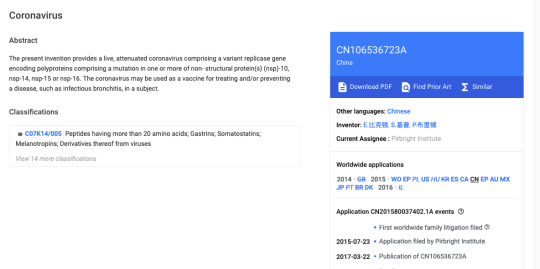
#attenuated live infections bronchitis virus
Text
How the Gates Foundation Hijacked COVID-19 for Profit
After much thought and a continuing stream of new evidence coming to light, I am going to do this. This is a timeline of events leading up to and surrounding the coronavirus outbreak in late 2019. I still cannot prove that the long-lasting, breath-shortening cold many people caught in December 2019 was in anyway related to COVID-19. I also cannot find information – most likely because in 2013,…
View On WordPress
#attenuated#attenuated live infections bronchitis virus#ChAdOx1#Chimp Adenovirus vector#Competition#coronavirus#Covid-19 vaccine#Dr Tess Lawrie#Erica Bickerton#Gates Foundation#human adenovirus#intellectual property#Inventor#Ivermectin#licensing#Livestock Antibody Hub#mutant spike protein#patents#pirbright institute#propaganda and censorship#Protease inhibitor#Responding to public#shutting down public speculation#viruses affecting farm animals and human health#Written Evidence to parliament
0 notes
Text
H1N1 Vaccines Market Size, Share, Outlook, and Opportunity Analysis, 2019– 2027

H1N1 Vaccines Market - Regional Insights
On the basis of geography, H1N1 Vaccines Market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. North America is expected to lead the H1N1 vaccines market during the forecast period. This is due to increasing government initiatives for prevention of H1N1 infection. U.S. which faced major epidemic in 2009. After this pandemic the U.S. has strengthen the preventive approach to control H1N1 outbreak by implementing vaccination program. According to statistics given by Centre for Disease Control and Prevention in February 2018, during October 1, 2017 and February 3, 2018, clinical laboratories tested 666,493 specimens for influenza virus, 124,316 (18.7%) of which tested positive for influenza virus. 84.3% of these tested positive for influenza A.
Furthermore Asia Pacific market is expected to exhibit the highest CAGR due to high prevalence of swine flu and increasing awareness about vaccination against H1N1. According to Ministry of Health and Family Welfare, Government of India, there were 0.1 million cases in India of H1N1 influenza during 2010 to 2017. Continuous changing nature of the virus creates challenges to overcome the H1N1 epidemic. For instance, in 2017 new Michigan strain of H1N1 swine flu virus was first identified in India. Government has played an important role in tackling swine flu by spreading awareness about H1N1 vaccine The Government of India has published guidelines for case management of influenza A/H1N1 which classifies influenza patients into three categories depending on severity. Vaccination was recommended in patient group which are susceptible to H1N1 fever in future.
* The sample copy includes: Report Summary, Table of Contents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/2056
H1N1 influenza virus causes viral disease, commonly known as swine flu, which mostly affects the respiratory system. Swine flu is characterized by infection from H1N1 strain. Symptoms of H1N1 flu are fever, cough, sore throat, runny or stuffy nose, watery and red eyes, body aches, headache, fatigue, and diarrhea. H1N1 spreads through contact with contaminated air or geographical locations infected with virus. H1N1 infection may worsen other chronic respiratory conditions such as bronchitis, pneumonia, COPD, and sometimes even death due to respiratory failure.
H1N1 vaccine is an important innovation to tackle the H1N1 pandemic since 2009. Initially, the vaccines developed or available were inactivated monovalent vaccines that could prevent infection from most of the strains of H1N1. There are two routes of administration such as intradermal for inactivated vaccine and intranasal for live attenuated vaccine. Both routes shown efficacy profile and are bioequivalent against preventing H1N1 infection. Global H1N1 vaccine market has presence of both multinational pharmaceutical companies and companies from emerging economies.
H1N1 Vaccines Market - Market Dynamics
One of the major factor propelling growth of H1N1 market is increased incidence of disease and episodes of pandemic worldwide from H1N1 infection. According to World Health Organization’s (WHO) 2018, factsheet estimates, 290,000 to 650,000 deaths are reported each year from all types of influenza infection (including Influenza A i.e. H1N1). Each year there are 3 to 5 million cases of severe illness from viral influenza infection globally, according to the same source.
Browse Research Report: https://www.coherentmarketinsights.com/ongoing-insight/h1n1-vaccines-market-2056
Government initiatives, both internationally and nationally, to tackle H1N1 pandemic would positively impact the market growth. Health institution such as World Health Organization (WHO) have been instrumental in developing vaccines and supporting efficient distribution of the vaccine. After 2009 pandemic of H1N1 influenza, the U.S. government initiated US$ 3 billion H1N1 vaccine project under which it contracted five major companies to develop vaccine for H1N1 influenza. Under the WHO’s Global Influenza Surveillance and Response System, National Influenza Centers and WHO Collaborating Centers are continuously monitoring the influenza viruses circulating in humans and updates the composition of influenza vaccines twice a year.
Furthermore, rising geriatric population worldwide would be another impetus for growth of the H1N1 vaccine market. Global population is crossing the age of 60 at annual rate of 3% according to 2017 UN report. Increased age, maximizes the risk of various respiratory tract related diseases and immune deficient diseases, thereby the risk for infection due to H1N1 virus rises. Awareness amongst patients about preventive healthcare approaches such as vaccination may lead to higher demand for H1N1 vaccines during forecast period.
However, seasonal nature of occurrence of H1N1 flu has led to meagre revenue for many of the key players. For instance, India-based Serum Institute, which produces intranasal live vaccine to treat swine flu had to destroy stock of 2 million doses in the year 2011 due to lack of demand. In contrast, if pandemic arises suddenly, supply of vaccine may be delayed due to slow production of vaccine, which may negatively affect health of many patients. Due to constant evolving nature of H1N1 virus, vaccine may prove to be ineffective in some parts of the world, which may deter the use of vaccines.
H1N1 Vaccines Market - Competitive Landscape
Key players present in the H1N1 vaccines market are Medimmune, AstraZeneca, GlaxoSmithKline, Novartis, Serum Institute of India, Abbott Healthcare, Sanofi Pasture, CSL Biotherapeutics, Sinovac, ID Biomedical Corporation, Cadila Healthcare, and Lupin Ltd.
Buy-Now this research report: https://www.coherentmarketinsights.com/insight/buy-now/2056
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
mailto:[email protected]
U.S. Office:
Name: Mr. Shah
Coherent Market Insights 1001 4th Ave,
# 3200 Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737
0 notes
Text
H1N1 Vaccines Market – Global Industry Trend, Growth, and Analysis, 2018–2026
H1N1 influenza virus causes viral disease, commonly known as swine flu, which mostly affects the respiratory system. Swine flu is characterized by infection from H1N1 strain. Symptoms of H1N1 flu are fever, cough, sore throat, runny or stuffy nose, watery and red eyes, body aches, headache, fatigue, and diarrhea. H1N1 spreads through contact with contaminated air or geographical locations infected with virus. H1N1 infection may worsen other chronic respiratory conditions such as bronchitis, pneumonia, COPD, and sometimes even death due to respiratory failure.
H1N1 vaccine is an important innovation to tackle the H1N1 pandemic since 2009. Initially, the vaccines developed or available were inactivated monovalent vaccines that could prevent infection from most of the strains of H1N1. There are two routes of administration such as intradermal for inactivated vaccine and intranasal for live attenuated vaccine. Both routes shown efficacy profile and are bioequivalent against preventing H1N1 infection. Global H1N1 vaccine market has presence of both multinational pharmaceutical companies and companies from emerging economies.
Get Free Request Sample Copy @ https://www.coherentmarketinsights.com/insight/request-sample/2056
One of the major factor propelling growth of H1N1 market is increased incidence of disease and episodes of pandemic worldwide from H1N1 infection. According to World Health Organization’s (WHO) 2018, factsheet estimates, 290,000 to 650,000 deaths are reported each year from all types of influenza infection (including Influenza A i.e. H1N1). Each year there are 3 to 5 million cases of severe illness from viral influenza infection globally, according to the same source.
Government initiatives, both internationally and nationally, to tackle H1N1 pandemic would positively impact the market growth. Health institution such as World Health Organization (WHO) have been instrumental in developing vaccines and supporting efficient distribution of the vaccine. After 2009 pandemic of H1N1 influenza, the U.S. government initiated US$ 3 billion H1N1 vaccine project under which it contracted five major companies to develop vaccine for H1N1 influenza. Under the WHO’s Global Influenza Surveillance and Response System, National Influenza Centers and WHO Collaborating Centers are continuously monitoring the influenza viruses circulating in humans and updates the composition of influenza vaccines twice a year.
Furthermore, rising geriatric population worldwide would be another impetus for growth of the H1N1 vaccine market. Global population is crossing the age of 60 at annual rate of 3% according to 2017 UN report. Increased age, maximizes the risk of various respiratory tract related diseases and immune deficient diseases, thereby the risk for infection due to H1N1 virus rises. Awareness amongst patients about preventive healthcare approaches such as vaccination may lead to higher demand for H1N1 vaccines during forecast period.
H1N1 Vaccines Market - Market Taxonomy - On the basis of vaccine type, the global H1N1 vaccines market is segmented into: Inactivated Vaccine, Live Attenuated Vaccine,. On the basis of route of administration, the global H1N1 vaccines market is segmented into: Injectable Vaccine, Intranasal Vaccine,. On the basis of distribution channel, the H1N1 vaccines market is segmented into: Retail Pharmacy, Hospital Pharmacy, Online Pharmacy,. On the basis of geography, the H1N1 vaccines market is segmented into: North America, Latin America, Europe, Asia Pacific, Middle East, Africa,.
Ask Questions to Expertise @ https://www.coherentmarketinsights.com/insight/talk-to-analyst/2056
Key players present in the H1N1 vaccines market are Medimmune, AstraZeneca, GlaxoSmithKline, Novartis, Serum Institute of India, Abbott Healthcare, Sanofi Pasture, CSL Biotherapeutics, Sinovac, ID Biomedical Corporation, Cadila Healthcare, and Lupin Ltd.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Coronavirus Infection Market Size, Share, Trends, Growth, Forecast Analysis Report by Product, by Application, by Segment, by Region – Global Forecast to 2024
Global Coronavirus Infection Market: Outline
Coronavirus is the type of virus that belongs to either of the two subfamilies, Coronavirinae or Torovirinae and it generally affects the respiratory tract of mammals. Coronavirus is thus called as it has a crown-like appearance and in Latin, the word ‘corona’ means crown. Human coronaviruses (HCoV) were discovered in the 1960s from the naval cavities of patients affected with common cold. These viruses cause a range of serious infections in humans from common cold to severe respiratory problems. Coronavirus is spread through droplets, i.e., by coughing or sneezing and there is a limited possibility that this large family of virus can also be spread by close contacts with an ability to pass from person to person. Sore throat, coughing, fever, respiratory infection and difficulty in breathing are some of the most common symptoms of coronavirus infection.
View Report : https://www.transparencymarketresearch.com/coronavirus-infection-market.html
Global Coronavirus Infection Market: Key Categories
Based on the infections caused by coronavirus infection, the market can be segmented as follows:
HCoV-229E: causes common cold, pneumonia, and bronchiolitis
HCoV-OC43: causes respiratory tract infection and pneumonia in infants
SARS-CoV: causes severe acute respiratory syndrome (SARS)
New Haven CoV: causes Kawasaki disease, aneurysmsof the coronary arteries
HKU1-CoV: causes acute respiratory distress and bilateral pneumonia
MERS-CoV (Middle East respiratory syndrome coronavirus): causes bronchial infections
There are few coronavirus vaccines available in the market such as inactivated coronavirus vaccine, live attenuated coronavirus vaccine and S-Protein based coronavirus vaccine. Various precautions and therapeutic drugs can also be taken to prevent or minimize the effect of this fatal infection. Some of the precautions are as follows:
Standard precaution: hand hygiene, eye protection, disinfection of equipments etc.
Droplet precaution: Use of medical mask and restricted patient movement
Airborne precaution: Use of PPE (personal protective equipment), gloves etc.
Global Coronavirus Infection Market: Trends and Opportunities
Major driving factors for the growth of coronary infection market are increase in the rate of communicable diseases, disposable income in emerging countries, increasing number of hospitals, and rising health hygiene of individuals across geographies. Technological advancement and innovation about respiratory infection treatment has also triggered the growth of the coronary infection market. On the other hand, huge amount of capital involved in the market would most likely impede the growth of this market.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) : https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=16940
Global Coronavirus Infection Market: Regional Outlook
Geographically, North America holds a leading position in the coronary infection market followed by Europe. The prime driving factors for the growth of this market in these regions are increasing awareness among people regarding infectious viruses such as SARS-CoV, shift to sophisticated treatment tools, and constantly increasing segment of the population who acquire coronavirus infection. According to the World Health Organization (WHO), around 1.1 million people in the United States were hospitalized with pneumonia and 50,000 people died from this disease. Asia-Pacific is one of the fastest growing regions and is the most lucrative market as this region is expected to show a rapid growth in the near future. Major factors that will boost the growth of the coronavirus infection market in Asia-Pacific are high population density in countries like China and India where there is high prevalence of contagious diseases, improved healthcare infrastructure, and demand for novel drug therapeutics related to coronavirus infected diseases.
Get an Idea about the Offerings of Our Market Research Report from this Brochure :
Bronchitis Treatment Market to Reach US$ 4.7 Bn by 2026, High Prevalence of Air Pollution Drives Growth:...
/PRNewswire/ -- The global Bronchitis Treatment Market is set to grow at a 3.6%, compounded…
Read more
Global Coronavirus Infection Market: Companies Covered under the Report
Some of the top major players operating in the coronavirus market are Inovio Pharmaceuticals, Inc., Novavax, Inc., Protein Potential, LLC, AlphaVax, Inc., Synairgen plc, NanoViricides, Inc., GGeneCure, LLC and many others.
0 notes
Text
The False Prop of ‘Get Your Flu Shot’ - LewRockwell
See on Scoop.it - COMPARE RISK COMMUNICATION
Walk in to any chain pharmacy or a Big Box store, and they are promoting flu shots as you enter the front door. Flu shots are big business now. The masses must be vaccinated. Scare tactics Relias Media, self-proclaimed as a “trusted source for healthcare information,” releases this headline story: “Given the nation’s antivaccine movement and the annual safety myths and efficacy quibbles about the seasonal influenza vaccine, public health officials are keeping it simple this year: CDC: Flu Vaccination Can Keep You Out Of The Hospital, Morgue.” In my lifetime, I have never known of a single person who died of influenza. Yet the annual mortal threat posed by the flu seems like an imagined pandemic every flu season. NBC-News posts a report entitled “Here’s why doctors urge people to get the flu shot every year” with an accompanying video story of a 4-year-old who became ill with flu and developed swelling of the brain and lost some of her vision, which may or may not be permanent, the report says. But the headline reads: “Mother’s warning after flu leaves 4-year old blind.” Video footage shows a child who certainly has some functional vision. The news report appears to be a scare tactic. A major news agency is doing the bidding of the vaccine makers. Another online political news portal, The Hill, ran with the NBC story with a headline that reads: “4-Year Old Who Wasn’t Vaccinated Against Flu Goes Blind From Virus.” And so it goes in our dystopian “fake” news era. Fiction becomes non-fiction. Andrew W. Saul Best Price: $17.94 Buy New $18.21 (as of 08:00 EST - Details) Meanwhile, other news agencies report flu vaccination is likely to be ineffective against a new flu strain. The overall blended message is “get a useless flu shot.” The essence of flu fakery But here is the essence of the annual flu fakery. It’s called “influenza-like illness.” At the end of December/2019 The Center for Infectious Disease Research & Policy (CIDRAP) reported 4.6 million flu illnesses, 39,000 hospitalizations and 2100 flu-related deaths in this flu season with outpatient visits for influenza-like illnesses spiking from 3.9% to 5.1% for the week ending Dec. 21. And therein lies the lie – – – influenza-like illnesses (ILI). ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza. The Centers For Disease Control (CDC) is said to collect and disseminate data from the “The U.S. Outpatient Influenza-Like Illness surveillance Network,” a collaboration of CDC, state and local health departments. What is this influenza-like illness? Where does it come from? Are medicines prescribed to treat the flu the same drugs to treat influenza-like illness? Pay close attention when you read accurate reports about the flu. When you read about the flu or hear news broadcasts about flu outbreaks, you are really seeing and hearing about ILI. Other infectious diseases mimic the flu MedScape reports 20-40% of influenza-like illness is caused by other respiratory tract viruses, particularly adenovirus or respiratory syncytial virus. The latter often causes a less intense fever and bronchitis, a symptom that is difficult to distinguish from the flu. Flu vaccines protect against strains of influenza virus, not other viruses. That means, if you opt for vaccination based upon symptoms, 20-40% of the time you have nothing to gain. The following chart provides a list of bacterial, viral and fungal infections that cause flu-like symptoms. Symptoms of the flu, influenza-like infections and side effects of anti-inflammatory/TNF-inhibitor drugs are much the same, making it difficult to discern one from the other. TNF-blocking drugs and the flu There are a lot more reasons why unwary patients may end up with flu-like symptoms. One of the most prominent is TNF-inhibiting drugs used to treat pain and suffering from autoimmune diseases like rheumatoid arthritis, psoriasis, ulcerative colitis, Crohn’s disease, eye inflammation (uveitis), multiple sclerosis. Rheumatoid arthritis patients are at increased risk of infection even in the absence of immunosuppressive TNF-inhibiting drugs. Given that TNF blockers produce symptoms that are similar to the flu, it is obvious that users of these immune-compromising drugs may be included in annual flu data and may comprise a sub-population at greater risk for hospitalization and death. Hundreds of thousands of patients are prescribed TNF inhibitors. TNF stands for tumor necrosis (“death”) factor. TNF is a component of the immune system. Infection with influenza virus induces expression of TNF-α in lung epithelial cells and TNF-α exerts powerful anti-influenza virus activity. However, TNF inhibitors can work too strongly and over-inhibit immunity. TNF drugs are known to actually increase risk for infectious disease including seasonal influenza (see chart below). Current expert opinion is that patients on any immune-suppression drugs are at increased risk for influenza. Therefore, doctors recommend patients with autoimmune disease update all their vaccinations prior to initiation of anti-TNF drugs. But the flu vaccine must be administered annually. And TNF-inhibitor drug users need to get booster shots of the flu vaccine because the first round of the flu shot may not produce sufficient antibodies. TNF-drug users may be on a tightrope. Infectious disease experts note that 71 healthy adults need to be vaccine to prevent 1 case of influenza. That represents a 1.4% reduced risk for the flu. Therefore, the chance that you will avoid the flu by getting vaccinated is nil (see chart below). MD JD Thomas E Levy Best Price: $9.85 Buy New $18.89 (as of 03:45 EST - Details) The rate of influenza infection in non-vaccinated individuals who take TNF-inhibiting drugs is 14.4%, reduced to 4.5% with flu vaccination prior to anti-TNF drug delivery. The science appears to warrant pre-vaccination of all patients receiving TNF-inhibiting drugs. In a report published in Arthritis Research & Therapy experts say: “Infections are most probably even more dangerous for patients under TNF blockade. The answer, therefore, is certainly not to leave vaccinations out, but to vaccinate more often, more consistently, and everybody with a rheumatic disease.” But this increases risk for side effects including flu infection itself. These numbers are only applicable when one anti-TNF drug is used. About a third of patients on anti-TNF drugs respond poorly and are candidates for a 2nd drug. Approximately half of the normal antibody levels are maintained one month after dual anti-TNF-blocking drugs are employed. Over-inhibition of TNF may place patients at risk for infection (namely tuberculosis and the flu). The recommendation is that patients with autoimmune disorders get vaccinated prior to initial anti-TNF treatment. However, there is a general warning for patients taking immune-suppressant drugs like anti-TNF inhibitors to avoid live attenuated vaccines (measles, MMR, polio, varicella/whooping cough, yellow fever, varicella-zoster and the live nasal instilled FluMist). The Immunization Action Coalition recommends patients with auto-immune diseases or who take immune inhibiting drugs be given inactivated vaccines and “should not be given ‘live’ vaccines.” Of note, nasally-instilled FluMist has been re-introduced for use in 2018. Senior adults comprise ~60% of patients hospitalized for the flu and 85% of flu deaths. This is especially important because vaccination against pneumonia does not result in reduce risk of death or hospitalization. Serious side effects and hospitalization from vaccination may result while the immune response is actually reduced due to use of anti-TNF drugs. This is highly possible because unwary patients may get vaccinated for the flu at local drug stores, bypassing medical risk assessment. It may not be fully recognized that TNF itself helps to prevent infection and death, but in high doses over-inhibit the immune response to the point of death, which has been shown in the animal lab. The following chart helps to understand the reduced risk for the flu produced by flu vaccination. The flu shot reduces risk in the healthy population from 2 to 1 in 100. Among individuals who take anti-TNF drugs, 14 will develop influenza and if they are pre-vaccinated prior to starting anti-TNF drugs the risk is reduced from 14 to 4. Source: Annals of Rheumatic Diseases, 2019 Other TNF-inhibiting factors There are natural TNF-inhibitors in foods, dietary supplements and Rx hormones. For example, polyphenols provided naturally from spices, grapes, berries, olives, or as dietary supplements (resveratrol, turmeric/curcumin, catechins/green tea, quercetin) are potent TNF inhibitors. Mega-doses of these natural TNF-inhibitors, which are much milder than TNF-blocking drugs, theoretically may combine with the drugs to worsen symptoms and increase risk for hospitalization and even death. No dietary supplements alone have been linked with influenza-like hospitalizations or deaths. It is the combination of TNF-blocking drugs with mega-dose natural anti-TNF supplements that is of concern. It should also be noted that cancer patients undergoing chemotherapy are immune-compromised and flu-like symptoms may result. Again, hospitalizations or deaths associated with TNF-blocking drugs and other anti-TNF agents may covertly contribute to the morbidity and mortality attributed to the flu. Analysis of US Food and Drug Administration (FDA) pharmacovigilance data reveals 714 cases of influenza reported in association with anti-TNF therapy in the 5-year period ending in 2008, including 7 deaths [FDA, 2009]. But the real numbers of cases and deaths are likely to be much higher. Rise of autoimmune disorders An unsettling concern is that autoimmune disorders are rising in the population at large. Between 2001 and 2009 the incidence of Type I autoimmune diabetes is reported to have increased by 23%. Other autoimmune diseases are also on the rise. One of the origins of autoimmune disease is believed to be molecular mimicry, that is, synthetic vaccines that utilize snippets of viruses that are structurally similar to microorganisms that cause infectious diseases may plausibly result in autoimmunity. Some vaccines are associated with autoimmune conditions. Autoimmune reactions to vaccines have been reported since the inception of vaccination programs. For example, clusters of paralyzing Guillain Barre Syndrome (GBS) are associated with flu vaccination (SM Journal Vaccine Research 2015). A 7.6-fold increased risk for GBS was revealed following 35 million flu vaccinations in 1976 which caused the National Influenza Immunization Program to be suspended in that year. (Note: for unknown reasons the journal cited above that reports this link between vaccination and autoimmunity cannot be accessed via the National Library of Medicine PubMed site. Nor can it be accessed via Google.). Vaccine makers have a dandy business going, fostering the need for more and more vaccines and inoculations. How many real cases of the flu? So exactly how many real cases of influenza infection (not influenza-like infection) occur during a typical flu season? In 2018 The Epoch Times published a chilling report entitled “How The CDC Uses Fear To Increase Demand For Flu Vaccines.” In that report, data from Johns Hopkins University School of Medicine researcher Peter Doshi was presented. Here is Doshi’s conclusive answer as to how many flu-confirmed deaths actually occur: Setting aside pneumonia and looking just at influenza-associated deaths from 1979 to 2002, the annual average according to the National Center for Health Statistics (NCHS) data was only 1,348. Illustrating the problem, Doshi observed that for the year 2001, the total number of reported pneumonia and influenza deaths was 62,034. Yet, of those, less than one half of one percent were attributed to influenza. Furthermore, of the mere 257 cases blamed on the flu, only 7 percent were laboratory confirmed. That’s only 18 cases of lab confirmed influenza out of 62,034 pneumonia and influenza deaths—or just 0.03 percent, according to the CDC’s own National Center for Health Statistics (NCHS). As a health journalist, the most common question I am asked is whether to get a flu shot or not. I hope this report authoritatively answers that question. If you still don’t understand all this medical terminology, the bottom-line answer is don’t get duped by the flu propaganda. The chances of you avoiding the flu are nil and the chance of developing a life-long autoimmune disorder is real. Most of my friends and acquaintances who opted to get a flu shot got sick and were bedridden for a couple of days. Supplemental vitamin D, C and zinc lozenges (preferably zinc acetate) may be helpful in the flu season. Taking 50,000 units of vitamin D per day in a single dose (not an overdose) often rapidly halts a runny-nose cold. Colds and flu occur in winter when sunshine vitamin D levels are low. Once flu symptoms arise, resveratrol pills may halt viral replication altogether.
0 notes
Text
Poultry Vaccines Market Size 2019 Industry Key Players, Growth, Trends, Analysis and Forecast to 2025
Brandessence Market Research is working on a new report title“Global Poultry Vaccines Market: Global Size, Trends, Competitive, Historical & Forecast Analysis, 2019-2025″. The rise in poultry population & poultry meat consumption and growing demand for poultry-related food products such as meat, eggs and chicken are likely to propel the growth of global poultry vaccines market.
Scope of Global Poultry Vaccines Market Reports –
The vaccines which are used to prevent and control contagious poultry diseases like Egg drop syndrome, Fowl Pox, Chicken Anaemia Virus Infection and Big Liver and Spleen Disease are called as Poultry Vaccines. This vaccine helps to avoid a specific disease by boosting the bird’s immune system to produce specific antibodies which will fight the causative agent. Many of the poultry vaccines are developed against various viral diseases. There are different routes of administration of Poultry vaccines such as intramuscular, subcutaneous, ocular, oral and nasal. There is also hygiene management in global poultry vaccines market which includes reduced air circulation, good air filters, adjustment to weather conditions and well conserved hatchery insulation system. These are is necessary for in vivo vaccination. A Poultry Vaccination Program are for broilers, broiler-breeders, commercial layers, turkeys, duck breeders, and commercial ducklings and it is depends on several factors such as type of production, level of biosecurity, local pattern of disease, status of maternal immunity, availability of various types of vaccines and its costs. During handling of poultry vaccines they must be transport in well-insulated cool boxes containing ice packs to keep the temperature constant, store in temperatures of between 2oC to 6oC and avoid exposing them to direct freezing, extreme heat and intense sunlight. So during the study of Global Poultry Vaccines Market, we have considered Global Poultry Vaccines like infectious bronchitis vaccines, Newcastle disease vaccines, Fowl Pox vaccines and infectious Bursal disease vaccines to analyze the Global Poultry Vaccines Market.
Get Full Report Overview @ https://www.brandessenceresearch.com/healthcare/poultry-vaccines-market/
Global poultry vaccines market is segmented on the basis of product type, dosage form, application, diagnostics, end user, and geography. On the basis of product type global poultry vaccines market is segmented into attenuated (live), inactivated (killed), recombinants, toxoids vaccines and others. On the basis of dosage form, global poultry vaccines market is segmented into liquid vaccine, freeze dried vaccine and dry form and other. On the basis of application global poultry vaccines market is segmented into newcastle disease, marek’s disease, infectious bronchitis, infectious bursal disease, fowl pox, chicken anaemia virus infection, big liver & spleen disease and other. On the basis of diagnostics global poultry vaccines market is segmented into enzyme linked sorbent assay (ELISA), rapid immune migration (RIM), agar gel immune diffusion (AGID) and other. On the basis of end user global poultry vaccines market is segmented into veterinary hospitals, poultry vaccination centers, poultry firms and others.
The regions covered in global poultry vaccines market report are North America, Europe, Asia-Pacific and Rest of the World. On the basis of country level, Global Poultry Vaccines Market sub divided in to U.S., Mexico, Canada, U.K., France, Germany, Italy, China, Japan, India, South East Asia, GCC, Africa, etc.
Key Players for Global Poultry Vaccines Market Reports –
Global poultry vaccines market report cover prominent players like Bayer, Ceva Animal Health, Heska Corporation, Lohmann Animal Health, Merck Animal Health, Merial Ltd., Protein Sciences Corporation, Zoetis Inc., QYH Biotech, Ringpu Biology, Yebio, Harbin Veterinary Research Institute, DHN, ChengDu Tecbond, FATRO, CAVAC, Vaksindo, S.C ROMVAC COMPANY S.A., Boehringer Ingelheim Vetmedica Inc., Intervet India Pvt. Ltd. Anicon GmbH, ASP Inc., Phibro Animal Health, Vetoquinol, Hester, Hipra, IDT Biologika, China Animal Husbandry, Biogenesis Bago, Tianjin Ringpu and Jinyu Bio-technology
Global Poultry Vaccines Market Dynamics –
The Global Poultry Vaccines Market is accelerated by the rising occurrence of zoonotic diseases like Bird flu and Avian influenza etc., rapid growth of poultry population, poultry vaccination, technological advancement and growing demand for poultry derived food products such as chicken, meat and eggs. Furthermore, launching of new vaccine by different manufactures such as, Merck Animal Health and Zoetis Inc., various vaccination programs by leading players, are some factors that propelling the growth of the Global Poultry Vaccines Market. However, increased competition for cultivated land, increase in storage cost for various vaccines like bacterial & viral vaccines and rising acceptance of vegetarian food may undesirably affect the growth of Global Poultry Vaccines Market. Furthermore, latest technologies for manufacturing of new vaccines and government initiatives for rising Global Poultry Vaccines Market are some key opportunities.
Global Poultry Vaccines Market Regional Analysis –
Geographically, North America holds the first position for the Global Poultry Vaccines Market owing to various technological advancement, increasing poultry inventories, growing demand for poultry derived products like eggs, chicken & meat and growing investment in animal health. Europe is the second largest market for Global Poultry Vaccines Market. Asia Pacific is likely to show lucrative growth rate for Global Poultry Vaccines Market due to increasing poultry production, government vaccination program and rising poultry health care expenditure. These factors are expected to boost the Asia Pacific market in the near future.
Key Benefits for Global Poultry Vaccines Market Reports –
Global Poultry Vaccines Market report covers in depth historical and forecast analysis.
Global Poultry Vaccines Market research report provides detail information about Market Introduction, Market Summary, Global market Revenue (Revenue USD), Global market sale (K Units), Global market Drivers, Market Restraints, Market opportunities, Competitive Analysis, Regional and Country Level.
Global Poultry Vaccines Market report helps to identify opportunities in market place.
Global Poultry Vaccines Market report covers extensive analysis of emerging trends and competitive landscape.
Poultry Vaccines Market Segmentation –
By Product Type
Attenuated (live)
Inactivated (killed)
Recombinants
Toxoids
Other
By Dosage Form
Liquid Vaccine
Freeze Dried Vaccine
Dry Form
Other
By Application
Newcastle Disease
Marek’s Disease
Infectious Bronchitis
Infectious Bursal Disease
Fowl Pox
Chicken Anaemia Virus Infection
Big Liver & Spleen Disease
Other
By Diagnostics
Enzyme Linked Sorbent Assay (ELISA)
Rapid Immune migration (RIM)
Agar Gel Immune diffusion (AGID)
Other
By End User
Veterinary Hospitals
Poultry Vaccination Centres
Poultry Firms
Others
By Region
U.S.
Mexico
Canada
UK
France
Germany
Italy
China
Japan
India
Southeast Asia
Brazil
GCC
Africa
Rest of Middle East and Africa
Poultry Vaccines Market Key Players
Anicon GmbH
ASP Inc.
Bayer
Biogenesis Bago
Boehringer Ingelheim Vetmedica Inc.
CAVAC
Ceva Animal Health
ChengDu Tecbond
China Animal Husbandry
DHN
For more information or any query mail at [email protected]
About us: Brandessence Market Research and Consulting Pvt. ltd.
Brandessence market research publishes market research reports & business insights produced by highly qualified and experienced industry analysts. Our research reports are available in a wide range of industry verticals including aviation, food & beverage, healthcare, ICT, Construction, Chemicals and lot more. Brand Essence Market Research report will be best fit for senior executives, business development managers, marketing managers, consultants, CEOs, CIOs, COOs, and Directors, governments, agencies, organizations and Ph.D. Students. We have a delivery center in Pune, India and our sales office is in London.
Contact us:
Alan Ruffalo
Business Development Manager
Kemp House, 152 – 160 City Road, London EC1V 2NX
Tel: +44-2038074155 / +91-7447409162
Email:[email protected]
Website:www.brandessenceresearch.biz
0 notes
Text
H1N1 Vaccines Market Opportunity Analysis, 2018 – 2026
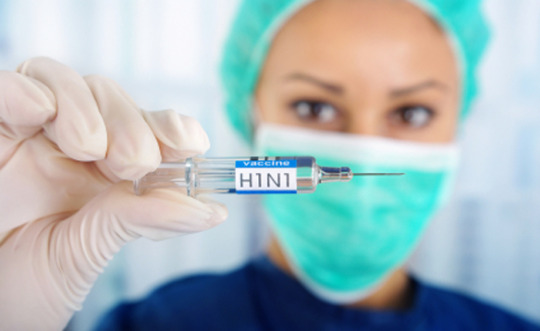
H1N1 influenza virus causes viral disease, commonly known as swine flu, which mostly affects the respiratory system. Swine flu is characterized by infection from H1N1 strain. Symptoms of H1N1 flu are fever, cough, sore throat, runny or stuffy nose, watery and red eyes, body aches, headache, fatigue, and diarrhea. H1N1 spreads through contact with contaminated air or geographical locations infected with virus. H1N1 infection may worsen other chronic respiratory conditions such as bronchitis, pneumonia, COPD, and sometimes even death due to respiratory failure.
Click To Continue Reading on H1N1 Vaccines Market
H1N1 vaccine is an important innovation to tackle the H1N1 pandemic since 2009. Initially, the vaccines developed or available were inactivated monovalent vaccines that could prevent infection from most of the strains of H1N1. There are two routes of administration such as intradermal for inactivated vaccine and intranasal for live attenuated vaccine. Both routes shown efficacy profile and are bioequivalent against preventing H1N1 infection. Global H1N1 vaccine market has presence of both multinational pharmaceutical companies and companies from emerging economies.
0 notes