#studycoordinator
Explore tagged Tumblr posts
Text










📊 Efficient Data Management in Clinical Trials!
Accurate and secure data management is essential for successful clinical trials. It ensures: ✅ Reliable study results through organized data handling. ✅ Compliance with regulatory standards like GCP & CFR 21 Part 11. ✅ Proper validation, storage, and reporting of trial data.
Key Data Management Steps: 📌 Collecting data via Case Report Forms (CRFs). 📌 Using Electronic Data Capture (EDC) systems. 📌 Regular validation to maintain accuracy. 📌 Secure storage and submission for regulatory approval.
Challenges? 🔹 Ensuring data security & privacy. 🔹 Managing large datasets from multiple sites. 🔹 Preventing entry errors & maintaining consistency.
🌐 Visit: ccrps.org
🔑 Takeaway: Mastering data management ensures trial success & regulatory compliance!
📢 Enhance your expertise—streamline clinical trial data efficiently!
#clinicaldata#clinicaltrials#clinicalresearch#datamanagement#EDC#CTMS#pharmaresearch#medicaldata#clinicalstudies#researchethics#dataintegrity#CRO#regulatorycompliance#GCP#datasecurity#clinicaltrialsuccess#medicalresearch#DrugDevelopment#biostatistics#healthcareinnovation#trialdata#studycoordinator#medicaltechnology#healthtech#researchexcellence#clinicaldatamanagement#PharmaLife
0 notes
Text
Get access to 150 GCP findings from this website https://www.gcpfinding.com/gcp-finding/
These findings will help: Clinical Research Associate – Ensuring GCP findings are captured during site monitoring visit
Clinical Trial Coordinator - To avoid GCP findings while completing study documents
Clinical Investigator- Keeping investigative site compliant with GCP requirements
#clinicalresearch #cra #clinicaltrials #clinicalresearchtraining #clinicalstudies #clinicalresearchcoordinator #clinicalresearchassociate #clinicalresearchcareer #clinicalresearchprofessionals #crc #clinicaltrialsguru #studycoordinator

2 notes
·
View notes
Text
Clinical Research Coordinator Certification
Clinical Research Coordinator Certification
Guide to Becoming a Clinical Research Coordinator
What is a Clinical Research Coordinator
Clinical research coordinators are usually supervised by clinical research managers. Their main task is to administer the clinical trials. Primary responsibilities normally include administering questionnaires, informing the participants about the objectives of the study, collecting data, and managing all the trials. They also have to adhere to all trail standards that have been set and also participate in recruitment of the subjects. Clinical research coordinators also have to engage with the subjects so that they can explain the things that are expected during the trial and also find out if they have any concerns. This means that a clinical research coordinator needs communicative and interpersonal skills.
The responsibilities
Clinical research coordinators have many responsibilities that need to be carried out to the best of their ability.
1. Maintaining records of all studies as per the guidelines
2. Sticking to all ethical standards
3. Sticking to all the regulatory standards set
4. Administering questionnaires
5. Managing the budget dedicated to the research
6. Overseeing the running of the trials as smoothly as possible
7. Understanding and engaging with the subjects so as to know all issues
8. Making sure that all equipment and supplies that are necessary for the success of the study are
working and in stock
9. Participating in the recruitment efforts of the participants
10. Working with the laboratories so as to share findings
Requirements
The qualifications of a clinical research coordinator usually depend on your locations or employer. In most cases, for you to access clinical research coordinator jobs you should:
Have an associate nursing degree or any related field
Experience of two years within the healthcare industry
Analytical mindset
Be attentive to detail
Have interpersonal skills which are exceptional
Be ready to continue learning even without being prompted to do so
Great skills in organizing
Have great verbal and written communication skills
2. Roles of a Clinical Research Coordinator
Roles And Duties Of Clinical Trial Coordinator
A clinical research coordinator or a clinical trial coordinator is in charge of managing clinical research at clinical research sites as per the protocol, ICH- GCP, and some other regulatory needs. The function of a study administrator in clinical research is very crucial. All the sites of a clinical trial have one or more study organizers relying on the workload at the research site. The clinical trial coordinator certification is required in such research sites.
The site-level clinical trial can roughly be classified into three stages:
Before starting the clinical trial: In this stage, the study organizer has to collect and finish questionnaires collected from different sponsors and various CROs. Clinical research coordinators also have to collect data from the principal investigator and dispatch them back to the people who had communicated to this site for the study. The sponsors choose locations based on responses submitted in the feasibility questionnaire and finally direct pre-site assortment visits to finalize the plots taking part in the conduct. The sites conducting the research work must have clinical trial coordinator certification to carry on with investigator meetings. These investigator meetings are held at the international level or national level. Before beginning with the trial, the clinical research conductors are usually busy in the submission of various documents to the ethics committee. Besides, subject diaries, investigators CVs, clinical research agreement, signature page of protocol, clinical trial coordinator certification, indemnification letter, insurance certificate, blank CRF's, various study logs, etc. are also required to be submitted. A clinical study organizer plays a vital role in these processes. The site-level can then initiate the clinical trial after getting permission from the ethics committee.
Conduct during the clinical trial: The clinical research conductor must have a good knowledge regarding the study protocol and should have a good idea of exclusion and inclusion criteria by the time clinical research is conducted. The study coordinator needs to have consent informed from the subject represented to do by the Principal Investigators. The study administrators need to collect subject pre-medical records, clinical trial coordinator certification, and according to investigation protocol, the person will have to manage issues programmed visits. Before visiting the item randomly, the clinical research coordinator must check exclusion and inclusion criteria, and after that, the entitled subjects are to be enrolled. After completing all the procedures of the visit, coordinators need to present information in the form of a case report. The clinical study coordinator should keep all relevant data and files up to date. In the next step, the CRC has to calculate the study drug accountability. Interactive web response system (IWRS) and Interactive voice response system (IVRS) are to be conducted to record the subject visit as per study requirements. The investigational product is the most crucial part of a clinical trial, and the study organizer has to reserve the same in the right condition and further maintain the essential temperature logs. Clinical trial coordinator certification is also kept up to record. Coordinators should collect all primary data such as stop and start date, severity, administration route, and consumption of medicine by the subject in case of serious adverse events (SAE’s) or adverse events (AE’s). The study coordinators must look after all central and local lab reports all over the clinical trial and take PI signatures on the news to keep it as a document that the PI reviewed them.
After closing the clinical trial: The study coordinators should check all the documents and clinical trial coordinator certification before closing the clinical trial and there on update all the documents. The Clinical research associate or CRA will review and verify all the materials on the day of closure of the clinical trial. The clinical research coordinator, after that, helps in archiving all the documents at the site, when the verification by CRA is complete. This site further maintains all the study associated records for about 15- 20 years.
So it is clear that the clinical research coordinator plays a crucial role at the site level in running a clinical trial, forming clinical trial coordinator certification, and acts as a connection in between EC, investigator site and the sponsor. All clinical trials have the approval of the present Institutional Review Board. Medical care and decisions are the duty of physicians, dentists, and health care professionals. Every individual working in a clinical trial is certified by experience, training, and education.
3. Education Requirements of a Clinical Research Coordinator
Steps to Become a Clinical Research Coordinator
A clinical research coordinator is someone who works under a primary investigator during a clinical trial. Other than the imminent amount of laboratory work that this job entails, you also have to have both managerial and communication skills. In a sense, Clinical Research coordinators have a vital role to play in all kinds of medical studies. Day to day clinical trial activities are not only supported and facilitated by Clinical Research coordinator, but they are also significant in making sure that all facets of the work happen smoothly. On the managerial aspect, the Clinical Research Coordinator is responsible for maintaining and gaining compliance from the federation state as well as the institution under which the clinical trial takes place.
If you want to become a Clinical Research coordinator, these are the steps that you will have to take:
Step 1: High School Graduation
To prepare for becoming a Clinical Research Coordinator, students must study subjects like physics, chemistry, biology, mathematics, statistics, and communication. Knowledge in these fields will help them prepare for the bachelor's degree that lies ahead.
Step 2: Bachelor's Degree
After this, a student must prepare for becoming a Clinical Research coordinator by looking for universities and colleges that offer bachelor's degrees in health sciences. These clinical research coordinator training programs provide student tools and methods that they will require in working in a laboratory as well as those needed for formulating medicines and running clinical trials and studies. You can choose from on campus as well as online courses that focus not only on the administrative but also on scientific methodologies required for Clinical Research and provide you with a solid base that will help you and becoming a Clinical Research coordinator. A typical course constitutes of such subjects as healthcare literature, bioethics, healthcare laws and regulations, and the basics of clinical management and medical studies. A bachelor's degree will help you to get entry-level positions in institutions as well as research laboratories. If you want a more robust salary and more professional Outlook, you can also go for additional education or internships.
Step 3: Work Experience
At this stage in your career, it is best to harvest a year or two gathering professional work experience and research laboratory and clinical trials to get hands-on experience. This is very important in getting National certification as a Clinical Research coordinator.
Step 4: Online Graduate Certificate
After this, a student usually may obtain an online graduate certificate in clinical research administration. These certificate courses typically extend for 18-credit-hour, which will go a long way in order to speak for the dedication and will strengthen your career. These courses will allow you to get the managerial and communication skills required by a Clinical Research coordinator. These credit hours can easily be transferred to the master's cause if both are under the same University. Such a course will help you to focus your attention on subjects like research design, site management, data management, statistics, teamwork and communication, participant safety considerations, biohazards, and more. They also offer additional courses in financial management in clinical trials, ethical Research, and other subjects included in drug and medical practices.
Step 5: Master's Degree ( Online or In site)
Obtaining a master's degree speaks volumes about your dedication and becoming a Clinical Research Coordinator and, at the same time, will advance your knowledge even further. If you are not comfortable with a traditional onsite course, you can also obtain your master's degree online from the plethora of colleges that offer this degree. These courses will develop your skills in subjects like fundamental regulatory affairs Clinical Research monitoring. At the end of this program, you will have all the knowledge that is typically useful in getting a certification as a Clinical Research Coordinator and also help you in becoming great at your job.
Step 6: Get the Certificate
You must get certification in order to become a certified Clinical Research coordinator. You can get your certificate from CCRPS, the trail of your certificate that is valid internationally. Students have to pass an exam to get the certificate.
4. Experience Requirements of a Clinical Research Coordinator
How to Become A Clinical Research Coordinator
There are so many clinical trials that are conducted these days. The job of a clinical research coordinator is to look after these trials, collect data from participants, and record the results. Not only do you need to get training to work as a Clinical Research Coordinator, but you also need to gain experience in this industry. In order to achieve your goals, you have to start early. Here are the steps that you need to take in order to fulfill your dream.
Obtain A Bachelors Degree or Associates Degree in Science
It is a long road to become a clinical research coordinator. There are certain degrees that you need to complete before you can become one. The first degree that you need to obtain is your training as a Clinical Research Coordinator. There is usually four-year-long degree course that will help you to get well versed in the field of human research. Apart from that, you will be able to know about the rules and ethics that you need to follow while conducting clinical research. While the initial years will help you to know more about the prerequisites, the later years will help you to focus on the main topic. Some of the common courses that you need to study are Microbiology, Human Anatomy, Organic Chemistry, and Microbiology. Apart from these Science subjects, you will also get to learn about scientific writing, Literature, data management, product development, and regulations.
Know More About What A Clinical Research Coordinator Does
There are employers who have specific requirements for hiring a clinical research coordinator. Some employers prefer individuals who, apart from completing a your training as a Clinical research coordinator, have a nursing background. In case you do, you can undertake a certificate program that will help you to move forward in your career. This program will help you to know more about research methodologies and statistics that you will need in a clinical research trial.
Gain Some Experience as A Clinical Research Assistant
This field requires individuals who have a good amount of experience under their wing. It is a requirement to take up clinical research related projects and internships in order to complete your training as a Clinical Research Coordinator
Even after this, you have to gain more experience in order to gain the role of a coordinator. Only then will your associates will be able to work underneath you. Hence, you have to work as an associate first in order to be a coordinator.
Certification for Clinical Research Coordinator
It is important for you to get certified in order to take your career as a clinical research coordinator forward. You will need evidence to show your work for a full-time research institution. There is a separate organisation where you have to apply to get your certification. Apart from your educational qualifications, you have to sit for an exam in order to become a certified clinical researcher. There are better chances of you becoming a coordinator when you are certified. Otherwise, you will be stuck as an associate forever. CCRPS provides this course online.
Advantages Of Becoming A Clinical Research Coordinator
There are several reasons why a lot of people want to complete their training as a clinical research coordinator. You will be able to earn a lot in case you decide to venture into this industry. In case you want to give more time to your family, you can do so while working. There are several clinical research coordinators who work from home. You can come up with your own schedule in case you are working part-time. Moreover, there is tremendous demand for clinical research these days. It helps to improve the quality of life, and hence, there are a lot of jobs available for you as an expert in clinical research. Not only doctors, but you will also be able to earn a handsome salary and benefits as a clinical research coordinator. You will get to work in clinics and hospitals properly.
What are you waiting for? There are a lot of students who are interested in this field these days. Hence, you have to act fast and start working on your dreams as soon as possible. It is not an easy route, but it is definitely rewarding. All you need is to meet the basic requirements, and you are good to go!
5. Clinical Research Coordinator Salary
HOW MUCH IS A CLINICAL RESEARCH COORDINATOR’S SALARY?
Clinical research coordinators have the responsibility to oversee all daily activities of staff that are given the responsibility to conduct research. They have to do their work alongside principal investigators so as to make a determination of whether the study being conducted is viable or not. They also have to work out the budget at hand. They also need to make a review of all research protocols, making sure that all the test subjects know the protocols. They help in carrying out the research.
The Salary
When a clinical research coordinator is experienced, pay has quite an encouraging trend. If a clinical research coordinator is a new entrant that has experience of less than five years, they earn a salary of around 43,000 dollars. This total is an average, according to salaries that were given by different anonymous users.
Usually, the compensation includes overtime payment, bonus payments, and even tips. In the mid of their careers, clinical research coordinators have experience of 5 to 10 years and they can expect a compensation of around 51,000 dollars. This is also an average that is based on different salaries submitted.
There is also the category of clinical research coordinators who have over 20 years’ experience in the field. For these professionals, the compensation is an average of 62,000 dollars and this is based on salaries submitted.
Clinical research coordinator salary by location
It is important to appreciate that different locations have different salary variations. There are some areas that pay more than the average, while others may pay less. If this is a career that you are willing to pursue, it is best that you find out as much as you can about the salary expected within your area. This information can help you negotiate for the best compensation for services rendered.
6. Free online clinical research coordinator training
Free courses for CRC training are available is specific subjects
The clinical researcher Coordinator is one of the most reputed posts in medical science. They are guided by the principal investigators whose job is helping with the managing, designing and conducting the inquiry of the clinical at a very high level. The clinical research coordinator must facilitate, organize, and also they must support in the regular activities in the clinical trials. Nowadays, there is some clinical research coordinator certification online; these online courses are introduced for the students for helping to get their certifications done early. The clinical trial manager works for the concurrence with the organization, institution, and sponsor to lead them via finances, personal issues, and compliance. There are many clinical research coordinator certifications online, which you must check out for the clinical researcher exams.
Online Courses For Clinical Research Coordinators
You can search for free courses on the following subjects to get less specific training that a CRC may benefit from. Overall, getting accredited certification from a trusted body such as CCRPS is the best option in showing employers your competency for coordinator roles.
Medical Ethics Course: - In this course, the professors introduce the values which are employed by the medical field of ethics, which include autonomy, non-maleficence, dignity, justice, and honesty. The students consider how to develop a framework for creating ethical decisions that were informed by laws and values. They discuss the ethical issues, which are a favorable cost-benefit ratio and the participant selection and also about confidentiality. It is one of the best clinical research coordinator certifications online courses which will help you to crack the exams.
Clinical Research Principles Course: - This clinical research coordinator certifications online course will provide a new overview of the process of clinical research, development, and the history of it. Here the students can quickly learn the management skill, which includes practice guidelines of clinics. They will make you learn about the roles of the research team member and the phase’s development of the clinical trials.
Medical Terminology Course: - This clinical research coordinator certification online courses cover up the standard medical terminology which uses up in the clinical research field. In this course, students learn about vocabulary, which is relevant for autonomy and the diagnostics and surgery, and many more. This knowledge will help you to enhance the effectiveness of managing the data and assuring you of quality control.
Health Information And The Law Course: - In this clinical research coordinator certifications online course, the students need to understand the overview of guidelines and the regulations which aim for protecting the human subjects, and it ensures about the research integrity. They will help you to learn about the obligations of the groups of regulatory and also the sponsors’ researches and also about principal investigators. Here students will even know to discuss the types of violations that are constituted by the scientific misconduct and also consider the consequences.
Introduction To The Health Records Courses: - This clinical research coordinator certifications online do explore the confidentially and also about the purpose. Here the students can quickly learn the management skill, which includes practice guidelines of clinics. This knowledge will help you to enhance the effectiveness of managing the data and assuring you of quality control. It will consist of how to use medical records for planning the caring of the patient and how to use laws for these records. Here patients are learned how to use the clinical records in an accurate way.
Study Of Financing Course: - This clinical research coordinator certifications online will provide you an overview of funding management of the study. Here students get to learn how to submit the proposals and how to create them. This course compiles the financial regulations, which consider the indirect and the direct cost.
Medical Device History: - In this clinical research coordinator certifications online course, it will provide you to explore the current trends which are affecting the research, which also implies the history of devices in medical. Students will be provided with a lot of case studies that can quickly help them to consider from the business, medical, and ethical and also in case of legal perspective.
These are a few free clinical research coordinator certifications online courses that you can go for preparing for your clinical research certifications. These courses can help you a lot in every perspective of exams, but the best option is to take a course that can provide you with in-depth accredited training.
7. How to get Clinical Research Coordinator Experience without Prior Experience?
How To Get Clinical Coordinator Certification?
The clinical coordinator or CRC is also known as the clinical trial manager. They play an essential role in the studies of medicine in all types. They work typically under the personal investigator who has a charge of managing and conducting the clinical trial in a higher level. CRC's job is to facilitate, support, and to organize the daily trial activities of the clinical. Getting a clinical coordinator certification online will take a lengthy procedure. There is some kind of the CRC at very different phases of various educational achievements. Let's look at the steps which you will need to get the clinical coordinator certification.
Step 1: Must Be Graduate From High School (4 Years)
For preparing for the clinical coordinator certification, you must begin with the high school courses like biology, chemistry, and physics, also with the maths and communications. This will found your form of the foundation which will be pursued for the college studies, which also be the four-year studies.
Step 2: - Obtain A Bachelor or Associates Degree in Science-related fields
When perusing the universities and the colleges, you must focus on the courses which offer you a bachelor's degree in health sciences in terms of clinical research administration. The students should dedicate them all the things to obtain this degree and must concentrate only on this for pursing the degree for full time. These programs generally provide you the clinical research of the professional with the tools which they will need to do the developing their medical sciences and conducting their trials and also studies. Both on the campus and the online programs on the clinical administrations focus on clinical research for placing to protect the human subjects.
The bachelor's degree can give you an entry-level position in the clinical organization or any institution. It will also help you with the exiting clinicians with the advance, which is in the current jobs. The students who wish to pursue more opportunities in terms of responsibility and also the salary.
Step 3: - Online Clinical Research Coordinator Certification
This online 120 module course is done for increasing the salary or obtaining your first 2 years of experience as a CRC. This is a 1-6 week course that is self paced and online. This course includes the clinical study of coordinators and as well as the data manager of the clinical, research assistants of the social science, and also about the clinical tech laboratories. Here you can explore many types of concepts of science and designing research, data management, professionalism, and leadership. After this course, the students will get some handsome salary and also be eligible for higher-grade jobs in clinical research. This will benefit you is pursuing the clinical coordinator certification.
Step 4: Apply and Get Real world Experience
Get The Certification
Now it's the time to hold the accreditation of your hard work, and it determines your success. There are some categories of certifications that will help you to understand which category you fall in.
In order to qualify for credentialing examination; a professional must:
Category 1: - It has two years of experience on a full-time basis as the clinical researcher professional.
Category 2: - It holds a degree of the clinical researcher of the full-year experience.
Category 3: - It holds an undergraduate degree or a graduate certificate in clinical research.
Conclusion: These are the certifications method, or you can call as the steps which you will need to clear out the clinical coordinator certification. You must check down the levels, and you must study sincerely as per the requirements. You have to reach for your dreams.
#clinical research coordinator#clinical research coordinator salary#clinical research coordinator jobs#clinical research coordinator certification#clinical research coordinator job description#clinical research coordinator resume#clinical research coordinator cover letter#clinical research coordinator interview questions#ucsf clinical research coordinator#clinical research coordinator training#entry level clinical research coordinator salary#how much does a clinical research coordinator make#how to become a clinical research coordinator#what does a clinical research coordinator do#assistant clinical research coordinator#average clinical research coordinator salary#clinical research coordinator jobs near me#clinical research coordinator salary nyc#senior clinical research coordinator salary#what is a clinical research coordinator#clinical research coordinator career path#clinical research coordinator career progression#clinical research coordinator course#clinical research coordinator memorial sloan kettering#clinical research coordinator salary florida#clinical research regulatory coordinator#lead clinical research coordinator salary#oncology clinical research coordinator#synexus clinical research coordinator#assistant clinical research coordinator salary stanford
0 notes
Text
How to Get a Clinical Research Coordinator (CRC) / Study Coordinator (SC) Entry Level Job
How to Get a #ClinicalResearchCoordinator (CRC) / #StudyCoordinator (SC) Entry Level Job in #ClinicalResearch
How to Get a Study Coordinator (SC) Job If you are looking to get into the clinical research field/ career, a “clinical research coordinator” (CRC) or “study coordinator” (SC) job can be one of the easiest ways to get your first clinical research job. If you search on “study coordinator” or “clinical research coordinator” you will find many different qualifying job descriptions and pay ranges.…
View On WordPress
#clinical research coordinator#clinical research coordinator job#CRC#how to get a clinical research job#how to get into clinical research field#study coordinator
0 notes
Text










⚠️ Handling Adverse Events in Clinical Trials!
Adverse events (AEs) are any unexpected medical occurrences during a clinical trial. They range from mild reactions to life-threatening complications and require strict monitoring and documentation.
🔎 Types of Adverse Events: ✔️ Mild – Minimal discomfort, no intervention. ✔️ Moderate – Requires treatment or observation. ✔️ Severe – Life-threatening or long-term impact. ✔️ Serious (SAE) – Hospitalization or fatal outcomes.
📋 Why Reporting Matters: 📌 Investigators must log all events. 📌 Sponsors analyze data for patterns. 📌 Regulators review SAEs for trial safety.
✅ Key Takeaway: Proper adverse event handling ensures participant safety and trial integrity. Stay vigilant, report accurately, and maintain ethical standards!
🌐 Visit: ccrps.org
#clinicaltrials#adverseevents#clinicalresearch#GCP#patientsafety#researchethics#pharmaresearch#clinicaltrialmonitoring#DrugSafety#healthcareinnovation#clinicalstudy#clinicaltrialawareness#medicalethics#SAE#pharmaceuticalindustry#medicalresearch#clinicalinvestigator#trialsafety#studycoordinator#bioethics#IRB#clinicaltrialdata#healthcarestudies#trustinresearch#pharmalife#clinicaltrialmanagement
0 notes
Text










📢 Understanding the Power of Informed Consent in Clinical Trials!
Informed consent is the foundation of ethical clinical research. It ensures participants: ✅ Fully understand the trial’s purpose, risks, and benefits. ✅ Can voluntarily decide without pressure. ✅ Have their rights and confidentiality protected.
Key Components of a Consent Form: 📌 Study purpose, duration, and procedures. 📌 Potential risks and benefits. 📌 Participant rights, including withdrawal at any time.
Challenges & Best Practices: 🔹 Language barriers? Use clear, translated materials. 🔹 Technical jargon? Simplify with visual aids. 🔹 Vulnerable populations? Apply ethical safeguards.
🔑 Takeaway: Ethical consent builds trust and enhances trial credibility.
🌐 Visit: ccrps.org
📢 Master the consent process & elevate your research standards!
#clinicaltrials#informedconsent#clinicalresearch#researchethics#GCP#IRB#medicalethics#patientrights#bioethics#pharmaresearch#healthcareinnovation#clinicalstudies#clinicaltrialsrecruitment#researchparticipants#consentprocess#ethicalresearch#medtech#studycoordinator#pharmalife#medicalresearch#DrugDevelopment#clinicalinvestigator#healthstudies#volunteerforscience#trustinresearch#clinicaltrialawareness
0 notes
Text










📑 Crafting Effective Clinical Trial Protocols A clinical trial protocol is the backbone of any study, ensuring consistency, compliance, and participant safety. It outlines study objectives, eligibility criteria, methodology, and data analysis plans. Regulatory bodies and ethics committees review and approve protocols to maintain trial integrity. 🔹 Why Are Protocols Important? ✅ Align research teams with clear guidelines ✅ Ensure ethical & regulatory compliance ✅ Protect participant safety & data integrity ✅ Support reproducibility & reliable results 💡 Challenges & Solutions 🚧 Complex designs? Use participant-centric approaches 🚧 Ambiguities? Engage multidisciplinary teams for clarity 🚧 Delayed approvals? Streamline amendment reviews 📌 Key Takeaway: A well-structured protocol enhances trial success! 🌐 Visit: ccrps.org
#clinicalresearch#clinicaltrialsmatter#clinicaltrialprotocol#researchethics#GCP#medicalresearch#researchstudy#regulatoryaffairs#pharmaresearch#drugdevelopment#patientsafety#IRB#ethicscommittee#goodclinicalpractice#ClinicalDevelopment#ResearchExcellence#FDAApproved#ClinicalTrialDesign#studycoordinator#CRO#clinicaloperations#BioPharma#clinicalinnovation#ClinicalStudy#medicalscience#healthcareresearch
0 notes
Text










🧑⚕️ Regulatory Foundations in Clinical Research 🏥
Clinical research thrives on strong regulations that protect participants and ensure trial integrity. 📜 Here’s what you need to know:
🔹 What Are Regulatory Foundations? ✔️ Guidelines that govern clinical trials ✔️ Ensure safety, efficacy, and compliance ✔️ Support drug approvals worldwide
🔹 Key Regulatory Bodies: 🇺🇸 FDA – U.S. drug and device oversight 🇪🇺 EMA – EU-wide medicine regulation 🇬🇧 MHRA – UK medicines & devices authority 🌍 ICH – Harmonizing global trial standards
🔹 Why Compliance Matters: ✅ Ensures trial consistency & safety ✅ Simplifies global research collaboration ✅ Builds public trust in new treatments
🌐 Visit: ccrps.org
📌 Stay informed, follow protocols, and master regulations to excel in clinical research! 💡
#clinicalresearch#GCP#clinicaltrials#FDA#EMA#MHRA#ICHGCP#regulatoryaffairs#CFR#drugdevelopment#medicalresearchmatters#IRB#researchethics#Pharma#biotech#clinicalstudy#goodclinicalpractice#researchcompliance#healthcareinnovation#clinicaltrialregulations#studycoordinator#medicalscience#regulatorycompliance#clinicaldevelopment#researchtraining#trialmanagement
0 notes
Text










Master the Core Principles of ICH GCP
The International Council for Harmonisation Good Clinical Practice (ICH GCP) is the global standard ensuring ethical, high-quality clinical research. It protects trial subjects, ensures data integrity, and sets guidelines adopted by regulatory bodies worldwide.
🔹 Key Principles ✔ Protect participants' rights and safety ✔ Maintain transparency with proper documentation ✔ Conduct trials with qualified professionals
🔹 Standard Practices ✔ Follow a detailed trial protocol ✔ Obtain informed consent ✔ Monitor and report adverse events
🔹 Why It Matters Mastering ICH GCP builds trust, enhances research credibility, and opens global career opportunities.
🌐 Visit: ccrps.org
📌 Start your journey today!
#clinicalresearch#ICHGCP#clinicaltrials#goodclinicalpractice#researchethics#pharmacist#biotechnology#CRO#clinicaltrialresearch#ICHGuidelines#MedicalResearch#ClinicalDataManagement#FDA#GCPTraining#clinicaltrialmanagement#LifeSciences#GCPCompliance#clinicalstudies#TrialMonitoring#HealthcareResearch#clinicaloperations#drugdevelopment#regulatoryaffairs#clinicalinvestigator#medicalscience#studycoordinator
0 notes
Text



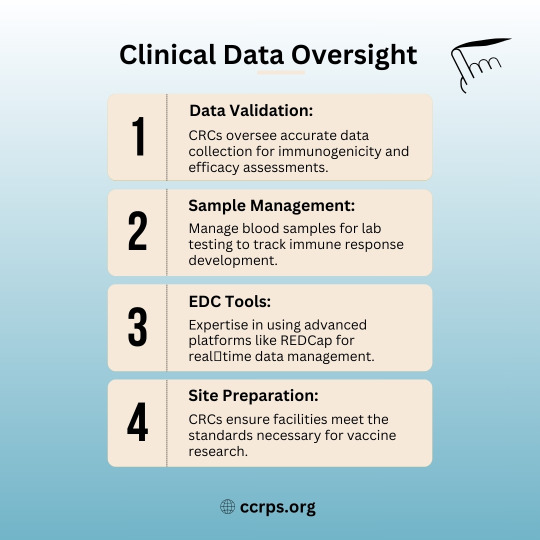






🩺 Why Clinical Research Coordinators (CRCs) Are Essential for Vaccine Trials! 💉
Vaccine development wouldn’t be possible without skilled CRCs! CRCs are the backbone of successful trials, from patient recruitment to ensuring regulatory compliance. 🚀
✅ Patient Recruitment – Finding diverse participants to ensure accurate data.
✅ Retention Mastery – Keeping participants engaged throughout multi-dose trials.
✅ Safety Reporting – Monitoring and documenting adverse reactions.
✅ Data Validation – Ensuring accuracy in immunogenicity & efficacy assessments.
✅ Community Outreach – Building trust to enhance participation.
Without CRCs, life-saving vaccines wouldn’t reach the public as quickly! ��
🌐 Visit: ccrps.org
👉 Want to play a crucial role in vaccine development? Enroll in the CCRPS Advanced Training Program today! Click the link in bio to get started! 🔗
#clinicalresearch #CRCs #vaccinedevelopment #clinicaltrials #researchcoordinator #pharmacareers #FDA #medicalresearch #healthcareheroes #vaccineresearch #patientcare #clinicaljobs #GCP #medicalscience #covid19research #lifesciences #HealthInnovation #ResearchMatters #ClinicalTrialCoordinator #PublicHealth #datamanagement #regulatoryaffairs #healthcareprofessionals #medicalcareers #pharmaceuticalresearch #studycoordinator #medicalethics
0 notes