#software development updates
Explore tagged Tumblr posts
Text
Real-World Evidence in Healthcare Decision-Making: Bridging the Gap
Introduction
Healthcare decision-making has traditionally relied on clinical trial data to shape treatment guidelines and policies. However, the emergence of real-world evidence (RWE) is transforming this landscape. This article explores the growing significance of RWE and its integration with clinical research blogs, software development updates, and clinical research latest updates.
Understanding Real-World Evidence
Real-world evidence encompasses data collected outside the controlled environment of clinical trials. It draws from sources like electronic health records, insurance claims, patient registries, wearable devices, and more. This treasure trove of information provides insights into how treatments perform in real-world settings.
The Role of RWE in Healthcare Decision-Making
Enhanced Patient-Centric Care: RWE reflects real patients' experiences, preferences, and outcomes. Clinical research blogs often emphasize the importance of patient-centric care, and RWE allows healthcare decisions to align more closely with individual patient needs.
Treatment Effectiveness: Clinical trials have strict inclusion criteria that may not represent the broader patient population. RWE helps evaluate how treatments perform across diverse patient groups, offering a more comprehensive view of their effectiveness.
Safety Monitoring: RWE aids in post-marketing surveillance by detecting potential adverse events that might not have been apparent during clinical trials. This is a key focus of clinical research latest updates.
Healthcare Economics: Clinical research blogs discuss the rising cost of healthcare. RWE informs cost-effectiveness analyses, enabling policymakers and providers to make informed decisions about resource allocation.
Integration with Clinical Research
Clinical research blogs, software development updates, and clinical research latest updates play pivotal roles in harnessing the potential of RWE:
Data Integration: RWE involves the management of vast and diverse datasets. Software development updates focus on creating tools that facilitate data integration and analysis, ensuring that RWE is usable and meaningful.
Data Quality: Ensuring the quality and reliability of real-world data is a constant challenge. Clinical research blogs often explore best practices for data quality assurance.
Advanced Analytics: The power of RWE lies in its analysis. Software development updates frequently introduce cutting-edge analytical techniques that help uncover meaningful insights from RWE.
Regulatory Compliance: RWE is becoming increasingly vital in regulatory decisions. Clinical research latest updates keep stakeholders informed about evolving regulatory requirements related to RWE.
Challenges and Future Directions
While RWE holds great promise, several challenges must be addressed:
Data Privacy and Security: Clinical research blogs discuss the importance of patient data privacy. Striking a balance between data access for research and protecting patient privacy is crucial.
Standardization: RWE comes from diverse sources, often lacking standardized formats. Efforts are ongoing to standardize data to ensure consistency and comparability.
Bias Mitigation: RWE can be prone to various biases. Methods to identify and correct biases are continually evolving, as discussed in clinical research latest updates.
Interoperability: Seamless integration of different data sources remains a challenge. Software development updates are focused on enhancing interoperability solutions.
Conclusion
Real-world evidence is reshaping healthcare decision-making, aligning it more closely with the needs and experiences of patients. As RWE gains prominence, its integration with clinical research blogs, software development updates, and clinical research latest updates becomes crucial for harnessing its full potential.
This evolving landscape offers a new era of healthcare decision-making, where treatment guidelines and policies are not solely based on controlled clinical trial data but also on the rich tapestry of real-world patient experiences. As challenges are overcome, RWE will continue to bridge the gap between research and practice, ultimately leading to more informed, patient-centered healthcare decisions.

0 notes
Text
Pharmacovigilance: Safeguarding Public Health Through Surveillance
Introduction
Pharmacovigilance is a vital component of healthcare systems worldwide, dedicated to monitoring the safety of medicinal products. This article explores the multifaceted role of pharmacovigilance in safeguarding public health through rigorous surveillance. We will delve into how pharmacovigilance helps identify, assess, and prevent adverse drug reactions (ADRs) while highlighting the importance of clinical research blogs, software development updates, and staying informed about clinical research latest updates in this critical field.
Understanding Pharmacovigilance
Pharmacovigilance, often referred to as drug safety surveillance, is the science and activity related to the detection, assessment, understanding, and prevention of ADRs and other medication-related problems. Its primary goal is to ensure that medicines on the market are safe and that the benefits outweigh the risks.
The Importance of Pharmacovigilance
Early Detection of ADRs: Pharmacovigilance serves as an early warning system for identifying previously unknown ADRs. By collecting and analyzing data from various sources, including healthcare professionals and patients, it can detect safety signals that may not have emerged during clinical trials.
Assessment of Risk-Benefit Profile: Through in-depth evaluation of ADR reports, pharmacovigilance experts assess the risk-benefit profile of medications. This analysis informs regulatory decisions, label updates, and even withdrawal of drugs if the risks are deemed excessive.
Preventing Harm: Pharmacovigilance plays a crucial role in preventing harm to patients. Timely identification of ADRs can lead to changes in medication usage guidelines, preventing unnecessary suffering and even fatalities.
Continuous Monitoring: Medications' safety profiles can evolve over time as more people use them. Pharmacovigilance ensures that this continuous monitoring takes place, adapting to emerging risks or new patient populations.
Clinical Research Blogs: A Knowledge Resource
Clinical research blogs are invaluable in disseminating information about pharmacovigilance:
Educational Content: These blogs provide educational content that explains the importance of pharmacovigilance in simple terms. This helps the public and healthcare professionals understand its significance.
Case Studies: Blogs often feature real-world case studies illustrating the impact of pharmacovigilance. These stories underscore the necessity of monitoring and the tangible benefits of early detection.
Regulatory Updates: Clinical research blogs keep readers informed about regulatory changes related to pharmacovigilance. This includes updates on reporting requirements and regulatory decisions based on ADR findings.
Best Practices: Blogs offer insights into best practices for pharmacovigilance reporting, encouraging healthcare professionals to actively participate in the process.
Software Development Updates: Enhancing Pharmacovigilance
The role of software in pharmacovigilance is indispensable:
Data Management: Robust software solutions are essential for managing the vast amount of data generated by ADR reports. These systems help streamline data collection, storage, and retrieval.
Signal Detection: Advanced software employs sophisticated algorithms to detect potential safety signals from the data. These signals are then investigated further to determine their clinical relevance.
Reporting Efficiency: Software tools simplify the reporting of ADRs to regulatory agencies. This streamlines the process, ensuring that essential information reaches those who need it promptly.
Data Visualization: Modern software enables the visualization of complex pharmacovigilance data, making it easier for experts to identify trends and patterns.
Staying Informed About Clinical Research Latest Updates
Keeping abreast of clinical research latest updates is pivotal for pharmacovigilance experts:
Emerging Risks: Staying informed helps professionals identify emerging risks associated with specific medications. This knowledge can lead to proactive safety measures.
Regulatory Changes: Regulatory agencies may revise guidelines and reporting requirements. Staying current ensures compliance and the dissemination of accurate information.
Technological Advancements: Pharmacovigilance software and data analysis techniques evolve. Being aware of these advancements can enhance pharmacovigilance practices.
Conclusion
Pharmacovigilance is the cornerstone of patient safety in healthcare. By systematically monitoring the safety of medications, it prevents harm, supports regulatory decisions, and ensures that the public can have confidence in the drugs they use. The role of clinical research blogs, software development updates, and staying informed about clinical research latest updates is pivotal in strengthening pharmacovigilance efforts. Through these channels, knowledge is shared, best practices are promoted, and emerging risks are addressed, ultimately contributing to safer and more effective healthcare worldwide. In an era of rapidly advancing medical science, pharmacovigilance remains a steadfast guardian of public health.
0 notes
Text
Decentralized Clinical Trials: Reshaping the Research Landscape
Decentralized Clinical Trials: Pioneering a New Era in Medical Research
In this article, we explore the paradigm shift brought about by decentralized clinical trials, illuminating how they are reshaping the research landscape. Additionally, we underscore the vital role of ethical considerations in clinical trials and emphasize the importance of staying informed through clinical research blogs, software development updates, and the latest trends in clinical research.
Decentralized Clinical Trials: A Game Changer
Enhanced Accessibility: Decentralized trials break down geographical barriers, allowing a more diverse and inclusive participant pool.
Patient-Centric: By enabling remote participation, these trials prioritize the convenience and comfort of patients, potentially improving recruitment and retention rates.
Real-World Insights: Decentralized trials provide a more accurate representation of how treatments work in everyday life, enhancing the relevance of research findings.
Ethical Considerations in Decentralized Clinical Trials
Informed Consent: Ensuring participants fully comprehend the remote trial process and associated risks is paramount, emphasizing the need for robust informed consent procedures.
Data Security: Protecting participants' data in remote settings is crucial to maintain trust and meet ethical standards.
Monitoring and Oversight: Ethical considerations mandate efficient remote monitoring and oversight mechanisms to guarantee data quality and participant safety.
Benefits of Ethical Considerations in Clinical Trials
Participant Trust: Ethical practices foster trust between researchers and participants, encouraging more individuals to engage in clinical trials.
Data Reliability: Ethical trials produce high-quality, reliable data, which is essential for making informed healthcare decisions.
Compliance and Reputation: Adherence to ethical guidelines safeguards against legal and reputational risks for research institutions and sponsors.
Preparing for the Future of Clinical Research
To prepare for the evolving landscape of clinical research, consider these steps:
Clinical Research Blogs: Stay updated on the latest trends and discussions in decentralized clinical trials through clinical research blogs.
Software Development Updates: Monitor software developments that enhance the security and efficiency of decentralized trial platforms.
Clinical Research Latest Updates: Continuously educate yourself about the latest innovations in decentralized trial methodologies and ethical considerations.
In conclusion, decentralized clinical trials are revolutionizing medical research by making it more accessible, patient-centric, and reflective of real-world scenarios. Ethical considerations are the cornerstone of responsible research, ensuring transparency, safety, and trust. By actively engaging with clinical research blogs, software development updates, and the latest clinical research trends, individuals can contribute to the ethical and impactful transformation of clinical trials, ultimately advancing healthcare for all.
0 notes
Text
Pediatric Clinical Trials: Bridging the Gap for Young Patients
In the ever-evolving landscape of clinical research blogs, software development updates, and clinical research latest updates, one crucial area of focus is often overlooked: pediatric clinical trials. These trials are not only essential for advancing children's healthcare but also demand ethical considerations to ensure their safety and well-being. In this blog post, we delve into the significance of pediatric clinical trials and the role of ethical considerations in bridging the gap for young patients.
1. Tailored Pediatric Treatments
Pediatric clinical trials provide a platform for developing treatments specifically tailored to children's unique physiological and medical needs. Ethical considerations are paramount in these trials, as they guide researchers to prioritize children's safety and welfare. In clinical research blogs, the emphasis on ethical practices ensures that young patients receive treatments designed with their well-being in mind, offering hope for improved health outcomes.
2. Advancements in Rare Pediatric Diseases
Many rare diseases predominantly affect children, and pediatric clinical trials offer a lifeline to those affected. Ethical considerations underscore the importance of researching and developing therapies for these rare conditions. In clinical research latest updates, these trials shine a light on the often-overlooked areas of medicine, fostering hope for affected families and driving innovation in the field.
3. Ethical Framework for Informed Consent
In pediatric clinical trials, informed consent takes center stage. Ethical considerations mandate that parents and guardians fully understand the potential risks and benefits of enrolling their child in a trial. This ensures that participation is voluntary and based on comprehensive information. In clinical research blogs and software development updates, this ethical framework provides a foundation of trust between researchers, families, and young patients, creating a safer and more transparent research environment.
4. Future-Proofing Children's Healthcare
Pediatric clinical trials not only provide immediate benefits but also future-proof children's healthcare. By conducting ethical research in young populations, we gather data that can inform healthcare practices for generations to come. In clinical research latest updates, the legacy of ethical pediatric trials is a commitment to advancing children's health and ensuring that their unique medical needs are addressed in a responsible and compassionate manner.
Conclusion
Pediatric clinical trials are a vital component of advancing children's healthcare, addressing rare diseases, and creating a brighter future for young patients. However, conducting these trials ethically is paramount to ensure the safety and well-being of children involved. In the world of clinical research blogs, ethical considerations serve as a guiding light, ensuring that research prioritizes the welfare of the most vulnerable among us. Through ethical practices, we can bridge the gap in pediatric healthcare, offering hope and improved outcomes for young patients now and in generations to come.
0 notes
Text
Voice Interface Development Trends: Shaping a Vocal Future
In today's fast-paced world of technology, where clinical research blogs and software development updates fuel innovation, Voice Interface Development is making remarkable strides. Similar to the precision and efficiency seen in clinical research latest updates, voice interfaces are transforming how we interact with digital devices, offering convenience and accessibility like never before.
Understanding Voice Interface Development
Voice Interface Development involves creating digital interfaces that users can interact with using voice commands. These interfaces rely on technologies like Natural Language Processing (NLP) and speech recognition to interpret and respond to spoken language. Just as clinical research blogs disseminate knowledge efficiently, voice interfaces aim to streamline digital interactions.
Key Trends in Voice Interface Development
Conversational AI: Conversational AI, powered by NLP, enables more natural and human-like interactions with devices and applications. It's akin to the fluid communication seen in clinical research updates.
Voice-Activated Assistants: Voice-activated virtual assistants like Siri, Alexa, and Google Assistant are becoming ubiquitous, offering a wide range of functionalities, much like the multifaceted tools used in clinical research blogs.
Voice Commerce: With the rise of e-commerce, voice commerce is on the rise, allowing users to shop and make transactions using voice commands, aligning with the convenience-driven practices in clinical research latest updates.
Multi-Modal Interfaces: These interfaces combine voice with other modes of interaction, such as touch or gestures, offering users flexibility and choice, similar to the multi-dimensional approaches in clinical research blogs and software development updates.
Advantages of Voice Interfaces
Accessibility: Voice interfaces make digital technology more accessible to individuals with disabilities, much like the inclusivity practices in clinical research latest updates.
Convenience: Users can perform tasks hands-free, enhancing convenience in various applications, similar to streamlining processes in clinical research blogs and software development updates.
Efficiency: Voice interfaces can speed up tasks like data entry and information retrieval, aligning with the efficiency-driven practices in clinical research latest updates.
Personalization: Advanced voice interfaces can learn user preferences and adapt responses, similar to personalized treatments and solutions in clinical research blogs.
Common Use Cases for Voice Interfaces
Smart Home Control: Users can control smart home devices like thermostats and lights using voice commands, aligning with the automation and optimization practices in clinical research updates.
Voice Search: Voice search is gaining popularity, allowing users to find information online quickly, similar to data retrieval in clinical research blogs.
In-Car Systems: Voice interfaces in cars enable drivers to stay connected and control various functions without taking their hands off the wheel, echoing the importance of safety in clinical research latest updates.
Challenges and Considerations
Privacy Concerns: Collecting and processing voice data can raise privacy issues, much like the ethical considerations in clinical research blogs.
Accuracy: Voice recognition technology is still evolving, and accuracy can vary, aligning with the need for precise data in clinical research latest updates.
Integration: Integrating voice interfaces into existing applications and systems can be complex, echoing the challenges of technology integration in clinical research blogs and software development updates.
Implementing Voice Interfaces in Your Projects
User-Centric Approach: Begin with a deep understanding of your target audience and their needs, similar to user-centric approaches in clinical research latest updates.
Testing and Validation: Thoroughly test voice interfaces to ensure they provide accurate responses and meet user expectations, much like validation and verification processes in clinical research and software development projects.
Privacy Compliance: Implement robust privacy and security measures to protect user data, aligning with data protection strategies in clinical research blogs.
In Conclusion
Voice Interface Development is reshaping how we interact with technology, much like the transformative technologies discussed in clinical research blogs and software development updates. By offering accessibility, convenience, and efficiency, voice interfaces are becoming integral parts of our daily lives. Just as clinical research's latest updates aim to provide efficient and accessible healthcare solutions, voice interfaces are ensuring that technology is more inclusive and user-friendly than ever before, bringing the power of voice to the forefront of digital innovation.
0 notes
Text
Revolutionizing Software Delivery: Unveiling the Impact of DevOps
In the ever-evolving realm of software development, the adoption of DevOps practices has ushered in a new era of efficiency and collaboration. As clinical research blogs and software development updates continue to shape the tech landscape, the influence of DevOps on software delivery is becoming increasingly significant. This paradigm shift is not only relevant to clinical research companies in Pune but also holds relevance across industries worldwide.
Understanding DevOps
DevOps, a portmanteau of "development" and "operations," represents a cultural and technical approach to software development that emphasizes collaboration, communication, and integration between development and IT operations teams. The goal is to automate and streamline the software development and deployment lifecycle, allowing for faster delivery of high-quality applications.
Synergy with Clinical Research
Clinical research, a domain that heavily relies on data analysis, software solutions, and compliance, finds itself at the crossroads of technology and healthcare. As clinical research blogs share the latest insights and clinical research latest updates drive innovation, the principles of DevOps can optimize software delivery within clinical research companies. This alignment ensures that applications for data analysis, management, and compliance are efficient, reliable, and adaptable.
Advantages of DevOps in Clinical Research
The adoption of DevOps practices within clinical research companies brings forth several advantages. Firstly, the integration of development and operations teams streamlines communication and reduces bottlenecks. Clinical research involves various stakeholders, from researchers to regulatory experts, and DevOps facilitates cross-functional collaboration, resulting in software that meets diverse requirements.
Enhanced Efficiency and Speed
DevOps places a strong emphasis on automation, reducing manual interventions and accelerating processes. In clinical research, where time-sensitive breakthroughs can shape medical advancements, the ability to deliver software updates and solutions rapidly is crucial. DevOps ensures that development, testing, and deployment are seamlessly interconnected, enabling clinical research companies in Pune to respond swiftly to evolving demands.
Quality Assurance and Compliance
In the context of clinical research, maintaining data integrity and compliance with regulations is of utmost importance. DevOps practices, such as continuous integration and continuous deployment (CI/CD), promote rigorous testing throughout the development lifecycle. This approach ensures that software solutions are thoroughly vetted, minimizing the chances of errors and enhancing the reliability of clinical research applications.
Agility in a Changing Landscape
As clinical research blogs shed light on the latest trends and breakthroughs, the adaptability of software solutions becomes paramount. DevOps provides the framework for creating applications that can quickly evolve in response to new insights and changing requirements. This agility aligns well with the dynamic nature of clinical research and empowers companies to stay at the forefront of innovation.
Pune's Role in the DevOps Revolution
Pune, renowned for its thriving clinical research companies and technological prowess, is a natural hub for embracing DevOps practices. With clinical research latest updates shaping the city's research landscape, the integration of DevOps can elevate Pune's clinical research industry to new heights. The amalgamation of clinical research and technology showcases Pune's potential as a breeding ground for innovation.
The Path Forward: A Collaborative Future
As clinical research blogs and software development updates continue to highlight the importance of innovation and collaboration, DevOps stands as a guiding principle that harmonizes these objectives. The ongoing evolution of both technology and healthcare underscores the need for efficient software delivery methods. DevOps bridges the gap between these domains, creating a symbiotic relationship that propels clinical research companies in Pune and beyond into a collaborative, technology-driven future.
In Conclusion
DevOps is reshaping the landscape of software delivery, and its impact extends far beyond clinical research companies. By embracing its principles, clinical research companies in Pune can harness the power of collaboration, automation, and efficiency to drive progress. As the synergy between DevOps and clinical research continues to evolve, it paves the way for a harmonious coexistence between technology and healthcare, where innovation thrives, and solutions are delivered with agility and precision.
0 notes
Text
The Basics of Clinical Trials: What You Need to Know
Clinical trials are the lifeblood of medical progress, driving innovation and shaping the future of healthcare. They are meticulously designed research studies that assess the safety and efficacy of new medical treatments, therapies, or interventions. In this article, we will delve into the fundamental aspects of clinical trials, offering insights from clinical research blogs, software development updates, and clinical research latest updates. Whether you're a prospective participant, a curious individual, or a healthcare enthusiast, understanding the basics of clinical trials is essential.
1. Why Clinical Trials Matter
Clinical trials are the bridge between scientific discovery and patient care. They play a pivotal role in:
Advancing Medicine: Clinical trials lead to the development of new drugs, treatments, and therapies.
Evidence-Based Care: They provide the evidence needed to determine whether a new approach to treatment is safe and effective.
Patient-Centered Care: Clinical trials focus on improving patient outcomes and enhancing the quality of healthcare.
2. The Phases of Clinical Trials
Clinical trials progress through several phases, each serving a specific purpose:
Phase I: These trials involve a small number of healthy volunteers and aim to assess safety, dosage, and side effects.
Phase II: In this phase, the focus shifts to a larger group of patients to evaluate the treatment's effectiveness and further assess safety.
Phase III: These trials involve an even larger patient population and compare the new treatment to existing standard treatments.
Phase IV: After approval, Phase IV trials monitor the treatment's long-term safety and effectiveness in real-world settings.
3. Informed Consent
Informed consent is a cornerstone of ethical clinical research. Before participating in a clinical trial, individuals must be provided with comprehensive information about the study, including its purpose, risks, benefits, and alternatives. Consent is entirely voluntary, and participants have the right to withdraw at any time without consequences.
4. The Role of Placebos
In some clinical trials, placebos are used to maintain scientific rigor. A placebo is a substance with no therapeutic effect. They are essential for ensuring that the treatment's benefits are not influenced by psychological factors and that any observed improvements are genuinely due to the treatment being tested.
5. The Importance of Control Groups
Control groups are a critical component of clinical trials. These groups receive either the current standard treatment or a placebo, allowing researchers to compare the new treatment's effectiveness. Control groups are essential for establishing the treatment's true impact.
6. Randomization and Blinding
Randomization involves randomly assigning participants to either the treatment or control group, reducing the risk of bias. Blinding, on the other hand, can be single-blind (participants are unaware) or double-blind (both participants and researchers are unaware of group assignments). These practices ensure the objectivity of trial results.
7. Ethical Oversight
Clinical trials are subject to rigorous ethical oversight. Institutional Review Boards (IRBs) or Ethics Committees review and approve trial protocols to ensure that the study is conducted safely and ethically. Protecting participants' rights and safety is of paramount importance.
8. The Inclusion and Exclusion Criteria
Clinical trials have specific inclusion and exclusion criteria to ensure that the participants are the right fit for the study. Inclusion criteria define who can participate, while exclusion criteria identify those who cannot. These criteria are crucial for patient safety and the validity of the trial results.
9. Monitoring and Data Collection
Clinical trials are closely monitored to ensure patient safety and data accuracy. Researchers collect data on the treatment's efficacy, side effects, and any adverse events. This data is meticulously analyzed to draw meaningful conclusions about the treatment's impact.
10. The Potential Risks and Benefits
Participating in a clinical trial comes with potential risks and benefits. Risks may include side effects, inconveniences, or the possibility that the treatment is ineffective. Benefits may include access to cutting-edge treatments, close medical monitoring, and the satisfaction of contributing to medical science.
11. Conclusion: The Road to Medical Advancement
Clinical trials are the foundation upon which medical progress is built. They are the culmination of years of scientific research and have the potential to improve patient care and save lives. By understanding the basics of clinical trials, you not only become an informed patient but also appreciate the dedication of researchers, the vigilance of ethical oversight, and the promise of a healthier future. Clinical research blogs, software development updates, clinical research companies in Pune, and medical professionals are all working tirelessly to ensure that clinical trials continue to drive innovation and bring hope to patients around the world.
0 notes
Text
The amount of people not buying a Switch 2 on launch gives me hope that there won't be any shortage/supply issues when it releases.
#nintendo#jacob blogs#everyones out here talking about how this console is going to sell over a million units the day it's released#like how?? if everyone's gonna basically boycott this next generation??#i mean i guess it would be worth it if it means the console drops $100 in the first year#idrc#im not a business owner#but ninty nickle and diming us over consoles hardware and software#is wayy more permissible in my eyes#compared to the dozens of game developers and publishers#that push for predatory microtransactions in unfinished games#and yearly releases of games with updated cosmetics and unecessary season passes
9 notes
·
View notes
Text
weekly reminder to update your software
i get it - it's annoying, it takes time and maybe there are changes (be it design or functionality... looking at you, discord) to the software you don't like. i get it. hell, these are things that annoy me too.
BUT it's also a security thing. old software and operating systems (windows or ios for example) is a safety risk. especially when you have it linked to accounts with your personal or financial information. cybercrime is no joke!! and your PC, laptop, smartphone or tablet can be virus-infected without you even noticing.
how can i be safe? i hear you asking. well, built-in security in operating systems is oftentimes enough - you don't need bloatware like avast or mcaffee or whatever they're called. they use unnecessary resources and can make older hardware slow.
but - and here is the catch - these built-in security measures (and even 3rd party software) are only safe when they're also kept UP TO DATE. windows defender needs to be updated with windows updates. so do them now, if you have been putting them up.
#ugh sorry. needed to get this out of my system#bc i talked to a friend a few days ago and they said their partner hates to update a software bc the developers changed the design and i -#- wanted to pull my own eyes out#i'm a IT specialist in training and i work at a place with high secruity levels#you have no idea how hard and labour intensive it is to keep data safe these days#it's ridiculous#addi.txt
5 notes
·
View notes
Text
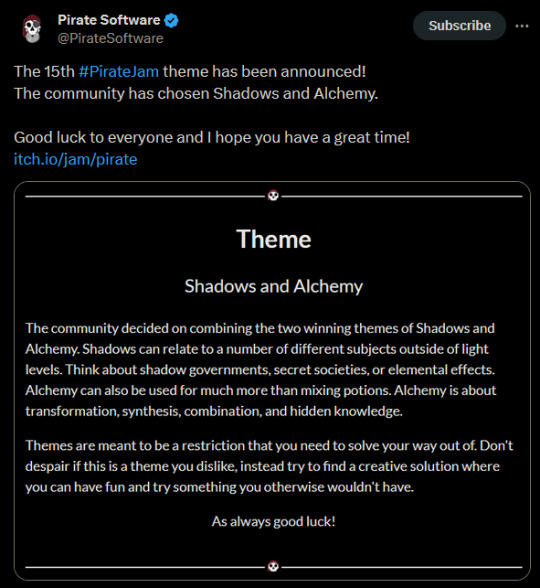
I'll be making a game for this game jam. ^^ It will include Zecharias but I'm not sure where the game would be in the canon yet. Look forward to seeing some teasers and drawings for the game. :D
I will work on the existing demos after this jam, and after the Murderboy jam.
#let me remind you vn#yandere#male yandere#visual novel#game dev#game development#indie game#game jam#pirate software#pirate software game jam#update
13 notes
·
View notes
Text
#hire shopify experts#shopify custom sections#shopify theme customization#shopify development services#shopify development company#Software development services#Software Development company#Hire software developers#Open Source Implementation#Ecommerce Factory#ERP Development#Refining Existing Applications#Conversion Rate Optimization#Don’t Just Update#Upgrade!#Startup Solutions#ADA Compliant Website#Online Marketplace Development#Theme Development#Platform Migration
2 notes
·
View notes
Text
Someone did NOT test before pushing to main.
#seriously though how did this happen#like#someone has to have fucked up super bad to not test if it KILLS WINDOWS before pushing an update to WINDOWS COMPUTERS.#software development#how tho#global outage
2 notes
·
View notes
Text
I love developing websites that no one but me will ever see <3
#i talk#I am making a website to help me keep track of story details and its so fun to develop#I'm making character pages rn and I need to start writing stuff about the world but that meands I have to make up names for everything#I am using an actual note compiling software called Obsidian for my writing but I am just jotting down ideas as they come to me#and I am not cleaning them up in case there's anything I will need later down the line#so to avoid trawling through dead and half finished ideas everything is getting refined into tha webbed site yippee!!#unfortunately this does mean I'd have to update the site whenever I make major changes to the characters and story#but I am soon getting to the point of story planning where big sweeping changes won't really be coming into play
3 notes
·
View notes
Text

Artificial Intelligence (AI):
Definition: AI refers to the broader concept of machines being able to carry out tasks in a way that we would consider “smart.” It encompasses various technologies that allow machines to mimic human intelligence.
Scope: Includes machine learning, natural language processing, robotics, and more.
Machine Learning (ML):
Definition: ML is a subset of AI that focuses on the ability of machines to learn from data, identify patterns, and make decisions with minimal human intervention.
Scope: Primarily involves algorithms and statistical models that enable computers to perform specific tasks without using explicit instructions.
#coding#programming#tech updates#cool tech#software#developer#tech#artificial intelligence#machine learning#ai/ml
2 notes
·
View notes
Note
im nosy what do you do for work beloved? <3

#answered#I haven’t used this pic in soooo long meu deus!#I work at a tech consulting company. sometimes im consulting…sometimes im software developing👩🏽💻 putergenius isn’t just my name that’s lif#haven’t done a life update in forever. not in school (phew…the drama.) working full time at a place I interned at#and planning a big move soon…it’s getting real over here…im GROWN. lmaoo
2 notes
·
View notes
Text
Welcome to InnovateHub TechTalk: Unleashing the Tech Frontier
Greetings, fellow tech enthusiasts, and welcome to the inaugural edition of InnovateHub TechTalk! I am Lucas Redford, your guide on this thrilling expedition into the boundless realms of technology. With each keystroke and pixel, we'll embark on a journey to unravel the mysteries, embrace the innovations, and discuss the trends that shape our digital world.
Charting New Horizons:
In the age of rapid technological advancement, it's impossible to ignore the transformative impact that technology has on our lives. From the moment we wake up to the time we rest our heads, technology surrounds us, empowering, entertaining, and evolving at an unprecedented pace.
Our Quest:
At InnovateHub TechTalk, our mission is simple yet profound: to ignite your curiosity and keep you informed about the dynamic world of technology. Whether you're a seasoned coder, a budding entrepreneur, a digital artist, or just someone intrigued by the possibilities, this platform is your haven.
What Awaits You:
As we embark on this voyage together, here's a glimpse of what you can expect from InnovateHub TechTalk:
Innovative Spotlights: Venture into the heart of innovation as we showcase groundbreaking technologies and inventions that are reshaping industries and society.
Tech Chats with Experts: Join me in engaging conversations with thought leaders, industry experts, and visionaries who are shaping the course of technology.
CodeCraft Corner: Whether you're a coding novice or a seasoned pro, our CodeCraft Corner will be your source for coding tips, projects, and insights to elevate your programming prowess.
FutureTalk: Delve into the crystal ball as we discuss emerging trends, speculative tech, and the potential future landscapes that await us.
Be a Part of the Conversation:
InnovateHub TechTalk is not just a blog; it's a community. Your thoughts, questions, and insights are the catalysts that will drive our discussions forward. Don't hesitate to jump into the comment section, share your perspectives, and connect with fellow tech aficionados.
With great excitement, I invite you to journey with me through the digital maze, the electronic wonderland, and the data-driven universe that defines our age. Together, we'll decode complexities, celebrate achievements, and ponder the limitless possibilities that lie ahead.
As we dive into the sea of 1s and 0s, remember that innovation knows no bounds, and at InnovateHub TechTalk, we're poised to explore it all.
Welcome aboard, tech voyagers!
Lucas Redford
Founder and Chief Explorer, InnovateHub TechTalk
#Technology Trends#Innovation Insights#Tech Enthusiasts#Digital Exploration#Future of Tech#Coding Tips#Emerging Technologies#Tech Conversations#User Experience Design#Digital Transformation#Artificial Intelligence#Internet of Things#Cybersecurity#Software Development#Gadgets and Devices#Web Technology#Virtual Reality#Blockchain#Tech News Updates#Data Privacy#Cloud Computing#Tech Industry#Online Innovation#Science and Technology#Technology Community#Tech Insights#Cutting-Edge Tech#Digital Evolution#Innovation Spotlight#Technology Exploration
2 notes
·
View notes