#Tumor Heterogeneity
Text
Personalized Approaches to Cutaneous Squamous Cell Carcinoma Treatment: Targeting Tumor Diversity
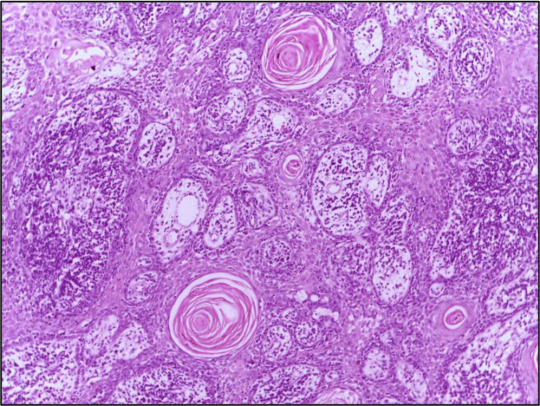
Cutaneous Squamous Cell Carcinoma (cSCC) is a heterogeneous disease characterized by diverse clinical and molecular features. Personalized treatment approaches that take into account the unique characteristics of individual tumors have emerged as a promising strategy to improve treatment outcomes and patient survival.
Understanding Cutaneous Squamous Cell Carcinoma: Cutaneous Squamous Cell Carcinoma (cSCC) is a type of skin cancer that arises from the malignant transformation of squamous cells in the epidermis or its appendages. It encompasses a spectrum of disease presentations, ranging from localized lesions to metastatic tumors with varying clinical behaviors.
Tumor Heterogeneity and Molecular Subtypes: Cutaneous Squamous Cell Carcinoma (cSCC) exhibits considerable heterogeneity at the molecular level, with distinct genetic alterations and signaling pathways driving tumor progression and metastasis. Molecular subtyping studies have identified different subgroups of cSCC tumors based on their genomic profiles, providing insights into tumor diversity and potential therapeutic targets.
Precision Medicine in cSCC Treatment: Precision medicine approaches aim to tailor treatment strategies to the specific molecular characteristics of individual tumors, allowing for more targeted and effective therapies. By identifying actionable mutations or biomarkers, clinicians can select therapies that are most likely to benefit patients while minimizing the risk of treatment-related toxicities.
Genomic Profiling and Biomarker Identification: Advances in genomic sequencing technologies have enabled comprehensive profiling of cSCC tumors, revealing recurrent mutations in genes involved in cell cycle regulation, DNA repair, and immune evasion. Biomarker identification efforts seek to identify predictive markers of treatment response and prognosis, guiding treatment decisions in personalized medicine.
Get More Insights On This Topic: Cutaneous Squamous Cell Carcinoma
#Cutaneous Squamous Cell Carcinoma#Skin Cancer#Tumor Heterogeneity#Precision Medicine#Molecular Subtypes#Targeted Therapy#Immunotherapy#Biomarker Identification#Personalized Treatment
0 notes
Link
Single-cell technologies are effective drug development and discovery tools, serving important functions at several phases. They aid in identifying potential therapeutic targets, streamline single-cell high-throughput screening, and support pharmacokinetic investigations of anti-tumor medications. New tools for single-cell research combine multiple molecular information, increase the size of data, and characterize the influence of genes on phenotypic outcomes. Shandong University researchers outlined emerging single-cell technologies in this review, which offers a thorough understanding of tumor biology. They also methodically summarised the applications of single-cell technologies in various sections of drug discovery for tumor treatment, including target identification, high throughput drug screening, and pharmacokinetic evaluation. It is anticipated that drug discovery based on single-cell technologies will enhance therapeutic approaches and enhance the clinical results of cancer patients.
Single-cell technologies, including transcriptomic, proteomic, epigenomic, and genomic technologies, offer a comprehensive understanding of biological systems with single-cell resolution. Large cell populations are enhanced by these technologies, which also show the variety of tumor tissues and paint a thorough picture of intricate biology. Nevertheless, certain cell subpopulations and states that are crucial for tumor growth and treatment response may be hidden by the signal averaging process.
Continue Reading
4 notes
·
View notes
Text
Activity 01
Following my review of the Gapminder study codebook, I have chosen to conduct an analysis of a global health concern: breast cancer. In conjunction with this primary focus, I will also explore the issue of suicide rates per hundred thousand individuals.
Question: Is breast cancer associated with suicide per 100th?
Variables: breastcancerper100th and suicideper100th
Hypothesis: Women diagnosed with breast cancer may be more likely to commit suicide compared to the general population.
Literature review:
Suicide After Breast Cancer: an International Population-Based Study of 723 810 Women
Catherine Schairer, Linda Morris Brown, Bingshu E. Chen, Regan Howard, Charles F. Lynch, Per Hall, Hans Storm, Eero Pukkala, Aage Anderson, Magnus Kaijser ... Show more
Summary: Few studies have examined long-term suicide risk among breast cancer survivors, and there are no data for women in the United States. We quantified suicide risk through 2002 among 723 810 1-year breast cancer survivors diagnosed between January 1, 1953, and December 31, 2001, and reported to 16 population-based cancer registries in the United States and Scandinavia. Among breast cancer survivors, we calculated standardized mortality ratios (SMRs) and excess absolute risks (EARs) compared with the general population, and the probability of suicide. We used Poisson regression likelihood ratio tests to assess heterogeneity in SMRs; all statistical tests were two-sided, with a .05 cutoff for statistical significance. In total 836 breast cancer patients committed suicide (SMR = 1.37, 95% confidence interval [CI] = 1.28 to 1.47; EAR = 4.1 per 100 000 person-years). Although SMRs ranged from 1.25 to 1.53 among registries, with 245 deaths among the sample of US women (SMR = 1.49, 95% CI = 1.32 to 1.70), differences among registries were not statistically significant ( P for heterogeneity = .19). Risk was elevated throughout follow-up, including for 25 or more years after diagnosis (SMR = 1.35, 95% CI = 0.82 to 2.12), and was highest among black women (SMR = 2.88, 95% CI = 1.44 to 5.17) ( P for heterogeneity = .06). Risk increased with increasing stage of breast cancer ( P for heterogeneity = .08) and remained elevated among women diagnosed between 1990 and 2001 (SMR = 1.36, 95% CI = 1.18 to 1.57). The cumulative probability of suicide was 0.20% 30 years after breast cancer diagnosis.
Topic: cancerheterogeneityearfollow-upscandinaviasurvivorsdiagnosissuicidebreast cancerlikelihood ratiosuicidal behaviortnm breast tumor stagingstandardized mortality ratio
Issue Section: Brief Communications
References:
(1) Rowland J, Mariotto A, Aziz N, Tesauro G, Feuer EJ, Blackman D, et al. Cancer survivorship — United States, 1971 – 2001. MMWR Morb Mortal Wkly Rep 2004 ; 53 : 526 – 9.
(2) Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al., editors. SEER cancer statistics review, 1975 – 2002. Bethesda (MD): National Cancer Institute; 2004. Available at: http://seer.cancer.gov/csr/1975_2002 . [Last accessed: September 22, 2005.]
(3) Yousaf U, Christensen M-LM, Engholm G, Storm HH. Suicides among Danish cancer patients 1971 – 1999. Br J Cancer 2005 ; 92 : 995 – 1000.
2 notes
·
View notes
Text


This week we are discussing imaging of brain tumors in adults. MRI with and without contrast is the imaging modality of choice for brain tumors, although many tumors are initially discovered at CT performed for other purposes.
Today’s case is a 75-year-old woman who presented with right-sided weakness. CT revealed a large, heterogeneous mass centered in the left thalamus / basal ganglia. Internal hypoattenuation is compatible with necrosis, while surrounding hypoattenuation is compatible with edema. There is obstructive hydrocephalus. MRI confirmed the findings and revealed peripheral, nodular enhancement and extension across the midline and along the brain stem.
Findings are highly suggestive of high-grade glioma. Stereotactic biopsy confirmed glioblastoma, IDH wild type.
Case courtesy of Bruno Di Muzio, Radiopaedia.org, rID: 40573
#TeachingRounds#Neurology#Radiology#Neurosurgery#Oncology#Neuroradiology#NeuroOncology#cancer#NeuroRad#MRI#MRIRad
10 notes
·
View notes
Text
What Are The Various Neuroendocrine Tumor Treatments?

Neuroendocrine tumors refer to a group of rare and heterogeneous neoplasms that arise from neuroendocrine cells. These tumors can occur in various parts of the body, including the lungs, pancreas, gastrointestinal tract, and others. The treatment options for neuroendocrine tumors depend on several factors, such as the location and stage of the tumor, as well as the patient's overall health. In recent years, there have been significant advancements in the management of these tumors, leading to improved outcomes and better quality of life for patients. For more information visit the below-mentioned source link.
Source: Neuroendocrine Tumor Treatments
3 notes
·
View notes
Text
Clinical Oncology Next Generation Sequencing Market 2024 : Size, Growth Rate, Business Module, Product Scope, Regional Analysis And Expansions 2033
The clinical oncology next generation sequencing global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.

Clinical Oncology Next Generation Sequencing Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size -
The clinical oncology next generation sequencing market size has grown rapidly in recent years. It will grow from $0.45 billion in 2023 to $0.52 billion in 2024 at a compound annual growth rate (CAGR) of 15.5%. The growth in the historic period can be attributed to genomic research advances, cancer biomarker discovery, technological advancements, regulatory approvals.
The clinical oncology next generation sequencing market size is expected to see rapid growth in the next few years. It will grow to $0.86 billion in 2028 at a compound annual growth rate (CAGR) of 13.2%. The growth in the forecast period can be attributed to growing cancer incidence, precision medicine, immuno-oncology, liquid biopsies. Major trends in the forecast period include comprehensive genomic profiling (cgp), immuno-oncology, tumor evolution and heterogeneity, ai and machine learning.
Order your report now for swift delivery @
https://www.thebusinessresearchcompany.com/report/clinical-oncology-next-generation-sequencing-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers -
The rise in the number of cancer cases across the globe is likely to contribute to the growth of the clinical oncology next-generation sequencing market during the forecast period. According to the American Cancer Society, there were 1.9 million new cases and 0.6 million cancer deaths in 2021 in the USA. The four most common types of cancer worldwide are lung, prostate, bowel, and female breast cancer, accounting for 43% of all the new cancer cases. Therefore, the rise in cancer incidence rate globally is anticipated to boost the demand for the growth of the clinical oncology next-generation sequencing market.
The clinical oncology next generation sequencing market covered in this report is segmented –
1) By Technology: Ion Semiconductor Sequencing, Pyro-Sequencing, Synthesis Sequencing, Real Time Sequencing, Ligation Sequencing, Reversible Dye Termination Sequencing, Nano-Pore Sequencing
2) By Application: Screening, Companion Diagnostics, Other Diagnostics
3) By End User: Hospital Laboratories, Clinical Research Organizations, Diagnostic laboratories
Get an inside scoop of the clinical oncology next generation sequencing market, Request now for Sample Report @
https://www.thebusinessresearchcompany.com/sample.aspx?id=3344&type=smp
Regional Insights -
North America was the largest region in the clinical oncology next-generation sequencing market in 2023. Asia-Pacific was the second largest region in the clinical oncology next-generation sequencing market. The regions covered in the clinical oncology next generation sequencing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa
Key Companies -
Major companies operating in the clinical oncology next generation sequencing market include Thermo Fisher Scientific, Oxford Nanopore Technologies Ltd., QIAGEN N.V., Myriad Genetics Inc., Illumina Inc., F. Hoffmann-La Roche Ltd., PerkinElmer Inc., Agilent Technologies Inc., Pacific Biosciences of California Inc., Caris Life Sciences, Paradigm Diagnostics, GATC Biotech AG, Macrogen Inc., DNASTAR Inc., Exosome Diagnostics Inc., Biomatters Ltd., Partek Inc., Foundation Medicine Inc., Becton Dickinson and Company (BD), Takara Bio Inc., Creative Biolabs, Mogene LC, Knome Inc., Genomatix Software GmbH, CLC bio, GnuBIO Inc., Bio-Rad Laboratories Inc., BGI Genomics Co. Ltd., Guardant Health Inc., Invitae Corporation, Natera Inc., NeoGenomics Laboratories Inc., Sysmex Corporation, Veracyte Inc., Zymo Research Corporation, ArcherDX Inc., Cepheid, Karius Inc., OncoDNA S.A., Personal Genome Diagnostics Inc., PierianDx Inc.
Table of Contents
1. Executive Summary
2. Clinical Oncology Next Generation Sequencing Market Report Structure
3. Clinical Oncology Next Generation Sequencing Market Trends And Strategies
4. Clinical Oncology Next Generation Sequencing Market – Macro Economic Scenario
5. Clinical Oncology Next Generation Sequencing Market Size And Growth
…..
27. Clinical Oncology Next Generation Sequencing Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
“Papillary Renal Cell Carcinoma”, Victor McKusick, Mendelian Inheritance in Man, 1966.
Here I present: “Papillary Renal Cell Carcinoma”, Victor McKusick, Mendelian Inheritance in Man’, 1966.
INTRODUCTION.
Papillary renal cell carcinoma (PRCC) is a malignant, heterogeneous tumor originating from renal tubular epithelial cells of the kidney, which comprises approximately 15% of all kidney neoplasms. Based on its morphological features, PRCC can be classified into two main…

View On WordPress
0 notes
Text
Tumor Heterogeneity, Tumor Evolution, and Emerging Cancer Genomics
National Cancer Institute, National Institutes of Health
????Postdoc Opportunity! Join our cutting-edge research team at NCI DCEG! We’re looking for talented individuals to explore cancer genomics.
See the full job description on jobRxiv: https://jobrxiv.org/job/national-cancer-institute-national-institutes-of-health-27778-tumor-heterogeneity-tumor-evolution-and-emerging-cancer-genomics-2/?feed_id=82099
#bioinformatics #cancer #cancer_genomics #computational_biology #tumor_evolution #ScienceJobs #hiring #research
0 notes
Text
Comprehensive Guide to Cell Isolation Techniques: Methods, Applications, and Advances
Cell isolation techniques are fundamental tools in biomedical research, diagnostics, and therapeutic applications. These methods allow scientists to extract and study specific cell types from a heterogeneous population, enabling a deeper understanding of cellular functions, disease mechanisms, and the development of targeted therapies. This article provides an overview of the most commonly used cell isolation techniques, their applications, and recent advancements in the field.

Download PDF Brochure
1. Density Gradient Centrifugation
Overview:
Density gradient centrifugation is a widely used technique for separating cells based on their size and density. Cells are suspended in a medium and subjected to centrifugation, causing them to migrate to different layers based on their density.
Applications:
Isolation of lymphocytes from blood samples.
Separation of cancer cells from a mixed cell population.
Advances:
Modern advancements include the development of more precise density gradients and the use of automated centrifugation systems, improving the efficiency and reproducibility of cell isolation.
2. Magnetic-Activated Cell Sorting (MACS)
Overview:
MACS utilizes magnetic beads coated with antibodies that specifically bind to target cells. When placed in a magnetic field, labeled cells are retained while non-labeled cells are washed away.
Applications:
Isolation of immune cells, such as T cells, for research and therapeutic purposes.
Separation of stem cells for regenerative medicine.
Advances:
Recent innovations in MACS include microchip-based platforms that allow for high-throughput and automated cell sorting, reducing time and labor.
3. Fluorescence-Activated Cell Sorting (FACS)
Overview:
FACS is a powerful technique that sorts cells based on their fluorescence characteristics. Cells are tagged with fluorescent markers and passed through a laser beam; the emitted light is detected and used to sort cells into different populations.
Applications:
Isolation of specific cell types for gene expression studies.
Sorting of transfected cells for gene therapy research.
Advances:
Recent developments in FACS include multi-parametric sorting, allowing for the simultaneous isolation of cells based on multiple characteristics, increasing the precision of cell separation.
Request Sample Pages
4. Immunomagnetic and Immunodensity Cell Isolation
Overview:
These techniques combine magnetic and density-based methods with antibody labeling to isolate specific cell populations. Immunomagnetic separation uses magnetic beads, while immunodensity separation employs density gradients combined with antibodies.
Applications:
Isolation of rare cell populations, such as circulating tumor cells (CTCs).
Enrichment of specific cell types for downstream analysis.
Advances:
Enhanced antibody specificity and the integration of automation have improved the accuracy and efficiency of these methods, making them more suitable for clinical applications.
5. Microfluidic-Based Cell Isolation
Overview:
Microfluidic technology enables the manipulation of cells in tiny channels, allowing for precise control over cell sorting based on size, shape, and other physical properties.
Applications:
Isolation of single cells for genomic and proteomic analysis.
Sorting of cells in personalized medicine applications.
Advances:
The integration of microfluidics with other technologies, such as real-time imaging and AI-driven analysis, has significantly enhanced the capabilities of cell isolation, making it faster and more efficient.
Conclusion
Cell isolation techniques are crucial for advancing our understanding of biology and improving therapeutic outcomes. As technology continues to evolve, these methods are becoming more precise, efficient, and accessible, enabling researchers and clinicians to explore new frontiers in cell-based research and therapy. Whether it's isolating immune cells for cancer treatment or separating stem cells for regenerative medicine, these techniques are paving the way for groundbreaking discoveries and innovations.
Content Source:
0 notes
Text
The Growing Frontier: An In-Depth Look at the Single-Cell Analysis Market
The Single-Cell Analysis Market was valued at USD 3.3 billion in 2023 and will surpass USD 8.7 billion by 2030; growing at a CAGR of 14.8% during 2024 - 2030. The field of life sciences is undergoing a transformative phase, with single-cell analysis emerging as a pivotal technique in modern biology and medicine. Unlike traditional bulk analysis methods that examine averages across populations of cells, single-cell analysis delves into the unique characteristics of individual cells, offering unprecedented insights into cellular diversity, disease mechanisms, and therapeutic targets. This article explores the current landscape, key drivers, and future prospects of the single-cell analysis market.
Single-cell analysis allows researchers to investigate the heterogeneity within a population of cells, which is crucial for understanding complex biological processes such as cancer progression, immune responses, and developmental biology. By examining individual cells, scientists can identify rare cell types, understand cell-to-cell variations, and gain insights into the dynamics of cellular networks. This level of detail is especially important in fields like oncology, immunology, and neurology, where subtle differences between cells can have significant implications for disease progression and treatment outcomes. The single-cell analysis market has experienced rapid growth over the past decade, driven by advancements in technology, increased research funding, and the growing recognition of the importance of cellular heterogeneity in biology and medicine.
Get a Sample Report: https://intentmarketresearch.com/request-sample/single-cell-analysis-market-3651.html
Key Drivers of Market Growth
Technological Advancements: Innovations in single-cell sequencing, microfluidics, and imaging technologies have significantly enhanced the accuracy, efficiency, and scalability of single-cell analysis. These advancements have made it easier for researchers to isolate, process, and analyze individual cells, driving adoption across various applications.
Rising Demand in Oncology: Cancer research is one of the primary areas driving the demand for single-cell analysis. The ability to identify and characterize rare cancer stem cells, understand tumor heterogeneity, and monitor the immune landscape of tumors has made single-cell analysis an indispensable tool in oncology.
Increased Funding and Collaborations: Governments, academic institutions, and private companies are increasingly investing in single-cell analysis research. Collaborative efforts between industry and academia are accelerating the development of new tools and applications, further fueling market growth.
Expansion of Applications: Beyond oncology, single-cell analysis is finding applications in immunology, neuroscience, stem cell research, and drug discovery. The versatility of this technology is broadening its appeal across multiple disciplines.
Challenges and Considerations
Despite its promising growth, the single-cell analysis market faces several challenges. The high cost of instruments and reagents remains a significant barrier for many research labs. Additionally, the complexity of data generated by single-cell analysis requires advanced bioinformatics tools and expertise, which can limit its accessibility to a broader range of researchers.
Furthermore, the standardization of protocols and data analysis methods is still evolving. Variability in sample preparation, sequencing techniques, and data interpretation can lead to inconsistencies in results, which is a critical issue that needs to be addressed to ensure the reliability of single-cell studies.
Get an insights of Customization: https://intentmarketresearch.com/ask-for-customization/single-cell-analysis-market-3651.html
Future Prospects
The future of the single-cell analysis market looks promising, with continued innovation and expansion into new research areas. Advances in artificial intelligence (AI) and machine learning (ML) are expected to play a significant role in improving data analysis and interpretation, making it easier for researchers to extract meaningful insights from complex datasets.
Moreover, as the cost of technology decreases and standardization improves, single-cell analysis is likely to become more accessible to a wider range of researchers, including those in smaller labs and developing countries. The integration of single-cell analysis with other omics technologies, such as proteomics and metabolomics, is also expected to open new avenues for research and personalized medicine.
Conclusion
The single-cell analysis market is at the forefront of a new era in biological research. As technology continues to advance and new applications emerge, this market is poised for substantial growth. The ability to study individual cells in detail is revolutionizing our understanding of health and disease, paving the way for more precise diagnostics, targeted therapies, and personalized medicine. For researchers, clinicians, and investors alike, the single-cell analysis market represents a dynamic and rapidly evolving frontier with significant potential to transform the life sciences landscape.
#Single-cell sequencing#Single-cell profiling#Single-cell RNA sequencing (scRNA-seq)#Single-cell genomics#Single-cell assay#Single-cell analytics
0 notes
Text
Scientists Design Machine Learning Model to Decipher the Microenvironment Communication Networks of Individual Tumors

Intercellular networks between key players in the tumor microenvironments make tumor cells invincible against the body’s natural defense mechanisms. Identifying and targeting these interactions can make tumors more susceptible to tumor invasions and their eventual retardation. This study demonstrates the use of Bayesian network models in designing a network that links together these interactions by incorporating data from both single-cell and bulk RNA-seq.
For a heterogeneous ecosystem such as a tumor microenvironment, the constituents that make it up and the interactions that drive its pathogenesis and progression are highly diverse. This diversity imparts the uniqueness of each tumor in its characteristic progression patterns, immune evasion strategies, and treatment responses. This makes it crucial to identify the said interactions that capture the core processes that underlie each individual tumor to tailor treatment regimens that counter it effectively.
Continue Reading
#bioinformatics#cancer#tumor#network#machinelearning#artificial intelligence#scicomm#science news#science side of tumblr
78 notes
·
View notes
Text
#cancers, Vol. 16, Pages 3057: Advanced Insights into Competitive Endogenous #RNAs (ce#RNAs) Regulated Pathogenic Mechanisms in Metastatic Triple-Negative Breast #cancer (mTNBC)
Triple-negative breast #cancer is aggressive and challenging to treat because of a lack of targets and heterogeneity among tumors. A paramount factor in the mortality from breast #cancer is metastasis, which is driven by genetic and phenotypic alterations that drive epithelial–mesenchymal transition, stemness, survival, migration and invasion. Many genetic and epigenetic mechanisms have been identified in triple-negative breast #cancer that drive these metastatic phenotypes; however, this knowledge has not yet led to the development of effective drugs for metastatic triple-negative breast #cancer (mTNBC). One that may not have received enough attention in the literature is post-translational regulation of broad sets of #cancer-related genes through inhibitory micro#RNAs and the complex competitive endogenous #RNA (ce#RNA) regulatory networks they are influenced by. This field of study and the resulting knowledge regarding alterations in these networks is coming of age, enabling translation into clinical benefit for patients. Herein, we review metastatic triple-negative breast #cancer (mTNBC), the role of ce#RNA network regulation in metastasis (and therefore clinical outcomes), potential approaches for therapeutic exploitation of these alterations, knowledge gaps and future directions in the field. https://www.mdpi.com/2072-6694/16/17/3057?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
The Role of Single-Cell Omics in Unraveling Complex Disease Mechanisms

The introduction of single-cell omics technology is a revolutionary step in the field of biological study. A variety of methods aimed at examining the molecular profiles of individual cells are referred to as “single-cell omics,” which provides before unattainable insights into cellular functioning and heterogeneity that were hidden in bulk investigations. With amazing accuracy, researchers can now uncover the intricacies of biological systems thanks to this method.
Single-Cell Omics: What Is It?
The study of genomic, transcriptomic, proteomic, and epigenomic data at the level of individual cells is known as single-cell omics. Single-cell omicsexamines the subtle changes between individual cells within a tissue or organism, in contrast to typical omics methodologies that study pooled cells and so disguise individual cell variations. Understanding how cells contribute to the overall variety and functionality of biological systems requires this level of detail.
Advances in Technology
Single-cell technology developments recently have completely changed the area. Researchers may quantify the gene expression patterns of individual cells using single-cell RNA sequencing (scRNA-seq), which reveals the dynamic and varied nature of gene activity. Comparably, single-cell DNA sequencing offers information on cellular genetic variants and mutations that are critical to comprehending cancer and other hereditary illnesses. These skills are further enhanced by single-cell proteomics and epigenomics, which provide single-cell resolution protein and epigenetic modification analysis.
Uses and Consequences
Single-cell omics has several significant uses. Single-cell sequencing has shown heterogeneity within tumors in cancer research, revealing subpopulations of cancer cells that could be involved in resistance and the advancement of the illness. Single-cell omics provides insights into how stem cells differentiate into different types of cells by tracing the cellular lineage in developmental biology. Single-cell investigations are useful in neurobiology because they map the variety of neuronal cell types and their roles in both healthy and diseased brains.
Furthermore, single-cell omics is essential for comprehending microbial populations, immunological responses, and complicated illnesses. Through analyzing the cellular makeup of these systems, scientists can discover disease causes and find new targets for treatment.
Obstacles and Prospects for the Future
Even with its revolutionary promise, single-cell omics has a number of drawbacks. Because of the intricacy of the data produced, analysis and interpretation need sophisticated computing techniques. Furthermore, a barrier to the general adoption of single-cell technologies may be their high cost and technical requirements.
Further insights might be obtained by combining single-cell omics with other high-throughput methods and cutting-edge technology. It is anticipated that developments in computational techniques, data integration, and technological downsizing would improve single-cell omics’ usability and accessibility across a range of study domains.
In summary
At the vanguard of biological study, single-cell omics provides an intricate and nuanced perspective of cellular dynamics that is unmatched by bulk analysis. The insights gleaned from single-cell omics will surely increase our knowledge of complex biological systems and aid in the development of targeted therapeutics and personalized medicine as technology and methodology continue to progress.
0 notes
Text
Extracellular Vesicles heterogeneity through the lens of multiomics
Extracellular vesicles (EVs) are heterogenous in size, biogenesis, cargo and function. Beside small EVs, aggressive tumor cells release a population of particularly large EVs, namely large oncosomes (LO). This study provides the first resource of label-free quantitative proteomics of LO and small EVs obtained from distinct cancer cell types (prostate, breast, and glioma). This dataset was integrated with a SWATH Proteomic assay on LO, rigorously isolated from the plasma of patients with metastatic prostate cancer (PC). Proteins enriched in LO, which were identified also at the RNA level, and found in plasma LO significantly correlated with PC progression. Single EV RNA-Seq of the PC cell-derived LO confirmed some of the main findings from the bulk RNA-Seq, providing first evidence that single cell technologies can be successfully applied to EVs. This multiomics resource of cancer EVs can be leveraged for developing a multi-analyte approach for liquid biopsy. http://dlvr.it/TC6T1H
0 notes
Link
Cancer is challenging to cure due to its complexity and variability. It involves numerous types of diseases, each with different characteristics, genetic mutations, and responses to treatment. Cancer cells can adapt, evolve, and develop resistance to therapies, making it difficult to target and eliminate them entirely without harming healthy cells. Cancer is difficult to cure for several key reasons: Cellular diversity: Cancer cells within a tumor can be genetically diverse, making uniform treatment challenging. Adaptability: Cancer cells can rapidly evolve and develop resistance to treatments. Similarity to normal cells: Cancer cells originate from normal cells, making it hard to target them without harming healthy tissue. Spread and metastasis: By the time cancer is detected, it may have already spread to other parts of the body. Complexity: Cancer involves multiple genetic and environmental factors, making it a highly complex disease. Immune evasion: Cancer cells can develop mechanisms to hide from or suppress the immune system. Heterogeneity between patients: Each person's cancer can be unique, requiring personalized treatment approaches. Cancer is Not a Single Disease Cancer is a term that encompasses a wide variety of diseases, each with its own unique characteristics and behaviors. Here are some examples to illustrate this diversity: Breast Cancer vs. Lung Cancer Breast Cancer: This type of cancer originates in the cells of the breast. It can be hormone receptor-positive, meaning it grows in response to hormones like estrogen or progesterone. Treatments often include hormone therapy, chemotherapy, radiation, and surgery. Lung Cancer: This cancer starts in the lungs and is often associated with smoking. It can be classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Treatments may include targeted therapy, immunotherapy, chemotherapy, and surgery. Despite both being cancers, the origins, behaviors, and treatments for breast cancer and lung cancer are quite different. Leukemia vs. Melanoma Leukemia: This is a cancer of the blood and bone marrow. It leads to the production of abnormal white blood cells. Leukemia is treated with chemotherapy, radiation therapy, and sometimes stem cell transplants. Melanoma: This is a type of skin cancer that develops from melanocytes, the cells that produce pigment. It is often caused by excessive exposure to ultraviolet (UV) radiation from the sun. Treatments include surgery, immunotherapy, targeted therapy, and radiation. Leukemia affects the blood and bone marrow, while melanoma affects the skin, highlighting the diverse nature of cancers. Prostate Cancer vs. Pancreatic Cancer Prostate Cancer: This cancer occurs in the prostate gland in men. It is often slow-growing and may not require immediate treatment. Options include active surveillance, surgery, radiation therapy, and hormone therapy. Pancreatic Cancer: This is a highly aggressive cancer that starts in the pancreas. It is often diagnosed at a late stage and has a poor prognosis. Treatments include surgery, chemotherapy, and targeted therapy Genetic Mutations and Variability Genetic mutations are changes in the DNA sequence of a cell. These mutations can lead to significant variability in how cancers develop and respond to treatment. Here are some examples to illustrate this concept: BRCA1 and BRCA2 Mutations in Breast Cancer BRCA1 and BRCA2 Genes: Mutations in these genes significantly increase the risk of developing breast and ovarian cancers. These genes normally help repair DNA damage, but when mutated, they fail to do so, leading to cancer development. Example: Angelina Jolie famously underwent a preventive double mastectomy after discovering she carried a BRCA1 mutation, which gave her an estimated 87% risk of breast cancer1. EGFR Mutations in Lung Cancer EGFR Gene: Mutations in the Epidermal Growth Factor Receptor (EGFR) gene are common in non-small cell lung cancer (NSCLC). These mutations cause cells to grow and divide uncontrollably. Example: Patients with EGFR mutations often respond well to targeted therapies like gefitinib or erlotinib, which specifically inhibit the EGFR protein2. KRAS Mutations in Colorectal Cancer KRAS Gene: Mutations in the KRAS gene are found in about 40% of colorectal cancers. These mutations lead to continuous cell growth and division. Example: KRAS mutations can make colorectal cancer resistant to certain targeted therapies, such as those targeting the EGFR pathway3. TP53 Mutations in Various Cancers TP53 Gene: Known as the “guardian of the genome,” the TP53 gene helps prevent cancer by repairing DNA or initiating cell death if the damage is irreparable. Mutations in TP53 are found in many types of cancer, including breast, lung, and colorectal cancers. Example: A TP53 mutation can lead to a loss of this protective function, allowing cancer cells to grow and spread unchecked Heterogeneity Within Tumors Tumor heterogeneity refers to the presence of a diverse population of cancer cells within a single tumor. This diversity can occur at multiple levels, including genetic, phenotypic, and functional differences among the cells. Here are some examples to illustrate this concept: Genetic Heterogeneity Example: In a single tumor, different regions may have distinct genetic mutations. For instance, one part of a lung tumor might have an EGFR mutation, while another part has a KRAS mutation. This genetic diversity can lead to varied responses to treatment within the same tumor. Phenotypic Heterogeneity Example: Cancer cells within a tumor can exhibit different physical characteristics, such as size, shape, and protein expression. In breast cancer, some cells might express high levels of hormone receptors (like estrogen receptors), while others do not. This can affect how the tumor responds to hormone therapy. Functional Heterogeneity Example: Different cancer cells within a tumor can have varying abilities to grow, invade tissues, and resist treatment. In glioblastoma, a type of brain cancer, some cells might be highly invasive, spreading quickly to other parts of the brain, while others might be more resistant to chemotherapy. Microenvironmental Heterogeneity Example: The tumor microenvironment, which includes surrounding blood vessels, immune cells, and other support cells, can vary within different regions of the tumor. In pancreatic cancer, some areas of the tumor might be well-supplied with blood, while others are hypoxic (low in oxygen). This can influence how different parts of the tumor respond to treatments like radiation therapy. Impact on Treatment The heterogeneity within tumors poses significant challenges for treatment: Resistance to Therapy: Different subpopulations of cancer cells may respond differently to the same treatment. For example, while chemotherapy might kill the majority of cancer cells, a small subset with specific mutations might survive and cause a relapse. Targeted Therapy Limitations: Targeted therapies are designed to attack specific genetic mutations. However, if a tumor has multiple mutations, a single targeted therapy might not be effective against all cancer cells. Adaptive Responses: Cancer cells can adapt to their environment and develop resistance mechanisms. For instance, if a tumor is treated with a drug that targets a specific pathway, cancer cells might activate alternative pathways to survive. Resistance to Treatment Cancer cells can develop resistance to treatments over time, making it challenging to achieve long-term remission. This resistance can occur through various mechanisms and can affect different types of cancer treatments, including chemotherapy, targeted therapy, and immunotherapy. Here are some examples to illustrate this concept: Chemotherapy Resistance Example: In ovarian cancer, patients often respond well to platinum-based chemotherapy initially. However, over time, the cancer cells can develop resistance, leading to a recurrence of the disease. This resistance can occur through several mechanisms, such as increased DNA repair capabilities of the cancer cells or changes in drug transport within the cells. Targeted Therapy Resistance Example: In chronic myeloid leukemia (CML), the drug imatinib (Gleevec) targets the BCR-ABL fusion protein, which is responsible for the uncontrolled growth of leukemia cells. While imatinib is highly effective initially, some patients develop resistance due to additional mutations in the BCR-ABL gene. These mutations alter the protein’s structure, preventing imatinib from binding effectively. Immunotherapy Resistance Example: In melanoma, immunotherapy drugs like pembrolizumab (Keytruda) work by enhancing the immune system’s ability to recognize and attack cancer cells. However, some melanoma cells can develop resistance by upregulating proteins that inhibit immune responses, such as PD-L1. This allows the cancer cells to evade detection and destruction by the immune system. Hormone Therapy Resistance Example: In hormone receptor-positive breast cancer, treatments like tamoxifen block estrogen receptors to prevent cancer cell growth. Over time, some cancer cells can become resistant by mutating the estrogen receptor or activating alternative growth pathways that do not rely on estrogen. Mechanisms of Resistance Cancer cells can develop resistance through various mechanisms, including: Genetic Mutations: New mutations can alter the target of the therapy, making the treatment less effective. Drug Efflux: Cancer cells can increase the expression of proteins that pump drugs out of the cell, reducing the drug’s intracellular concentration. DNA Repair: Enhanced DNA repair mechanisms can allow cancer cells to survive despite the DNA-damaging effects of chemotherapy. Alternative Pathways: Cancer cells can activate alternative signaling pathways to bypass the blocked pathway targeted by the therapy. Impact on Treatment Resistance to treatment poses significant challenges for cancer therapy: Relapse: Even if a treatment is initially effective, resistance can lead to a relapse of the disease. Combination Therapies: To overcome resistance, doctors often use combination therapies that target multiple pathways simultaneously. However, this approach can increase the risk of side effects. Personalized Medicine: Understanding the specific mechanisms of resistance in individual patients can help tailor treatments to overcome resistance and improve outcomes. Cancer’s Ability to Spread Cancer’s ability to spread, known as metastasis, is one of the most challenging aspects of the disease. Metastasis occurs when cancer cells break away from the primary tumor and travel to other parts of the body, forming new tumors. Here are some examples and explanations to illustrate this process: Breast Cancer Metastasis Example: Breast cancer cells can spread to various parts of the body, including the bones, liver, lungs, and brain. When breast cancer spreads to the bones, it can cause pain and fractures. If it spreads to the liver, it can lead to liver dysfunction and jaundice. Mechanism: Breast cancer cells can enter the bloodstream or lymphatic system, which acts as a highway for these cells to travel to distant organs. Once they reach a new site, they can establish a new tumor by adapting to the local environment. Lung Cancer Metastasis Example: Lung cancer often spreads to the brain, bones, liver, and adrenal glands. Brain metastases can cause neurological symptoms such as headaches, seizures, and cognitive changes. Mechanism: Lung cancer cells can invade nearby blood vessels and travel through the bloodstream to distant organs. They can also spread through the lymphatic system, which drains fluid from tissues and returns it to the bloodstream. Colorectal Cancer Metastasis Example: Colorectal cancer commonly spreads to the liver and lungs. Liver metastases can lead to liver enlargement, pain, and impaired liver function. Mechanism: Colorectal cancer cells can spread through the portal vein, which carries blood from the intestines to the liver. This direct connection makes the liver a common site for metastasis. Prostate Cancer Metastasis Example: Prostate cancer frequently spreads to the bones, particularly the spine, pelvis, and ribs. Bone metastases can cause severe pain, fractures, and spinal cord compression. Mechanism: Prostate cancer cells can spread through the bloodstream or lymphatic system. They often target bones because the bone microenvironment provides factors that promote cancer cell growth. Factors Influencing Metastasis Several factors contribute to the ability of cancer cells to spread: Genetic Changes: Mutations in certain genes can enhance the ability of cancer cells to invade tissues and spread to distant sites. Tumor Microenvironment: The surrounding environment of the tumor, including blood vessels, immune cells, and support cells, can influence the ability of cancer cells to metastasize. Cell Adhesion: Cancer cells can lose their ability to stick to each other, making it easier for them to break away from the primary tumor and travel through the body. Angiogenesis: The formation of new blood vessels (angiogenesis) can provide cancer cells with the nutrients and oxygen they need to grow and spread. Impact on Treatment Metastasis significantly complicates cancer treatment: Multiple Sites: Treating cancer that has spread to multiple sites requires a more comprehensive approach, often involving systemic therapies like chemotherapy, targeted therapy, or immunotherapy. Resistance: Metastatic cancer cells can be more resistant to treatment compared to the primary tumor, making it harder to achieve remission. Prognosis: The presence of metastasis generally indicates a more advanced stage of cancer and is associated with a poorer prognosis. Impact on the Immune System Cancer can significantly impact the immune system, both by evading immune detection and by actively suppressing immune responses. Here are some examples to illustrate how cancer interacts with the immune system: Immune Evasion Example: Melanoma cells can express high levels of PD-L1, a protein that binds to the PD-1 receptor on T-cells (a type of immune cell). This interaction inhibits T-cell activity, allowing the cancer cells to evade immune detection and destruction. Mechanism: By expressing PD-L1, melanoma cells effectively “turn off” the immune response against them, making it difficult for the body to recognize and attack the cancer. Immune Suppression Example: In ovarian cancer, the tumor microenvironment can be rich in regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs). These cells suppress the activity of other immune cells that would normally attack the cancer. Mechanism: Tregs and MDSCs release cytokines and other factors that inhibit the function of cytotoxic T-cells and natural killer (NK) cells, which are crucial for targeting and killing cancer cells. Chronic Inflammation Example: Chronic inflammation, such as that caused by hepatitis B or C infections, can lead to liver cancer. The persistent inflammatory environment promotes genetic mutations and cancer cell growth. Mechanism: Inflammation can cause DNA damage and create a microenvironment that supports cancer cell survival and proliferation. Immune cells that are constantly activated can also produce growth factors that aid in tumor development. Immunotherapy and Immune Checkpoints Example: Immunotherapy drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) target immune checkpoints such as PD-1 and CTLA-4. These drugs block the inhibitory signals, allowing T-cells to attack cancer cells more effectively. Mechanism: By inhibiting the checkpoints, these drugs enhance the immune system’s ability to recognize and destroy cancer cells. This approach has shown success in treating cancers like melanoma, lung cancer, and renal cell carcinoma. Impact on Treatment The interaction between cancer and the immune system has significant implications for treatment: Immunotherapy: Leveraging the immune system to fight cancer has become a promising approach. Immunotherapies, such as checkpoint inhibitors and CAR-T cell therapy, aim to boost the body’s natural defenses against cancer. Combination Therapies: Combining immunotherapy with other treatments, like chemotherapy or radiation, can enhance the overall effectiveness. For example, radiation can increase the visibility of cancer cells to the immune system, making immunotherapy more effective. Personalized Medicine: Understanding the specific immune landscape of a patient’s tumor can help tailor immunotherapy treatments to achieve better outcomes. Side Effects of Cancer Treatments Cancer treatments, while effective at targeting cancer cells, can also affect healthy cells and tissues, leading to various side effects. Here are some examples of common cancer treatments and their associated side effects: Chemotherapy Example: Chemotherapy drugs target rapidly dividing cells, which include both cancer cells and healthy cells like those in the bone marrow, digestive tract, and hair follicles. Side Effects: Bone Marrow Suppression: This can lead to a decrease in blood cells, causing anemia (fatigue), leukopenia (increased risk of infection), and thrombocytopenia (increased risk of bleeding). Gastrointestinal Issues: Nausea, vomiting, diarrhea, and mouth sores are common due to the impact on the digestive tract lining. Hair Loss: Damage to hair follicles can result in temporary hair loss. Radiation Therapy Example: Radiation therapy uses high-energy particles or waves to destroy or damage cancer cells. It can also affect nearby healthy tissues. Side Effects: Skin Changes: Redness, blistering, and peeling of the skin in the treated area, similar to a sunburn. Fatigue: A common side effect due to the body’s response to radiation. Organ-Specific Effects: Depending on the area treated, radiation can cause specific side effects, such as difficulty swallowing (if the throat is treated) or urinary issues (if the pelvic area is treated). Surgery Example: Surgical removal of tumors can be an effective treatment but comes with risks and side effects. Side Effects: Pain: Post-operative pain is common and can be managed with medications. Infection: There is a risk of infection at the surgical site. Functional Impairment: Depending on the surgery, there may be a loss of function or changes in appearance (e.g., mastectomy for breast cancer). Hormone Therapy Example: Hormone therapy is used to treat cancers that are sensitive to hormones, such as breast and prostate cancer. Side Effects: Hot Flashes: Common in both men and women undergoing hormone therapy. Bone Thinning: Long-term use can lead to osteoporosis. Mood Changes: Hormone therapy can affect mood and emotional well-being. Targeted Therapy Example: Targeted therapies are designed to specifically target cancer cells with certain genetic mutations. Side Effects: Skin Problems: Rashes, dry skin, and changes in skin color. Liver Problems: Elevated liver enzymes indicating liver damage. Gastrointestinal Issues: Diarrhea and nausea. Immunotherapy Example: Immunotherapy boosts the body’s immune system to fight cancer. Side Effects: Immune-Related Side Effects: Inflammation of healthy tissues, such as colitis (inflammation of the colon), pneumonitis (inflammation of the lungs), and hepatitis (inflammation of the liver). Flu-Like Symptoms: Fever, chills, and fatigue. Managing Side Effects Managing the side effects of cancer treatments is crucial for maintaining the quality of life for patients. Here are some strategies: Monitoring and Adjustment: Regular monitoring of side effects allows healthcare providers to adjust treatment plans as needed to minimize adverse effects. Medications: Anti-nausea drugs, pain relievers, and other medications can help manage specific side effects. Supportive Care: Nutritional support, physical therapy, and counseling can help address the broader impacts of treatment. Ongoing Research and Hope Despite the challenges in curing cancer, ongoing research is making significant strides in understanding the disease and developing new treatments. Here are some examples of promising areas of research and the hope they bring: Targeted Therapy Example: Targeted therapies are designed to attack specific genetic mutations or proteins that drive cancer growth. For instance, drugs like trastuzumab (Herceptin) target the HER2 protein in certain breast cancers, significantly improving outcomes for patients with HER2-positive breast cancer. Hope: By focusing on the unique characteristics of cancer cells, targeted therapies can be more effective and have fewer side effects compared to traditional chemotherapy. Immunotherapy Example: Immunotherapy harnesses the body’s immune system to fight cancer. Checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), have shown success in treating cancers like melanoma, lung cancer, and renal cell carcinoma. Hope: Immunotherapy offers the potential for long-lasting responses and even cures in some cases, as it helps the immune system recognize and attack cancer cells more effectively. CAR-T Cell Therapy Example: CAR-T cell therapy involves modifying a patient’s T-cells to express a receptor that targets cancer cells. This approach has been particularly successful in treating certain types of blood cancers, such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). Hope: CAR-T cell therapy represents a personalized treatment approach that can lead to complete remissions in patients who have not responded to other treatments. Liquid Biopsies Example: Liquid biopsies are blood tests that detect cancer-related genetic mutations and other biomarkers. They offer a non-invasive way to monitor cancer progression and response to treatment. Hope: Liquid biopsies can provide real-time insights into a patient’s cancer, allowing for more precise and timely adjustments to treatment plans. Cancer Vaccines Example: Cancer vaccines aim to stimulate the immune system to recognize and attack cancer cells. The HPV vaccine, for instance, prevents infections with human papillomavirus, which can lead to cervical and other cancers. Hope: Preventive vaccines can reduce the incidence of certain cancers, while therapeutic vaccines are being developed to treat existing cancers by boosting the immune response. Gene Editing Example: CRISPR-Cas9 is a gene-editing technology that allows scientists to precisely modify DNA. Researchers are exploring its use to correct genetic mutations that cause cancer or to enhance the immune system’s ability to fight cancer. Hope: Gene editing holds the promise of directly targeting the genetic causes of cancer, potentially leading to more effective and lasting treatments.
0 notes
Text
Understanding the Dynamics of the Germ Cell Tumor Market: Drivers, Barriers, and Future Outlook
A germ cell tumor is a type of tumor that originates in the germ cells, which are cells that form sperm in males and eggs in females. These tumors can occur in various parts of the body, including the ovaries, testes, and areas along the midline of the body such as the brain, chest, and abdomen.
Germ Cell Tumor Market Drivers:
Increasing Incidence Rates: The prevalence of germ cell tumors has been gradually rising, primarily due to factors like improved diagnostic techniques and increased awareness leading to early detection.
Advancements in Diagnosis and Screening: Technological advancements in diagnostic tools, such as imaging techniques like MRI, CT scans, and biomarker tests, facilitate early detection and accurate diagnosis of germ cell tumors. This contributes to a larger pool of patients seeking treatment.
Growing Pipeline of Therapies: Pharmaceutical companies are actively engaged in research and development efforts to bring novel therapies to the market. This includes targeted therapies, immunotherapies, and combination treatments, which offer potential improvements in efficacy and reduced side effects compared to traditional treatments.
Emerging Personalized Medicine Approaches: The trend toward personalized medicine, driven by developments in genomics and molecular profiling, enables the identification of specific genetic mutations or biomarkers associated with germ cell tumors. This allows for tailored treatment regimens that are more effective and better tolerated by patients.
Increasing Healthcare Expenditure: Rising healthcare expenditure globally, coupled with improved access to healthcare services in emerging economies, provides patients with greater access to advanced treatments for germ cell tumors.
Germ Cell Tumor Market Barriers:
Limited Awareness and Diagnosis Challenges: Despite advancements in diagnostic techniques, many cases of germ cell tumors still go undetected until they reach advanced stages. Limited awareness among patients and healthcare providers about the symptoms and risk factors may delay diagnosis and initiation of treatment.
High Cost of Treatment: The cost of novel therapies for germ cell tumors, especially targeted therapies and immunotherapies, can be prohibitively high. This poses a barrier to access, particularly in regions with limited healthcare resources or inadequate insurance coverage.
Drug Development Challenges: Developing new therapies for germ cell tumors faces challenges, including the rarity and heterogeneity of these tumors, as well as the complexity of the underlying biology. Clinical trials for these treatments often require large patient populations and long-term follow-up, which can prolong the drug development process.
Resistance to Conventional Treatments: While chemotherapy and surgery remain standard treatments for germ cell tumors, some patients may develop resistance to these therapies over time. This necessitates the development of alternative treatment options, which may not be readily available or accessible to all patients.
Regulatory Hurdles: Regulatory approval processes for new treatments can be lengthy and rigorous, delaying the availability of innovative therapies to patients. Additionally, variations in regulatory requirements across different regions can further complicate the market entry of new drugs.
Germ Cell Tumor Market Analysis:
The germ cell tumor treatment market is expected to witness steady growth in the coming years, driven by factors such as increasing incidence rates, advancements in diagnostic technologies, and the emergence of novel therapeutic approaches. However, challenges related to high treatment costs, limited awareness, and regulatory complexities may temper the market growth to some extent.
Market players, including pharmaceutical companies, biotechnology firms, and academic research institutions, are actively investing in R&D efforts to address unmet needs in germ cell tumor treatment. Collaboration among stakeholders, including healthcare providers, patient advocacy groups, and regulatory agencies, will be crucial in overcoming barriers and accelerating the development and adoption of innovative therapies.
Evolving Germ Cell Tumor Treatment Outlook
Chemotherapy: Chemotherapy remains a cornerstone of treatment for many germ cell tumors, including both localized and metastatic disease. Platinum-based chemotherapy regimens are commonly used, often in combination with other agents.
Surgery: Surgical intervention plays a crucial role in the management of germ cell tumors, particularly in cases of localized disease or for debulking purposes. Surgical resection may involve removal of the primary tumor and affected tissues, lymph nodes, or metastases.
Radiation Therapy: Radiation therapy is employed in certain cases of germ cell tumors, either as a primary treatment modality or as adjuvant therapy following surgery or chemotherapy. It may be used to target residual tumor tissue or to manage symptoms in cases of metastatic disease.
Targeted Therapies: Increasingly, targeted therapies are being investigated for the treatment of germ cell tumors, particularly in cases of refractory or relapsed disease. These therapies aim to specifically target molecular pathways or genetic aberrations implicated in tumor growth and progression.
Immunotherapy: Immunotherapy, including immune checkpoint inhibitors and other immunomodulatory agents, holds promise in the treatment of germ cell tumors. These therapies harness the body's immune system to recognize and attack cancer cells, potentially leading to durable responses in some patients.
Role of Companies in the Germ Cell Tumor Market
In the Germ Cell Tumor market, companies such as Takeda, Novartis, Bristol Myers Squibb, Merck & Co., Inc., Roche, Bayer, and others play a pivotal role in driving innovation, research, development, and the provision of treatments and therapies for individuals suffering from this chronic inflammatory skin condition. These companies encompass pharmaceutical giants, biotechnology firms, medical device manufacturers, and healthcare service providers, each contributing uniquely to the advancement of Germ Cell Tumor management. Pharmaceutical companies lead the charge in developing novel drugs, ranging from topical corticosteroids to biologics targeting specific immune pathways implicated in Germ Cell Tumor pathogenesis.
Germ Cell Tumor Market Outlook - Key Conclusion and Analysis
The Germ Cell Tumor market is undergoing a transformative period, driven by advances in research, innovation in therapeutic approaches, and shifting treatment paradigms. While significant progress has been made in improving outcomes for patients with Germ Cell Tumor, several barriers continue to challenge the market's expansion, including high treatment costs, safety concerns, and regulatory hurdles. Looking ahead, personalized medicine, novel therapeutic targets, and digital health solutions are poised to shape the future of Germ Cell Tumor management, offering new hope for patients and caregivers alike. Efforts to address these challenges and capitalize on emerging opportunities will be critical in advancing the field and ultimately improving the lives of individuals living with Germ Cell Tumor.
Get a more detailed overview, at: Germ Cell Tumor Market Outlook and Forecast
Trending Reports by DelveInsight:
Bullous Pemphigoid Market | Congenital Myasthenic Syndromes Market | Cryopyrin-associated Periodic Syndrome Market | Glycogen Storage Disease Market | Inflammatory Breast Cancer Market | Lag-3-next Generation Immunotherapy Market | Metastatic Colorectal Cancer Market | Secondary Hyperparathyroidism Market | Dermatomyositis Market | Tenosynovial Giant Cell Tumors Market
dyspepsia market | monoclonal gammopathy of undetermined significance market | oral electrolyte solutions market | ventral hernia market | gene therapy in CNS disorder market | myeloproliferative neoplasms market | nk cell therapy market | spinal implants market | thyroid cancer market | attention deficit hyperactivity disorder adhd market
About DelveInsight:
DelveInsight is a prominent business consultant and market research firm specializing in the life sciences sector. With a focus on supporting pharmaceutical companies, DelveInsight provides end-to-end solutions to enhance their performance.
Connect with DelveInsight:
LinkedIn | Facebook | Twitter
Contact Us:
Kritika RehaniTeam Lead, Marketing
+91-9650213330
www.delveinsight.com
0 notes