDA had issued "Guidance for Industry: In Vitro Companion Diagnostic Devices," to assist syndicates recognize the necessity for companion diagnostics at an initial stage in the drug development procedure and to strategize for co-development of the drug and companion diagnostic test.
On July 15, 2016, FDA introduced the draft regulation, "Principles for Co-development of an In Vitro Companion Diagnostic Device with a Therapeutic Product." This guidance text is envisioned to be a practical guide to support therapeutic product promoters and IVD sponsors in evolving a therapeutic product and an associated IVD companion diagnostic. The scientific progressions in the in-situ hybridization and automated silver-enhanced in-situ hybridization (SISH) for the monitoring of genes is trending in the global companion diagnostics market.
Schedule a Call
Companion Diagnostic Market Players Perspective:
Some of the key players influencing the global market are:- Abbott Laboratories, Agilent Technologies, biomerieux, Bio-Genex Laboratories, Danaher Corporation, GE Healthcare, Myriad Genetics, Inc., QIAGEN N.V., R-Biopharm AG, and Roche Diagnostics.
In April 2017, Abbott acquired Alere for a new price of about $5.3 billion. Alere is the global leader in point of care diagnostics focused on the areas of infectious disease, molecular, cardiometabolic and toxicology. The collective business will offer the biggest point of care menu of infectious disease, molecular, cardiometabolic and toxicology testing. Abbott's platforms will be expanded to comprise benchtop and rapid strip tests.
Buy Now
Companion Diagnostic Market Research Scope:
The base year of the study is 2018, with forecast done up to 2025. The study presents a thorough analysis of the competitive landscape, taking into account the market shares of the leading companies. It also provides information on unit shipments. These provide the key market participants with the necessary business intelligence and help them understand the future of the companion diagnostic market. The assessment includes the forecast, an overview of the competitive structure, the market shares of the competitors, as well as the market trends, market demands, market drivers, market challenges, and product analysis. The market drivers and restraints have been assessed to fathom their impact over the forecast period. This report further identifies the key opportunities for growth while also detailing the key challenges and possible threats. The key areas of focus include the various diagnostics in companion diagnostic market, and their specific advantages.
#companion diagnostics market#companion diagnostics market size#companion diagnostics market share#companion diagnostics market forecast#companion diagnostics market report#drugs#chronic diseases#treatment#">
0 notes
Text
Kuiken said his concern about the risk that infected raw milk poses is not so much that the practice might somehow help the virus to mutate in ways that would allow it to spread easily to and among people — in other words, trigger a pandemic. But he believes it would likely seriously sicken people who drink raw milk from an H5N1-infected cow. Reports of the amount of virus present in infected udders is higher than anything he’s seen in studies where he’s experimentally infected animals with H5N1 to chart the illness the virus wreaked, Kuiken said.
Jürgen Richt, a veterinarian and director of the Center of Excellence for Emerging and Zoonotic Animal Diseases at Kansas State University’s College of Veterinary Medicine, spoke with a note of disbelief in his voice about the amount of dead viruses or viral particles being found in commercial milk that tested positive for the virus.
“From [results] I have seen, I wouldn’t want to drink raw milk,” Richt said. “And I wouldn’t feed it to my cats, nor my dogs, nor my calves, if I’m on a farm.”
The FDA is urging consumers not to drink raw milk or eat raw milk cheeses. That is a position the agency has long held, because of the other health risks these products hold, but it has re-emphasized it in the current context.
It has also recommended the dairy industry not “manufacture or sell raw milk or raw milk products, including raw milk cheese, made with milk from cows showing symptoms of illness, including those infected with avian influenza viruses or exposed to those infected with avian influenza viruses.”
Kuiken said he is less concerned about raw milk cheeses, saying the various processes involved in cheesemaking are “not conducive to survival of infectious virus.” He did suggest, though, that raw milk cheesemakers could be at risk, if they were inadvertently using milk laced with H5N1 virus.
Whether herds owned by farmers who sell raw milk have been infected by the virus isn’t publicly known. While authorities and scientists believe outbreaks are occurring over a much broader swathe of the country than has been detected, the U.S. Department of Agriculture has only confirmed infections of 34 herds in nine states — Texas, Kansas, Michigan, New Mexico, Idaho, Ohio, South Dakota, North Carolina, and Colorado. It has not given any details about the operations on which infected animals were found.
But the USDA has admitted some farmers have been refusing to test their animals. And analysis of the genetic sequences of viruses retrieved from cows combined with evidence of H5N1 RNA in commercial milk found in a number of U.S. markets — the FDA said Thursday that about 1 in 5 samples tested for H5N1 from across the country have been positive — bolster the argument that this has been going on for longer than has been recognized and likely involves far more herds than have tested positive.
The testing of commercial milk was done by polymerase chain reaction, or PCR. In PCR testing, the concentration of a pathogen is estimated by how many cycles the test has to run to find it. The lower the cycle threshold — known as a “Ct value” — the higher the concentration. Anything with a cycle threshold of 29 or lower is considered a strongly positive result. Some milk testing has shown a Ct value of below 10, Kuiken said.
2 notes
·
View notes
Text
Forensic Technologies Market: Innovations and Trends Shaping the Future
Forensic Technologies Market Overview: A lot of factors, such as geographic growth, segmentation, and market size by value and volume, are taken into account in the SkyQuest Technology Group research to provide a full and accurate analysis of the global Forensic Technologies market. This outstanding research study was created specifically to provide the most latest data on significant aspects of the global Forensic Technologies Industry. Numerous market estimates are provided in the analysis, including those for market size, output, revenue, consumption, CAGR, gross margin, price, and other critical factors. The best primary and secondary research methods and tools on the Forensic Technologies market were used to build it. Numerous research studies are included in it, including ones on pricing analysis, production and consumption analysis, company profile, and manufacturing cost analysis.
The competitive environment is a crucial element that every key factor needs to be aware of. The study explains the market's competitive landscape so that readers may gauge the degree of both domestic and global rivalry. Additionally, market researchers have provided summaries of each significant firm in the global Forensic Technologies industry, taking into consideration crucial elements including operational areas, production, and product portfolio. When analyzing the organizations in the study, significant factors including business size, market share, market growth, revenue, production volume, and profitability are also taken into account. The study report uses both qualitative and quantitative data to offer a thorough view of the market. It examines and forecasts the global market in a number of critical industries. The research provides a thorough overview of the industry by segmenting the Forensic Technologies market into groups based on application, end-user, and location. A thorough research of each market segment was conducted, taking into consideration current and upcoming market trends.
Global Forensic Technologies Market size was valued at USD 14.78 billion in 2022 and is poised to grow from USD 16.61 billion in 2023 to USD 41.92 billion by 2031, growing at a CAGR of 12.28% in the forecast period (2024-2031).
Chance to get a free sample @ https://www.skyquestt.com/sample-request/forensic-technologies-market
Detailed Segmentation and Classification of the report (Market Size and Forecast - 2031, Y-o-Y growth rate, and CAGR):
The Forensic Technologies Market can be segmented based on several factors, including product type, application, end-user, and distribution channel. Understanding these segments is crucial for companies looking to target specific markets and tailor their offerings to meet consumer needs.
Product
Digital & Computer Forensics, Ballistic Forensics, DNA Testing, Biometrics, Others
Application
Pharmacogenetics, Biodefense & Bio-Surveillance, Judicial, Law Enforcement, Others
Service
Laboratory Forensics (DNA Testing, Drug Testing, Biometrics, Others), Forensic Consulting
Technique
Polymerase Chain Reaction (PCR), Capillary Electrophoresis, Next-Generation Sequencing, Rapid DNA Analysis, Automated Liquid Handling Technology, Microarrays, Others
Location
Laboratory Forensic Technology, Portable Forensic Technology
Get your Customized report @ https://www.skyquestt.com/speak-with-analyst/forensic-technologies-market
Following are the players analyzed in the report:
GE Healthcare
Thermo Fisher Scientific Inc.
Agilent Technologies Inc.
LGC Limited
Danaher Corporation
Eurofins Scientific SE
Forensic Technology WAI Inc.
NMS Labs Inc.
SCIEX
Bruker Corporation
Bode Technology
Morpho (Safran)
IDEMIA Group
Qiagen N.V.
NicheVision Forensics Co.
Foster + Freeman Ltd.
FLIR Systems Inc.
Sirchie
Firearm and Toolmark Examination Unit
Forensic Pathology Services
Motives for purchasing this report-
- A full understanding of customer experiences, upcoming trends, and growth drivers may be obtained by market category analysis.
-Forensic Technologies Market participants will be able to quickly decide on their course of action in order to achieve a competitive advantage thanks to the essential information provided in this area.
The factors affecting the sales prospect are carefully examined by SkyQuest Technology Group across several important categories.
- Analysing market categories can provide detailed insights into consumer experiences, upcoming trends, and growth-promoting factors. A thorough analysis of market manufacturing trends is a crucial component of the study.
-These observations offer crucial information on the ways in which market participants are reacting to the most recent developments that are oversaturating the market.
-An in-depth analysis of the numerous organic
Buy your full Market Report now: https://www.skyquestt.com/buy-now/forensic-technologies-market
FAQs:
1. What are the main vendors' points of strength and weakness?
2. What are the primary business plans of the leading important players for the near future?
3. What will the market size and growth rate be for Forensic Technologies in the upcoming year?
4. Which prevailing global trends are affecting the Forensic Technologies market shares of the leading regions? What effect does Covid19 have on the Industry right now?
1 note
·
View note
Text
Latin America Molecular Diagnostics Market Overview: Challenges and Growth Opportunities
According to a recent report by Meticulous Research®, the Latin America molecular diagnostics market is forecasted to reach $2.50 billion by 2031, expanding at a compound annual growth rate (CAGR) of 6.3% from 2024 to 2031. The market growth is propelled by several key factors, including the increasing global geriatric population, rising prevalence of communicable and noncommunicable diseases, rapid advancements in molecular diagnostics technology, and a surge in healthcare spending across the region.
The rising demand for molecular diagnostics in Latin America is further supported by opportunities such as the expanding scope in emerging economies, increased focus on companion diagnostics, and the growing popularity of direct-to-consumer (DTC) testing. Despite these growth drivers, challenges like the shortage of skilled professionals, unfavorable regulatory frameworks, and the high costs of molecular diagnostic tests could restrain the market’s growth.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759
Market Segmentation and Key Insights
The Latin America molecular diagnostics market is segmented based on offerings, test types, technologies, applications, and end users.
By Offering: In 2024, the kits and reagents segment is expected to dominate the market. This is attributed to the widespread availability of diagnostic reagents and consumables, disease-specific test kits and assays, and increasing awareness about the importance of early disease diagnosis.
By Test Type: The laboratory tests segment is projected to account for the largest share in 2024. Laboratory tests remain the preferred choice for hospitals, diagnostic laboratories, and academic institutions, driven by their wide availability and the significant developments in diagnostic testing within clinical settings.
By Technology: The polymerase chain reaction (PCR) segment is expected to lead the market in 2024. PCR's dominant position is due to its versatility in multi-drug resistance testing, widespread use in clinical and research laboratories, and applications in areas such as DNA fingerprinting, bacterial and viral detection (particularly HIV/AIDS), and genetic disorder diagnosis.
By Application: The infectious diseases segment is anticipated to hold the largest share of the market in 2024. The increasing prevalence of infectious diseases, rising funding for the development of innovative diagnostic tools, and the heightened focus on diagnostic technologies during the COVID-19 pandemic are driving the demand for molecular diagnostics in infectious disease management.
By End User: Hospitals and clinics are expected to dominate the market by end user in 2024. The growing number of hospitalizations due to a variety of diseases requiring molecular diagnosis, coupled with the proliferation of healthcare facilities in countries such as Brazil, Mexico, Chile, and Colombia, is contributing to the expansion of molecular diagnostic product utilization in this segment.
Key Industry Players
The competitive landscape of the Latin America molecular diagnostics market is shaped by several prominent global and regional players. Leading companies in the market include:
Bio-Manguinhos (Brazil)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Hologic, Inc. (U.S.)
Illumina, Inc. (U.S.)
OmicronLab (Mexico)
QIAGEN N.V. (Netherlands)
Danaher Corporation (U.S.)
Abbott Laboratories (U.S.)
Agilent Technologies, Inc. (U.S.)
These companies are at the forefront of innovation and technological advancements in molecular diagnostics, driving market growth through product development, partnerships, and strategic expansions.
Opportunities and Challenges
The Latin America molecular diagnostics market is ripe with opportunities, particularly in emerging economies where there is increasing demand for advanced diagnostic tools. The rising focus on companion diagnostics and the expanding DTC testing market offer significant growth prospects. However, challenges such as the high cost of molecular diagnostic tests and regulatory hurdles may hinder market expansion.
Despite these challenges, the region’s healthcare landscape is undergoing rapid transformation, with increasing healthcare investments and advancements in diagnostic technologies expected to fuel further growth.
Read Full Report @ https://www.meticulousresearch.com/product/latin-america-molecular-diagnostics-market-5759
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes