#and scientists are sticking human genes into yeast
Text
you can make gelatin out of human bones
which means you can make people jello
#don’t me ask me why i looked this up#but it’s perfectly possible to do#and scientists are sticking human genes into yeast#to make people gelatin#because of diseases or something?#it’s supposed to be safer#but i’d rather risk getting sick with cow bones#than eating gmo human yeast jello
2 notes
·
View notes
Text
@a-bisexual-teenager here are some mitochondria facts to wow your teachers on all your biology and general skills tests to come (one of these actually helped me on the SAT):
• Mitochondria are double membraned organelles
• The prominent theory for how eukaryotes got them is the endosymbiotic theory (which is also the prominent theory for how plants got chloroplasts, but this would have been earlier than mitochondria). The endosymbiotic theory is basically that a eukaryote was doing just fine doing glycolysis, then engulfed (functionally ate) a bacterium capable of doing cellular respiration (or something very very similar). The bacterium processed the cell’s nutrients and got to multiply within the cell. The cell got much more energy because cellular respiration is like 18x more efficient than glycolysis, so it got to grow and divide more. Because these cells had a huge advantage, they multiplied a lot and beat out all the other eukaryotes for survival.
• The main support for this theory is that mitochondria have their very own DNA (37 genes) that is structured like bacterial DNA, have their own ribosomes, and replicate themselves independently from the cell cycle.
• Because of the way human reproduction works, all your mitochondria come from your mom. The mtDNA also does not change significantly across generations. This is especially valuable for anthropologists because they can trace SPECIFICALLY maternal lines by analyzing mtDNA. (there is a way to trace paternal lines too, but it has nothing to do with mitochondria)
• Mitochondria have all those folds because they increase surface area, meaning it can fit more transport proteins per cubed unit space, and therefore perform more reactions per unit volume per unit time. This is seen in most organelles involved in protein synthesis because in biology, Surface Area:Volume ratio is a really good predictor of cell efficiency. (why cells have to divide before they get too big)
• Mitochondria are unlike bacteria in that they can change shape and fuse, then redivide with other mitochondria. This is because of a peptide scientists call a “diaper pin peptide” that can change shape to either “open” and let the mitochondria stick together or “close” and make them separate.
• All the mitochondria in a cell share their Nucleoids. They get passed around like pens in a classroom. It’s thought that the Endoplasmic Reticulum may trigger copying of the Nucleoids and subsequent mitochondrial fission.
• Yeast cells can sometimes get mutant mitochondria that divides unchecked, resembling cancer. But instead of cells dividing unchecked, harming the organism as a whole, it’s mitochondria dividing unchecked, harming a single cell.
• Mitochondria and chloroplasts are the only organelles that are colored.
• Glucose has to be broken down into pyruvate before entering the mitochondria, but fatty acids can enter right away.
• Mitochondria regulate programmed cell death
20 notes
·
View notes
Text
Spider glue's sticky secret revealed by new genetic research
by Sarah Stellwagen

What do all of the over 45,000 described spider species on Earth have in common? Each makes at least one type of silk. And there are an awful lot of types out there.
An individual orb weaving spider – the kind that spins the classic two-dimensional aerial spiral webs that seem to always be suspended at human face-height – can produce seven different silks, each with unique material properties.
Dragline silk forms the frame of an orb web and is famous for its strength and toughness, comparable to that of steel. The capture spiral is made of a highly stretchy version called flagelliform silk. Orb weaving spiders use an additional type of silk to wrap prey and create web decorations.
But there’s another kind that, on the surface, doesn’t resemble silk at all: the sticky glue with which some spiders cover their silk capture threads. It doesn’t look like the classic threads that come to mind when thinking of spider silk, but the gluey substance from these webs is in fact a silk protein.
For many years, researchers have been uncovering the secrets of spider glue, which stays wet in its open air environment and sticky over many rounds of attachment and release. Its genetic blueprint has remained elusive, however, meaning scientists haven’t been able to think about setting up large-scale production of this potentially useful biomaterial.
Using new technology, my colleague and I have been able to sequence the first full genetic sequences that code for spider glue proteins.
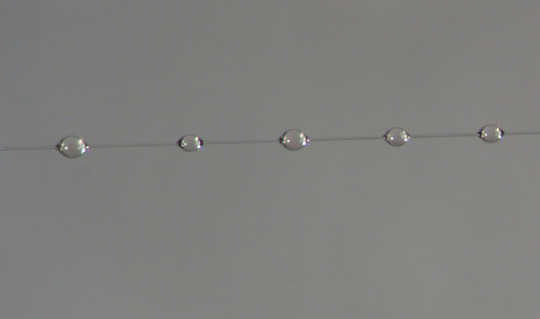
Spider glue drops spread along a strand of capture spiral silk. Sarah Stellwagen, CC BY-ND
A silk that’s really a sticky glue
Under a microscope, orb weaver glue resembles beads on a string – little glistening spheres along a strand of stretchy support silk. Instead of being spun into a fiber as it leaves the spider’s body like other silks, the glue proteins are extruded as a jumbled mass. Their job is to stickily retain prey that get caught in the web.
Different spider species produce glue tailored to their habitat’s conditions and prey.
The glue of tropical orb weaving species is sticky in the spider’s wet habitat, but downgrades to just tacky in low humidity. The glue of orb weavers from dry regions becomes dilute and thin if the humidity is too high.
Bolas spiders forgo the orb web, and instead produce a large globule of glue at the end of a long strand of silk that they whirl rapidly through the air. The glue of this sticky snare is specialized for capturing moths covered with loose scales.
Widow spiders produce vertical, glue-covered trip lines that detach from the ground when encountered by an unsuspecting victim, springing the prey into the air where it hangs suspended. Unlike orb weaver glue, widow glue is resistant to fluctuating humidity.
These various specialized adhesive properties have intrigued biomaterials researchers who can dream up plenty of uses for artificial versions of spider glues. But without knowing the genes that code for these proteins, there hasn’t been a clear road map for how to produce synthetic spider glues.

Their sticky glue is part of what makes spiders’ webs so hard to escape. Robert Mutch/Shutterstock.com
Cracking a long, repetitive code
Surprisingly, researchers have only sequenced around 20 full-length spider silk genes despite the incredible diversity of spiders and decades-old interest in silk as a useful biomaterial.
It turns out that not only are the properties of spider silk amazing, but so is the DNA code that stores the instructions for making the protein. Spider silk genes are extremely large; in itself that’s not a problem, but the bulk of their sequence is made from repeats of the same small DNA bits.
Imagine that the sentence “THE QUICK BROWN FOX JUMPED OVER THE LAZY DOG” is a sequence of DNA that encodes for a protein, but whose exact order of letters is still unknown.
In order to discover this sequence, the main method of DNA sequencing technology available today has three main steps. Once a DNA sample is collected, many copies of the sentence are randomly broken up into small pieces. For example, you might end up with a collection of fragments like “THE QU” “QUICK B” “BROWN FO” “WN FOX J” “AZY DOG” and on and on.
Then a DNA sequencing machine discovers each letter of each piece. The final step is stitching all the short pieces, technically called “reads,” back together in one sequence to figure out the original sentence.
For the sentence above, this is an easy task. The sequence of letters is unique, and as long as there are at least five characters in each read, it’s possible to figure out where one fits relative to another.
Now imagine a similar sentence: “THE QUICK BROWN FOX JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS JUMPS OVER THE LAZY DOG.” Given many random short reads from the middle region like “UMPS J” or “S JUMP,” no matter how you slice and dice, it’s impossible to use this method to figure out the number of “JUMPS” in the complete sentence.
Sequencing a long read of DNA in one go
For many years DNA sequencing has been limited to this short-read strategy: breaking a gene into bits and then reassembling into one cohesive sequence.
Setting aside some difficult and expensive techniques that are out of reach for standard labs, the best way to fully discover a long, repetitive gene is to sequence the repetitive part from start to finish in one go. Fortunately, emerging technology, while still in its infancy, is starting to allow this long-read sequencing by getting around the chemistry limitations of the short-read method. For those that study super-repetitive DNA this is excellent news: New types of DNA sequencers are finally resolving the “JUMPS.”
Now that two spider glue genes are fully sequenced, the first step towards making a synthetic version is complete. Researchers can now insert the genes into other organisms, like bacteria or yeast, to make the glue in bulk.
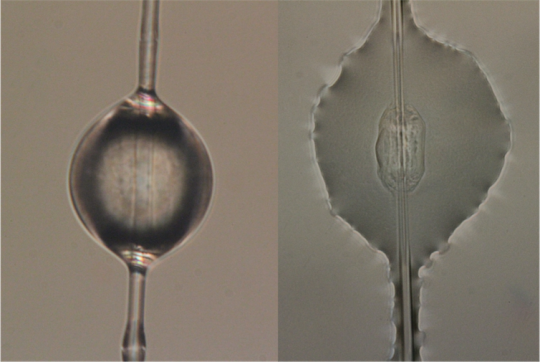
Droplet of spider glue suspended on capture spiral silk (left) and after adhering to a glass slide (right). Sarah Stellwagen, CC BY-ND
Unlike solid silks, the glue proteins do not have to be transformed from a liquid to a solid fiber, something spiders do effortlessly but that scientists have trouble replicating. The glue has the potential for many unique applications and is biodegradable, water soluble and stays sticky for months or even years.
Imagine safer pest control or washable filters. Or frat boys wrestling in a kiddie pool of the stuff. Either way, someday soon it might be possible to reach your hand into a bucket of spider glue – the tricky part will be not sticking to whatever you touch next.

About The Author:
Sarah Stellwagen is a Postdoctoral Researcher in Biological Sciences at the University of Maryland, Baltimore County
This article is republished from our content partners at The Conversation under a Creative Commons license.
The main image at the top of this article was changed to spare our arachnophobic friends the shock as they scrolled through the site.
16 notes
·
View notes
Text
Are Household Products Killing Us?
Story at-a-glance
In Europe, more than 1,300 chemicals are banned from personal care products; in the US, just 11 are banned
Common sources of toxic chemicals include fragrances in cleaning and personal care products, vinyl products, stain-resistant furniture and clothing, and more
Chemicals in household dust may be contributing to obesity while the plastics chemical bisphenol-A (BPA) has been linked to prostate cancer
By Dr. Mercola
In Europe, more than 1,300 chemicals are banned from use in lotions, soaps, toothpaste, cosmetics, and other personal care products. Contrast that to in the US, where just 11 are banned.1
Adding insult to injury, the US Food and Drug Administration (FDA) tasks the companies that manufacture and market cosmetics and other personal care products with ensuring their safety.
Not only does this pose an obvious conflict of interest, but “neither the law nor FDA regulations require specific tests to demonstrate the safety of individual products or ingredients.”2
The average US women uses 12 personal care products and/or cosmetics a day, containing 168 different chemicals, according to the Environmental Working Group (EWG). There are other chemicals risks as well, like those lurking in your household cleaning products, food packaging, furniture, and carpeting.
Dr. Julia Brody, executive director of the Silent Spring Institute, is among those speaking out against environmental chemicals and the risk they pose to human health, and in particular to women’s health.
About 80 percent of the women who develop breast cancer, for instance, have no family history of the disease. Environmental chemicals, including those that disrupt your body’s hormone systems (endocrine-disrupting chemicals) are thought to play a significant role.3
Which Household Products Should You Avoid?
Silent Spring has identified multiple chemicals groups that you’re better off avoiding to protect your health. This includes chemicals common in household items you may currently be using everyday:
1.Fragrances in Cleaning and Personal Care Products
Fragranced products are almost always loaded with synthetic chemicals that have been linked to cancer, reproductive toxicity, allergies, and more. Some common offenders lurking in “fragrance” include:
Parabens: Synthetic preservatives known to interfere with hormone production and release.
Phthalates: Another synthetic preservative that’s carcinogenic and linked to adverse reproductive effects (decreased sperm counts, early breast development, and birth defects) and liver and kidney damage.
Synthetic musks: These are linked to hormone disruption and are thought to persist and accumulate in breast milk, body fat, umbilical cord blood, and the environment.
According to the Environmental Working Group (EWG):4
“An analysis of the chemical contents of products reveals that the innocuous-looking ‘fragrance’ often contains chemicals linked to negative health effects.
Phthalates, used to make fragrances last longer, are associated [with] damage to the male reproductive system, and artificial musks accumulate in our bodies and can be found in breast milk. Some artificial musks are even linked to cancer.
And if you’ve got asthma, watch out – fragrance formulas are considered to be among the top 5 known allergens, and can trigger asthma attacks.
The same kinds of chemicals are often used for fragrances in cleaning products, scented candles, and air fresheners. To avoid those unpleasant side effects, choose fragrance-free products, but beware labels that say ‘unscented.’ It may only mean that the manufacturer has added yet another fragrance to mask the original odor.”
2.Vinyl Products
You know the smell that seeps out when you take a brand new plastic or vinyl shower curtain out of its package? That’s due to the polyvinyl chloride (PVC) it’s made out of.
This is a significant source of exposure to chemicals known as phthalates, which are used as plasticizers in everything from vinyl flooring to detergents, hoses, raincoats, adhesives, air fresheners, and toys — and even in some soaps, shampoos, lotions, and nail polish.
Phthalates are one of the groups of “gender-bending” chemicals causing males of all species to become more female.
These chemicals have disrupted the endocrine systems of wildlife, causing testicular cancer, genital deformations, low sperm counts, and infertility in a number of species, including polar bears, deer, whales, and otters, just to name a few. Scientists believe phthalates are responsible for a similar pattern in humans as well, and they have been linked to:
Impaired ovulatory cycles and polycystic ovary disease (PCOS) “Decreased dysgenesis syndrome”: A syndrome involving cryptorchidism (undescended testicles), hypospadias (birth defect in which opening of urethra is on the underside of the penis instead of at the end), and oligospermia (low sperm count), and testicular cancer Interference with sexual differentiation in utero Enlarged prostate glands Disturbed lactation Numerous hormonal disruptions Early or delayed puberty Breast cancer and uterine fibroids
Research conducted by the U.S. Centers for Disease Control and Prevention (CDC) discovered high levels of phthalates in all 289 adult Americans tested, and the levels of some phthalates in women of childbearing age exceeded the government’s safe levels set to protect against birth defects, leading scientists to conclude phthalate exposures are “much higher and more common than previously suspected.5
This is why it makes sense to choose a fabric shower curtain (or install glass doors) in lieu of a vinyl one, as well as avoid other common vinyl products in your home.
3.Antimicrobials (Triclosan)
Antibacterial soap and certain toothpastes contain an antibacterial chemical called triclosan, which has been linked to concerns over antibiotic resistance and endocrine disruption.
Some animal studies showed that triclosan caused fetal bone malformations in mice and rats, which may hint at hormonal effects. Triclosan has also been found to cause estrogenic activities in human breast cancer cells, which may stimulate the growth and development of cancer cells.6
Further, as noted by Professor Caren Helbing Ph.D. at the University of Victoria in Canada, the chemical structure of triclosan is similar to thyroid hormones and polychlorinated biphenyls (PCBs).
This similarity allows it to attach to hormone receptors. Helbing’s research shows that tadpoles exposed to triclosan suffered stunted development and leg deformations. The metamorphic process these frogs undergo is mediated by thyroid hormones.
Her findings were published in the Journal of Aquatic Toxicology in 2006, which concluded, “Exposure to low levels of triclosan disrupts thyroid hormone-associated gene expression and can alter the rate of thyroid hormone-mediated postembryonic anuran development.”7
4.Stain-Resistant Furniture Sprays and Clothing
Perfluorinated compounds (PFCs) include PFOA, which was widely used to make non-stick cookware, and PFOS, which was a key ingredient in stain-resistant fabrics. These chemicals have been linked to so many health problems – cancer, miscarriages, thyroid problems, and more – that they’ve been phased out in the US and essentially banned in Europe.
The problem is that PFCs, which are scientifically known as poly- and perfluoroalkyl substances (PFASs), are a family of chemicals, and PFOA and PFOS make up only two of them. The products being used in their place are structurally similar and likely pose many of the same health and environmental risks. EWG’s report on these global contaminants revealed numerous health risks, including:
Cancer Hypothyroidism Reproductive problems Birth defects Immune system problems Organ damage
5.Parabens
Parabens are chemicals found in deodorants and other cosmetics that have been shown to mimic the action of the female hormone estrogen, which can drive the growth of human breast tumors. A study published in 2012 suggested that parabens from antiperspirants and other cosmetics indeed appear to increase your risk of breast cancer.8
The research looked at where breast tumors were appearing and determined that higher concentrations of parabens were found in the upper quadrants of the breast and axillary area, where antiperspirants are usually applied. Parabens inhibit the growth of bacteria, yeast, and molds, and are used as preservatives in countless consumer products, including:
Deodorants and antiperspirants Shampoos and conditioners Shaving gel Toothpaste Lotions and sunscreens Make-up / cosmetics Pharmaceutical drugs Food additives
Bisphenol-A (BPA) Linked to Prostate Cancer
BPA, widely used in plastics, cash register receipts, and canned goods, has been linked to a number of health concerns, particularly in pregnant women, fetuses, and young children, but also in adults, including:
Structural damage to your brain Changes in gender-specific behavior and abnormal sexual behavior Hyperactivity, increased aggressiveness, and impaired learning Early puberty, stimulation of mammary gland development, disrupted reproductive cycles, ovarian dysfunction, and infertility Increased fat formation and risk of obesity Stimulation of prostate cancer cells Altered immune function Increased prostate size and decreased sperm production
BPA coats about 75 percent of cans in North America, which means if you eat canned foods, it’s likely a major source of BPA exposurefor you. Even BPA-free cans and plastics may not be safe, as they often contain a similar chemical known as BPS. However, aside from being a known endocrine disrupter, BPA also appears to play a role in prostate cancer. Research involving an “organoid” grown from embryonic stem cells, which has all the same biomarkers as an adult organ, found low-dose exposure to BPA lead to a proliferation of prostate stem cells.9
An abnormally high number of stem cells is a known risk factor for cancer development. Study author Professor Gail Prins, from the University of Illinois in Chicago, told Yahoo:10
“The higher number of stem cells we saw in developing organoids given very low doses of BPA may be the underlying mechanism by which BPA increases the risk for prostate cancer… This is as definitive as it gets, when it comes to the effect of BPA on the developing prostate. It produces an abnormally high number of prostate stem cells in the tissue, and these nests are a strong candidate for why exposure to BPA during development has been linked to prostate cancer later in life.”
Chemicals in Household Dust Linked to Obesity
When your home is filled with goods that contain potentially toxic chemicals, where do you think those chemicals end up when they come out of carpeting, couch cushions, and the like? Many of them end up in household dust, which is why those dust bunnies accumulating in the corners can be among the most toxic concoctions of all. Young children, in particular, may ingest about 50 milligrams of household dust a day, making it an important pathway by which people are exposed to environmental contaminants.11
New research published in Environmental Science & Technology even revealed that 28 of 30 semi-volatile compounds commonly found in indoor dust were PPARgamma (peroxisome proliferator-activated nuclear receptor gamma) antagonists. This means they could bind to and activate PPARgamma, which is involved in regulating fat metabolism, cell proliferation, and cell death.12 The researchers believe such chemical exposures may play a key role in the development of obesity. As reported by Futurity:13
“The researchers found signs of significant PPARgamma activation in more than half of the 25 dust samples collected from homes, offices, and gyms, at a level of exposure that would be similar to a child’s daily dose.”
Watch Out for Chemicals in Children’s School Supplies
Even back-to-school items geared toward children are not free from environmental chemicals, and such items may even be among the worst offenders. Shiny plastic backpacks are often made from PVC, for instance, and phthalates are widespread in backpacks and 3-ring binders.14 BPA is commonly used in lunchboxes and plastic water bottles for students, and even BPA-free models may contain similar endocrine disruptors.
Even some crayons imported from China may be contaminated with asbestos (best to stick with US-made crayons to avoid this). Finally, resist the urge to send your child to school with hand sanitizer, as many contain antimicrobial chemicals that may harm thyroid function and encourage antibiotic resistance. Teach your child that washing with soap and water is best. When selecting school supplies for your children, EWG recommends the following safer options:15
Natural fabric backpacks Stainless-steel lunchboxes Glass water bottles Notebooks and binders made from recycled cardboard or other natural fibers (look for “no PVC” on the label) Recycled paper products Water-based glues, glue sticks or “school glue” in lieu of stronger adhesives Plain wooden pencils made from sustainable wood or recycled newspaper Crayons made from soy or beeswax
19 More Tips to Reduce Your Chemical Exposure at Home
A great way to identify harmful chemicals on a personal care product label, learn what the research says, and to begin choosing safer alternatives is to take the women’s health challenge from Naturally Savvy.
Additionally, implementing the following measures will help you avoid the worst endocrine-disrupting culprits as well as other chemicals from a wide variety of sources. To sum it up, try to stick with whole foods and natural products around your home. The fewer ingredients a product contains, the better, and try to make sure anything you put on or in your body – or use around your home – contains only substances you’re familiar with. If you can’t pronounce it, you probably don’t want it anywhere near your family.
As much as possible, buy and eat organic produce and free-range, organic meats to reduce your exposure to added hormones, pesticides, and fertilizers. Also avoid milk and other dairy products that contain the genetically engineered recombinant bovine growth hormone (rBGH or rBST).
Rather than eating conventional or farm-raised fish, which are often heavily contaminated with PCBs and mercury, supplement with a high-quality purified krill oil, or eat smaller fish or fish that is wild-caught and lab tested for purity. Wild-caught Alaskan salmon is about the only fish I eat for these reasons.
Buy products that come in glass bottles or jars rather than plastic or canned, since chemicals can leach out of plastics and into the contents.
Store your food and beverages in glass rather than plastic, and avoid using plastic wrap.
Use glass baby bottles and avoid plastic sippy cups for your little ones.
Eat mostly raw, fresh foods. Processed, prepackaged foods (of all kinds) are a common source of chemicals such as BPA and phthalates.
Replace your non-stick pots and pans with ceramic or glass cookware.
Filter your tap water — both for drinking and bathing. If you can only afford to do one, filtering your bathing water may be more important, as your skin absorbs contaminants. To remove the endocrine-disrupting herbicide Atrazine, make sure the filter is certified to remove it. According to the Environmental Working Group (EWG), perchlorate can be filtered out using a reverse osmosis filter.
Look for products that are made by companies that are earth-friendly, animal-friendly, green, non-toxic, and/or 100% organic. This applies to everything from food and personal care products to building materials, carpeting, paint, baby items, upholstery, and more.
Use a vacuum cleaner with a HEPA filter to remove house dust, which is often contaminated with traces of chemicals.
When buying new products such as furniture, mattresses, or carpet padding, ask what type of fire retardant it contains. Be mindful of and/or avoid items containing PBDEs, antimony, formaldehyde, boric acid, and other brominated chemicals. As you replace these toxic items around your home, select those that contain naturally less flammable materials, such as leather, wool, and cotton.
Avoid stain- and water-resistant clothing, furniture, and carpets to avoid perfluorinated chemicals (PFCs).
Minimize your use of plastic baby and child toys, opting for those made of natural wood or fabric instead.
Only use natural cleaning products in your home or make your own. Avoid products that contain 2-butoxyethanol (EGBE) and methoxydiglycol (DEGME) — two toxic glycol ethers that can damage fertility and cause fetal harm.16
Switch over to organic brands of toiletries such as shampoo, toothpaste, antiperspirants, and cosmetics. You can replace many different products with coconut oil and baking soda, for example. EWG has a great database17 to help you find personal care products that are free of phthalates and other potentially dangerous chemicals. I also offer one of the highest quality organic skin care lines, shampoo, and conditioner, and body butter that are completely natural and safe.
Replace feminine hygiene products like tampons and sanitary pads with safer alternatives.
Avoid artificial air fresheners, dryer sheets, fabric softeners, or other synthetic fragrances.
Look for products that are fragrance-free. One artificial fragrance can contain hundreds – even thousands – of potentially toxic chemicals.
Replace your vinyl shower curtain with one made of fabric.
We carry a complete line of natural, non-toxic household cleaning products.
Start saving today by purchasing the Thieves Starter Kit. Join and get 24% off for life.


from WordPress http://ift.tt/2GcRrfW
via IFTTT
1 note
·
View note
Text
Just Three Micrometers Can Paint a Picture of Skin Cancer
With new advancing technologies, aspiring scientists, and countless new human diseases needed to be diagnosed, the study of biomedicine has made much progress over the past years. It is important to note that scientific and biomedical research would not have been possible without the utilization of model organisms, essential in understanding and diagnosing certain human disorders and illnesses. Working in a laboratory this summer and experimenting with small organisms and cells, I discovered the correlation between the genes in model organisms and in human illnesses, for example, skin cancer.
A day outside in the sweltering heat and scorching sun not only can make one sweat and feel extremely dehydrated, but can have a major impact on the skin. According to the Skin Cancer Foundation, approximately 9,500 people in the United States are diagnosed with skin cancer daily, and “more than two people die of the disease every hour” [Skin Cancer Foundation]. Exposure to ultra-violet radiation is one of the most common causes of skin cancer as UV rays from the sun can harm DNA in skin cells. The chemical bond in thymine is altered in which the bond forces two thymines to stick to each other, producing a dimer. Cancerous cells are produced when a cell has an abundant amount of dimers.
Sunlight is not the only factor of skin cancer, however. A study in Denmark, conducted in 2013, showed the increased risk of skin cancer with mobile users [NCBI] due to radio-frequency radiation. Due to this form of radiation being non-ionizing, it has a low frequency, so scientists are still researching the harmful effects of cellular devices. But, research shows that blue light, emitted by smartphones, is linked to prostate and breast cancer [Lieber], so limiting the amount of time you spend on your phone in the dark may be a good idea.
During my time at the laboratory, I conducted an experiment with yeast in order to identify the impact of sunlight on the growth of yeast colonies in a petri dish, and to determine effective protective measures to block harmful UV radiation. My team and I tested a wild-type yeast strain (experimental control) and the UV-sensitive mutant yeast strain (DNA repair enzyme) to identify the different reactions of the two distinct yeast types from the exposure of UV light. In this investigation, we tested aluminum foil, shade, and sunscreen as protectants. The results showed that the yeast strains which experienced more cell growth (more colonies) were protected against UV radiation (with the protectants) and the yeast with dead cells did not have efficient protection (in real life, this could result in skin cancer or other affiliated diseases). It came to no surprise that the sunscreen proved to result in a lawn of cells, however, to take this experiment further, different levels of sunscreen SPF could be tested [Reeves]. The yeast cells which were not damaged by the sun stretched to large quantities as these cells reproduce every 90 minutes, while kept in Fetal Bovine Serum (nutrients for the cells). This experiment was a direct connection to the effect of UV radiation on humans and is essential in determining effective and valid protection against UV radiation.
With this experiment, I not only began to realize how essential model organisms are in our daily lives, but was shocked with the fact that a cell, just three micrometers big, could paint a whole picture of skin cancer…
Written by: Shreya Papneja
0 notes
Text
New Peanut Allergy Patch Could Be Coming to Pharmacies This Year
Visit Now - http://zeroviral.com/new-peanut-allergy-patch-could-be-coming-to-pharmacies-this-year/
New Peanut Allergy Patch Could Be Coming to Pharmacies This Year
Vaccines have long been hailed as one of our greatest public health achievements. They can be made to protect us from infections with either viral or bacterial microbes. Measles and smallpox, for example, are viruses; Streptococcus pneumoniae is a bacterium that causes a range of diseases, including pneumonia, ear and sinus infections, and meningitis. Hundreds of millions of illnesses and deaths have been prevented due to vaccines that eradicated smallpox and significantly reduced polio and measles infections. However, some misunderstanding remains regarding how vaccines are made, and why some scary-sounding ingredients [PDF] are included in the manufacturing process.
The production of our vaccines has greatly evolved since the early days, when vaccination was potentially dangerous. Inoculating an individual with ground-up smallpox scabs usually led to a mild infection (called “variolation”), and protected them from acquiring the disease the “regular” way (via the air). But there was always a chance the infection could still be severe. When Edward Jenner introduced the first true vaccination with cowpox, protection from smallpox became safer, but there were still issues: The cowpox material could be contaminated with other germs, and sometimes was transmitted from one vaccinated person to another, leading to the inadvertent spread of blood-borne pathogens. We’ve come far in the last 200 years.
There are different kinds of vaccines, and each requires different processes to move from the laboratory to your physician’s office. The key to all of them is production of one or more antigens—the portion of the microbe that triggers a host immune response.
LIVE ATTENUATED VACCINES AND DEAD, “INACTIVATED” VACCINES
There are several methods to produce antigens. One common technique is to grow a virus in what’s called a cell culture. Typically grown in large vats called bioreactors, living cells are inoculated with a virus and placed in a liquid growth medium that contains nutrients—proteins, amino acids, carbohydrates, essential minerals—that help the virus grow in the cells, producing thousands of copies of itself in each infected cell. At this stage the virus is also getting its own dose of protective medicine: antibiotics like neomycin or polymyxin B, which prevent bacterial and fungal contamination that could kill the cells serving as hosts for the virus.
Once a virus completes its life cycle in the host cell, the viruses are purified by separating them from the host cells and growth media, which are discarded. This is often done using several different types of filters; the viruses are small and can pass through holes in the filter that trap larger host cells and cell debris.
This is how “live attenuated vaccines” are created. These vaccines contain viruses that have been modified so that they are no longer harmful to humans. Some of them are grown for many generations in cells that aren’t human, such as chicken cells, so that they have mutated to no longer cause harm to humans. Others, like the influenza nasal mist, were grown at low temperatures until they lost the ability to replicate in the warmer temperatures of the lungs. Many of these vaccines you were probably given as a child: measles, mumps, rubella (“German measles”), and chickenpox.
Live attenuated vaccines replicate briefly in the body, triggering a strong—and long-lasting—response from your immune system. Because your immune system kicks into high gear at what it perceives to be a major threat, you need fewer doses of the vaccine for protection against these diseases. And unlike the harmful form of the virus, it is extremely unlikely (because they only replicate at low levels) that these vaccines will cause the host to develop the actual disease, or to spread it to other contacts. One exception is the live polio vaccine, which could spread to others and, extremely rarely, caused polio disease (approximately one case of polio from 3 million doses of the virus). For this reason, the live polio virus was discontinued in the United States in 2000.
Scientists use the same growth technique for what are known as “killed” or “inactivated” vaccines, but they add an extra step: viral death. Inactivated viruses are killed, typically via heat treatment or use of a chemical such as formaldehyde, which modifies the virus’s proteins and nucleic acids and renders the virus unable to replicate. Inactivated vaccines include Hepatitis A, the injected polio virus, and the flu shot.
A dead virus can’t replicate in your body, obviously. This means that the immune response to inactivated vaccines isn’t as robust as it is with live attenuated vaccines; replication by the live viruses alerts many different types of your immune cells of a potential invader, while killed vaccines primarily alert only one part of your immune system (your B cells, which produce antibodies). That’s why you need more doses to achieve and maintain immunity.
While live attenuated vaccines were the primary way to make vaccines until the 1960s, concerns about potential safety issues, and the difficulty of making them, mean that few are attempting to develop new live attenuated vaccines today.
COMBINATION, BACTERIAL, AND GENETICALLY ENGINEERED VACCINES
Other vaccines aren’t made of whole organisms at all, but rather bits and pieces of a microbe. The combination vaccine that protects against diphtheria, pertussis, and tetanus—all at once—is one example. This vaccine is called the DTaP for children, and Tdap for adults. It contains toxins (the proteins that cause disease) from diphtheria, pertussis, and tetanus bacteria that have been inactivated by chemicals. (The toxins are called “toxoids” once inactivated.) This protects the host—a.k.a. you, potentially—from developing clinical diphtheria and tetanus disease, even if you are exposed to the microorganisms. (Some viruses have toxins—Ebola appears to, for example—but they’re not the key antigens, so they’re not used for our current vaccines.)
As they do when developing live attenuated or inactivated vaccines, scientists who create these bacterial vaccines need some target bacteria to culture. But because the bacteria don’t need a host cell to grow, they can be produced in simple nutrient broths by vaccine manufacturers. The toxins are then separated from the rest of the bacteria and growth media and inactivated for use as vaccines.
Similarly, some vaccines contain just a few antigens from a bacterial species. Vaccines for Streptococcus pneumoniae, Haemophilus influenzae type B, and Neisseria meningitidis all use sugars that are found on the outer part of the bacteria as antigens. These sugars are purified from the bacteria and then bound to another protein to enhance the immune response. The protein helps to recruit T cells in addition to B cells and create a more robust reaction.
Finally, we can also use genetic engineering to produce vaccines. We do this for Hepatitis B, a virus that can cause severe liver disease and liver cancer. The vaccine for it consists of a single antigen: the hepatitis B surface antigen, which is a protein on the outside of the virus. The gene that makes this antigen is inserted into yeast cells; these cells can then be grown in a medium similar to bacteria and without the need for cell culture. The hepatitis B surface antigen is then separated from the yeast and serves as the primary vaccine component.
OTHER INGREDIENTS IN VACCINES (AND WHY THEY’RE THERE)
Once you have the live or killed viruses, or purified antigens, sometimes chemicals need to be added to protect the vaccine or to make it work better. Adjuvants, such as aluminum salts, are a common additive; they help enhance the immune response to some antigens by keeping the antigen in contact with the cells of the immune system for a longer period of time. Vaccines for DTaP/Tdap, meningitis, pneumococcus, and hepatitis B all use aluminum salts as an adjuvant.
Other chemicals may be added as stabilizers, to help keep the vaccine working effectively even in extreme conditions (such as hot temperatures). Stabilizers can include sugars or monosodium glutamate (MSG). Preservatives can be added to prevent microbial growth in the finished product.
For many years, the most common preservative was a compound called thimerosal, which is 50 percent ethylmercury by weight. Ethylmercury doesn’t stick around; your body quickly eliminates it via the gut and feces. (This is different from methylmercury, which accumulates in fish and can, at high doses, cause long-lasting damage in humans.) In 2001, thimerosal was removed from the vaccines given in childhood due to consumer concerns, but many studies have demonstrated its safety.
Finally, the vaccine is divided into vials for shipping to physicians, hospitals, public health departments, and some pharmacies. These can be single-dose or multi-dose vials, which can be used for multiple patients as long as they’re prepared and stored away from patient treatment areas. Preservatives are important for multi-dose vials: bacteria and fungi are very opportunistic, and multiple uses increase the potential for contamination of the vaccine. This is why thimerosal is still used in some multi-dose influenza vaccines.
Though some of the vaccine ingredients sound worrisome, most of these chemicals are removed during multiple purification steps, and those that remain (such as adjuvants) are necessary for the vaccine’s effectiveness, are present in very low levels, and have an excellent track record of safety.
0 notes