#coprecipitating
Text
Short Stories :: Sneaky Link Vibes
Amature Interracial creampie
White Wives Black Cock Compilation
Lovely massage girl stuffs her lustful mouth with thick dick
Chubby and skinny matures have a dirty German threesome
Graziella Diamond baise avec un couple libertin
True amateur teen first time I desired to be the one who sated the
asian milf at porn store wanking off in video booth with dildos
Alia Bhatt Dual Cumshot tribute by Chandrashekhar
Gorda Se MAsturba
#Quantrill#spermology#Wardle#nodicorn#unstrained#self-offence#stellerid#antichthones#Dungannon#sea-traveling#cohort#Fanagalo#reenjoyed#insociableness#retinic#chihuahuas#accumulate#redknees#coprecipitating#fermenting
0 notes
Text
COALS Controls Model for FGD System
COALS Controls Model
In order to develop a COALS Controls Model for FGD system, we first have to understand the power plant in question. Then, we need to understand the emission levels and associated control methods. This will enable us to develop a model that is applicable to a wide range of power plants.
The COALS Controls Model can provide useful guidance to design an efficient FGD system for a WTE plant. By modeling the emissions, COALS can estimate the concentration of a range of trace elements. The simulated data can be used to estimate FGD wastewater concentrations more closely.
The COALS Controls Model simulates the total mass of trace elements entering the FGD system and the mass entering the coal-fired boilers. It then multiplies the total mass of coal by the county-level distribution of trace elements. COALQual data provides the best nationwide coverage.
Wet Circoclean(r)
Wet Circoclean(r) system is designed to remove pollutants from flue gas in WTE plants. It is suitable for plants burning biomass, refuse-derived fuels, domestic waste, or industrial waste. The system uses a circulating fluidised bed to separate acid gases, dioxins, and heavy metals. It also uses a high-velocity process that results in high separation efficiency.
Organosulfides for metals precipitation
Metals can be precipitated in a wastewater treatment plant (WTE) using a variety of chemicals. Typically, lime, dolomite, sodium carbonate, and sodium hydroxide are used. Other chemicals that can precipitate metals include calcium salts and fluoride.
Metals can also be precipitated using a variety of organic compounds, including sodium sulfide, potassium sulfide, or sodium hydrosulfide. These agents are used in continuous or batch processes. They are usually slightly soluble.
DTCs can precipitate various metals by altering their chemical structures. Increasing the number of dithiocarbamate groups in the DTC can improve the efficiency of the process. In a study published by Fu et al., the use of disodium N,N-bis-(dithiocarboxy)piperazine improved heavy metal precipitation in water with a high concentration of recycled sludge.
Researchers found a correlation between copper ion removal and BDP/Cu2+. A 1:1 ratio of BDP to Cu2+ allowed copper to be reduced from 50 to 0.04 mg/L. These results indicated that the precipitation process can be efficient in a wide pH range. In addition, further studies revealed that BDP was effective at removing dyes.
Iron coprecipitation
Iron coprecipitation is a process whereby iron oxide nanoparticles grow in a hydrogel network. These particles perturb the local and gradient magnetic fields and dephase water proton spins. The final density of the iron oxide nanoparticles can be tuned by varying the concentration of iron chlorides.
To model the process accurately, coupled reaction-diffusion equations must be solved. They must account for the diffusive transport and removal of OH-1 ions during the in situ coprecipitation process. The finite capacity of effective sinks complicates the analytic evaluation of the diffusion constant. Once the iron precursor has been converted into iron oxide, it no longer serves as a sink for the OH-1 ions. To address these limitations, numerical models of the process are used.
Another technique that is useful for monitoring the iron coprecipitation process is magnetic resonance imaging (MRI). This technique allows visualization of the growth of iron oxide nanoparticles in the hydrogel network. MRI images reveal a dark contrast due to the growth of iron oxide nanoparticles, which are nucleated by diffusion of precipitating agent into the gel.
0 notes
Text
Trametinib inhibits ezrin-mediated migration involving hepatocellular carcinoma cellular material
Also, quotations associated with foliar predawn drinking water potential and gasoline change components, in addition to regarding night time flowered water possible associated with C. spinosa expanded upon hills over the subway monument have been high and also mirrored your performance associated with origins inside maintaining acquisition of normal water via heavy dirt tiers, throughout the dry season #Link# . (Chemical) 2015 Elsevier Limited. All protection under the law earmarked.A lot of methods for plasmid is purified are already designed, along with the whole process has to be built to eliminate the web host RNA, proteins, genomic Genetic make-up as well as endotoxin. Presently, plasmid is usually filtered by simply #Link# time-consuming chromatographies. As a substitute, a fresh plasmid filtering technologies along with cetyltrimethylammonium bromide (CTAB) can be explained. Following lysis along with alkali, the CTAB has been right titrated in to the supernatant pertaining to plasmid rainfall, then a coprecipitated pellets ended up managed Three or more Meters KAc and TritonX-114. High quality discovery demonstrated that your filtered plasmids were free from the particular toxic contamination regarding sponsor RNA. Inside One particular milligram filtered plasmid, the microbial genomic DNA, sponsor endotoxin and also necessary protein had been under 12 mu g/mg, 60 EU/mg and Ten mu g/mg, respectively. The number of OD(260)/OD(Two hundred eighty) was among A single.75 : 1.85, a lot more than 90% of the well prepared plasmid presented inside the supercoiled type. Further check revealed that the actual pcDNAlacZ purified along with CTAB and well-respected endotoxin-free plasmid System experienced the same transfection efficiency throughout vivo as well as in vitro. CTAB can be used plasmid purification; the primary the best-selling DNAs purified with CTAB are the deterrence involving animal-derived nutrients, poisonous compound like chloroform along with phenol. More attractive is that the entire course of action contains the predominance regarding low cost.Your recognition and also depiction involving supporters, cis-elements and also transcription aspects tend to be critical for researching gene appearance during growth and development in almost any affected person. Use of specific recommends is surely an absolute requirement for the actual appearance of international genetics inside vegetation inside a developmentally, spatially and/or temporally managed fashion. Your YY2 cDNA has been shown to always be expressed specifically in the tapetum cells involving almond #Link# anther. Within this examine, a 968 bp upstream regulation sequence (GenBank accession absolutely no. FJ957881) from the gene had been singled out utilizing genome walking strategy. The actual singled out fragment ended up being shown to push anther-specific expression associated with GUS press reporter gene throughout grain as well as in the particular dicot place Arabidopsis thaliana. Erasure analysis said that the same to 500 blood pressure 5' regulation place is enough to consult anther-specific phrase regarding GUS gene. (C) 09 Elsevier Munster Limited. Most legal rights set aside.Background and objectives Blood vessels components must be kept under manipulated temperatures conditions, for motives associated with element good quality and also safety. Even so, solutions when components could possibly be exposed to problems outwith the identified limits.
0 notes
Text
The particular Chart kinase chemical Long-chain-fatty-acid-CoA ligase minimizes genetic lack of stability inside the autoimmune encephalomyelitis SJL/J-mouse model of ms
The problem is caused by abnormal continuing development of your ductal menu, your embryonic forerunners to the interlobular bile ducts. It has rarely been recently documented inside veterinary clinic types, and contains never recently been documented throughout pet dogs. This post identifies 5 instances of a new ductal menu malformation within dogs consistent with hereditary hepatic fibrosis. In gentle microscopy, most A few livers had severe connecting fibrosis using a notable surge in the amount of tiny bile ductwork, which regularly got unpredictable, dilated profiles reminiscent of the establishing ductal dish. Additionally, 80% (Four associated with 5) of cases lacked common site abnormal vein single profiles. Cytokeratin Seven along with growing mobile atomic antigen immunohistochemistry was performed around the 3 cases in which paraffin-embedded tissues was available. Your bile air duct single profiles were clearly optimistic with regard to cytokeratin Seven in every Several circumstances, and they were unfavorable regarding proliferating cellular atomic antigen or simply had rare positive tissue. Almost all Your five dogs assigned medical signs of website high blood pressure levels. Hereditary hepatic fibrosis should be considered within the differential medical diagnosis throughout youthful puppies that present with website blood pressure and also wounds that may have already been viewed since linking biliary hyperplasia as well as extrahepatic biliary blockage.Permanent magnetic poly(methyl methacrylate) (PMMA) microspheres had been served by double-miniemulsion polymerization. 1st, oleic acid sprayed magnetite allergens #Link# created by using coprecipitation ended up dispersed straight into octane to secure a #Link# ferrofluid. Your ferrofluid and also MMA ended up #Link# emulsified to create O/W emulsion, correspondingly. Eventually a pair of miniemulsions were put together collectively for polymerization. Your obtained permanent magnet polymer-bonded debris were seen as a, Fourier enhance ir spectroscopy, tranny electron microscopy, deciphering electron microscopy, X-ray powdered ingredients diffraction, as well as thermogravimetry. The outcomes indicated that oleic acidity coated magnetite contaminants ended up well summarized in PMMA. The effects of initiator dosage along with monomer attention to your alteration regarding Fighting were also researched. (Chemical) 2009 Wiley Magazines, Inc. J Appl Polym Sci 112:89-98, 2009Your trophic reputation with the eastern Gulf of mexico involving Finland, the place that the most significant Baltic town E. Petersburg rests on the mouth with the biggest Baltic river Neva, can be raised but current recommendations on water safety measures are generally controversial. In this examine, the effects of nutrient weight reductions about this environment were projected with a new three-dimensional combined hydrodynamic-biogeochemical model. As a guide, the particular contemporary seasonal character have been simulated along with source of nourishment information equivalent to the present quotes associated with stage and also riverine sources. To be able to remove the effects of normal inter-annual variants, the computations ended up manage under persistent annual making for several many years, right up until quasi steady-state seasonal dynamics had been achieved.
0 notes
Text
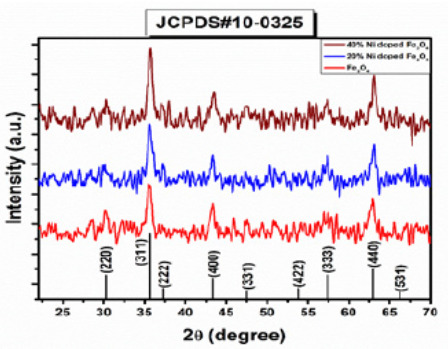
Synthesis and Characterization of Nickel Doped Iron Oxide Nano Particles for Biomedical Application _ Crimson Publishers
Synthesis and Characterization of Nickel Doped Iron Oxide Nano Particles for Biomedical Application by Arshad Javid Muhammad in Integrative Journal of Conference Proceedings
Abstract
The objective of this work is to synthesize and characterization of nickel ferrite as MRI contrast agent to improve the signal intensity of T2 weighted images for biomedical application. For structural analysis, XRD was revealed that Ni-doped Fe2O4 have a cubic spinal structure Having Miller Indices (hkl) values of (220), (311), (222), (400), (331), (442), (333), (440) and (531). From XRD data, the grain size of Ni Fe2O4 was observed to be (17.12nm) after 20wt.% Ni-doped Fe2O4, and its further increases up to19.36nm for 40wt.% Ni Fe2O4, respectively. The XRD pattern confirmed that doping of Ni metal increased the grain size of nanoparticles. SEM was performed to study the morphology of prepared samples. EDX was performed to confirm the elemental analysis. EDX spectra depicted the desired peaks of Fe, O, Cl, and Ni for Ni-doped Fe3O4. Saturation magnetization (Ms) was improved with concentration of dopant material Ni (20wt.%, 40wt.% ) in magnetic nanoparticles. In essence, this study demonstrates the very easy way of synthesis of Ni doped iron oxides nanoparticles for biomedical applications as MRI contrast agents
Keywords: Nickel ferrites; Magnetic nanoparticles; MRI contrast agents; T2-Weighted
Introduction
Magnetic nanoparticles were significantly studied for biomedical research such as drug delivery, hyperthermia in cancer, protein separation, biosensing and Magnetic Resonance Imaging (MRI) [1-3]. Since the late 1990s, iron oxide-based nanoparticle contrast agents have been explored and clinically used as T2-weighted contrasts agents. They compose magnetic nanoparticle core and biocompatible coating material, preventing aggregation and sedimentation and allowing high biological tolerance [4]. Recently, researchers have focused on nickel ferrite nanoparticles as MRI contrast agents due to their high magnetic susceptibility, biocompatibility, biodegradability and nontoxicity characteristics [5]. Several studies have investigated the nickel-based nanoparticles as an alternative to gadolinium for reducing the risk of toxicity [6]. Nickel metal also possesses a high spin quantum number and proton exchange kinetics [7]. MRI has several blessings over unique imaging modalities due to excessive spatial selection, amazing clean tissue evaluation and non-utilization of radioisotopes. Paramagnetic gadolinium complexes are commonly used as MRI contrast agent [8]. However, gadolinium-based complexes have low sensitivity and have toxic outcomes that incorporate Nephrogenic Systemic Fibrosis (NSF) [9]. Moreover, most gadolinium complexes are designed to circulate time, precluding excessive decisions and focusing on MRI quickly. The signal intensity is a function of T2 relaxation, i.e., I∼Mo e-t⁄T2 was used to determine the T2 relaxation times. In this research work, nickel dopped iron oxide nanoparticle have been synthesized using co-precipitation method to enhance its sensitivity as T2-W contrast agents.
Experimental
Ferric chloride hexahydrate (FeCl3.H2O), ferrous chloride tetrahydrate (FeCl2.H2O), nickel chloride hexahydrate (NiCl2.H2O) and ammonium hydroxide (NH4OH) were used for the preparation of Fe3O4 and NiFe2O4 superparamagnetic nanoparticles using coprecipitation method. Distilled water was used as a solvent to remove the impurities in the final product. Oleic acid was used as a surfactant [3]. First, the solution of NiCl2.6H2O was prepared in distilled water and stirrered for 1 hour at at 50 ℃ approximately. Then the solution of FeCl2.4H2O was prepared in the distilled water and stirrered for 1 hour at 50 ℃. Then solutions of NiCl2.6H2O and FeCl2.4H2O were mixed with continuous stirring at 70 ℃. Then NaOH was added drop wise upto pH 12. Oleic acids were added in the same solution as a capping agent and surfactant. The precipitation was washed out with distilled water and dried in the oven at 80 ℃ for 6 hours. The synthesized nickel ferrites were grinded into a fine powder. The chemical reaction of the NiFe2O4 has been mentioned as in equation-1
Results and Discussion
X-Rays Powder Diffraction (XRPD)
Nidoped ferrites was analyzed with X-ray diffractometer using Cu as a targeted source of X-rays production with Kα_1 radiation having the wavelength of λ=1.54 Å. The powder sample was evaluated in the angle range of 2θ=10110° at the scanning speed of 0.02°/min and step size of 0.031° and step time of 0.3 sec. Powder samples of undoped and Ni-doped ferrites showed crystalline nature as shown in Figure 1. XRD pattern for Ni-doped iron oxide Ni0.2 Fe2.8 O4 and Ni0.4 Fe2.6 O4. was shown in Figure 1. Diffracting peaks of all prepared samples were depicted in Figure 1 at 2θ = 29.94°,35.57°,37.13°,43.32°,47.33°,54.11°,57.21° and 62.95° with miller indices (220), (311), (222), (400), (331), (422), (333) and (440) respectively (Table 1).
The XRD diffraction peaks of the Ni0.2 Fe2.8 O4 (S2), and Ni0.4 Fe2.6 O4 (S3), belongs to the FCC structure, which can be well-matched with (JCPDS) card no (000100325). Diffraction peaks and their sharpness define the degree of crystallinity. There are no other extra secondary phases, suggesting that the ions of Ni2+ are entirely diffused into the Asite which is Fe2+ in Fe3O4. For the calculation of the lattice parameter following relation was used:
SEM analysis
The surface morphology of Nidoped Fe3O4 was studied through SEM model Instrument JSM5910, Japan at 20.0kV. SEM confirmed that particles are spherical in shape and most of them are in flask shape [10]. The density of the particles was also increased with the increase in the concentration of Ni in Fe (Figure 2).
Vibrating Sample Magnetometer (VSM)
Magnetic properties of prepared samples such as saturation magnetization were measured at room temperature using Dexing Magnet Tech Co, Model (VSM100), China. Nidoped Fe2O4 nanoparticles did not depicted hysteresis curve. This saturation magnetization confirmed that all the samples have superparamagnetic behavior in nature. The magnetization curve showed high saturation magnetization and low coercive force. The saturation magnetization was increased from 48.96emu/ cm3 to 126.7emu/cm3. When high concentration of Ni2+ shifts the Fe3+ ions from tetrahedral site to the octahedral site, then the tetrahedral site-to-octahedral site interactions increases, and the octahedral-to-octahedral interactions decreases. The total magnetic moment of the system is increased and therefore the magnetization of the system also increases. It was observed that the coercivity of the system decreases with the increasing content of Ni substitution. When the 20wt.% and 40wt.% nickel is incorporated in ferrite the maximum saturation magnetization was increased up to 115.55emu/cm3 and 126.7emu/cm3 respectively also the remanence was increased to 19.92emu/cm3 and then decrease to 19.50emu/cm3 for 20% Ni dope Ni and 40% Ni doped ferrite, respectively. There is no detectable change observed in coercive field values that are 0.0094 and 0.0095 T for 20% Ni-doped Ni and 40% Ni-doped ferrite, respectively. The Maximum saturation magnetization (Ms), Remanence (Mr), the ration of Mr/Ms, and coercive field values for undoped and Ni doped ferrites was listed in Table 3.
Where Hc represents the coercive field and Ms shows saturation magnetization, while anisotropy constant value K depends upon the concentration of dopant material. It means that the anisotropy constant of the system increases with the increasing content of Ni (Figure 3).
Conclusion
In this study, Nidoped iron oxides nanoparticles were prepared using co-precipitation method at room temperature. The structural conformation was done with XRD which exhibit spinal cubic structure of magnetic nanoparticles. From XRD data, the average grain size of Ni Fe2O4 from 17.12nm to 19.36nm after doping of Ni with 20wt.% and 40wt.%, respectively. The surface morphology of samples revealed that particles depicted the flat surface and have negligible agglomeration in SEM analysis. The saturation magnetization for NiFe2O4 was enhanced 115.55, to 126.7emu/cm3 after Ni doping with 20wt.% and 40wt.% Ni, respectively. Therefore, this study concludes that nickel ferrites may be used in diagnostic modality to see the pathology of the organ as T2-W contrast agents for biomedical applications.
https://crimsonpublishers.com/icp/fulltext/ICP.000549.php
For more open access journals in Crimson Publishers
please click on https://crimsonpublishers.com/
For more articles in open access Integrative Journal of Conference Proceedings
please click on: https://crimsonpublishers.com/icp/
Follow On Publons: https://publons.com/publisher/6342/crimson-publishers
Follow On LinkedIn: https://www.linkedin.com/company/crimsonpublishers
#Crimson Publishers LLC#crimson publishers#Crimson ICP#conference proceedings#Integrative Journal of Conference Proceedings#Open Access Journal in Conference Proceedings
0 notes
Text
Lupine Publishers| Monitoring Time-Progression of Structural, Magnetic Properties of Ni Nano Ferrite During Synthesis

Abstract
We present time-progression of structural, magnetic properties of NiFe2O4 nano ferrite during its synthesis via sol-gel auto combustion technique, monitored by x-ray diffraction XRD, and magnetic measurements. XRD patterns of the samples collected between 18-52 minutes shows the formation of the nano spinel phase (grain diameter: 15.4 nm-28.6 nm), presence of a-Fe2O3phase was also detected. Samples collected between 8-14 minutes show the amorphous nature of the samples. Time-progression studies show: a) sample taken after 20 minutes shows a sharp decrease of specific surface area (range between 39.01 m2/g to 72.73 m2/g), b) non-equilibrium cationic distribution for samples taken between 16-20 minutes with a continuous increase of Fe3+ ions population on B-site with simultaneous decrease of Ni2+ population, c) for samples taken after 22, 52 minutes, cationic distribution is close to its ideal value of (Fe3+) [Ni2+Fe3+], d) alteration of a degree of inversion (d), oxygen parameter (u), modification of A-O-B, A-O-A, B-O-B super-exchange interactions, e) ferrimagnetically aligned core, and spin disorder on the surface with a thickness between 1.9 nm to 3.6 nm, reducing the saturation magnetization (ranging between 11.7 - 25.5 Am2/kg), as compared to bulk Ni ferrite (55 Am2/kg), f) low squareness ratio values (0.15-0.22) shows the presence of multi-domain nanoparticles, with coercivity between 111-157 Oe.
Keywords: Time-evolution of properties; Sol-gel auto combustion synthesis; XRD; Nano Ni ferrite; Cationic distribution; Magnetic properties
Introduction
Spinel ferrites with general formula Me2+O.Fe3+2 O3, [Me: Divalent metal ion e.g. – Ni2+, Zn2+, Mg2+ Co2+ etc.], display face-centered cubic (fcc) structure, with two inter-penetrating sub-lattices: tetrahedrally coordinated (A site), octahedrally coordinated (B site) [1]. Nickel ferrite (NiFe2O4) has inverse spinel structure expressed as: (Fe3+) [Ni2+Fe3+] [1]. Allocation of cations on A, B site is crucial in determining properties of spinel ferrites [2,3], can be effectively used to achieve desired properties. Literature gives Ni ferrite synthesis using various methods including mechanical milling [4], coprecipitation [5], hydrothermal synthesis [6], sol-gel auto combustion method [7], showing the effect of the technique on structural, magnetic properties. Literature also reports real-time monitoring (in-situ studies) of properties [8,9], require special, sophisticated equipment, may not be available in all laboratories. Ex-situ monitoring of properties [10], describing the time-evolution of structural, magnetic properties, is a rather simple, more convenient way to perform experiments by utilizing standard laboratory equipment available in many laboratories. Ni ferrite is used in magnetic resonance imaging (MRI) agents [5], photocatalysis for water purification, antimicrobial activity [11], etc.) hence tuning its properties are preferred for improved efficiency.So, in this work, we present the time-development of structural, magnetic properties of NiFe2O4 nano ferrite during its synthesis via sol-gel auto combustion technique. Prepared samples are investigated via x-ray diffraction 'XRD,' vibration sample magnetometry, to get complimentary information on structural, magnetic properties.
Experimental Details
NiFe2O4 ferrite samples were synthesized by the sol-gel auto-combustion protocol, as described in detail in [12], by utilizing AR grade -nitrate/acetate-citrate precursors: Nickel acetate - Ni(CH₃CO₂)₂·4H₂O, Ferric nitrate (Fe(NO3)3.9H2O), Citric acid - C6H8O7]. The precursors were mixed in the stoichiometric ratio, were dissolved in 10 ml de-ionized water by keeping metal salts to fuel (citric acid) ratio as 1:1. At the same time, the solution pH was maintained at 7. Now the solution was heated at ~110 ̊C. As dry gel starts to form (taken as 0 minutes) small part of the sample is taken out from the reaction vessel (in an interval of 8, 10, 12, 14, 16, 18, 22, and 52 minutes), and were immediately ice-quenched to room temperature. Powder samples were used for Cu-K- X-ray diffraction 'XRD' measurements (Bruker D8 diffractometer), hysteresis loops by vibrating sample magnetometer. Full-profile XRD analysis was done by MAUD Rietveld refinement software [13] to obtain the lattice parameter (apex.). XRD analysis gives Scherrer's crystalline size D (calculated by the integral width of 311 peak, corrected for instrumental broadening), specific surface area (S), inversion parameter (d), oxygen parameter (u). XRD data was also analysed to get cationic distribution via Bertaut method [14], This provides cationic distribution by comparing experimental and computed intensity ratio of planes I(220)/I(400) and I(400)/I(422), susceptible to cationic distribution [12]. Cationic distribution was used to calculate theoretical or Néel magnetic moment at 0K (Ms(th)), theoretical lattice parameter (ath.), bond angles (θ1, θ2, θ3, θ4, θ5) as shown in [3]. Coercivity (Hc), saturation magnetization (Ms), remanence (Mr), squareness ratio (Mr/Ms) was obtained from hysteresis loops. (Figure 1) gives the schematic of sample synthesis and characterization.
Figure 1: Schematic of sample synthesis and characterization.
Results and Discussion
(Figure 2) (a) gives XRD-patterns of the studied NiFe2O4 samples collected after 18, 20, 22, and 52 minutes, confirm the formation of the spinel phase. XRD patterns also show the presence of a-Fe2O3 phase, ascribable to sample synthesis at a reasonably lower temperature (~110̊C), as reported in [15], while its disappearance is seen after higher sintering temperature. Figure 1(a) inset shows XRD patterns of samples collected after 8, 10, 12, 14 minutes show the amorphous nature of the samples. Only in the sample collected after 14 minutes, there is the start of spinel phase formation (indicated by a dotted circle). Illustrative Rietveld refined XRD pattern (Figure 2) b) of NiFe2O4 sample taken after 20 minutes also validates the cubic spinel ferrite phase formation. (Figure 2)(c) shows a variation of D (range between 15.4 nm to 28.6 nm) and S (range between 39.01 m2/g to 72.73 m2/g) for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. A perusal of (Figure 2) (c) shows a well-known inverse relationship shown by the expression: [S = [6/(D ´rXRD)], where rXRDis x-ray density, as was also reported in[2]. (Figure 2) (c) shows that for samples taken after 22, 52 minutes, D sharply increases with concurrent reduction of S, is ascribable to significant changes in cationic distribution via migration of Ni2+ions to B site with simultaneous migration of Fe3+ ions on A site(as can be seen in Table 1). (Figure 2 )(c) inset display linear relation between d and u as was also observed earlier [3], shows that reduction of the degree of inversion (d) leads to a reduction of oxygen parameter (u), a measure of disorder in the studied system, is expected to affect the properties of the studied samples. Table 1 depicts the variation of experimental and theoretical lattice parameter (aexp., ath. ), inversion parameter (d), oxygen parameter (u), Cation distribution (for A, B site), and calculated, observed intensity ratios for I400/422, I220/400 plane for the studied samples. The observed variation of aexp. is consistent with changes in cationic distribution, and variation of the degree of inversion (d). Close agreement between observed, calculated aexp., ath. suggests that the computed cationic distribution agrees well with real distribution [16]. Close matching of calculated, observed intensity ratios for I400/422, I220/400 signifies an accurate cationic distribution among A, B site [17]. Cationic distribution illustrates that as we go from NiFe2O4 samples taken after 16, 18, and 20 minutes, the population of Fe3+ ions on B site increases from 1.2 to 1.5 with a concurrent decrease of Ni2+ ions from 0.80 to 0.50. For samples taken after 22, 52 minutes Fe3+ population on B site decreases, while Ni2+ ion population increases up to 0.98, which is close to the ideal inverse cationic distribution of (Fe3+) [Ni2+Fe3+] [1].
Figure 2: (a): XRD patterns of the studied NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes showing the formation of the spinel phase. Inset: XRD patterns of the studied samples taken after 8, 10, 12, 14 minutes. (b): Illustrative Rietveld refined XRD pattern of NiFe2O4 sample taken after 20 minutes (* - Experimental data, Solid line - theoretically analyzed data, |- Bragg peak positions, Bottom line- Difference between experimental, and fitted data). (c) variation of grain diameter (D) and specific surface area (S) for NiFe2O4samples taken after 16, 18, 20 22, 52 minutes. Line connecting points guide to the eye. Inset: variation of inversion parameter (d) with oxygen parameter (u). The straight line is a linear fit to the experimental data.
Figure 3 depicts the variation of bond angles between cations, cation-anion q1, q2,q3, q4and q5, for the studied samples taken between 16 - 52 minutes. In samples taken after 8, 10, 12, and 14 minutes, due to the absence of the spinel phase, bond angles could not be computed. Bond angles provide information on super-exchange interaction (A-O-B, A-O-A, B-O-B), mediate by oxygen. (Figure 3) shows that for samples taken after 16, 16, 20 minutes q1, q2, q5, decreases while q3, q4increases, indicates a weakening of A–O–B, A– O–A and strengthening B–O–B super-exchange interaction as is also observed earlier [16]. For samples taken after 22, 52 minutes q1, q2, q5, increases, and q3, q4decreases reveals strengthening of A-O-B, A-O-A, and weakening of B-O-B super-exchange interaction, reported in the literature with compositional changes [3]. Samples taken after different times, there is a modification of A-O-B, A-O-A, B-O-B super-exchange interactions, are attributed to changes in dand u as shown in(Table 1), observed with compositional changes [3,16]. Observed A-O-B, A-O-A, B-O-B super-exchange interactions should mirror in magnetic properties, matches well with reported literature [3,16]. Thus, collecting samples after different times during synthesis is analogous to compositional changes in spinel ferrites, affects structural, magnetic properties [3, 12, 16, 18].
Figure 3: Dependence of bond angles (q1A-O-B,q2A-O-B, q3B-O-B,q4B-O-B, q5A-O-A) for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Line connecting points guide to the eye.
Figure 4 depicts hysteresis loops, reveal changes in Ms(exp.)samples taken after 16, 18, 20 22, 52 minutes, attributable to alteration of B-O-B, A-O-B, and A-O-A interaction, depends on bond angles, as shown in (Figure 3), and cationic distribution, as shown in (Table 1). (Figure 4) inset displays hysteresis loops of the samples taken after 8, 10, 12, 14 minutes, showing very low magnetization, attributable to the fact that in these samples ferrite phase is not formed, as was also observed in XRD data shown inset of (Figure 2) (a). Observed lower values of Ms(exp.) (ranging between 11.7 - 25.5 Am2/kg) as compared to the multi-domain bulk Ni ferrite (55 Am2/kg) is attributed to the two-component nanoparticle system as described in [19]consisting of a spin-disorder on the surface layer and ferrimagnetically aligned spins within the core. Computed magnetic dead layer thickness as described in [20,21]for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes are respectively 2.3, 1.8, 1.9, 2.5 and 3.6 nm. They confirm the contribution of 'dead layer thickness' in the reduction of Ms(exp.), apart from B-O-B, A-O-B, and A-O-A super-exchange interaction and cationic distribution.
Figure 4: Hysteresis loops of the studied NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Inset: Hysteresis loops of the samples taken after 8, 10, 12, 14 minutes.
Table 1: Variation of experimental and theoretical lattice parameter (aexp., ath.), inversion parameter (), oxygen parameter (u), Cation distribution (for A, B site), and observed, calculated intensity ratios for I400/422, I220/400 plane for the studied samples.
Figure 5(a) depicts Variation of Ms(exp.), Ms(th.)for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. A perusal of figure 5(a) shows that observed behaviour is attributable to alteration of B-O-B, A-O-B, and A-O-A super-exchange interaction, depends on bond angles (see figure 3), and cationic distribution (see Table 1). Non-similar trend of Ms(exp.), Ms(th.)in (Figure 5)(a), shows that the magnetization behaviour is governed by Yafet-Kittel three sub-lattice model, described in [22], confirmed by the computed canting angle (aY-K) values for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes, which are respectively 52.7, 56.6, 46.2, 55.7, 46.9 ̊. The canting angle provides information on spin canting on the surface, is so-called 'magnetic dead layer,' leads to a reduction of Ms(exp.), which is lower than bulk saturation magnetization of Ni ferrite (55 Am2/kg). Inset of Figure 5 (a) shows the variation of Ms(exp.) with oxygen parameter 'u'(which is a measure of disorder in the samples [1]). Figure 5 (a) shows the disorder-induced enhancement of Ms(exp.),as was also reported in [3]. (Figure 5) (b) depicts the Coercivity(Hc) variation for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Obtained Hcand related Dvalues imply that studied samples lie in the region with overlap between single or multi-domain structures, as reported earlier [3]. (Figure 5) (b) Inset depicts the variation of Mr/Ms for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Mr/Ms values ranging between 0.15-0.22 reveal enhanced inter-grain interactions suggesting isotropic behavior of the material [23] reveal multi-domain particles with no preferential magnetization direction. Time-dependent tunable structural, magnetic properties during synthesis are valuable in achieving optimal properties of Ni ferrite for their usage in magnetic resonance imaging [5], hyperthermia [24] for cancer treatment, photocatalysis for water purification [11].
Figure 5: (a) Variation of Ms(exp.), Ms(th.)for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Inset: Dependence of Ms(exp.)on oxygen parameter (u), line connecting points in Inset are linear fit to the experimental data.; (b) Coercivity(Hc) variation for NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes. Inset: Variation of Mr/Msfor NiFe2O4 samples taken after 16, 18, 20 22, 52 minutes.
Summary
To summarize, the sol-gel auto combustion technique is used to observe the time-development of structural, magnetic properties of Ni ferrite. Changes in cationic distribution lead to modification of structural properties, magnetic interactions, responsible for observed magnetic properties. Time-progression of properties are of use to alter structural, magnetic properties of Ni ferrite as a material for its prospective usage in heterogeneous catalysis, water purifications, biomedical applications.
Acknowledgments
Authors thank Dr. M. Gupta-L. Behra, UGC-DAE CSR, Indore for XRD measurements. Work supported by UGC-DAE CSR, Indore project (No.: CSR-IC-ISUM-25/CRS-308/2019-20/1360, dated March 5, 2020).
Conflicts of Interest
Author
Author Contributions
Conception: SNK; Sample synthesis, RV, SNK; Measurements, analysis of data: SNK, RV; Supervision, Resources, supervision, project management: SNK; Writing the manuscript: SNK, RV. All authors approve the draft and participate in reviewing.
For more Lupine Publishers Open Access Journals Please visit our website:
wt u have given that link add For more Modern Approaches on Material Science articles Please Click Here:
https://lupinepublishers.com/material-science-journal/
#lupinepublishers#lupinepublishersjournals#materialsciencejournal#compositematerials#openaccessjournals
0 notes
Text
Bioequivalent Drugs: Towards A Needed Holistic Paradigm Shift? | Lupine Publishers Journal of Drug Designing | Lupine Publishers

Opinion
The author of this short communication has been working in and teaching drug development for the last 20 years. He has been involved in more than 100 drug development projects, including drug delivery, medical device, biologics, innovators and generic drugs. He has also been involved in all the steps that are needed to file properly to different governmental agencies investigational new drug applications (IND), clinical trial applications (CTA), abbreviated and new drug applications/submissions (ANDA\S; NDA/S), 505b2, 510k and biologics legal applications (BLA). After several other interactions with all the other actors, such as Health Canada (HC), the Food and Drug Agency (FDA), the European Medicines Agency (EMA), pharmacovigilance companies and consultants, public relation companies, insurance companies and especially patients, the author has decided to gather all the comments in order to initiate a kind of a new debate on the innovators and generic drugs. However, the goal of this current expert opinion is not to generate conflicts, or to compare generic versus innovator drugs in the sense that one is better than the other. The author has already been involved in several bioequivalence studies, comparing two innovator products where the results showed lack of bioequivalence….or in bioequivalence studies evaluated with clinical endpoint where generic drug products were more potent than the innovators…The goal of this paper should be more based on the following question: are the current methods used to assess bioequivalence are suitable and reliable enough to “stamp” that generic drugs are bioequivalent, are as stable, reliable form a quality, a safety and efficacy standpoints. Based on the literature generic drugs can be defined as copies of innovator drugs and should contain the same dose strength, the same indication, pharmacodynamical effects, adverse events, safety profile and route of administration than the brand drug. Basically, generic drugs should behave the same than the innovator drugs. To achieve this equivalent profile, drug substance should be formulated and mimic as much as possible the formulation of the brand. It means that drug products ideally should show the same composition from both a chemical but also from a physical point of view. If these last comments are achieved then the biopharmaceutical phase, therefore the phase where the active pharmaceutical ingredient (API) should become available to the body, this phase should be equivalent between the generic and the brand. To help formulators developing bioequivalent formulation, several tools and development methods have been developed over the last decades. The preformulation step represents one of the most crucial steps in order to achieve a reliable, stable and bioequivalent formulation. In the quest of the nanomolar efficacy, small molecules became more and more difficult to formulate, making most of them difficult to solubilize and thus even more difficult to be absorbed because of their low solubilities or bad permeability behavior. Furthermore, all the “easy ones” have already been genericized. The bioavailability and thus the achievement of bioequivalence became then more challenging. In some cases, solid-sate chemistry could be held responsible for this lack of solubility/absorption pattern. Literature has already shown several times that crystalline structure was the most selected structure because sponsors rather have to select a molecule that shows better stability over the time than a higher rate of solubility (by selecting the amorphous structure) for the simple reason of a constant stability, reliability, efficacy over the time. But molecules became more and more insoluble and did not give sponsors the option of working only with the amorphous structure or changing the crystalline structure to amorphous structure (spray-drying, solid dispersion, coprecipitate) in order to get reasonable solubility and then maybe a better bioavailability without changing the stability. This actually represented the highest challenge. The HIV protease inhibitor Ritonavir was one of the best examples [1]. It is a lipophilic molecule with a high molecular weight of 721g.mol-1, that shows low solubility values of 0,001mg/ml at pH 6.8 and 0.4mg/ml in 0.1 N HCl. Because of these last characteristics and its dose of 1,2g per day, it became easy to predict that the absorption would be limited by its poor dissolution profile. It represented than an ideal candidate for solid dispersion. The sponsor then had problems with Ritonavir and had to stop the solid dosage form manufacturing because of the tendency of the drug substance to recrystallize over the time, generating dissolution and absorption problems [2]. It is then easy to imagine that the monitoring of solid-state chemistry became more popular and preformulation department got more and more equipped with state-of-the-art equipment (such X-Ray powder diffraction, differential scanning calorimetry, Raman, …) to monitor such phenomenon, not only form a preformulation standpoint, but also form a whole formulation/manufacturing development standpoint. But the chemistry of the solid-state became also extremely important from an intellectual property (IP) standpoint. And a lot of people will say that for a brand company, IP represents the “crux of the matter” in formulation development...in other words, how far can it be possible to deviate from the patent (without infringing it) by keeping plus or less the same qualitative and quantitative composition, in order to maintain bioequivalence, stability and reliability over the time. For example, the percentage of crystallinity/amorphous of an API in a formulation could become “patentable” knowing that below or above this threshold, it would become extremely difficult to formulate and to become bioequivalent. Atorvastatin calcium is a good example where both amorphous and crystalline structures coexist [3]. The monitoring of these formulations showing different solid-state characteristics were monitored in the marketed Atorvastatin tablets and as described above such differences may have impacted the stability of these tablets. The author had the chance to be involved in several atorvastatin formulation developments (more than 20) and other drug products showing solid-state chemistry issues to achieve bioequivalence between the brand and the generic (in progress) and in the large majority of cases, solid-state chemistry could be held responsible for not achieving bioequivalence. One of the reasons was that solid-state chemistry was not monitored over the time and formulation development steps, such as wet granulation, blending, roller compacting, grinding may have impacted the chemistry of the solid state and thus change the whole drug product behavior, from both a quality and a pharmacokinetic (PK) standpoint.
Bioequivalence…. What Does It Mean?
The literature [4] is telling that us that bioequivalence is the property wherein two drugs with identical active ingredients or two different dosage forms of the same drug possess similar bioavailability and produce the same effect at the site of physiological activity. Of course, this definition can be challenged, and “purists” are more than invited to argue from a semantic standpoint. In this article the focus on bioequivalence assessment will be on PK parameters, and not on clinical endpoints. Bioequivalence is mostly evaluated with PK parameters, the mains being the API maximum concentration in plasma. (Cmax) and the area under the curve that represents the concentration of API in the body following the dosing of a drug product. From a general standpoint, a generic formulation (or test product) is considered bioequivalent to a brand formulation if the ratio test/reference product for Cmax and AUC 0-t (form time 0 to a time t) are within 80.00-125.00%. For the Food and Drug Agency (FDA) [5] and European Medicines Agency (EMA) [6], the 90% confidence interval (CI) geometric mean ratios is expected for these two PK parameters whereas for Health Canada, the 90% CI is expected for the AUC only [7]. From a physiological standpoint, Cmax is more variable than the AUC therefore it can be predicted that form a biostatistical point of view, if the 90%CI is not requested on the Cmax and only the ratio of 80-125%, a smaller sample size (or volunteers) may be expected to successfully achieve bioequivalence. It does not mean that the generic formulation will be less potent and/or safe but in some cases, for certain class of drug products, but some variability when changing from the brand to a first generic and then to a second generic (and so on) may generate side effects or adverse events. Of course, it is expected that the smallest the difference will be in bioequivalence between the brand and the generic, side effects may not be considered significant form a clinical point of view.That being said concerning the bioequivalence definitions and how it is evaluated and by going back to all the above in this current short communication, it can be expected that physico-chemical characteristics may impact drastically the bioequivalence between tests and reference products. In order to minimize bias as much as possible it becomes then crucial that chemistry manufacturing and controls (CMC) should not be held responsible for non-achievable bioequivalence. Let’s make it clear: a bioequivalence study compares two formulations behavior therefore CMC represents the cornerstone of generic development.It should be kept in mind that bioequivalence studies are carried out:a) On healthy volunteers (unless patients are targeted and mandatory according the guidance).b) On a very small sample size that may not represents the targeted population. It is then difficult to extrapolate on a bigger sample size, on subpopulations. Specialists are relying on phase 4/pharmacovigilance data of the brand (since no such studies are carried out on generic compounds)c) Most of the time, single doses are evaluated, and even if steady-state is requested by agencies, it will never mimic chronic uses of a drug product (when the drug product is taken over several years). Here again lack of data are available in that regards since such studies are not performed neither.d) A new generic formulation will always be compared to the reference, and not to the previous generic available in the market. Therefore, for example if the first generic passed on the Cmax with a ratio of 86%, and the second one with a ratio of 117%, some adverse events may be expected. After discussion with worldwide specialists in that domain, most of them will say that these differences will not affect the patient and will be lost once the steady-state will be achieved. It is clear that pharmacovigilance results are difficult to consult. Hence it is difficult to conclude that differences in terms of safety and efficacy have been monitored, even though a quick survey on the population will show you some interesting results with regards toi. the perception of generic drugsii. whether they have noticed differences by interchanging their brands to generic andiii. whether they have noticed a difference with regards to safety and efficacy when they have been switched from a first generic to a second generic.
So…Finally Can Generic Drugs Be Considered on the Same Footing Than Innovators?
From an academic standpoint, after several presentations given by the author dealing with drug development in general, the interchangeability, and all the fields plus or less linked with generic and innovator drugs, it has been noted that a lot of healthcare professionals are not aware and are not well trained in that regards. Several of them told the author the following: “we understand what you are saying but we have not been trained accurately in that sense at all”. Of course, since the author is teaching it is plus or less true, knowing the propaedeutic of their undergraduate and graduate studies, and the several continuous upgrading trainings they have to follow. Even though the sample size needed for the carrying out of bioequivalence studies, which the author refers, may not be statistically relevant and do not reflect the reality, the goal of this paper was to crystallize an idea about the perception of generic drugs versus innovators that, down the road will hopefully generate a paradigm shift in the perception of these drug products. And then, when new tools may be created and will be helpful to enhance the fact that generic and innovator could be considered equal, from a safety, efficacy, stability and reliability standpoint.
For more Lupine Publishers Open Access Journals Please visit our website: https://lupinepublishersgroup.com/
For more journal of Drug Designing Please Click Here: https://lupinepublishers.com/drug-designing-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
0 notes
Text
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE NANOPARTICLES BY SOL-GEL PROCESS
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE NANOPARTICLES BY SOL-GEL PROCESS
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE
NANOPARTICLES BY SOL-GEL PROCESS
CHAPTER ONE
INTRODUCTION
1.1 BACKGROUND OF THE RESEARCH
Miniaturization is a general aim of the technological development that is taking place to produce smaller, faster, lighter and cheaper devices with greater functionality, while using less raw materials and consuming less energy. Research on…
View On WordPress
#cerium oxide nanoparticles applications and prospects in nanomedicine#iron oxide nanoparticles coated with dextran#iron oxide nanoparticles coprecipitation method#iron oxide nanoparticles dextran coating#metal oxide nanoparticles in organic solvents synthesis formation#zinc oxide nanoparticles for selective destruction of tumor#zinc oxide nanoparticles in modern sunscreens
0 notes
Text
Bulk Metal Alloys as a Precursor of Dispersed Particles Catalyzing Growth of Carbon Nanofibers Abstract Bulk metal alloys can be considered as a precursor of self-organized catalysts consisting of dispersed metal particles fixed within the structure of carbon nanofibers, which were grown as a result of catalytic chemical vapor deposition of halogen-substituted hydrocarbons. Being subjected to interaction with aggressive reaction mixture, the bulk alloy precursors undergo relatively fast process of metal dusting, which ends with complete wastage of initial metal item. The catalysts thus obtained are characterized with excellent long-term stability of operation along with high activity in decomposition of hydrocarbons of different nature. The approach described opens new horizons for a synthesis of nanostructured carbon materials including carbon-carbon composites of bimodal structure. Keywords: Bulk metal alloys; Metal dusting; Self-disintegration; Carbon nanofibers; Catalytic chemical vapor deposition Go to Introduction Industrially manufactured bulk metal alloys mostly being the resistive materials are widely applied in the heating systems (as a heating element), in the explosives and fireworks industry (as a bridge wire in igniters), in ceramic (as an internal support structure), etc. These materials are quite stable under both oxidative and reductive atmospheres, but undergo fast self-disintegration if exposed to carbon-containing (carburizing) medium at elevated temperature. The process of self-disintegration of metal items, also known as a metal dusting, is considered as an actual problem of modern chemical industry, which results in a wastage of chemical reactors [1-9]. Thus, steel reactors operated at 400-800 °C during hundreds hours become almost completely disintegrated into a dust consisting of dispersed metal particles and carbon. The situation becomes more complicated if the aggressive medium of halogen-substituted hydrocarbons is used [10,11]. There are a lot of papers devoted to the study on the mechanism of the metal dusting [1,3,6,7,9]. According to Grabke [1], the process goes through the following stages: Decomposition of carbon-containing precursor with subsequent transfer of carbon into solid solution up to oversaturation. Nucleation of metal carbide phase at surface and grain boundaries and its growth with protrusions into the bulk metal phase. Nucleation of the graphite phase. Decomposition of carbide to metal and graphite. Nano particle formed plays a role of catalytic sites for carbon nanofibers (or nanotubes) growth. Formed catalytic sites (dispersed metallic or alloyed particles), being stabilized within the structure of carbon nanofibers, catalyze further formation of nanostructured carbon via catalytic chemical vapor deposition. Thereby, the process of self-disintegration (metal dusting) can be proposed as a novel approach to prepare dispersed metal particles from bulk metal precursors for the synthesis of nanostructured carbon [12-17]. Go to Discussion During the last two decades the process of metal dusting as a way to obtain nanostructured carbon materials attracts more and more attention [12-24]. Carbon nanotubes and nanofibers of various morphologies were produced over stainless steel, iron- and nickel-containing alloys. In all these cases, the surfaceof bulk metals was intentionally subjected to controlled process of metal dusting yielding carbon deposits. As it was already mentioned, the process of self-disintegration is characterized with prolong induction period (up to few hundred hours), when no visible changes take place. At the same time, if the halogenated hydrocarbon is used as a carbon source, the process accelerates significantly [10,11,25,26]. Thus, interaction of bulk nickel alloys (nichrome, chromel, alumel, etc.) with vapors of chlorinated hydrocarbons results in a fast metal dusting with induction period no longer than 3 hours at 550 °C. It should be noted that induction period shortens along with temperature increase. At the end of induction period, the weight of the sample sharply grows up, and the system became inverted into an ensemble of dispersed metal particles fixed within the structure of grown carbon nanofibers. These particles can be considered as self-organized catalyst, and can be applied for decomposition of any other carbon-containing substrates with formation of nanostructured carbon. Thereby it can be concluded that halogen contained in a molecule of substrate affects crucially the rate of metal dusting. HCl emerged in hydrogen-containing reaction mixture provides the occurrence of reversible chlorination-dechlorination process, which leads to quick chemical corrosion of bulk alloy surface. It should be also emphasize that the addition of hydrogen excess into reaction mixture is required for dechlorination of the alloy surface [10]. The duration of induction period of the self-disintegration process can be shortened by using the pretreatment procedures [25,26]. Among such procedures, the most efficient are etching in a mixture of mineral acids (HCl/HNO3=3/1, 2-3min) and redox activation (altering of oxidative and reductive treatment at 500 °C, 3 cycles, 90min).These methods make a rough reconstruction of the alloy surface that eases further formation of dispersed active particles. While the considered alloys are resistive materials, they can be heated up to desired temperature by a direct supply of current. Decomposition of 1,2-dichloroethane in such regime is reported in Ref. [27]. In this case, besides the processes described above, hydrodechlorination and dechloro-coupling reactions take place leading to formation of ethane, ethylene and butenes within the gas phase products. At the resistive heating, the process of substituted hydrocarbon decomposition proceeds through the stages of C-H bond breakage, and hydrogenolysis and breakage of C-C and C-Cl bonds [28]. Unsaturated hydrocarbons and corresponding radical species formed as a result of hydrogenolysis undergo further pyrolysis over nickel crystallites. Table 1 summaries the data on carbon yield resulted from catalytic chemical deposition of 1,2- dichloroethane during 2 hours over different industrially manufactured alloys. As seen, among the studied alloys, nichrome (Ni-80%, Cr-20%) occupies the leading position. It allows one to obtain the carbon yield of 67.5 gC/gAl, which surpasses the values for other samples in one order of magnitude. If the resistive heating is applied (Table 1, row #2), the carbon yield over the same type of nichrome decreases in approximately three times, which is connected, as it was mentioned above, with occurrence of parallel processes [29]. The lowest carbon yield (0.5gC/gAl) was observed in the case of stainless steel, thus indicating that 2 hours is not long enough time for such kind of bulk precursor. Click here to view Large Table 1 *gC -weight of carbon product; gAl -weight of alloy sample Besides the industrially manufactured alloys, similar solid solutions of predefined composition can be especially prepared via multi-stage route involving coprecipitation of salt followed by high-temperature reduction in hydrogen, or via single-stage route of mechanical alloying of metal powders in a planetary mill [30-32]. An advantage of the first approach is a guaranteed formation of solid solution with desired ratio of the metals and homogeneous distribution. The formation of solid solutions was confirmed by precise powder X-ray diffraction analysis [33- 38]. Data on carbon yield over such alloys are shown in Table 2. As it follows, the nature and loading of an alloying metal affect noticeably the productivity of bulk precursor towards carbon. Thus, addition of iron worsens the catalytic behavior of the resulted alloy, while molybdenum, oppositely, significantly enhances it. It should be mentioned that these especially prepared alloy samples being subjected to complete self-disintegration process under aggressive medium of 1,2-dichloroethane can also be considered as self-organized catalyst for further usage in decomposition of various organic substrates [39]. Click here to view Large Table 2 *gC–weight of carbon product; gAl–weight of alloy sample. Self-organized catalysts obtained using both types of bulk precursors (industrially manufactured and especially prepared) were found to be quite active and extremely stable in catalytic decomposition of various hydrocarbons and their mixtures (C2H6, C2-C4 mix, C6H6) [40]. Additionally, it was shown that even chlorofluorocarbons can be efficiently decomposed, but in this case introduction of odd hydrogen into reaction feed is of great importance in order to bind the halogen atoms into HF and HCl. Surprisingly, the interaction of CF2Cl2 with submicron Ni crystals formed during the self-disintegration of bulk alloy precursor and fixed within the structure of carbon nanofibers causes their secondary disintegration which leads to formation of unique bimodal carbon nanomaterial. All processes of catalytic chemical vapor deposition with formation of carbon nanofibers described above were considered within a concept of flow-through or ‘open’ systems, when the reagent(s) continuously inlets into the reaction volume and the gas phase products pass the volume away. On the other hand, application of so called ‘closed’ reaction volume system was recently shown to be perspective for synthesis of various nanostructured inorganic materials [41-46]. Regarding the decomposition of substituted hydrocarbons, the process has the same requirements as in the case of ‘open’ system: both halogen and hydrogen source substrates should present in a reaction volume. Thus, for example, the use of halogenated organics (hexafluorobenzene, hexachlorobenzene, 1-bromobutane 1-iodobutane, etc.) together with hexamethylbenzene (as an inner hydrogen source) was shown to initialize the metal dusting process of bulk NiCr alloy with formation of nanostructured product [46]. Depending on the nature of halogen-containing substrate, the starting temperature of active particles formation detected by means of a ferromagnetic resonance spectrometry was different. The approach additionally provides a possibility to study the metastable reaction intermediates, which is inaccessible in the flow-through regime. Go to Conclusion Preparation of the catalysts for various catalytic processes remains to be a challenge task. At the same time, creation of the appropriate, in most cases aggressive, conditions for the catalyst’s precursor might shift the system towards state of self-organization. One of the most illustrative examples of this phenomenon is a nickel-based catalytic system for chemical vapor deposition of halogen-substituted hydrocarbons resulting in formation of nanostructured carbon fibers. Conventionally used for this process Ni-containing catalysts supported on oxide or carbonaceous supports are known to be rapidly deactivated under the reaction conditions. Oppositely, bulk metal alloys subjected to interaction with aggressive halogencontaining medium were shown to undergo the metal dusting process, which ends with complete wastage of initial bulk item. The dispersed metal particles formed as a result of such selfdisintegration were found to be quite stable and extremely active in decomposition of any other substituted and unsubstituted hydrocarbons that allows one to consider them as self-organized catalysts. The main regularities of the process are similar for the cases of external and direct (resistive) heating of the bulk alloy precursor, and if compare ‘open’ reaction volume systems with ‘closed’ one. For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/ for more details click on the juniper publishers material scienc
#Juniper Publishers#juniper publishers group#material science#composite materials#biological materials
0 notes
Text
Immunostimulatory activity of Y-shaped DNA nanostructures mediated through the activation of TLR9.
Immunostimulatory activity of Y-shaped DNA nanostructures mediated through the activation of TLR9.
Biomed Pharmacother. 2019 Feb 21;112:108657
Authors: Yang G, Koo JE, Lee HE, Shin SW, Um SH, Lee JY
Abstract
Immunostimulatory oligodeoxynucleotides (DNAs) have been widely studied in pharmaceutical and biomedical research fields for applications in cancer immunotherapy and vaccination. Toll-like receptors (TLRs) are critical for the instruction and orchestration of the host immune system composed of innate and adaptive immunity. In particular, TLR9 responds to DNAs with unmethylated deoxycytosine-deoxyguanosine (CpG) motifs, thereby inducing the activation of innate immune cells, such as dendritic cells, and consequently, adaptive immune cells. In this study, we developed two kinds of Y-shaped double-stranded DNA nanostructures (Y-DNAs), including a single unit composed of three DNA strands (YS-DNA) and a ligated multiunit complex formed by crosslinking each YS-DNA (YL-DNA), and investigated whether they have immunostimulatory activity in innate immune cells. YS-DNA and YL-DNA induced the production of immune cytokines such as IL-12 and TNF-α and the expression of costimulatory molecules such as CD80 and CD86 in primary mouse dendritic cells and macrophage cells (RAW264.7 cells). A Coprecipitation study demonstrated that YL-DNA was directly associated with TLR9. The induction of immune cytokines by YS-DNA and YL-DNA was abolished in TLR9-deficient primary mouse dendritic cells. The results demonstrated that Y-DNAs induced the activation of dendritic cells and macrophages mediated by the activation of TLR9, as shown by the expression of immune cytokines and costimulatory molecules. The results suggest that Y-DNA nanostructures provide a beneficial strategy for immunotherapy by modulating the immune system.
PMID: 30798119 [PubMed - as supplied by publisher] http://dlvr.it/QzdRmz
0 notes
Text
Microwave and Infrared for Ceramic
Ceramic materials have different properties because of different components. As a structural material, ceramic materials have been widely used in various industries.

Far infrared ceramics are characterized by their ability to emit more far infrared radiation (higher infrared emissivity) than normal objects. Using this special property, the application of far infrared ceramics can be divided into two aspects: the application in high temperature zone and the application in normal temperature zone. It is mainly used in heating of boilers, baking paint, heating and drying of wood and food in high temperature zone, and in manufacturing various far-infrared thermal insulation materials in normal temperature zone, such as far-infrared ceramic powder, far-infrared ceramic fibers, far-infrared ceramic polyester, and far-infrared functional ceramics. For example, some far-infrared ceramic materials have been applied in sports training and rehabilitation, energy saving of oil-fired stoves, indoor air purification and human health care. Infrared radiation from far infrared ceramics can reduce the viscosity and surface tension of fuel, which is beneficial to atomization and full combustion. Far-infrared ceramic coatings (including nano-titanium oxide coatings) have catalytic oxidation function. OH-, which can effectively remove benzene, formaldehyde, sulfide, ammonia and odorous substances indoors, can be produced under sunlight (especially ultraviolet radiation), and have bactericidal function. All kinds of far-infrared ceramic coatings will improve people’s living environment by popularizing and applying in living rooms, public buildings and transportation.

Far-infrared ceramics are a kind of artificial light radiation source. They can radiate light in a specific band according to the wavelength people need. Moreover, they have strong penetration and little loss when they penetrate the atmosphere. In all aspects of health care and medical treatment, a variety of products have been developed, such as Far Red Outer Health Care Garments, Heart-saving Cards, Patches and so on. They are used to promote human blood circulation, regulate physical health and treat many chronic diseases. They have been verified and marketized in many ways. The electromagnetic wave it emits is also called “life wave” and the Japanese also call it “nurturing light-line”. In recent years, the application of far infrared ceramic materials has extended to many aspects of environmental protection.

With the further development of microwave and infrared for ceramic, many new preparation methods are emerging. Such as: coprecipitation method, hydrolysis precipitation method, hydrothermal method, sol gel method, microemulsion method (reverse micelle method), etc. Some researchers even explored new ideas for preparing far-infrared ceramic ultrafine powders, such as high-temperature spray pyrolysis, spray induced coupling ion method, etc. The production process of these methods is quite different from the traditional chemical pulverizing process. It is an efficient way to combine the decomposition, synthesis, drying and even calcination processes. However, these methods are not yet mature and need further research and exploration.
Far-Infrared Ceramics
0 notes
Text
Magnesium Oxide for Battery
The glassy carbon electrode made of nano magnesium oxide has a lot of characters. For example, good stability, high conductivity, high purity. And there is no gas in the electrode, regeneration is easier, the price is more suitable, etc. At present, as a professional magnesium oxide manufacturer, Meishen Technology gave an outline of nano magnesium oxide for battery.
At first, the lithium ion battery selects to add insoluble solid particle that diameter is between 0.05μm to 10μm, such as TiO2, SiO2, Cr2O3, ZrO2, CeO2, Fe2O3, BaSO, SiC, MgO and so on. It has many advantages, for example, high efficiency, high specific capacity, stable cycle performance, etc.
Next, for lithium-ion battery cathode material, taking nano magnesium oxide as electric dopant, and made to anode material with nanostructure. The actual discharge capacity can achieve 240mAh/g. This kind of new type anode material is high energy, safety, and low price. It is suitable for liquid battery and colloform battery, especially for the power battery with super power.
And then, optimizing the capacity and cycle performance of spinel manganic acid lithium. Taking spinel manganic acid lithium as anode material, adding nano magnesium oxide as acid removal agent to remove acid. The additive amount is from 0.5 to 20 percent of bath solution amount. After disacidifying bath solution, the HF content of free acid in the bath solution can decrease below 20ppm.
The first step: taking nano magnesium oxide as PH regulator, and adding it into the mixed solution of cobalt salt and nickel salt.
The second step: adding lithium hydrate in the Ni-CO compound hydroxide, and heat treating the mixture under 280℃ to 420℃.
The third step: the product from the second step is treated under the temperature of 650℃ to 750℃. Related with coprecipitation time, the mean grain size of lithium composite oxide will be decreased or bulk density will be increased. When the lithium composite oxide is taken as anodic active material, it can get a lithium ion secondary battery with high electric capacity. In addition, the actual additive amount of magnesium oxide should depend on the detail mixture list.
0 notes
Text
The role of vimentin in the tumor marker Nup88-dependent multinucleated phenotype
Abstract
Background
Nucleoporin Nup88, a component of nuclear pore complexes, is known to be overexpressed in several types of tumor tissue. The overexpression of Nup88 has been reported to promote the early step of tumorigenesis by inducing multinuclei in both HeLa cells and a mouse model. However, the molecular basis of how Nup88 leads to a multinucleated phenotype remains unclear because of a lack of information concerning its binding partners. In this study, we characterize a novel interaction between Nup88 and vimentin. We also examine the involvement of vimentin in the Nup88-dependent multinucleated phenotype.
Methods
Cells overexpressing tagged versions of Nup88, vimentin and their truncations were used in this study. Coprecipitation and GST-pulldown assays were carried out to analyze protein-protein interactions. Vimentin knockdown by siRNA was performed to examine the functional role of the Nup88-vimentin interaction in cells. The phosphorylation status of vimentin was analyzed by immunoblotting using an antibody specific for its phosphorylation site.
Results
Vimentin was identified as a Nup88 interacting partner, although it did not bind to other nucleoporins, such as Nup50, Nup214, and Nup358, in HeLa cell lysates. The N-terminal 541 amino acid residues of Nup88 was found to be responsible for its interaction with vimentin. Recombinant GST-tagged Nup88 bound to recombinant vimentin in a GST-pulldown assay. Although overexpression of Nup88 in HeLa cells was observed mainly at the nuclear rim and in the cytoplasm, colocalization with vimentin was only partially detected at or around the nuclear rim. Disruption of the Nup88-vimentin interaction by vimentin specific siRNA transfection suppressed the Nup88-dependent multinucleated phenotype. An excess amount of Nup88 in cell lysates inhibited the dephosphorylation of a serine residue (Ser83) within the vimentin N-terminal region even in the absence and presence of an exogenous phosphatase. The N-terminal 96 amino acid residues of vimentin interacted with both full-length and the N-terminal 541 residues of Nup88.
Conclusions
Nup88 can affect the phosphorylation status of vimentin, which may contribute to the Nup88-dependent multinucleated phenotype through changing the organization of vimentin.
https://ift.tt/2IeqQmb
0 notes
Text
Preparation of NiFe2O4 - TiO2 nanoparticles and study of their photocatalytic activity
Preparation of NiFe2O4 – TiO2 nanoparticles and study of their photocatalytic activity
Do, Quoc Hung Nguyen, Kim Thanh In this article, the authors describe the method for preparation of NiFe2O4 – TiO2 magnetic nanoparticles and present the results on study of their photocatalytic activity. NiFe2O4 nanoparticles have been prepared by coprecipitation using spraying technique with subsequent hydrothermal processing. NiFe2O4-TiO2 composite nanoparticles were prepared by covering thin…
View On WordPress
0 notes
Text
Nutritional value of soya protein and milk coprecipitates in sausage products
http://dlvr.it/PT7nCN
0 notes
Text
Bioequivalent Drugs: Towards A Needed Holistic Paradigm Shift? | Lupine Publishers
Journal of Drug Designing | Lupine Publishers

Opinion
The author of this short communication has been working in and teaching drug development for the last 20 years. He has been involved in more than 100 drug development projects, including drug delivery, medical device, biologics, innovators and generic drugs. He has also been involved in all the steps that are needed to file properly to different governmental agencies investigational new drug applications (IND), clinical trial applications (CTA), abbreviated and new drug applications/submissions (ANDA\S; NDA/S), 505b2, 510k and biologics legal applications (BLA). After several other interactions with all the other actors, such as Health Canada (HC), the Food and Drug Agency (FDA), the European Medicines Agency (EMA), pharmacovigilance companies and consultants, public relation companies, insurance companies and especially patients, the author has decided to gather all the comments in order to initiate a kind of a new debate on the innovators and generic drugs. However, the goal of this current expert opinion is not to generate conflicts, or to compare generic versus innovator drugs in the sense that one is better than the other. The author has already been involved in several bioequivalence studies, comparing two innovator products where the results showed lack of bioequivalence….or in bioequivalence studies evaluated with clinical endpoint where generic drug products were more potent than the innovators…The goal of this paper should be more based on the following question: are the current methods used to assess bioequivalence are suitable and reliable enough to “stamp” that generic drugs are bioequivalent, are as stable, reliable form a quality, a safety and efficacy standpoints.
Based on the literature generic drugs can be defined as copies of innovator drugs and should contain the same dose strength, the same indication, pharmacodynamical effects, adverse events, safety profile and route of administration than the brand drug. Basically, generic drugs should behave the same than the innovator drugs. To achieve this equivalent profile, drug substance should be formulated and mimic as much as possible the formulation of the brand. It means that drug products ideally should show the same composition from both a chemical but also from a physical point of view. If these last comments are achieved then the biopharmaceutical phase, therefore the phase where the active pharmaceutical ingredient (API) should become available to the body, this phase should be equivalent between the generic and the brand. To help formulators developing bioequivalent formulation, several tools and development methods have been developed over the last decades. The preformulation step represents one of the most crucial steps in order to achieve a reliable, stable and bioequivalent formulation. In the quest of the nanomolar efficacy, small molecules became more and more difficult to formulate, making most of them difficult to solubilize and thus even more difficult to be absorbed because of their low solubilities or bad permeability behavior. Furthermore, all the “easy ones” have already been genericized. The bioavailability and thus the achievement of bioequivalence became then more challenging. In some cases, solid-sate chemistry could be held responsible for this lack of solubility/absorption pattern. Literature has already shown several times that crystalline structure was the most selected structure because sponsors rather have to select a molecule that shows better stability over the time than a higher rate of solubility (by selecting the amorphous structure) for the simple reason of a constant stability, reliability, efficacy over the time. But molecules became more and more insoluble and did not give sponsors the option of working only with the amorphous structure or changing the crystalline structure to amorphous structure (spray-drying, solid dispersion, coprecipitate) in order to get reasonable solubility and then maybe a better bioavailability without changing the stability. This actually represented the highest challenge. The HIV protease inhibitor Ritonavir was one of the best examples [1]. It is a lipophilic molecule with a high molecular weight of 721g.mol-1, that shows low solubility values of 0,001mg/ml at pH 6.8 and 0.4mg/ml in 0.1 N HCl. Because of these last characteristics and its dose of 1,2g per day, it became easy to predict that the absorption would be limited by its poor dissolution profile. It represented than an ideal candidate for solid dispersion. The sponsor then had problems with Ritonavir and had to stop the solid dosage form manufacturing because of the tendency of the drug substance to recrystallize over the time, generating dissolution and absorption problems [2]. It is then easy to imagine that the monitoring of solid-state chemistry became more popular and preformulation department got more and more equipped with state-of-the-art equipment (such X-Ray powder diffraction, differential scanning calorimetry, Raman, …) to monitor such phenomenon, not only form a preformulation standpoint, but also form a whole formulation/manufacturing development standpoint. But the chemistry of the solid-state became also extremely important from an intellectual property (IP) standpoint. And a lot of people will say that for a brand company, IP represents the “crux of the matter” in formulation development...in other words, how far can it be possible to deviate from the patent (without infringing it) by keeping plus or less the same qualitative and quantitative composition, in order to maintain bioequivalence, stability and reliability over the time. For example, the percentage of crystallinity/amorphous of an API in a formulation could become “patentable” knowing that below or above this threshold, it would become extremely difficult to formulate and to become bioequivalent. Atorvastatin calcium is a good example where both amorphous and crystalline structures coexist [3]. The monitoring of these formulations showing different solid-state characteristics were monitored in the marketed Atorvastatin tablets and as described above such differences may have impacted the stability of these tablets. The author had the chance to be involved in several atorvastatin formulation developments (more than 20) and other drug products showing solid-state chemistry issues to achieve bioequivalence between the brand and the generic (in progress) and in the large majority of cases, solid-state chemistry could be held responsible for not achieving bioequivalence. One of the reasons was that solid-state chemistry was not monitored over the time and formulation development steps, such as wet granulation, blending, roller compacting, grinding may have impacted the chemistry of the solid state and thus change the whole drug product behavior, from both a quality and a pharmacokinetic (PK) standpoint.
Bioequivalence…. What Does It Mean?
The literature [4] is telling that us that bioequivalence is the property wherein two drugs with identical active ingredients or two different dosage forms of the same drug possess similar bioavailability and produce the same effect at the site of physiological activity. Of course, this definition can be challenged, and “purists” are more than invited to argue from a semantic standpoint. In this article the focus on bioequivalence assessment will be on PK parameters, and not on clinical endpoints. Bioequivalence is mostly evaluated with PK parameters, the mains being the API maximum concentration in plasma. (Cmax) and the area under the curve that represents the concentration of API in the body following the dosing of a drug product. From a general standpoint, a generic formulation (or test product) is considered bioequivalent to a brand formulation if the ratio test/reference product for Cmax and AUC 0-t (form time 0 to a time t) are within 80.00-125.00%. For the Food and Drug Agency (FDA) [5] and European Medicines Agency (EMA) [6], the 90% confidence interval (CI) geometric mean ratios is expected for these two PK parameters whereas for Health Canada, the 90% CI is expected for the AUC only [7]. From a physiological standpoint, Cmax is more variable than the AUC therefore it can be predicted that form a biostatistical point of view, if the 90%CI is not requested on the Cmax and only the ratio of 80-125%, a smaller sample size (or volunteers) may be expected to successfully achieve bioequivalence. It does not mean that the generic formulation will be less potent and/or safe but in some cases, for certain class of drug products, but some variability when changing from the brand to a first generic and then to a second generic (and so on) may generate side effects or adverse events. Of course, it is expected that the smallest the difference will be in bioequivalence between the brand and the generic, side effects may not be considered significant form a clinical point of view.
That being said concerning the bioequivalence definitions and how it is evaluated and by going back to all the above in this current short communication, it can be expected that physico-chemical characteristics may impact drastically the bioequivalence between tests and reference products. In order to minimize bias as much as possible it becomes then crucial that chemistry manufacturing and controls (CMC) should not be held responsible for non-achievable bioequivalence. Let’s make it clear: a bioequivalence study compares two formulations behavior therefore CMC represents the cornerstone of generic development.
It should be kept in mind that bioequivalence studies are carried out:
a) On healthy volunteers (unless patients are targeted and mandatory according the guidance).
b) On a very small sample size that may not represents the targeted population. It is then difficult to extrapolate on a bigger sample size, on subpopulations. Specialists are relying on phase 4/pharmacovigilance data of the brand (since no such studies are carried out on generic compounds)
c) Most of the time, single doses are evaluated, and even if steady-state is requested by agencies, it will never mimic chronic uses of a drug product (when the drug product is taken over several years). Here again lack of data are available in that regards since such studies are not performed neither.
d) A new generic formulation will always be compared to the reference, and not to the previous generic available in the market. Therefore, for example if the first generic passed on the Cmax with a ratio of 86%, and the second one with a ratio of 117%, some adverse events may be expected. After discussion with worldwide specialists in that domain, most of them will say that these differences will not affect the patient and will be lost once the steady-state will be achieved. It is clear that pharmacovigilance results are difficult to consult. Hence it is difficult to conclude that differences in terms of safety and efficacy have been monitored, even though a quick survey on the population will show you some interesting results with regards to
i. the perception of generic drugs
ii. whether they have noticed differences by interchanging their brands to generic and
iii. whether they have noticed a difference with regards to safety and efficacy when they have been switched from a first generic to a second generic.
So…Finally Can Generic Drugs Be Considered on the Same Footing Than Innovators?
From an academic standpoint, after several presentations given by the author dealing with drug development in general, the interchangeability, and all the fields plus or less linked with generic and innovator drugs, it has been noted that a lot of healthcare professionals are not aware and are not well trained in that regards. Several of them told the author the following: “we understand what you are saying but we have not been trained accurately in that sense at all”. Of course, since the author is teaching it is plus or less true, knowing the propaedeutic of their undergraduate and graduate studies, and the several continuous upgrading trainings they have to follow. Even though the sample size needed for the carrying out of bioequivalence studies, which the author refers, may not be statistically relevant and do not reflect the reality, the goal of this paper was to crystallize an idea about the perception of generic drugs versus innovators that, down the road will hopefully generate a paradigm shift in the perception of these drug products. And then, when new tools may be created and will be helpful to enhance the fact that generic and innovator could be considered equal, from a safety, efficacy, stability and reliability standpoint.
For more Lupine Publishers Open Access Journals Please visit our website:https://lupinepublishersgroup.com/
For more Journal of Drug Designing Please Click Here: https://lupinepublishers.com/drug-designing-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
0 notes