#irb protocol software
Explore tagged Tumblr posts
Text
What Skills Do You Gain from Pursuing a Career in Clinical Research?
One area of the pharmaceutical and healthcare industries that is expanding quickly is clinical research. The need for qualified experts in clinical research has grown dramatically as more novel therapies and drugs are created. What skills do you get by pursuing a career in clinical research? Is a question you may have if you're thinking about taking clinical research courses?
Let’s explore the core competencies and transferable skills that make clinical research a dynamic and rewarding career path.
1. Analytical and Critical Thinking Skills
Analytical thinking is one of the fundamental abilities you acquire in clinical research. To ascertain the efficacy and safety of novel medications or therapies, clinical trials entail gathering, evaluating, and analysing enormous data sets. You will get knowledge of how to: • accurately interpret clinical data; • Use statistical software to analyse outcomes; Analyse possible risks and rewards; find patterns and trends in clinical outcomes; these abilities contribute to the validity, dependability, and action ability of study findings.
2. Attention to Detail
A high degree of precision is required for clinical research. Your attention to detail is essential whether you're organizing case report forms, recording patient replies, or making sure ethical compliance is maintained. Clinical research courses help you to: • Excellent documentation abilities • the practice of cross-checking and error-checking • Adherence to legal requirements; • Regularity in data collection since a single mistake in paperwork can affect the entire trial, accuracy is one of the most highly regarded qualities in this field.
3. Ethical and Regulatory Understanding
In clinical research, adhering to Good Clinical Practice (GCP) and ethical standards is non-negotiable. You’ll gain a thorough understanding of:
Informed consent procedures
Patient rights and confidentiality
Institutional Review Board (IRB) processes
International guidelines like ICH-GCP, FDA, and EMA standards
These skills ensure that you can conduct trials ethically and legally across diverse settings.
4. Communication and Interpersonal Skills
Clinical research is a collaborative field. Whether you’re coordinating with doctors, interacting with participants, or reporting to sponsors, strong communication is vital. During your training, you’ll build:
Verbal and written communication skills
Report writing and documentation expertise
Conflict resolution and patient counselling abilities
Team collaboration strategies
Clear communication improves study outcomes and participant trust.
5. Project Management and Organizational Skills
Managing a clinical trial requires juggling multiple responsibilities, from budgeting and resource allocation to timeline tracking. Clinical research courses equip you with:
Time management strategies
Budget and resource planning
Task delegation and workflow coordination
Monitoring and reporting progress
These skills are especially beneficial if you pursue roles like Clinical Research Associate (CRA) or Project Manager.
6. Technical Proficiency
Modern clinical research relies heavily on technology. You’ll gain hands-on experience with tools and platforms used for:
Electronic Data Capture (EDC)
Clinical Trial Management Systems (CTMS)
Statistical software like SPSS or SAS
Remote monitoring tools
This technical foundation enhances your efficiency and employability.
7. Problem-Solving and Decision-Making Abilities
Unexpected issues often arise in clinical trials—participant dropouts, protocol deviations, or data inconsistencies. Clinical research training helps you:
Identify problems quickly
Develop logical solutions
Make informed decisions under pressure
Adapt to changes and stay compliant
These capabilities are crucial in dynamic and high-stakes environments.
8. Career-Ready Professionalism
Beyond technical skills, clinical research courses also nurture professional behaviour. You learn to:
Maintain confidentiality
Act responsibly and ethically
Work independently and as part of a team
Uphold a high standard of integrity
These soft skills are key to building long-term success and credibility in the field.
Conclusion
What abilities can a job in clinical research provide, then? The answer is: a broad range of interpersonal, analytical, ethical, and technical abilities that set you up for success in one of the most important sectors of healthcare innovation. Clinical research courses provide a strong basis for a rewarding career if you have a strong interest in science, patient care, and advancing medical knowledge. Regardless of your professional goals—clinical research coordinator, CRA, data manager, or regulatory specialist—the abilities you gain will provide access to a wide range of public and private sector employment options.
0 notes
Text
Fresher Research Consultant Vacancies in Bangalore | Advarra Job Opportunities Are you a fresh graduate looking to start your career in clinical research? Advarra, a leading player in the clinical research industry, is offering exciting opportunities for fresher Research Consultants in Bangalore. This role is perfect for candidates with a degree in clinical research or related fields who are passionate about advancing human health. Read on to learn more about the vacancy, qualifications, and how to apply. About Advarra Advarra is a global leader in clinical research and ethical review services, dedicated to improving human health through innovation and collaboration. With a strong focus on aligning patients, sites, sponsors, and CROs in a connected ecosystem, Advarra accelerates clinical trials and brings impactful therapies to the market faster. Job Overview: Research Consultant Role As a Fresher Research Consultant at Advarra, you will play a crucial role in supporting the Institutional Review Board (IRB) services. This position involves working with Advarra’s Clinical Trial Management Software (CTMS) and the CIRBI system to enhance research effectiveness and streamline study startup timelines. You will be responsible for various tasks, including translation management, safety reporting, ongoing reviews, and IRB submissions. Key Responsibilities CTMS and IRB Support: Develop familiarity with Advarra’s Clinical Trial Management Software and CIRBI system to assist in study startup activities. Customer Escalation Management: Monitor and resolve customer escalations promptly to ensure a smooth study startup process. Documentation and Reporting: Utilize internal case management and reporting software to track and complete daily/weekly work assignments. Team Collaboration: Actively participate in team meetings and contribute to discussions on customer cases and protocols. [caption id="attachment_100896" align="aligncenter" width="640"] Fresher Research Consultant Vacancies in Bangalore | Advarra Job Opportunities[/caption] Qualifications Required Basic Qualifications: Knowledge of clinical research methodology, industry regulations, and Good Clinical Practice (GCP) guidelines. Strong organizational and administrative abilities. Familiarity with MS Office and business software. Preferred Qualifications: 0-1 year of internship experience in clinical trial coordination, study startup, regulatory affairs, or clinical data management. Excellent communication skills and a proactive attitude. Ability to work independently and as part of a team. Location and Work Environment This position is based in Bangalore, India. The role requires on-site presence, where you will work in a dynamic and collaborative environment. Advarra values its employees and fosters an inclusive culture that encourages growth, teamwork, and innovation. Apply Now to Join Advarra If you are a fresh graduate eager to contribute to the world of clinical research, don’t miss this opportunity to join Advarra as a Research Consultant in Bangalore. Apply now
0 notes
Text

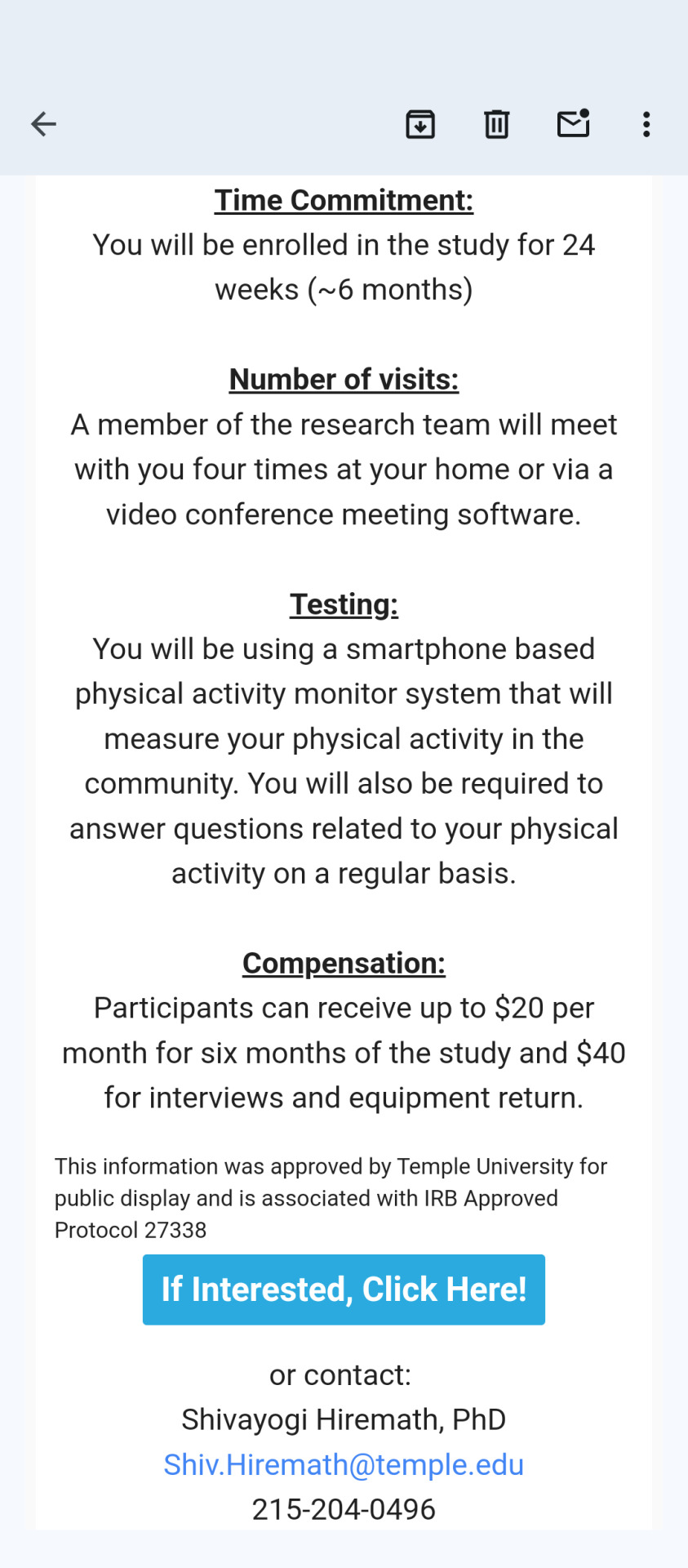
To all of my SCI family, colleagues, and friends!
Please participate in a study to help individuals with spinal cord injury
improve their physical activity levels in the community!
If you are interested, here are the details..
If you are an individual with spinal cord injury, you may be eligible to participate if you...
are between 18 and 75 years of age
are at least 6 months post-injury and medically stable
use a manual or power wheelchair in the community
use you upper arms for exercise
are able to use a smartphone and a smartwatch independently
are interested in starting an exercise program
do not have health conditions that medically restrict you from physical activity
Time Commitment:
You will be enrolled in the study for 24 weeks (~6 months)
Number of visits:
A member of the research team will meet with you four times at your home or via a video conference meeting software.
Testing:
You will be using a smartphone based physical activity monitor system that will measure your physical activity in the community. You will also be required to answer questions related to your physical activity on a regular basis.
Compensation:
Participants can receive up to $20 per month for six months of the study and $40 for interviews and equipment return.
This information was approved by Temple University for public display and is associated with IRB Approved Protocol 27338
If Interested, Click Here!
or contact:
Shivayogi Hiremath, PhD
215-204-0496
Twitter
Facebook
Website
Copyright © 2024, North American Spinal Cord Injury Consortium, All rights reserved.
1 note
·
View note
Text
The Basics of Clinical Trials: What You Need to Know
Clinical trials are the lifeblood of medical progress, driving innovation and shaping the future of healthcare. They are meticulously designed research studies that assess the safety and efficacy of new medical treatments, therapies, or interventions. In this article, we will delve into the fundamental aspects of clinical trials, offering insights from clinical research blogs, software development updates, and clinical research latest updates. Whether you're a prospective participant, a curious individual, or a healthcare enthusiast, understanding the basics of clinical trials is essential.
1. Why Clinical Trials Matter
Clinical trials are the bridge between scientific discovery and patient care. They play a pivotal role in:
Advancing Medicine: Clinical trials lead to the development of new drugs, treatments, and therapies.
Evidence-Based Care: They provide the evidence needed to determine whether a new approach to treatment is safe and effective.
Patient-Centered Care: Clinical trials focus on improving patient outcomes and enhancing the quality of healthcare.
2. The Phases of Clinical Trials
Clinical trials progress through several phases, each serving a specific purpose:
Phase I: These trials involve a small number of healthy volunteers and aim to assess safety, dosage, and side effects.
Phase II: In this phase, the focus shifts to a larger group of patients to evaluate the treatment's effectiveness and further assess safety.
Phase III: These trials involve an even larger patient population and compare the new treatment to existing standard treatments.
Phase IV: After approval, Phase IV trials monitor the treatment's long-term safety and effectiveness in real-world settings.
3. Informed Consent
Informed consent is a cornerstone of ethical clinical research. Before participating in a clinical trial, individuals must be provided with comprehensive information about the study, including its purpose, risks, benefits, and alternatives. Consent is entirely voluntary, and participants have the right to withdraw at any time without consequences.
4. The Role of Placebos
In some clinical trials, placebos are used to maintain scientific rigor. A placebo is a substance with no therapeutic effect. They are essential for ensuring that the treatment's benefits are not influenced by psychological factors and that any observed improvements are genuinely due to the treatment being tested.
5. The Importance of Control Groups
Control groups are a critical component of clinical trials. These groups receive either the current standard treatment or a placebo, allowing researchers to compare the new treatment's effectiveness. Control groups are essential for establishing the treatment's true impact.
6. Randomization and Blinding
Randomization involves randomly assigning participants to either the treatment or control group, reducing the risk of bias. Blinding, on the other hand, can be single-blind (participants are unaware) or double-blind (both participants and researchers are unaware of group assignments). These practices ensure the objectivity of trial results.
7. Ethical Oversight
Clinical trials are subject to rigorous ethical oversight. Institutional Review Boards (IRBs) or Ethics Committees review and approve trial protocols to ensure that the study is conducted safely and ethically. Protecting participants' rights and safety is of paramount importance.
8. The Inclusion and Exclusion Criteria
Clinical trials have specific inclusion and exclusion criteria to ensure that the participants are the right fit for the study. Inclusion criteria define who can participate, while exclusion criteria identify those who cannot. These criteria are crucial for patient safety and the validity of the trial results.
9. Monitoring and Data Collection
Clinical trials are closely monitored to ensure patient safety and data accuracy. Researchers collect data on the treatment's efficacy, side effects, and any adverse events. This data is meticulously analyzed to draw meaningful conclusions about the treatment's impact.
10. The Potential Risks and Benefits
Participating in a clinical trial comes with potential risks and benefits. Risks may include side effects, inconveniences, or the possibility that the treatment is ineffective. Benefits may include access to cutting-edge treatments, close medical monitoring, and the satisfaction of contributing to medical science.
11. Conclusion: The Road to Medical Advancement
Clinical trials are the foundation upon which medical progress is built. They are the culmination of years of scientific research and have the potential to improve patient care and save lives. By understanding the basics of clinical trials, you not only become an informed patient but also appreciate the dedication of researchers, the vigilance of ethical oversight, and the promise of a healthier future. Clinical research blogs, software development updates, clinical research companies in Pune, and medical professionals are all working tirelessly to ensure that clinical trials continue to drive innovation and bring hope to patients around the world.
0 notes
Text
"Aside from the ways in which any survey software will collect and maintain survey data, the researcher will also need to provide information about how the data, once retrieved from the survey software provider, will be maintained (i.e. – on password protected computers, on password protected cloud storage [What are the Terms Of Service for cloud storage company], etc). This information is necessary for the IRB to assess the level of security and subsequent risk to participants data being divulged. Information regarding data security would need to be included in the protocol submission and in the consent form"
In order for any research to pass review to be able to get participants they have to inform the board of how they'll keep data secure and the board has to agree it's sufficient

265 notes
·
View notes
Text
Research Assistant for Cognitive Development in Infancy and Early Childhood Lab at the University of California Davis
Dr. Lisa Oakes with the Center for Mind and Brain at the University of California, Davis seeks to fill a research assistant position at the Junior Specialist level starting as soon as June 1, 2021, depending on funding. This is a 100% position and is for one year. There may be a possibility for extension depending on funding. The successful candidate will participate in all aspects of research in cognitive development in infancy and early childhood, from stimulus creation to data analysis to presentation of research findings in seminars, conferences, and manuscripts submitted for publication. The position will involve developing, in collaboration with the PI and grad students, new experimental designs, stimuli, and procedures. The person hired is expected to independently analyze data, create materials for presentation at conferences, and present research at national and international conferences, such as the biennial meeting of the International Congress of Infant Studies in Ottawa in July 2022.
The position will include the following academic duties: The junior specialist will be actively and significantly involved in publishable research activities. Their roles and duties will include (80%):
Designing research and developing research materials
Read original literature related on infant cognitive development and participate in weekly discussions of recent and classic findings, as well as methodological and theoretical issues.
Participate in meetings and discussions with the PI and graduate students to make decisions about experimental design, stimulus creation, and development of materials and experimental protocols.
Prepare stimulus and other experimental materials using Matlab, Python, Photoshop, or other software and programming environments
Conducting research
Participate in data collection by interviewing and obtaining consent from parents and by testing infant and child research participants, both observing behavior in the moment and using automatic eye-tracking systems
Oversee the day-to-day running of the lab, including prepare and maintaining IRB materials and ensuring compliance with those protocols, ordering supplies, and other miscellaneous tasks
Disseminating the results of research
Use R, Matlab, excel, and SPSS to process and analyze data, prepare figures and text for presentation at research conferences and for manuscripts to be submitted for publication
Contribute to the writing of manuscripts and preparation of conference presentations
The Junior Specialist will be engaged in presenting the results of work at national and international societies and in preparing manuscripts for publication (5%):
The Junior Specialist will attend national and international conferences, held by major professional societies. In April 2022, the Junior Specialist may attend the biennial meeting of the Cognitive Development Society in Madison, WI, and in July 2022 the Junior Specialist may attend the biennial meeting of the International Congress on Infant Studies in Ottawa, Canada. If appropriate, the Junior Specialist will present research at one or both of these conferences.
As appropriate, the junior specialist will participate in the preparation of manuscripts for publication. This may involve creation of figures, writing of procedure and methods, reporting of statistics, and contribution to other sections of manuscripts.
The Junior Specialist will be actively involved in service to the university through training and mentoring undergraduate students, and public service through the dissemination of information about infant cognitive development through forums such as facebook and twitter (15%):
The Junior Specialist will train and mentor undergraduate students in experimental procedures, data collection, and coding of behavior from video.
The Junior Specialist will train and mentor undergraduate students in data processing, analysis, and visualization
The Junior Specialist will contribute to a facebook page, twitter account, and Instagram account all designed to disseminate information about research findings and to promote scientists in the field to a broad audience. These social media efforts are targeted at providing a resource for parents and for students.
Position qualifications include:
Required: Bachelor’s degree in Psychology or related field, experience with human subjects in a laboratory setting, knowledge of excel, experience with programming environments such as matlab, python, or R, strong organizational skills and attention to detail. Preferred: Experience with testing infants or young children in behavioral research, knowledge of experimental methods to study early cognitive development, experience with and knowledge of infrared automatic eye-tracking systems, familiarity with human subjects protocols, experience conducting interview or consenting potential research participants, knowledge of most or all of the following software: R, SPSS, Adobe Photoshop, Adobe Director, Filemaker, Matlab and/or Python.
Applicants should submit a cover letter of interest describing relevant skills, a statement of contributions to diversity, a C.V., and provide contact information for 2-3 referees. Submit application materials here: https://recruit.ucdavis.edu/JPF04078.
The position will remain open until filled. For full consideration, please apply by April 7, 2021. Starting salary is $44,500 annually plus benefits. If hired, a background check is required
The University of California is an Equal Opportunity/Affirmative Action Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, age or protected veteran status. For the complete University of California nondiscrimination and affirmative action policy see: http://policy.ucop.edu/doc/4000376/NondiscrimAffirmAct.
Under Federal law, the University of California may employ only individuals who are legally able to work in the United States as established by providing documents as specified in the Immigration Reform and Control Act of 1986. Certain UCSC positions funded by federal contracts or sub-contracts require the selected candidate to pass an E-Verify check. More information is available http://www.uscis.gov/e-verify.
UC Davis is a smoke & tobacco-free campus (http://breathefree.ucdavis.edu/).
If you need accommodation due to a disability, please contact the recruiting department.
Questions: Please direct any questions to [email protected].
Lisa M. Oakes Professor, Department of Psychology Faculty Researcher, Center for Mind and Brain University of California, Davis 267 Cousteau Place Davis, CA 95618 phone: 530 754-4523 OR 530 754-8304
#psychology#university jobs#davis#university of california#mind#development#job#cognitive#cognitivedevelopment
3 notes
·
View notes
Text
Should You Microdose to Treat Depression?
May 8, 2020 7 min read
Opinions expressed by Entrepreneur contributors are their own.
The following article is written by Ben Angel. Author of the book, Unstoppable: A 90-Day Plan to Biohack Your Mind and Body for Success. Buy it now from Amazon | Barnes & Noble | iBooks | IndieBound. And be sure to order The Unstoppable Journal, the only journal of its kind based on neuroscience, psychology and biohacking to help you reach your goals.
If you asked 100 people about psychedelics, you’d most likely get 100 opinions based on their firsthand experience, strong condemnation or stories from their adventures at Woodstock in the ’60s. No matter what people might know or think they know about psychedelics, the 40-year moratorium that closed down related research in the ’70s is now coming to an end. Psychiatrists are beginning to realize that strategic, supervised use of these psychopharmacological drugs is helping people with mental disorders including obsessive-compulsive disorder, post-traumatic stress disorder, alcoholism, depression and cluster headaches. Still, are there enough scientific studies to warrant the use of these drugs in mainstream society?
I’ll admit that talk of psychedelic therapy to treat depression makes me nervous. In researching my book, Unstoppable, I looked at other key triggers that can mimic psychological disorders like depression and anxiety, such as inflammation, nutritional deficiencies, hormonal changes, side effects from medications, gut imbalances and food sensitivities. The reality is, depression is complex. What works for one may not work for another. Any successful treatment must first identify the root cause of one’s depression successfully, which can be a complex process if not done under the right medical care. A psychedelic treatment isn’t suddenly going to fix a nutritional deficiency, for example, but it may help target other symptoms and behaviors that correspond with depression. This is why it was critical to set my own biases aside and speak to an expert.
Related: There Will Be 4 Identity Types in This Recovery. Which One Are You?
Image credit for photo of Dr. Sportelli: Jen Vitale Photography
I was fortunate enough to interview Dr. Domenick J. Sportelli, who is board-certified by the American Board of Neurology and Psychiatry for General Psychiatry and fellowship-trained and Board Certified in Child and Adolescent Psychiatry. He also specializes in human behavior and psychopharmacology. I wanted to get the most current information on the use of psychedelics in treatment for depression, anxiety and PTSD, so I first asked him first to clarify what psychedelics were.
“The term ‘psychedelic substance’ refers to an exogenous substance [derived outside the body] that, when taken into the body in various ways, physiologically, neurologically and psychologically manifest an internal personal experience of altered states of consciousness,” he explains. “This includes perceptual distortions, hallucinations, synesthesia [a mixing of the senses], altered sense of time and space, as well as potentially inducing what researchers call a ‘mystical experience’ — a sense of oneness, of noetic experience and an undefinable but profoundly spiritual quality.”
Is there enough evidence to support psychedelic therapy?
Sportelli wants to make clear that the most researched psychedelics — LSD, psilocybin (mushrooms), peyote, MDMA, DMT and ketamine — have different mechanisms of action and even induce subtle, subjective experiential differences. Although each is grouped under the term “psychedelics,” they are quite disparate.
Dr. Sportelli is cautiously optimistic about the multitudes of large-scale, university-based testing and prior research compiled decades ago, but worries about the abiliity to circumvent bureaucracy and conduct safe, credible and substantial testing today. He does add that recent testing of psilocybin, LSD, ketamine and MDMA in particular has generated cause for optimism, and that they will likely have a place not only in continued, diverse research design and protocol, but eventually in therapeutic use.
What types of depression can psychedelics treat?
If we were to look at the onset of most mental illnesses, the majority start to become evident between the ages of 11 and 24, according to the National Institute of Health. With only 42 percent of people getting treatment, most typically do not seek out assistance until a secondary mental illness occurs several years later.
When asked how broadly psychedelics might be able to help treat people with depression, Sportelli concedes that, “Unfortunately, research hasn’t determined the level of scientific data to specify the type of depression or mood disorder that psychedelic therapy will benefit.” But he does add that research and data are beginning to show statistically significant improvements in mood, reduced anxiety, change in positive personality traits over time, the possibility of reducing addictive behaviors, reduction in suicidal tendencies and increased personal insight.
Do psychedelics treat the symptoms or the cause?
According to Dr. Sportelli, depression stems from a mix of genetic, biological, neurological, psychological and sociological factors. Recent research has demonstrated how the chemical breakdown of psilocybins closely resembles that of serotonin, and indicated the promising interplay of select hormone transmission. Dr. Sportelli stresses the critical role that these drugs might offer in mood disorders is at the forefront of the pharmaceutical quest for treatment.
“We have never seen substances like these that can potentially change the way that we look at our life and change perspective with lasting results,” he says, noting that they might be able to help “supercharge psychotherapy.”
Is this ultimately a recommend reatment, and where does one turn for it?
“At this time, in the U.S., I would only recommend this treatment be a part of, and under the close supervision of, a university-based IRB [Institutional Review Board]-monitored clinical trial,” Sportelli emphasizes. Before any psychiatric treatment, Dr. Sportelli also recommends a full medical and neurological evaluation to rule out any of the multitudes of medical circumstances that can manifest as a primary mood disorder, and reiterates that significant and often profoundly adverse outcomes associated with such powerful, mind-altering chemicals need to be weighed further as well. That’s why, as part of any regulated trial, all the necessary medical workups would be completed before participation.
Is the stigma around psychedelic therapy warranted?
Sportelli acknowledges that there is a safety concern associated with psychedelics, and does not condone their recreational or illict use. But he does believe that regulated clinical trials, judicious and ethical research methodology and the progression for therapeutic intervention should not be overlook based on previous stigma and possible misclassification.
Related: 50,000 Entrepreneurs Tell Us How to Avoid Stress and Anxiety
I’ve never been one to throw the baby out with the bathwater. After interviewing Dr. Sportelli, I hold hope for the future, but also a concern for those who may seek out this kind of treatment without an accurate medical diagnosis. My number-one hesitation remains — that is we simply do not have the studies to show which types of depression psychedelic therapy successfully treats, which may result in people attempting to use a hammer when in fact they need a nail.
Either way, if you are to venture into this arena, find someone who specializes in it. The risk of going it alone could come at too a high price.
Are you ready to become unstoppable?
Visit www.areyouunstoppable.com and take your FREE 60-second online quiz now. By answering a series of simple questions, my software will analyze your results and provide you with a comprehensive report that will indicate your identity type and lead you to the tools and tips you need to close that gap between who you are and who you could become. Take the quiz to get started!
Website Design & SEO Delray Beach by DBL07.co
Delray Beach SEO
source http://www.scpie.org/should-you-microdose-to-treat-depression/ source https://scpie1.blogspot.com/2020/05/should-you-microdose-to-treat-depression.html
2 notes
·
View notes
Text
SCRO Stem Cell Research Oversight Protocol Software
Key Solutions offers SCRO stem cell research oversight protocol software which is responsible for developing guidelines on issues like donors, registries etc.
Key Solutions offers research administration and compliance software which are integrated with modules like iacuc and irb.
The SCRO module ensures that any stem cells research conducted in an organization is in compliance with the organization’s regulatory policies by streamlining the approval workflow between the investigators, the coordinators, and the review committee.
1 note
·
View note
Text
Lessons from the COVID-19 swab crisis -- LiveScience.Tech

A major crisis that accompanied the rise of the pandemic was lack of availability of the nasopharyngeal swab — necessary for testing for COVID-19, which in turn, was necessary to get a grip on the pandemic. An account of how one group addressed that crisis is published this week Journal of Clinical Microbiology, a journal of the American Society for Microbiology.
“We met the challenge by creating all-new swabs, which were ready and clinically tested in just three weeks,” said Ramy Arnaout, M.D., D.Phil., Associate Professor of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, and Associate Director of the Clinical Microbiology Laboratories, Beth Israel Deaconess Medical Center (BIDMC).
“Handling crises successfully requires a different set of skills than the everyday,” said Dr. Arnaout. “Competition and secrecy are out. Cooperation and openness are in. Resolving the swab crisis was a case study in these and other valuable lessons.”
As the first wave of COVID-19 broke out across the United States, BIDMC, which had the largest in-house COVID-19 testing center in Boston, found themselves with only a week’s supply of swabs. “More manufacturing was the only lasting solution,” Dr. Arnaout said. He and his colleagues began reverse engineering swabs, to determine if they could make them from scratch.
Swabs must be engineered to be neither too stiff, nor too flexible, and must be individually packaged and sterile. BIDMC needed around ten thousand a week; the country needed roughly ten million.
The first week, group members floated, shot down, resurrected, and repurposed various ideas, said Dr. Arnaout. Ultimately, the team saw two options: to find a scalable means to assemble swabs, or else to “find a way to make a stripped-down swab in a single go, without the need for assembly.” 3D printing had “advantages in speed of development and in the variety of structures it can make.”
Dr. Arnaout had previously demonstrated that open and collaborative crowdsourcing is a viable route to solving complex computational problems, specifically his work in computational immunology. He put this lesson to work in the COVID crisis.
“We navigated our… networks, letting manufacturers know about the swab crisis and what we needed from them to solve it,” said Arnaout. “We set up a free publicly viewable knowledge base online in the form of a GitHub repository — a type of website usually used by software engineers to collaborate on coding projects — to share everything we knew with everyone who might want to know it. This was critical for lowering the activation energy for anyone who wanted to join the effort… By the end of the first week, prototypes were rolling in.”
Over the course of the second week, the team tested more than 150 prototypes. “We were giving manufacturers feedback and suggestions one day and receiving new prototypes the next,” Dr. Arnaout said. “We put our protocols and results online.”
The team spoke often with BIDMC’s Institutional Review Board, “whose help and quick feedback were indispensable for cutting through red tape,” Dr. Arnaout said. “We put our IRB-approved protocol online as well.” BIDMC’s technology ventures office assured the team that the evaluation and feedback they were providing to manufacturers would not constitute intellectual property, thereby avoiding any haggling over ownership, which could have wasted precious time.”
By the fourth week, the team had validated four prototypes for clinical use. By summer’s end, “millions of the swabs our coalition helped design, vet, and mass-produce had been sold and used for COVID-19 testing” across the United States and in Europe, Dr. Arnaout wrote.
That experience suggested five lessons:
Define the mission — “a simple, clear, and concrete unifying goal for the entire team,” said Dr. Arnaout.
Establish norms for behavior. At BIDMC, that took place “mostly via conversations, repetition of the main message and personal example,” said Dr. Arnaout. “Conversations often began or ended with an explicit acknowledgment of the temptation to go it alone… and a reminder that we were not going to give in to such temptation.”
Leverage expertise. “At BIDMC, the clinical trials office handled paperwork that the investigators would have handled themselves under normal circumstances.”
Practice open and clear communication, “by eliminating the friction of gatekeeping access to information,” said Dr. Arnaout.
Stay positive.
“Perhaps we all can take the opportunity afforded by this trying time to improve how we meet our everyday challenges,” said Dr. Arnaout. “By doing so, we might find ourselves further along, more capable, and better prepared for when the next crisis inevitably hits.”
New post published on: https://livescience.tech/2021/08/19/lessons-from-the-covid-19-swab-crisis-livescience-tech/
0 notes
Text
Live Webinar: Quality Management of Electronic Systems in Clinical Trial Investigations: A Comparison of FDA and EMA Guidance
Live Webinar: Quality Management of Electronic Systems in Clinical Trial Investigations: A Comparison of FDA and EMA Guidance When: Friday September 3, 2021 - 01:00 PM Duration: 60 Minutes.
Register Now

As clinical researchers, we are challenged to fully integrate the management systems needed across functional areas within our electronic systems. Most frequently, the IT group and vendor manage the technology, but software AND process needs to be managed and understood by other cross functional areas when conducting day to day trial activities to support human subject protection and data integrity. This includes pre-study protocol development, vendor and site selection activities, subject safety surveillance, data management, site monitoring, protocol management, project management, and more. LEARNING OBJECTIVES By attending this interactive session, you will be able to… Identify specific quality systems elements to support the use of electronic systems regarding sponsor, site, and IRB/EC regulatory responsibilities Avoid the most common misconceptions related to use of electronic records Recognize the process of ensuring the use of electronic records supports the predicate rules for sponsors, investigators, and IRBs/ECs
0 notes
Text
Overcome Challenges in Cancer Research & Improve Decision-Making Using a Cloud-Based Informatics Solution

The fight against cancer has remained a great challenge for patients, their families and physicians as well as oncology researchers due to the dynamic and versatile nature of the disease. To date, there are over 100 different types of cancer that have been identified and they affect different cells in the body. They include carcinomas, sarcomas, leukemias, myelomas, melanomas, and lymphomas among others. Consequently, oncology researchers are constantly dealing with an avalanche of data and metadata (e.g., omics data or radiology data), which require robust infrastructure to collect, manage and interpret. It becomes even more difficult when these data sets have to be shared across different teams while maintaining compliance and other industry standards.
Why is Cancer Research Important?
Cancer research involves the discovery of cancer biology and genetic diversity and translating this knowledge to improve cancer therapy. Often, this involves the use and reuse of data from disparate sources (databases and samples). It may be necessary to get this data on one streamlined platform to allow oncology researchers faster access to the data and also facilitate collaboration, compliance, and maximize the value of research. This helps shorten the time to diagnosis and treatment and expedite the entry of drugs into the market.
Cancer treatment, from diagnosis to treatment, is always a race against time.
Cancer research sites and institutions often have limited access to research staff, facilities, and study participants or samples. Therefore, it is necessary to form networks where data, knowledge and samples can be shared collaboratively which is often a massive undertaking that requires advanced technological solutions. Multiple technical interfaces (interoperability) have to come in place since the data is usually found in varying documentation systems and at different sites. Data privacy, in this case, can be easily compromised if adequate measures are not put in place. Hence, appropriate technological solutions for pseudonymization have to be created and integrated into existing workflows to support compliance as well as yield acceptable and reproducible research results. Modern IT solutions can be used to help cancer research laboratories to maximize the value of cancer research.
As much as IT solutions will not necessarily cure patients; advances in medical IT research will go a long way in accelerating cancer research and facilitate timely treatments and consequently improve patient outcomes. With all forms of cancer, time is always of the essence.
How can a LIMS Help with Cancer Research?
The prototypical nature of cancer research requires the processing and integration of datasets in a robust technological environment. An appropriate LIMS for Cancer Research should support the whole process from tissue and sample collection and annotation to data analysis and reporting. A LIMS helps to collate data from multiple sources and studies without data redundancy, providing researchers access to high-quality data for fast decision-making. A LIMS for cancer research also helps in automating and monitoring complex workflows and flags steps that deviate from defined SOPs.
A LIMS for cancer research should be flexible, adaptive, and compliant. It needs to be able to manage data flow across different disciplines and allow the researchers to easily share and receive data, metadata, and results with other researchers in real time.
Integrating a LIMS with the different instruments such as DNA sequencers, visual tools, and other software, such as EMR, EHR, pathology systems, statistical tools, will provide the oncologist with a holistic view of the cancer patient in a real-life situation.
Ensuring Compliance in Cancer Research
Compliance is a top priority for every cancer laboratory. The Health Insurance Portability and Accountability Act (HIPAA) regulates the use and sharing of protected health information such as data related to the operation of cancer registries and registry-supported cancer research.
The lab’s LIMS should support the HL7 standards for data exchange in the cancer research industry. It also helps with consent management and meeting HIPAA requirements for safeguarding patient privacy & IRB protocols for tissue acquisition and data sharing.
Conclusion
With the complexity of cancer research and treatment, researchers can easily get overwhelmed by the multiform data that they are required to process. Deciphering the significance or irrelevance of data and metadata and drawing conclusions from the generated data sets requires the support of an intelligent and automated LIMS software. Such a software offers broader insights that can easily be translated into therapy at a faster turnaround time.
A cloud-based LIMS that manages large datasets and facilitates data access, visualization, analysis, and transmission can be integrated with other systems to facilitate informed decision-making. A cloud solution, besides storing data, may be able to execute algorithms that can be used by other researchers to gain insights from data without needing deep technical knowledge. This data can be easily accessed from different sources and locations at any given time, thereby overcoming the time constraints which is a major challenge in cancer research. This, hopefully, will speed up the decision-making process, improve patient outcomes, and clinical care of patients.
Originally published at https://cloudlims.com.
0 notes
Text
O que você precisa saber e acompanhar nesta quarta

Aqui estão as notícias para você começar o dia Alexandre Cassiano/Agência O Globo Produção de derivados da Petrobras avança 6,6% A produção de derivados nas refinarias da Petrobras no terceiro trimestre cresceu 17,8% em relação ao segundo trimestre, chegando a uma média de 1,93 milhão de barris/dia. Em relação ao terceiro trimestre de 2019, a alta foi de 6,6%. A estatal elevou sua projeção de produção de óleo e gás para 2020 de 2,7 milhões de barris diários de óleo equivalente (BOE/dia) para 2,84 milhões de BOE/dia. Desse total, a produção de petróleo deve chegar a 2,28 milhões de barris por dia, ante 2,2 milhões da previsão inicial. Receita anuncia arrecadação de setembro A Receita Federal anuncia, às 14h30, o resultado da arrecadação de tributos federais e contribuições previdenciárias de setembro. Em agosto, a arrecadação total das receitas federais atingiu R$ 124,505 bilhões, registrando acréscimo real de 1,33% em relação a agosto de 2019. No período acumulado de janeiro a agosto, a arrecadação alcançou o valor de R$ 906,461 bilhões. Federal Reserve comunica Livro Bege O Federal Reserve comunica, às 15h (de Brasília), o "Livro Bege", um sumário das condições econômicas atuais que servirá de base para as discussões de política monetária no encontro programado para os dias 4 e 5 de novembro. Bolsonaro visita instalações do Sirius em Campinas (SP) O presidente Jair Bolsonaro embarca, às 8h, para Campinas (SP). Às 10h40, visita o Laboratório de Enriquecimento Isotópico (LEI) em Iperó (SP) e, às 11h15, o Laboratório de Geração de Energia Nucleoelétrica (Labgene). Às 14h45, visita as instalações do Sirius; às 15h30 participa de cerimônia alusiva à abertura da primeira linha de luz do Sirius e, às 17h15, embarca de volta para Brasília. CCJ do Senado sabatina indicado ao STF A Comissão de Constituição e Justiça (CCJ) do Senado sabatina às 8h o desembargador Kassio Marques, indicado para o Supremo Tribunal Federal (STF). Neogrid protocola prospecto para IPO A companhia de software Neogrid protocolou prospecto preliminar para sua oferta pública inicial de ações (IPO, na sigla em inglês) . A oferta será primária e secundária. Totvs estende validade de proposta pela Linx A Totvs estendeu a validade da proposta de combinação de negócios com a Linx para 31 de dezembro. No último dia 8, a companhia já havia estendido o prazo para 17 de novembro, em comunicado em que criticava a postura dos conselheiros independentes da Linx durante a condução do negócio. BRF suspende abate de frango em Carambeí (PR) A BRF vai paralisar temporariamente os abates de frango de sua unidade em Carambeí, no Paraná, para ajustes operacionais, visando adequação da produção à demanda do mercado. Diretor da C&A deixa o cargo O diretor-presidente da C&A, Paulo Correa Junior, deixou o cargo que ocupava no conselho de administração da companhia. O conselheiro Petrus Zegger, que integra o conselho desde 2019 e é responsável pela área financeira da Cofra Holding, empresa da família que controla a varejista, também deixou o posto. A companhia afirma que as mudanças fazem parte de um movimento de reorganização do conselho de administração. IRB tem prejuízo de R$ 65,4 milhões O IRB Brasil Re registrou prejuízo líquido de R$ 65,4 milhões em agosto, segundo dados mensais não auditados enviados à Susep. Neoenergia tem lucro 36% maior O lucro da Neoenergia cresceu 36% no terceiro trimestre, na comparação anual, chegando a R$ 814 milhões. A receita líquida da companhia, controlada pela espanhola Iberdrola, somou R$ 7,98 bilhões, o que representa uma alta de 11% na mesma base de comparação. Romi vê lucro subir 30% A fabricante de máquinas e equipamentos Romi teve lucro de R$ 36 milhões no terceiro trimestre, o que representa um aumento de 30% frente ao registrado no mesmo intervalo de 2019. A receita da companhia avançou 1,6%, chegando a R$ 250,5 milhões. EDP vai reajustar tarifas em SP A Agência Nacional de Energia Elétrica (Aneel) autorizou o reajuste tarifário da EDP São Paulo a partir de 23 de outubro. O reajuste sentido pelo consumidor será de 4,82%, e R$ 355,8 milhões referentes à Conta Covid serão revertidos como componente financeiro negativo. O que você precisa saber e acompanhar nesta quarta
0 notes
Text
O que você precisa saber e acompanhar nesta quarta

Aqui estão as notícias para você começar o dia Alexandre Cassiano/Agência O Globo Produção de derivados da Petrobras avança 6,6% A produção de derivados nas refinarias da Petrobras no terceiro trimestre cresceu 17,8% em relação ao segundo trimestre, chegando a uma média de 1,93 milhão de barris/dia. Em relação ao terceiro trimestre de 2019, a alta foi de 6,6%. A estatal elevou sua projeção de produção de óleo e gás para 2020 de 2,7 milhões de barris diários de óleo equivalente (BOE/dia) para 2,84 milhões de BOE/dia. Desse total, a produção de petróleo deve chegar a 2,28 milhões de barris por dia, ante 2,2 milhões da previsão inicial. Receita anuncia arrecadação de setembro A Receita Federal anuncia, às 14h30, o resultado da arrecadação de tributos federais e contribuições previdenciárias de setembro. Em agosto, a arrecadação total das receitas federais atingiu R$ 124,505 bilhões, registrando acréscimo real de 1,33% em relação a agosto de 2019. No período acumulado de janeiro a agosto, a arrecadação alcançou o valor de R$ 906,461 bilhões. Federal Reserve comunica Livro Bege O Federal Reserve comunica, às 15h (de Brasília), o “Livro Bege”, um sumário das condições econômicas atuais que servirá de base para as discussões de política monetária no encontro programado para os dias 4 e 5 de novembro. Bolsonaro visita instalações do Sirius em Campinas (SP) O presidente Jair Bolsonaro embarca, às 8h, para Campinas (SP). Às 10h40, visita o Laboratório de Enriquecimento Isotópico (LEI) em Iperó (SP) e, às 11h15, o Laboratório de Geração de Energia Nucleoelétrica (Labgene). Às 14h45, visita as instalações do Sirius; às 15h30 participa de cerimônia alusiva à abertura da primeira linha de luz do Sirius e, às 17h15, embarca de volta para Brasília. CCJ do Senado sabatina indicado ao STF A Comissão de Constituição e Justiça (CCJ) do Senado sabatina às 8h o desembargador Kassio Marques, indicado para o Supremo Tribunal Federal (STF). Neogrid protocola prospecto para IPO A companhia de software Neogrid protocolou prospecto preliminar para sua oferta pública inicial de ações (IPO, na sigla em inglês) . A oferta será primária e secundária. Totvs estende validade de proposta pela Linx A Totvs estendeu a validade da proposta de combinação de negócios com a Linx para 31 de dezembro. No último dia 8, a companhia já havia estendido o prazo para 17 de novembro, em comunicado em que criticava a postura dos conselheiros independentes da Linx durante a condução do negócio. BRF suspende abate de frango em Carambeí (PR) A BRF vai paralisar temporariamente os abates de frango de sua unidade em Carambeí, no Paraná, para ajustes operacionais, visando adequação da produção à demanda do mercado. Diretor da C&A deixa o cargo O diretor-presidente da C&A, Paulo Correa Junior, deixou o cargo que ocupava no conselho de administração da companhia. O conselheiro Petrus Zegger, que integra o conselho desde 2019 e é responsável pela área financeira da Cofra Holding, empresa da família que controla a varejista, também deixou o posto. A companhia afirma que as mudanças fazem parte de um movimento de reorganização do conselho de administração. IRB tem prejuízo de R$ 65,4 milhões O IRB Brasil Re registrou prejuízo líquido de R$ 65,4 milhões em agosto, segundo dados mensais não auditados enviados à Susep. Neoenergia tem lucro 36% maior O lucro da Neoenergia cresceu 36% no terceiro trimestre, na comparação anual, chegando a R$ 814 milhões. A receita líquida da companhia, controlada pela espanhola Iberdrola, somou R$ 7,98 bilhões, o que representa uma alta de 11% na mesma base de comparação. Romi vê lucro subir 30% A fabricante de máquinas e equipamentos Romi teve lucro de R$ 36 milhões no terceiro trimestre, o que representa um aumento de 30% frente ao registrado no mesmo intervalo de 2019. A receita da companhia avançou 1,6%, chegando a R$ 250,5 milhões. EDP vai reajustar tarifas em SP A Agência Nacional de Energia Elétrica (Aneel) autorizou o reajuste tarifário da EDP São Paulo a partir de 23 de outubro. O reajuste sentido pelo consumidor será de 4,82%, e R$ 355,8 milhões referentes à Conta Covid serão revertidos como componente financeiro negativo.
Leia o artigo original em: Valor.com.br
Via: Blog da Fefe
0 notes
Text
Senior Clinical Research Assistant for Child Stress and Trauma Project at Bradley Hospital
SUMMARY:
Work with a multidisciplinary team of developmental and clinical psychologists who (1) conduct longitudinal developmental and applied psychological research with children and families (2) offer community-based consultation services (3) conduct program evaluation of early childhood interventions. Under the general supervision of the Principal Investigator of Research on children's stress and trauma assist in the acquisition and analysis of participant information for clinical research projects and interview participants in community settings to gather information. Prepare and maintain study record. Enter data and achieve inter-rater reliability to code observational assessment of parent and child behavior. Assist with coordination of clinical and consultation activities. Participate in the pre and post-award administration of research projects. May research literature in the field and assist in the analysis and preparation of research data for presentation and publication. Opportunities to participate in presentations and posters may be available.
RESPONSIBILITIES:
Coordinate and manage clinical research studies including recruitment and retention of participants and tracking of longitudinal study assessments.
Community-Based Data Collection:
Provide supervision to research assistants and volunteers as needed.
Oversee and assist with data entry data processing and analysis. Code videotapes of child behavior and parent-child interaction.
May assist with coordination of early childhood clinical and consultation services.
Responsible for compliance with hospital and university IRB regulation and oversight including working with the team to complete continuing review and other progress reports or preparation of new proposals.
Review literature pertaining to research being conducted.
May assist with grant and contract pre-award activities including completion of funding agency proposal documents and creation of budgets working closely with the principal investigator to gather and maintain all additional materials to be submitted in the proposal packet.
May assist with post-award financial oversight and management budgeting tracking monthly grant and contract expenditures cost control and analysis forecasting and compliance monitoring.
Recruit participants from community settings.
Complete research assessments with children and families in the home environment.
Assist in identification and follow-up of participants meeting criteria for inclusion in clinical research studies. Ensure protocol eligibility requirements are met.
Establish and/or maintain study record for each participant. Interview participant and/or family to explain nature of study. Obtain signed consent forms and schedule research assessments. According to established protocol may administer standardized and non-standardized research observations and assessments.
May participate in data collection at a research summer camp.
May conduct classroom assessments at local childcare sites.
BASIC KNOWLEDGE:
Demonstrated knowledge and skills necessary to interact with participants prospective patients and community partners with consideration of child development in context human development stages and cultural patterns.
REQUIREMENTS:
Bachelor's Degree in Psychology Behavioral Sciences or related area including courses in research methodologies and statistics. Master's Degree preferred.
Must have valid driver's license and reliable vehicle to commute to and from home visits throughout the state of Rhode Island and neighboring areas. Mileage will be reimbursed.
Availability to regularly work some evenings and Saturdays is required.
Experience with children and/or home visiting preferred.
Experience with conversational Spanish required.
Thorough knowledge of software including Microsoft Word Excel and PowerPoint as well as Adobe Acrobat for PDF creation and manipulation. Knowledge of Microsoft Access SPSS and REDCap preferred. Personal traits including excellent interpersonal skills ability to work effectively as part of a team excellent organizational skills ability to multi-task and solve problems creatively.
EXPERIENCE:
Two to three years' experience working in a research environment including direct work with children and families in a research capacity.
Experience supervising staff in human subject's research preferred.
WORK ENVIRONMENT AND PHYSICAL REQUIREMENTS:
Work is performed in a typical office environment as well as in the field including the homes of research participants or other community settings.
INDEPENDENT ACTION:
Performs independently within department policies and practices. Refers specific complex problems to supervisor where clarification of departmental policies and procedures may be required.
TO APPLY:
https://jobs.lifespan.org/search/jobdetails/sr-clinical-research-asst/1a6516f0-0548-43b5-9ac1-5f06047441f2
2 notes
·
View notes
Text
RSC Protocol Software | Radiation Safety Committee in USA
Key Solutions offers RSC protocol software that involve the use of radioactive materials which affect lab equipment, study subjects, or environment at large.
Key Solutions offers Research administration and compliance software which are integrated with modules like iacuc and irb.

0 notes
Text
Document Processing Specialist
Our Document Processors are responsible for processing clinical study research documents while utilizing our proprietary software program. Over time you will have the opportunity to rotate between various functions and gain new skills, and increase your pay! Although having knowledge of medical terminology is helpful, it is not required. If you’re looking for a back-office administrative type position, this role might be for you. This position is internally called IRB Operations Specialist. Job Location; Puyallup, WA On-site THIS IS NOT A REMOTE OR VIRTUAL POSITION... Required Skills: Ability to quickly proofread and detect errors in formatting. Intermediate to expert skills with Micro-soft word and Micro-soft outlook. Someone with editing abilities but this isn't an editor role. Essential Duties/Responsibilities The primary responsibility will be to collect, revise, and process clinical trial and protocol documents in preparation for our board’s review. Review and edit the documents to comply with company formatting standards. Enter data into our proprietary software. Work effectively in a queue with high volumes and frequent changes, all while meeting rigid deadlines. Maintain both speed and accuracy requirements. Overtime is often required in order to meet deadlines and to help clinical trial participants. We estimate 0-10 hours of overtime weekly, depending on company needs. What this job is not: We are not editors. Our review process is focused on the content of the clinical study, which is submitted to us by our clients. Education Requirements: HS Diploma is required Qualifications/Experience: Bachelors degree or two+ years of office experience where proofing documents and attention to detail were essential elements of your job requirements. Intermediate skills with Microsoft Word and Microsoft Outlook. A background in utilizing specialized database systems is strongly desired. Ability to quickly proofread and detect errors in formatting. Our Benefits: Robust medical, dental and vision plans - within 30 days of your employment. Competitive salary: Starting pay is $17.50 an hour. After training (6-9 months on average) the rate of pay will increase to $19.25. 20 PTO days and 10 paid holidays annually Matching 401K program No parking fees Tuition reimbursement Document Processing Specialist jobs Source: http://jobrealtime.com/jobs/technology/document-processing-specialist_i5491
0 notes