#Remdesivir for injection 100 mg
Photo
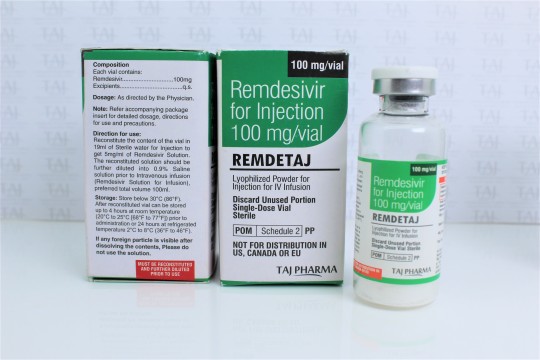


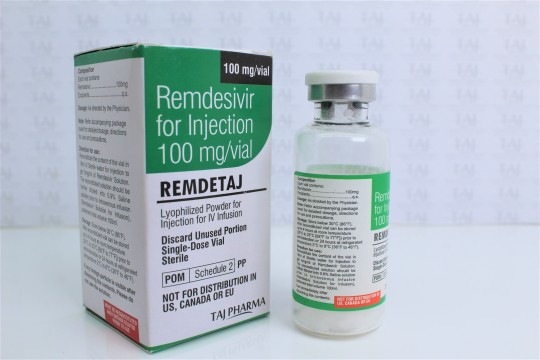

For treatment of suspected or laboratory confirmed corona virus disease 2019 (COVID-19) in adults and children hospitalized with moderate to severe disease.
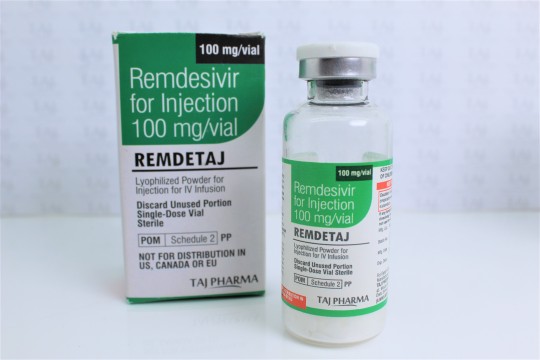
Remdesivir for Injection 100 mg/vial
Lyophilized Powder for Injection for IV Infusion
Lyophilized Powder
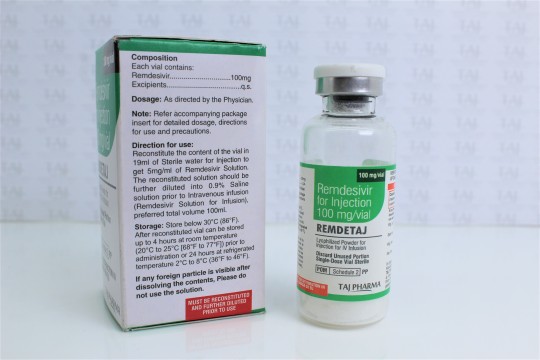
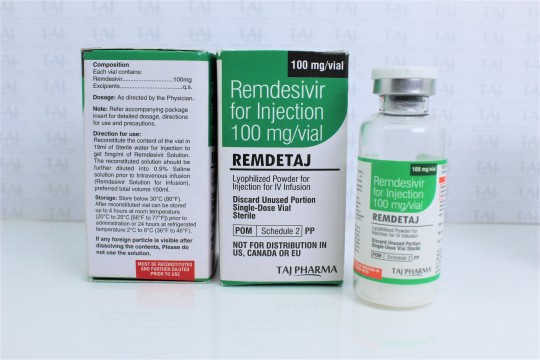
Remdesivir for injection, 100 mg, is a sterile, preservative-free lyophilized powder that is to be reconstituted with 19 mL of Sterile Water for Injection and diluted into 0.9% saline prior to administration by intravenous infusion. Remdesivir for injection, 100 mg, is supplied in a single-dose clear glass vial. The appearance of the lyophilized powder is white to off-white to yellow.
Inactive Ingredients
The inactive ingredients are sulfobutylether-β-cyclodextrin sodium salt (SBECD), Water for Injection, USP, and may include hydrochloric acid and/or sodium hydroxide for pH adjustment. Remdesivir for injection, 100 mg, contains 3 g SBECD.
General Information
The optimal dosing and duration of treatment are unknown. The suggested dose and duration may be updated as data from clinical trials become available.
Adult and pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing of remdesivir.
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir.
Remdesivir should be administered via intravenous (IV) infusion only. Do not administer as an intramuscular (IM) injection.
Adult Patients
The dose of the drug for adults should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
Remdesivir is to be administered via intravenous infusion in a total volume of up to 250 mL 0.9% saline over 30 to 120 minutes [For further information see Dosage and Administration].
Extension of administration of the drug beyond 5 days to 10 days is not recommended.
All adult patients must have creatinine clearance determined before dosing [For further information see Dosage and Administration].
Hepatic laboratory testing should be performed in all patients before starting remdesivir and daily while receiving remdesivir dosing [For further information see Dosage and Administration].
Pediatric Patients
Dosing in pediatric patients is based upon physiologically-based (PBPK) modeling and simulation of pharmacokinetic data from healthy adult subjects. The recommended pediatric dose for pediatric patients weighing between 3.5 kg and <40 kg should be calculated using the mg/kg dose according to the patient’s weight [For further information see Dosage and Administration];
The dose of the drug for pediatric patients weighing more than 40 kg should be a single dose of 200 mg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 100 mg, infused intravenously over 30-120 minutes for 4 days.
The dose for pediatric patients with bodyweight between 3.5 kg and less than 40 kg should be a single dose of 5 mg/kg infused intravenously over 30-120 minutes on day 1 followed by a once-daily maintenance dose of 2.5 mg/kg, infused intravenously over 30-120 minutes for 4 days.
Extension of administration of the drug beyond 5 days to 10 days is not recommended.
Pediatric patients (>28 days old) must have an eGFR determined and full-term neonates (≥7 days to ≤28 days old) must have serum creatinine determined before dosing [For further information see Dosage and Administration].
COVID-19: coronavirus disease 2019
0 notes
Text
0 notes
Text
#Remdesivir 100 mg #injections which is very essential #LifeSavingDrug in the #hospitalized #ICU #COVID #patients. Our company carries expertise in the production of Remdetaj Injection #OmicronVariant
Lyophilized.#tajpharma #EmergencySupplies. 👉More: https://bit.ly/3BsZTqj.
Ready stock available.
#manufacturing#tajpharma#injection#emergencyresponse#product#pharmaceutical#supplier#exporter#india#maldives#oman#bahrain#cambodia#saudiarabia#lebanon#uganda#africa#iran#middleeast#country
0 notes
Text
Asia-Pacific Generic Drugs Market 2021: Top Industry Players, Growth Report to 2027
Asia-Pacific generic drugs market is estimated to grow at a CAGR of nearly 8.0% during the forecast period. A significant rise in the prevalence of chronic diseases is the major factor accelerating the demand for generic medicines in the region. As per the estimation by the World Health Organization (WHO), the number of new cancer incidences expected to be reported in china was nearly 4.6 million in 2020. Further, the National Cancer Registry Programme estimates that there will be 13.9 lakh new cancer incidences reported in India in 2020, which is expected to rise to 15.7 lakh by 2025.
(Get 15% Discount on Buying this Report)
Get Report Sample Copy @ https://www.omrglobal.com/request-sample/asia-pacific-generic-drugs-market
This, in turn, is leading to increasing demand for generic drugs in these countries. Cost is the major benefit associated with the use of generic drugs. The cost of generic drugs can be up to 85% lower compared to the brand-name drug. Generic drug firms do not have to invest in high initial drug development costs which results in the low cost of these drugs compared to branded drugs. The low cost of generics is acting as a major factor accelerating market growth.
A Full Report of Asia-Pacific Generic Drugs Market is Available at: https://www.omrglobal.com/industry-reports/asia-pacific-generic-drugs-market
Scope of the Asia-Pacific Generic Drugs Market
Market Coverage
Market number available for 2019-2026
Base year- 2019
Forecast period- 2020-2026
Segment Covered- By Application and Route of Administration
Regions Covered- China, India, Japan, and Rest of Asia-Pacific
Competitive Landscape- Mylan N.V., Teva Pharmaceutical Industries Ltd., Lupin Ltd., Aurobindo Pharma Ltd., and Sun Pharmaceutical Industries Ltd.
Recent Strategic Initiatives in the Asia-Pacific Generic Drugs Market
In July 2020, Cipla declared the launch of its generic version of antiviral drug remdesivir, Cipremi, at $53.3 per 100 mg vial. This makes it among the lowest cost versions for COVID-19 treatment and Cipla is looking to deliver more than 80,000 vials within the first month.
In July 2020, Mylan launched a generic version of Remdesivir drug, Desrem, in India for treatment of COVID-19 patients. This drug will be available at a price of nearly $64.2 per 100 mg vial as against Hetero’s Covifor at nearly 72.2 per 100 mg vial and Cipla’s Cipremi costing nearly $53.3 per 100 mg vial.
Asia-Pacific Generic Drugs Market-Segmentation
By Application
Cancer
CVD
Musculoskeletal Diseases
Infectious Diseases
Neurology
Diabetes
Others
By Route of Administration
Oral
Topical
Injectable
Inhaler
Asia-Pacific Generic Drugs Market– Segment by Country
China
Japan
India
Rest of Asia-Pacific
Reasons to Buying From us –
1. We cover more than 15 major industries, further segmented into more than 90 sectors.
2. More than 120 countries are for analysis.
3. Over 100+ paid data sources mined for investigation.
4. Our expert research analysts answer all your questions before and after purchasing your report.
For More Customized Data, Request for Report Customization @ https://www.omrglobal.com/report-customization/asia-pacific-generic-drugs-market
About Orion Market Research
Orion Market Research (OMR) is a market research and consulting company known for its crisp and concise reports. The company is equipped with an experienced team of analysts and consultants. OMR offers quality syndicated research reports, customized research reports, consulting and other research-based services.
Media Contact:
Company Name: Orion Market Research
Contact Person: Mr. Anurag Tiwari
Email: [email protected]
Contact no: +91 780-304-0404
0 notes
Photo

ONE HELD FOR ILLEGALLY PROCURING & SELLING REMDESIVIR INJECTION “Redyx-L” IN BLACK MARKET ON HIGHER PRICE On 07-05-2021, on credible information the sleuths of Commissioner’s Task Force, North Zone Team laid a trap at H.No. 2-2-57/3. Pan Bazar in the limits of Mahankali PS and apprehended one person by name Akula Mehul Kumar who is illegally procuring and selling Remdesivir Injections “ Redyx-L” in black market on higher price to needy customers which are being used for Antiviral medicine for Covid-19 patients to gain easy money illegally. Particulars of accused:- Akula Mehul Kumar S/o. M.Vijay Kumar, age. 26 yrs,Occ. Pvt job R/o. Pan Bazar, James Street, Secunderabad, Seized material – Covid Medicine : 1. (04) Remdesivir Injections “Redyx-L” each 100 MG 2. One cell phone Brief facts of the case :- The accused Akula Mehul Kumar is residing in the limits of James Street, Secunderabad. He is employee in HSBC bank at Hi-Tech City. His father M.Vijay Kumar is running Medical shop in the limits of Pan Bazar, Secunderabad. Due to Covid 19 pandemic situation huge demand of the Remdesivir “Redyx-L” injections which are being used for Antiviral medicine for Covid-19 patients. Accused hatched a plan to gain easy money by illegally procuring the Remdesivir injections in low price thorough known sources and selling the same in black market on high price without any valid documents to needy customers. Today i.e on 07-05-2021 the accused illegally procured (04) Remdesivir “Redyx-L” injections and set a deal with a customer for selling Rs. 35,000/- each and trying to deliver the same, meanwhile on tip of apprehended the accused Akula Mehul Kumar and seized (4) Remdesiver injection “Redyx-L” & one cell phone from his possession. The apprehended accused person along with seized material handed over to S.H.O. Mahankali PS for taking further action. The arrest was made under the supervision of Sri.P.Radhakishan Rao, Dy. Commissioner of Police (OSD), Commissioner’s Task Force, Hyderabad lead by K.Nageswar Rao, Inspector of Police, Commissioner’s Task Force, North Zone and North Zone Task Force https://www.instagram.com/p/COlCp2NLwXC/?igshid=4rbaq2o0ov5i
0 notes
Text
Identify fake Remdesivir through IPS officer Monika Bhardwaj’s 9-point tutorial

IPS officer Monika Bhardwaj has pointed out nine 'errors' present on the package of the fake version of Remdesivir injection that can help differentiate it from the genuine jab.
The Delhi Police arrested at least four persons for alleged black marketing of Remdesivir, an injection prescribed for Covid-19 patients with severe illness. The drug is reported to be out of stock at several hospitals and is being sold at highly inflated prices on the black market.
Taking advantage of the short supply of Remdesivir, some people were found to be selling fake Remdesivir. Police have arrested some people in Uttar Pradesh and Madhya Pradesh for selling fake Remdesivir injections.
Now, IPS officer Monika Bhardwaj, DCP of the Crime Branch of the Delhi Police, has posted tweets “for educational purposes” detailing how to differentiate genuine Remdesivir from a fake vial.
Monika Bhardwaj points out nine 'errors' present on the package of the fake version of Remdesivir injection that can help differentiate it from the genuine jab.
1. Fake Remdesivir packages do not have “Rx” written on it just before the name of the injection.
2. A capitalisation error in the third line written on the package. The genuine package reads as “100 mg/Vial” while the fake one has “100 mg/vial” written on it.
3. There is alignment error in the brand name of the product. Note the gap on the package of fake and genuine Remdesivir injections. The fake vial has an increased gap.
4. There is another capitalisation error below the brand name in “Vial/vial” on the fake package.
5. One more capitalisation error is found at the bottom of the front side of the fake Remdesivir package. “For use in” written on the genuine package becomes “for use in” on the fake drug package.
6. On the back of the box, the “Warning” label is in red on the genuine package. The fake one has a black warning label.
7. Just below the warning label, key information “Covifir [brand name] is manufactured under the licence from Gilead Sciences, Inc” is missing on the fake injection package.
8. There is a capitalisation error in the text identifying the drug-maker, Hetero Labs. The fake Remdesivir package reads India as “india”.
9. There is a spelling error in the full address on the package containing the fake Remdesivir injection. It spells Telangana as “Telagana”.
Read more here: https://www.indiatoday.in/.../identify-fake-remdesivir...
0 notes
Text
Gilead files COVID-19 drug remdesivir with FDA

Gilead has filed its COVID-19 drug remdesivir with the FDA, to treat patients with severe disease, under the brand name Veklury.
The drug is currently available to US patients under an Emergency Use Authorisation for treatment of hospitalised patients with severe COVID-19.
This filing is the last part of a rolling submission with the FDA that the company began in early April and could lead to a full licence for the drug, which was the first to show significant improvements in recovery time in patients with severe disease.
The filing is supported by data from two randomised, open-label, multi-centre phase 3 clinical studies of Veklury conducted by Gilead.
It also includes the phase 3 randomised, placebo-controlled study of Veklury conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
Data from the studies showed treatment with Veklury led to faster time to recovery compared with placebo and that a 5-day or 10-day treatment duration led to similar clinical improvement.
Across studies, Veklury was generally well tolerated in both the 5-day and 10-day treatment groups, with no new safety signals identified.
The filing with the FDA follows approval by several regulators across the world, including in the European Union and Japan.
Originally intended as a treatment for Ebola virus, Veklury is a nucleotide analogue with broad-spectrum antiviral activity both in vitro and in vivo in animal models against several emerging viruses.
There are ongoing phase 3 trials testing its safety and efficacy in patients infected by the SARS-CoV-2 coronavirus that causes COVID-19, including combination trials with other drugs.
Veklury must be administered via intravenous (IV) infusion and is supplied two ways: Veklury (remdesivir) for injection, 100 mg, lyophilised powder, or Veklury (remdesivir) injection, 100 mg/20 mL (5 mg/mL), concentrated solution.
Last week Pfizer signed a multi-year agreement with Gilead to manufacture and supply the COVID-19 antiviral remdesivir, which is also being tested in combination with other drugs in an effort to fight the pandemic.
Under the terms of the agreement Pfizer will become one of several external partners who manufacture the investigational treatment for the disease.
Pfizer will provide contract manufacturing services at its facility in McPherson, Kansas, to supply the drug to Gilead.
Several other drugs are being tested as a treatment for the disease: one of the most promising is the off-patent steroid dexamethasone, which lowered mortality in a UK-based phase 3 trial and is much cheaper than Veklury.
Feature image courtesy of Rocky Mountain Laboratories/NIH
The post Gilead files COVID-19 drug remdesivir with FDA appeared first on .
from https://pharmaphorum.com/news/gilead-files-covid-19-drug-remdesivir-with-fda/
0 notes
Text
Generic version of REMDESIVIR launched for the treatment
Generic version of REMDESIVIR launched for the treatment
Medication firm Jubilant Life Sciences said it has propelled the conventional adaptation of antiviral medication remdesivir in India for the treatment of COVID-19 patients. The injectable medication, under the brand name ‘JUBI-R’, is evaluated at Rs 4,700 for every vial of 100 mg.
The organization will make the medication accessible to more than 1,000 clinics giving COVID-19 treatment, Jubilant…
View On WordPress
#COVID-19#COVID-19 Vaccine#india corona curve#JUBI-R#jubilant life sciences#Remdesivir Drug#Rs 4700 for 100mg vial#treatment
0 notes
Text
Jubilant Life Sciences launches generic version of remdesivir for COVID-19 treatment
Jubilant Life Sciences launches generic version of remdesivir for COVID-19 treatment
[ad_1]

Image Source : FILE
The injectable drug, under the brand name ‘JUBI-R’, is priced at Rs 4,700 per vial of 100 mg.
Drug firm Jubilant Life Sciences on Monday said it has launched the generic version of antiviral drug remdesivir in India for the treatment of COVID-19 patients. The injectable drug, under the brand name ‘JUBI-R’, is priced at Rs 4,700 per vial of 100 mg. The company…
View On WordPress
0 notes
Photo
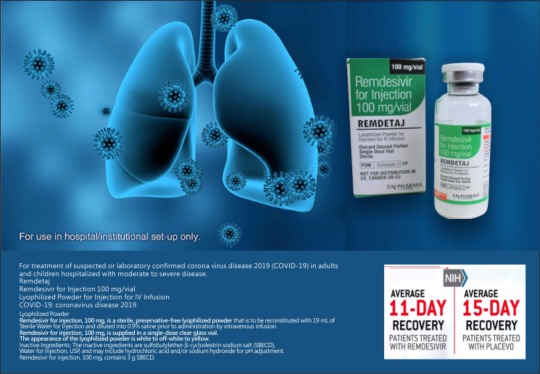
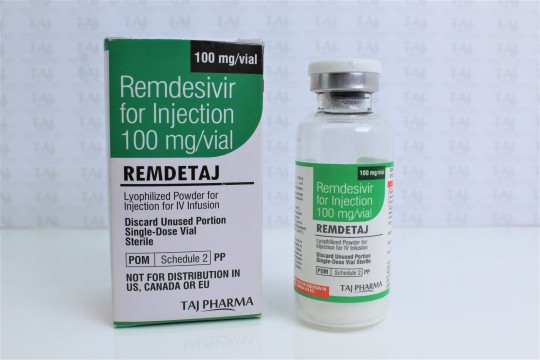
For treatment of suspected or laboratory confirmed corona virus disease 2019 (COVID-19) in adults and children hospitalized with moderate to severe disease. #Remdesivir for Injection 100 mg/vial Lyophilized Powder for #Injection for IV Infusion #COVID-19: coronavirus disease 2019
0 notes
Photo

Jubilant Life Sciences launches generic version of remdesivir for COVID-19 treatment Image Source : FILE The injectable drug, under the brand name 'JUBI-R', is priced at Rs 4,700 per vial of 100 mg.
#coronavirus drug#COVID19#Generic#Jubilant#Jubilant Life Sciences#launches#life#remdesivir#Sciences#Treatment#version
0 notes
Text
Jubilant Life Sciences launches remdesivir for injection at ₹4,700 per vial
Jubilant Life Sciences launches remdesivir for injection at ₹4,700 per vial
Jubilant Life Sciences on Monday announced that its arm, Jubilant Generics, launched remdesivir for injection under the brand name ‘JUBI-R’ in the Indian market at a price of ₹4,700 per vial of 100 mg (lyophilized injection).
Jubilant Generics will make the drug available to over 1,000 hospitals providing Covid-19 treatment in India through its distribution network.
In May 2020, Jubilanten…
View On WordPress
0 notes
Text
COVID-19: Hetero, Cipla get nod to manufacture, market antiviral drug remdesivir
COVID-19: Hetero, Cipla get nod to manufacture, market antiviral drug remdesivir
By: PTI | New Delhi |
Up to date: June 21, 2020 10:48:26 am

The drug, administered within the type of injection, needs to be given at a dose of 200 mg on day one adopted by 100 mg day by day for 5 days. (File)
India’s Drug Regulator on Saturday gave permission to Hetero and Cipla to fabricate and market antiviral…
View On WordPress
#Coronavirus#Coronavirus medicines#coronavirus treatment#coronavirus vaccine#how remdesivir works#indian express#remdesivir#remdesivir covid19#remdesivir dose#remdesivir explained#remdesivir india#remdesivir side effects#remdesivir tablets coronavirus#remdesivir uses
0 notes
Text
Jubilant Life gets DCGI nod to manufacture generic Remdesivir
Jubilant Life gets DCGI nod to manufacture generic Remdesivir
[ad_1]
Jubilant’s Remdesivir will be marketed under the brand name ‘Jubi-R’ in India and will be made available in 100 mg vials (injectable), it added. (Representative image)
Jubilant Life Sciences has received the Drug Controller General of India (DCGI) approval to manufacture generic version of antiviral drug Remdesivir 100 mg per vial for restricted emergency use for treatment of severe…
View On WordPress
0 notes
Text
Zydus Cadila's Remdesivir at Rs 2,800 cheapest so far; triggers price war in COVID-19 treatment
Zydus Cadila’s Remdesivir at Rs 2,800 cheapest so far; triggers price war in COVID-19 treatment
[ad_1]
KEY HIGHLIGHTS
Zydus’ drug is priced at Rs 2,800 per 100 mg vial
Its Remdac will be the most economical Remdesivir brand in India
Cipla’s Cipremi costs Rs 4,000, Hetero Healthcare’s Covifor Rs 5,400, Mylan’s version Rs 4,800 and Jubilant’s JUBI-R Rs 4,700
Ahmedabad-based Zydus Cadila has launched the cheapest Remdesivir injection for COVID-19 treatment in India so far, triggering a…
View On WordPress
0 notes
Text
Jubilant Life hits 52-week high on DCGI nod for Remdesivir; Rakesh Jhunjhunwala raises stake in June quarter
Jubilant Life hits 52-week high on DCGI nod for Remdesivir; Rakesh Jhunjhunwala raises stake in June quarter
The company#39;s subsidiary Jubilant Generics has received the approval from Drug Controller General of India (DCGI) to manufacture and market investigational drug Remdesivir 100 mg/vial (lyophilized injection).
View On WordPress
0 notes