#Process Validation for Medical Devices - Iziel
Text
Demystifying Medical Device Software Validation: Ensuring Safety and Compliance
Briefly introduce the importance of medical device software validation.
Explain that the blog post will provide an overview of the key concepts, processes, and regulations related to medical device software validation.
I. Understanding Medical Device Software
Define what medical device software is and its significance in healthcare.
Discuss the different types of medical device software (e.g., embedded, standalone, mobile apps).
Emphasize the increasing reliance on software in modern medical devices.
II. Why Software Validation Matters
Explain the critical role of software validation in ensuring patient safety.
Discuss the potential risks and consequences of inadequate software validation.
Highlight real-world examples of medical device software failures and their implications.
III. Regulatory Framework
Introduce the regulatory bodies and standards governing medical device software validation (e.g., FDA, ISO 13485, IEC 62304).
Explain the classification of medical devices and how it affects validation requirements.
Provide an overview of the FDA's Software as a Medical Device (SaMD) guidance.
IV. The Software Validation Process
Outline the key steps in the software validation process:
Requirements analysis and specification
Risk assessment and management
Design and development
Testing and verification
Validation and documentation
Emphasize the importance of traceability and documentation.
V. Common Challenges and Solutions
Discuss common challenges in medical device software validation (e.g., changing requirements, evolving technology).
Provide practical solutions and best practices for overcoming these challenges.
VI. Case Studies
Present real-world case studies of successful medical device software validation projects.
Highlight the benefits of proper validation, including improved patient outcomes and reduced liability.
VII. Future Trends in Medical Device Software Validation
Explore emerging trends and technologies in medical device software validation (e.g., artificial intelligence, machine learning).
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in design and development of highly sophisticated implantable devices at industry leading companies, with direct expertise in software V&V. This team, with the support of IZiel’s regulatory and clinical experts, are decidedly equipped to handle complex software validation activities for medical device manufacturers. Integrating risk assessments into the validation lifecycle and documenting the basis for what was done also provides a level of assurance to management and regulatory authorities that the system was properly defined, designed, built, tested, operated, and maintained.
0 notes
Link
Iziel Healthcare is offering complete solution in Process Validation for Medical Devices. Our team is Design for Six Sigma (DFSS - Black Belt Level) trained and is fully competent to conduct requirements management, process validations, that are ISO13485, US FDA & CE approval compliant
0 notes
Text
Ensuring Safety and Efficacy: Process Validation and Verification Services for Medical Devices
In the fast-evolving world of medical devices, ensuring safety and efficacy is paramount. Manufacturers must adhere to strict regulatory requirements and industry standards to bring their products to market. This is where process validation and verification services play a crucial role. In this blog post, we will explore the importance of these services in the medical device industry and how they contribute to product quality and patient safety.
What is Process Validation?
Process validation is a systematic approach to ensuring that a manufacturing process consistently produces medical devices that meet their specifications and intended use. It is a critical step in quality assurance and regulatory compliance. Validation involves a series of activities to demonstrate that a manufacturing process can consistently produce devices that meet predefined quality standards.
Key Elements of Process Validation:
Process Design: Before production begins, manufacturers must establish the process design, including equipment, materials, and operating parameters.
Process Qualification: This phase involves conducting experiments and collecting data to demonstrate that the process consistently produces devices within specifications.
Continued Process Verification: Ongoing monitoring and periodic revalidation ensure that the process remains in a state of control throughout the product's lifecycle.
Importance of Process Validation:
Patient Safety: Validated processes reduce the risk of defects and ensure that medical devices perform as intended, minimizing harm to patients.
Regulatory Compliance: Regulatory bodies, such as the FDA and CE, require manufacturers to validate their processes to ensure product quality and safety.
Cost Savings: Identifying and addressing issues early in the manufacturing process can prevent costly recalls and rework.
What is Process Verification?
Process verification is the ongoing monitoring and confirmation that a manufacturing process remains in a state of control. It involves collecting and analyzing data to ensure that the process consistently produces devices that meet quality standards. Process verification is a crucial component of post-market surveillance and is required by regulatory authorities.
Key Elements of Process Verification:
Data Collection: Manufacturers must collect data related to critical process parameters and product characteristics.
Data Analysis: Statistical tools and methods are used to analyze the collected data and identify trends or deviations.
Corrective Actions: If deviations are detected, manufacturers must take appropriate corrective actions to bring the process back into control.
Importance of Process Verification:
Continuous Improvement: By continuously monitoring the manufacturing process, manufacturers can identify opportunities for improvement and make necessary adjustments.
Regulatory Compliance: Demonstrating ongoing process verification is a regulatory requirement to maintain product approvals.
Quality Assurance: Process verification ensures that the quality of medical devices remains consistent over time, enhancing patient safety and satisfaction.
Conclusion:
Process validation and verification services are essential for ensuring the safety, efficacy, and quality of medical devices. Manufacturers must adhere to rigorous standards and regulatory requirements to bring their products to market and keep them there. By implementing robust validation and verification processes, manufacturers can not only meet these requirements but also enhance patient safety and reduce the risk of costly recalls. In the dynamic and highly regulated world of medical devices, these services are critical for success.
IZiel’s Onshore-Offshore Model works with 1 Onsite Member supported by a team of 3-4 engineers from our Technical Center in India. Typically, the data is collected, evaluated and evidence is developed from the process design stage throughout production. Our Model enables the company to complete the project faster and in a cost-effective manner.
0 notes
Text
Configuration Management for Medical Devices
Configuration management for medical devices is a critical process that involves the systematic management of the various components, specifications, and changes related to medical devices throughout their lifecycle. This process ensures that medical devices are developed, manufactured, and maintained in a controlled and traceable manner, conforming to regulatory standards and meeting quality and safety requirements.
Here are some key aspects of configuration management for medical devices:
1. Requirements Management: Capture and document all requirements for the medical device, including functional, performance, safety, and regulatory requirements. These requirements will serve as the foundation for the design and development process.
2. Version Control: Implement a version control system to manage different iterations and versions of the device's design, software, and documentation. This allows for easy tracking of changes and ensures that the latest approved version is being used.
3. Change Management: Establish a formal change management process to assess and approve proposed changes to the device's design, components, software, or documentation. This process should include impact assessments, risk evaluations, and regulatory considerations.
4. Configuration Identification: Clearly define and identify all components, sub-components, software modules, and documentation associated with the medical device. Each configuration item should have a unique identifier.
5. Baseline Establishment: Create baselines at key points in the device's development lifecycle, such as after design verification and validation, to establish a reference point for future changes.
6. Traceability: Establish traceability between requirements, design elements, verification and validation activities, and any changes made throughout the device's lifecycle. This ensures that all aspects of the device are aligned and that changes are properly documented.
7. Documentation Management: Maintain accurate and up-to-date documentation for the device's design, manufacturing processes, software, and any changes made. This documentation should be organized and easily accessible.
8. Risk Management: Integrate risk management processes into configuration management to assess the impact of changes on the device's safety and performance. This is especially important for medical devices, where patient safety is paramount.
9. Regulatory Compliance: Ensure that the configuration management process adheres to relevant regulatory standards, such as ISO 13485 for medical device quality management systems, and the requirements of regulatory bodies like the FDA (U.S. Food and Drug Administration) or the European Medicines Agency (EMA).
10. Collaboration: Facilitate communication and collaboration among cross-functional teams involved in the device's development, manufacturing, and maintenance. This helps prevent miscommunication and ensures that everyone is working with the correct and approved configurations.
11. Validation and Verification: Implement rigorous validation and verification processes for any changes made to the device's design, components, or software. This helps ensure that changes do not negatively impact the device's safety, performance, or regulatory compliance.
Configuration management for medical devices is an integral part of ensuring product quality, patient safety, and compliance with regulatory requirements. It requires careful planning, documentation, and adherence to established processes to effectively manage changes and maintain the integrity of the device throughout its lifecycle.
IZiel has highly trained configuration managers who will be able to create, coordinate and implement the Configuration Management Plan (CMP – includes responsibilities and resources, (including personnel), training requirements, administrative meeting guidelines (including a definition of procedures and tools), baselining processes, configuration control and configuration-status accounting, naming conventions, audits and reviews, subcontractor/vendor configuration management requirements, regulatory requirements) for Product Creation Process (PCP) projects in co-operation with the Project Managers and Operations Department.
0 notes
Text
Design Verification and Validation for Medical Devices
Design Verification and Validation (V&V) for medical devices is a critical process that ensures the safety, efficacy, and compliance of the device before it is released to the market. These processes are essential to minimize the risks associated with medical devices and to meet regulatory requirements. Here's an overview of the steps involved in Design Verification and Validation for medical devices:
1. Design Verification:
Design verification ensures that the device's design meets the specified requirements and functions correctly. It focuses on confirming that the device was designed correctly and that it meets the intended performance standards. Verification activities include:
- Requirements Verification: Ensure that all design requirements are clear, well-defined, and traceable throughout the development process.
- Design Reviews: Regularly scheduled reviews with cross-functional teams to assess design progress, identify potential issues, and ensure alignment with user needs and regulatory requirements.
- Testing and Analysis: Perform various tests such as functional testing, performance testing, stress testing, and reliability testing to verify that the device operates as intended and meets its performance specifications.
- Software Verification: If the device includes software components, perform thorough software testing to validate its functionality, reliability, and security.
- Prototyping and Simulation: Use prototypes or simulations to validate the device's functionality and behaviour in controlled environments.
- Risk Assessment: Evaluate potential risks associated with the device's design and verify that risk mitigation strategies are effectively implemented.
2. Design Validation:
Design validation focuses on ensuring that the device meets the needs and expectations of its intended users and performs reliably under real-world conditions. Validation activities include:
- Usability Testing: Conduct usability studies with representative users to validate that the device is user-friendly, intuitive, and can be effectively operated by its intended users.
- Clinical Trials: For devices that require clinical use, conduct well-designed clinical trials to demonstrate the safety and efficacy of the device in the intended patient population.
- Human Factors Engineering: Evaluate the device's interaction with users and its environment to identify and mitigate potential use-related hazards.
- Field Testing: Conduct field testing in real-world scenarios to validate the device's performance, reliability, and safety under various conditions.
- Performance Evaluation: Assess the device's performance metrics against predetermined criteria to ensure it meets the intended outcomes.
3. Documentation and Reporting:
Thorough documentation of all verification and validation activities is essential for regulatory compliance and accountability. Documentation should include test protocols, test results, risk assessment reports, usability studies, clinical trial data, and any other relevant documentation that demonstrates the device's safety and effectiveness.
4. Regulatory Compliance:
Throughout the verification and validation processes, it's crucial to adhere to relevant regulations and standards, such as ISO 13485 (Quality Management Systems for Medical Devices), ISO 14971 (Risk Management for Medical Devices), and FDA guidelines (for devices marketed in the United States).
By following a systematic approach to Design Verification and Validation, medical device manufacturers can ensure that their devices are safe, effective, and compliant with regulatory requirements, thereby minimizing risks to patients and users.
At IZiel, our team has successfully completed and worked with various client teams to support in developing the Design and Development Plan, Design Verification, Validation Protocol and Reports, Design Transfer Plan and other related documents to complete your design control document.
0 notes
Text
MDD to MDR Transition for Medical Devices
The transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) marks a significant change in the regulatory framework for medical devices in the European Union (EU). The MDR came into full effect on May 26, 2021, replacing the MDD and introducing more stringent requirements for the marketing and oversight of medical devices.
Here are some key points to consider regarding the transition from MDD to MDR for medical devices:
1. Scope and Classification: The MDR has a broader scope and includes a wider range of medical devices than the MDD. It introduces new classification rules, which might result in the reclassification of some devices.
2. Conformity Assessment: The MDR emphasizes a risk-based approach to conformity assessment. It introduces stricter requirements for clinical evidence and post-market surveillance. Manufacturers need to provide more comprehensive data on safety, performance, and clinical benefit.
3. Unique Device Identification (UDI): The MDR mandates the use of Unique Device Identifiers (UDIs) for better traceability of devices throughout their lifecycle. UDIs provide information about the device's identity, origin, and production history.
4. Post-Market Surveillance: The MDR places greater emphasis on post-market surveillance and vigilance. Manufacturers are required to actively monitor the performance of their devices on the market and report any incidents or safety concerns.
5. Economic Operators: The MDR introduces new roles for economic operators in the supply chain, including importers and distributors. These entities have specific responsibilities related to device oversight and reporting.
6. Clinical Data Requirements: The MDR sets higher standards for clinical data and requires more extensive clinical evaluation for devices, especially for high-risk devices. Clinical data must support the device's safety and performance claims.
7. Notified Bodies: Notified Bodies play a crucial role in the conformity assessment process. The MDR requires stricter criteria for designation and oversight of Notified Bodies to ensure consistency and reliability in the assessment process.
8. Transitional Period: There was a transition period for manufacturers to comply with the MDR. Devices certified under the MDD could continue to be placed on the market until May 26, 2024, if the MDD certificate remained valid. However, for new devices, the MDR requirements are applied immediately after implementation.
9. Legacy Devices: After the transition period, devices that were certified under the MDD and placed on the market could still be used, provided they continue to meet their intended purpose and do not compromise patient safety. However, any modifications to these devices may trigger the need for MDR compliance.
It's important for manufacturers, importers, distributors, and other stakeholders in the medical device industry to understand the new requirements outlined in the MDR and ensure compliance to continue marketing and using medical devices in the EU market. The transition involves comprehensive changes, including adjustments to processes, documentation, and quality management systems to align with the new regulation. It's recommended to consult with legal experts and regulatory consultants to navigate the transition successfully.
IZiel team of specialists and quality professionals look forward to support more medical device companies to file their devices under the MDR 2017/745.
0 notes
Text
Process Validation IQ, OQ, PQ for Medical Device
Process Validation is a crucial aspect of the medical device manufacturing process to ensure that the products consistently meet the desired quality standards. It involves three main stages: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These stages help establish the reliability and robustness of the manufacturing process.
1. Installation Qualification (IQ):
IQ involves documenting and verifying that all equipment, utilities, facilities, and supporting systems are correctly installed and operational according to the manufacturer's specifications and intended use. The key steps in IQ include:
- Verifying that equipment is correctly installed and located as per design specifications.
- Confirm that all required utilities (power, water, gases, etc.) are properly connected and functioning.
- Ensuring that calibration and maintenance schedules are in place for all equipment.
- Documenting all installation activities and results.
2. Operational Qualification (OQ):
OQ focuses on testing the equipment and processes to ensure that they operate within defined parameters and are capable of consistently producing the desired results. The main steps in OQ include:
- Defining the operating ranges and limits for critical process parameters.
- Conducting tests to demonstrate that equipment functions within specified parameters.
- Performing worst-case scenario testing to validate the process's robustness.
- Collecting and analysing data to verify that equipment consistently meets specifications.
- Documenting all test protocols, results, and any deviations along with corrective actions taken.
3. Performance Qualification (PQ):
PQ demonstrates that the manufacturing process consistently produces products that meet all quality requirements and specifications. It involves testing the entire process using actual production materials and conditions. The key steps in PQ include:
- Conducting production runs using actual materials under normal operating conditions.
- Monitoring and collecting data throughout the production runs to ensure consistency.
- Analysing the data to confirm that the process consistently meets specifications.
- Addressing any deviations or out-of-spec results and implementing corrective actions.
- Documenting the entire PQ process, including protocols, data, and conclusions.
Throughout the IQ, OQ, and PQ stages, it's essential to maintain thorough documentation of all activities, tests, results, and corrective actions. This documentation serves as evidence that the manufacturing process is validated and can consistently produce safe and effective medical devices.
Remember that the specific requirements and guidelines for process validation can vary based on the regulatory environment, the type of medical device, and the standards applicable in your region. It's crucial to consult relevant regulatory guidelines (such as FDA's Quality System Regulation or ISO 13485) and work closely with your quality and regulatory teams during the validation process.
IZiel’s Onshore-Offshore Model works with 1 Onsite Member supported by a team of 3-4 engineers from our Technical Center in India. Typically, the data is collected, evaluated and evidence is developed from the process design stage throughout production. Our Model enables the company to complete the project faster and in a cost-effective manner.
0 notes
Text
Verification and Validation in Software Testing for medical device
Verification and validation are critical processes in software testing, especially for medical devices, where safety and reliability are of utmost importance. Let's delve into the concepts of verification and validation in the context of software testing for medical devices.
1. Verification:
Verification ensures that the software meets its specified requirements and is developed according to design specifications. It is essentially a quality control process that focuses on the correctness and completeness of the software at each stage of its development. In the context of medical devices, verification involves confirming that the software is built in accordance with the regulations and standards set by regulatory bodies (such as the FDA in the United States or the EU MDR in Europe).
Key steps in verification for medical device software:
- Requirements Analysis: Ensure that the software's requirements are well-defined, accurate, and traceable. This involves defining the intended functionality, performance, and safety requirements.
- Design Verification: Confirm that the software design meets the specified requirements. This involves reviewing design documents, code, and other artefacts to ensure they align with the intended functionality.
- Code Review and Inspection: Conduct thorough reviews of the code to identify defects, ensure coding standards compliance, and verify that the code accurately implements the design.
- Unit Testing: Test individual components or units of the software in isolation to ensure they behave as expected. Unit testing helps catch defects at an early stage.
- Integration Testing: Verify the interactions between different software components or modules to ensure they work together as intended.
- Static Analysis: Use automated tools to analyse code for potential issues such as coding standards violations, potential security vulnerabilities, and other defects.
2. Validation:
Validation ensures that the final software product meets the needs and intended use of the end users. It confirms that the software, when used in its intended environment, produces the desired outcomes, and operates safely and effectively. In the context of medical devices, validation is critical to ensure patient safety and product efficacy.
Key steps in validation for medical device software:
- User Requirements: Clearly define and document the user requirements, including the intended use, expected performance, and safety aspects of the software.
- Software Testing: Perform comprehensive testing to demonstrate that the software meets its intended use and requirements. This includes functional testing, performance testing, usability testing, and more.
- Risk Management: Identify and mitigate potential risks associated with the software's use, such as safety hazards, software failures, and other potential issues.
- Clinical Validation: For medical devices that directly impact patient care, clinical validation involves testing the software in a clinical environment to ensure it performs as intended and poses no harm to patients.
- Documentation: Keep detailed records of the validation process, including test results, risk assessments, and any deviations or corrective actions taken.
- Regulatory Compliance: Ensure that the validation process complies with relevant regulations and standards for medical device software, such as ISO 13485 and IEC 62304.
It's important to note that verification and validation are ongoing processes throughout the software development lifecycle. Regular updates, changes, and improvements to the software may require iterative rounds of verification and validation to ensure its continued safety and effectiveness. Additionally, involving domain experts, software engineers, and quality assurance professionals is essential to the successful verification and validation of medical device software.
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in the design and development of highly sophisticated implantable devices at industry-leading companies, with direct expertise in software V&V.
0 notes
Text
Step-by-Step Guide to Developing a CER
Developing a Clinical Evaluation Report (CER) involves a systematic process to assess the safety and performance of a medical device. Here's a step-by-step guide to help you in developing a CER:
Step 1: Define the Scope and Objectives
- Clearly define the scope of your CER, including the medical device, its intended use, and any specific regulatory requirements.
- Determine the objectives of the CER, such as demonstrating compliance with relevant regulations, evaluating post-market data, or supporting a new device submission.
Step 2: Identify Applicable Regulations and Guidelines
- Familiarize yourself with the applicable regulatory requirements, such as Medical Device Regulation (MDR) in the European Union or the US FDA's guidance documents.
- Identify relevant guidelines, such as MEDDEV 2.7/1 rev. 4, that provide detailed instructions on CER preparation.
Step 3: Collect and Review Data
- Gather all available data on the medical device, including preclinical and clinical studies, post-market surveillance data, complaints, adverse events, and any other relevant information.
- Review the collected data to identify potential gaps, inconsistencies, or areas requiring further investigation.
Step 4: Perform a Clinical Evaluation
- Conduct a systematic and comprehensive clinical evaluation of the medical device.
- Assess the device's safety, performance, and intended use against the defined scope and objectives.
- Evaluate the clinical data and compare it with the device's risk-benefit profile.
Step 5: Analyze and Summarize Data
- Analyze the collected data to identify any trends, patterns, or safety concerns.
- Summarize the key findings from the clinical evaluation, including the device's intended use, indications, contraindications, and identified risks.
Step 6: Prepare the CER
- Structure the CER according to the applicable guidelines, including the required sections and subsections.
- Include an executive summary, introduction, device description, clinical evaluation methodology, data analysis, and conclusion sections.
- Address any specific requirements outlined in the regulations or guidelines.
Step 7: Include Post-Market Clinical Follow-up (PMCF)
- Assess the need for post-market clinical follow-up studies based on the device's risk class and available clinical data.
- If required, plan and conduct PMCF activities to gather additional clinical evidence to support the ongoing safety and performance of the device.
Step 8: Review and Validate the CER
- Ensure that the CER is accurate, complete, and compliant with the applicable regulations and guidelines.
- Have the CER reviewed by subject matter experts, regulatory professionals, and other relevant stakeholders.
- Validate the data and conclusions presented in the CER with supporting evidence.
Step 9: Update and Maintain the CER
- Periodically update the CER as new data becomes available or when changes in regulations or guidelines occur.
- Keep a record of the updates made to the CER, including the rationale and supporting documentation.
- Maintain a well-organized and up-to-date CER for regulatory inspections and audits.
It's important to note that developing a CER can be a complex and resource-intensive process. Seeking expert advice and staying updated with the latest regulatory requirements will greatly contribute to the success of your CER development.
IZiel provides a unique solution for developing Clinical Evaluation Plan (CEP), and Clinical Evaluation Report (CER) and thereafter provides the physician’s certificate. Our partners have a network of 40+ National Board-Certified Physicians that conduct the risk-benefit analysis and provide the necessary certification.
0 notes
Text
How USFDA Consulting Firms Help Companies Navigate Regulatory Challenges
Selecting a medical devices consultant to help your organization with GMP, quality and compliance concerns can be daunting for any Quality executive. This is especially true for new or smaller companies that do not have existing relationships with consulting firms or solo consultants.
Selecting the right medical devices consulting firm is often one of the most important decisions an executive will make, mostly because projects in this functional area are so critical to a company’s business performance, and a project’s success is contingent on the selected firm’s competency and ability to achieve desired outcomes.

The following are some points for consideration.
· Specialization
· Thought Leadership
· Activeness in Industry
· Resources
· In-person Discussion
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
Choosing the Right USFDA Consulting Firm for medical devices
The medical device industry is rapidly growing, and with it, the importance of regulatory compliance. The United States Food and Drug Administration (USFDA) is responsible for the regulation and approval of medical devices in the United States. USFDA Consulting Firms and USFDA Regulatory Consultants can assist medical device companies in navigating the complex regulatory landscape to ensure compliance with USFDA regulations.

USFDA regulatory consultants are experts in the field of medical device regulations. They provide a range of services to medical device companies, including regulatory compliance, product registration, and product approval. Consulting firms can help companies navigate the complex and ever-changing FDA regulatory landscape, ensuring that their products meet the necessary standards.
This article will explore the factors to consider when choosing the right USFDA Consulting Firm for medical devices. We will discuss the importance of experience, expertise, and reputation in selecting a consulting firm. Additionally, we will highlight the key services provided by USFDA Consulting Firms and how they can benefit medical device companies.
Experience is a critical factor to consider when choosing a USFDA consulting firm. Look for a firm that has a proven track record of successfully guiding medical device companies through the regulatory process. A firm with a long history of working with medical device companies is likely to have a deep understanding of the regulatory requirements and challenges associated with the industry.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
IQ, OQ, PQ - A Guide to Process Validation
Process Validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications/requirements and quality characteristics. This enables to ensure the complete safety & efficacy of medical devices.
· Installation Qualification (IQ) - Installation qualification is used to ensure that the installation of any necessary equipment, piping, services, or instrumentation has been executed in accordance with the manufacturer's requirements.
· Operational Qualification (OQ) - During operational qualification, the equipment should be tested to determine process control limits, potential failure modes, action levels, and worst-case scenarios.
· Performance Qualification (PQ) - In the performance qualification phase, the goal is to demonstrate that the process will consistently produce acceptable results under normal operating conditions.
When do you use IQ, OQ and PQ?
Asked below mentioned questions to get the answer.
IQ – Is everything installed correctly?
OQ – Is everything operating correctly? & What are the operating limitations of the device?
PQ – Does this process produces the right results? & Is the process safe and consistent?

IZiel provides complete expertise in process validation and assists medical device manufacturers to consistently meet required parameters through their optimized manufacturing process.
IZiel’s Onshore-Offshore Model works with 1 Onsite Member supported by a team of 3-4 engineers from our Technical Center in India. Typically, the data is collected, evaluated and evidence is developed from the process design stage throughout production. Our Model enables the company to complete the project faster and in a cost-effective manner.
0 notes
Text
When should you start a QMS?
“When do I need a Quality Management System (QMS)?” is the most common question that we get from new clients who are just entering the medical device field. The answer depends on your target market and your exit plan.
QMS is a quality management system as the name suggests this is essential for any simplest medical device too. There are two aspects of QMS. One initial is to build a system wherein you will document the system mostly as per ISO standard 13485. System build means, writing down the procedure for each section of ISO stating what, how, when and where you are going to do. At this stage, you are only developing a strategy or system but not doing anything in action.
In the second stage, you will carry out the action and create evidence of action through records of your system. This is an explanation of what QMS one must do.
This is just a basic need. Then comes regulatory requirements which are different for different countries. These are must requirements, and you have no choice to follow them or not.
In theory, a medical device is supposed to be developed using design control as described in ISO 13485 and 21 CFR 820. In the US once clearance or approval is granted for a device it can be legally placed on the market and the FDA may inspect the manufacturing facility to ensure the required quality system is in place. During an inspection, the auditor looks for evidence that the QMS is being obeyed and this most often comes in the form of records, or more specifically manufacturing records.
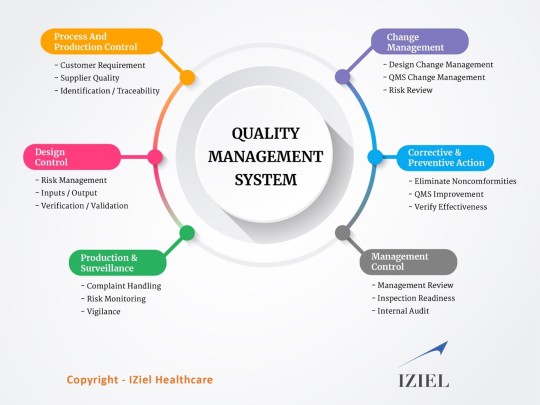
IZiel covers all the product-specific requirements for all components, manufacturing processes, verification & validation along with corrective and preventative actions (CAPA) for assessing customer satisfaction, product non-conformance, assessing and improving quality policies and procedures, carrying out and assessing the results of internal audits, and implementing systems for continuous improvement.
IZiel works with clients to make the QMS simple yet effective and flexible to allow changes to keep up with the changing regulatory requirements.
#Quality Management System#QMS Certificate#QMS Documentation#ISO 13485 Certification#ISO 13485 Consultants
0 notes
Text
Process Validation: Medical Device Vs. Pharma
Process validation is a formal methodology that allows companies to manufacture products on approved and qualified equipment. A defined process leads to products that consistently meet their predetermined specifications and quality requirements.
Process Validation for medical devices is conducted to ensure consistent delivery of quality products meeting their predetermined specifications/requirements and quality characteristics. This enables to ensure the complete safety & efficacy of medical devices.
Pharma Vs. Medical Device Process Validations, Points to be considered as bellow.
1. Qualification Strategy
2. Timing of Critical Parameters
3. Measurement Method
4. Process
5. Equipment
6. Batch Production
7. Process Validation
Pharmaceuticals and Medical Devices must be manufactured to the highest quality levels. Product testing by itself does not guarantee the quality of the product. Process Validation assists in building the quality into the product and has proven to be an important tool for quality management of Pharmaceuticals and Medical Devices.
IZiel has successfully delivered various projects through the Onshore-Offshore Work Model, completing projects faster and with major cost savings. IZiel team works together with the client to plan, execute, compile reports, and receive stakeholder approvals for all process validation projects.
0 notes
Text
Do medical devices need to be approved by the FDA?
Medical Device Manufacturers require USFDA Approvals to sell their products in USA. USFDA differentiates product approvals in Class I, II &III depending upon the risk associated. The submissions include self-certification, 510(k) and PMA depending upon the class of the product.
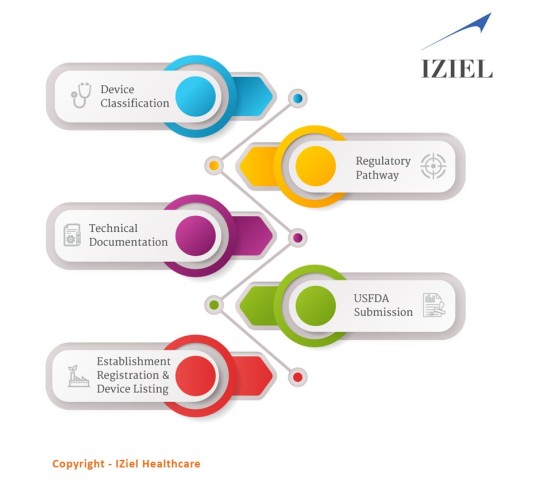
The FDA medical devices have been classified into 3 classes.
Class I: They are low-risk devices.
Class II: They are medium-moderate risk devices.
Class III: They are of high risk, generally life supporting and life-sustaining.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Text
Things You Need to Know About Medical Device Software Validation
Software Verification and Validation (Software V&V) is an integral part of software design that spans all the development stages as specified in IEC 62304 which addresses the Software Development Life Cycle (SDLC) of medical software and software embedded within medical devices. Software Verification is to test if the software was designed as per requirements and Software Validation is to test if the right product was built for the user. Medical device software validation generally occurs during or at the end of the development cycle.
The difference between Software Validation & Software Verification can be answered by asking these mentioned questions:
· Verification: Are we building the product, right?
· Validation: Are we building the right product?
Software validation is a process of checking if the product will meet the customer’s actual needs, while verification involves procedures for making certain that the software is well-engineered, free of errors, and functional.
Following are the steps for Software Validation for Medical Devices
· Create a software validation plan
· Determine system requirements
· Create a validation protocol and test specifications
· Conduct and document tests
· Establish procedures and write your final report
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in the design and development of highly sophisticated implantable devices at industry-leading companies, with direct expertise in software V&V.
0 notes