#BioPharma
Explore tagged Tumblr posts
Text
Boost your sales performance with Healthark Insights' tailored enablement services, including CRM setup, lead generation, and strategic outreach.
0 notes
Text
#ATMPs#AdvancedTherapies#CellTherapy#GeneTherapy#TissueEngineering#RegenerativeMedicine#Biotech#Pharma#GMP#ClinicalTrials#Biopharma#GxPCompliance#LifeSciences#InnovativeMedicines#ATMPManufacturing
0 notes
Text
Biobetters Market Overview: Exploring Key Innovations Driving Next-Generation Biologic Therapies and Global Growth Trends
The Biobetters Market Overview highlights a transformative shift in the pharmaceutical industry, where enhanced biologic drugs—biobetters—are outperforming first-generation biologics in efficacy, safety, and patient compliance. Unlike biosimilars, biobetters offer modified structures and improved mechanisms of action, creating a lucrative path for both innovation and long-term healthcare solutions.

Understanding Biobetters: Beyond Biosimilars
Biobetters, also referred to as biosuperiors, are next-generation biologics developed to improve upon existing biologic therapies. These drugs share a common origin with their predecessors but are chemically modified to deliver superior clinical outcomes. The improvements range from prolonged half-life and reduced immunogenicity to simplified administration and lower side-effect profiles.
Unlike biosimilars, which are essentially generic versions of biologics, biobetters aim for therapeutic advancement. This strategic distinction offers pharmaceutical companies stronger patent protection and premium pricing opportunities, making biobetters a crucial focus in long-term R&D investment.
Key Innovations Transforming the Biobetters Market
1. Protein Engineering and Molecular Design
The cornerstone of biobetter innovation lies in protein engineering technologies. Through site-directed mutagenesis, glycoengineering, and PEGylation, researchers are creating biologics with improved solubility, stability, and bioavailability. These innovations not only enhance therapeutic efficacy but also allow for less frequent dosing—greatly benefiting patients with chronic conditions like rheumatoid arthritis and diabetes.
2. Advanced Drug Delivery Mechanisms
Another major leap in biobetters stems from novel drug delivery platforms. Innovations such as subcutaneous injections, auto-injectors, and depot formulations are simplifying the administration process for patients, replacing intravenous infusions with more patient-friendly alternatives. This not only boosts adherence but also reduces the burden on healthcare infrastructure.
3. AI and Computational Biology
Artificial intelligence and machine learning are playing a pivotal role in designing more effective biobetters. AI-driven models help predict protein folding, simulate drug-receptor interactions, and identify optimal structural modifications. These computational advances are dramatically shortening development cycles and increasing the probability of clinical success.
Market Drivers Fueling Global Expansion
Several factors are propelling the biobetters market forward:
Patent Expiry of First-Generation Biologics: As key biologics lose exclusivity, the door opens for biobetters to enter with improved therapeutic profiles.
Rising Demand for Chronic Disease Management: Biobetters offer sustained relief and higher compliance in managing long-term diseases like cancer, autoimmune disorders, and metabolic conditions.
Regulatory Encouragement: Regulatory agencies like the FDA and EMA are facilitating fast-track approvals for innovative therapies with proven improvements over existing treatments.
Investment in Biotech R&D: Major pharmaceutical companies are ramping up their investment in biobetter pipelines to strengthen portfolios and achieve market differentiation.
Regional Insights and Growth Dynamics
North America
North America currently leads the global biobetters market, owing to strong R&D ecosystems, robust healthcare infrastructure, and favorable reimbursement policies. The presence of major industry players and rising consumer awareness also contribute to the region's dominance.
Europe
Europe follows closely, supported by aggressive adoption of biologic treatments and initiatives promoting biosimilar and biobetter alternatives to reduce healthcare costs. Countries like Germany and the UK are hotspots for clinical trials and regulatory innovation.
Asia-Pacific
Asia-Pacific is emerging as a high-growth region, particularly due to rising healthcare expenditure, expanding patient populations, and increasing biopharma collaborations in countries like China, India, and South Korea. Government support for biotech innovation is further catalyzing regional market expansion.
Competitive Landscape and Strategic Moves
The biobetters market is moderately consolidated, with several key players leveraging their biologics expertise to develop next-gen therapies. Major strategies include:
Strategic Collaborations: Companies are forming partnerships with research institutes and biotech firms to access cutting-edge technologies and speed up drug development.
Portfolio Diversification: Many players are expanding their biobetter offerings across multiple therapeutic areas to capture broader market segments.
Licensing Agreements: Out-licensing and in-licensing of biobetter candidates are becoming common to reduce risk and share development costs.
Market Challenges and Future Outlook
Despite its promising outlook, the biobetters market faces some hurdles:
High Development Costs: Developing a biobetter is both time-consuming and expensive, requiring intensive research and large-scale clinical trials.
Regulatory Complexity: Although agencies support innovation, navigating the nuanced regulatory pathways for approval remains a challenge.
Market Penetration: Gaining market share against entrenched biologics or biosimilars can be difficult without clear clinical superiority or cost advantages.
Looking ahead, the future of the biobetters market appears robust. Advances in genomics, personalized medicine, and biologic delivery will continue to push the envelope. As healthcare systems worldwide emphasize value-based care, biobetters will become increasingly central to treatment strategies across therapeutic domains.
#Biobetters#BiologicsInnovation#PharmaceuticalTrends#HealthcareInnovation#ProteinEngineering#NextGenTherapies#DrugDevelopment#Biopharma#GlobalHealthcare#ChronicDiseaseManagement#BiotechTrends#FutureOfMedicine
0 notes
Text
The Tough Path to Industrial-Grade MSC Exosomes – And How We’re Fixing It
If you’re in #biotech or #CellTherapy, you know: taking MSC-derived exosomes (MSC-Exos) from small-scale research to mass production is hard.
The big challenges? 🔸 Scalability – Flasks & static culture don’t cut it for large batches. 🔸 Batch differences – Slight changes = inconsistent exosome quality. 🔸 Storage risks – Exosomes are fragile; keeping them stable is tricky.
Our new approach: ✔ Microcarriers → Better MSC adhesion & growth ✔ Single-use stirred bioreactors → Smooth scale-up ✔ Harvesting from supernatant → Higher yields, easier purification
This could be a game-changer for #RegenerativeMedicine and #DrugDelivery! What do you think—would this work for your research?
1 note
·
View note
Text
Investment Opportunities in the Acute Myeloid Leukemia Treatment Market
The global acute myeloid leukemia treatment market size was estimated at USD 3.47 billion in 2024 and is projected to grow at a CAGR of 10.6% from 2025 to 2030. Rising incidences of acute myeloid leukemia and increasing need for advanced therapeutics are attributed to factors such as genetic mutations, unhealthy lifestyles, continued exposure to hazardous chemicals such as benzene, and radiation exposure. Moreover, the increasing geriatric population base and growing unmet healthcare needs are expected to boost market growth further.

Advancements in targeted therapies have been at the forefront of the progress in Acute Myeloid Leukemia (AML) treatment so far. In recent years, there has been substantial development in therapies such as BCL2 inhibitors, IDH inhibitors, and FLT3 inhibitors. These therapies provide personalized treatment options tailored to specific genetic mutations, resulting in improved outcomes for patients. For instance, in July 2023, the FDA approved quizartinib (Vanflyta) for newly diagnosed FLT3-ITD positive acute myeloid leukemia in the U.S., enhancing treatment options alongside standard chemotherapy.
Combinations such as venetoclax with azacitidine or other hypomethylating agents have shown promising results in enhancing survival rates for AML patients. Additional research interest focuses on incorporating venetoclax into the traditional 7+3 chemotherapy regimen, which aims to maximize treatment efficacy. Such innovative approaches indicate a growing commitment to developing multifaceted treatment strategies that promise to improve patient outcomes.
Moreover, the exploration of immunotherapies, including CAR-T cell therapies, represents a transformative trend in AML treatment. These therapies are designed to bolster the immune system’s response to cancer cells, thus providing new hope for patients who have limited options. The advancements in the understanding of genetic mutations associated with AML have further contributed to personalized treatment strategies, such as targeting specific mutations such as KMT2A and NPM1 with menin inhibitors.
For More Details or Sample Copy please visit link @: Acute Myeloid Leukemia Treatment Market
Key Acute Myeloid Leukemia Treatment Company Insights
Some key companies operating in the market include Astellas Pharma Inc.; Bristol-Myers Squibb Company; DAIICHI SANKYO COMPANY, LIMITED; among others. The market is characterized by a dynamic competitive landscape, with pharmaceutical giants, biotechnology innovators, diagnostics companies, and healthcare institutions vying for market share-pharmaceuticals who are launching innovative targeted therapies, immunotherapies, and personalized treatments.
#AcuteMyeloidLeukemia#AML#LeukemiaTreatment#CancerTherapeutics#OncologyDrugs#HematologyMarket#TargetedTherapies#ChemotherapyAlternatives#PrecisionOncology#Biopharma#PharmaMarketTrends#HealthcareInvestments#GlobalOncologyMarket
0 notes
Text
#AntibodyProduction#Biopharma#MonoclonalAntibodies#Biotechnology#TherapeuticAntibodies#AntibodyEngineering#BiologicDrugs#LifeSciences#AntibodyResearch#BiotechInnovation#PharmaceuticalR&D#ProteinExpression#Bioprocessing
0 notes
Text










📑 Crafting Effective Clinical Trial Protocols A clinical trial protocol is the backbone of any study, ensuring consistency, compliance, and participant safety. It outlines study objectives, eligibility criteria, methodology, and data analysis plans. Regulatory bodies and ethics committees review and approve protocols to maintain trial integrity. 🔹 Why Are Protocols Important? ✅ Align research teams with clear guidelines ✅ Ensure ethical & regulatory compliance ✅ Protect participant safety & data integrity ✅ Support reproducibility & reliable results 💡 Challenges & Solutions 🚧 Complex designs? Use participant-centric approaches 🚧 Ambiguities? Engage multidisciplinary teams for clarity 🚧 Delayed approvals? Streamline amendment reviews 📌 Key Takeaway: A well-structured protocol enhances trial success! 🌐 Visit: ccrps.org
#clinicalresearch#clinicaltrialsmatter#clinicaltrialprotocol#researchethics#GCP#medicalresearch#researchstudy#regulatoryaffairs#pharmaresearch#drugdevelopment#patientsafety#IRB#ethicscommittee#goodclinicalpractice#ClinicalDevelopment#ResearchExcellence#FDAApproved#ClinicalTrialDesign#studycoordinator#CRO#clinicaloperations#BioPharma#clinicalinnovation#ClinicalStudy#medicalscience#healthcareresearch
0 notes
Text

🌟 Exciting News from Eurofins Biopharma Product Testing(BPT), India! 🌟 We are proud to announce that our BPT Lab has successfully met and exceeded the stringent requirements for accreditation under the QAI Testing Laboratory Accreditation Programme as per the globally recognized 𝐈𝐒𝐎 / 𝐈𝐄𝐂 17025:2017 standard! This #accreditation reaffirms our commitment to providing high-quality and reliable testing services. It signifies that our processes meet the international standards for accuracy, consistency, and technical proficiency. A huge 𝐜𝐨𝐧𝐠𝐫𝐚𝐭𝐮𝐥𝐚𝐭𝐢𝐨𝐧𝐬 𝐭𝐨 𝐨𝐮𝐫 𝐢𝐧𝐜𝐫𝐞𝐝𝐢𝐛𝐥𝐞 𝐭𝐞𝐚𝐦 at the lab for their hard work, dedication, and commitment to excellence, which has made this achievement possible! We look forward to continuing to deliver world-class testing services and further strengthening our partnerships with our valued clients.
0 notes
Text
nologhttps://vidgastech.com/about-us/
0 notes
Text

France Alopecia (Hair Loss) Therapeutics Market Analysis
The France Alopecia (Hair Loss) Therapeutics Market was valued at US $226 Mn in 2022, and is predicted to grow at (CAGR) of 6.5% from 2023 to 2030, to US $373 Mn by 2030. The key drivers of this industry include the surge in the prevalence of alopecia (hair loss), increased disposable income, lifestyle changes, and others. The industry is primarily dominated by players such as Johnson & Johnson, Eli Lilly, Merck, Pfizer, Cipla, Sun Pharmaceuticals, and iRestore, among others.
#FranceAlopeciaMarket#HairLossTherapeutics#AlopeciaTreatment#PharmaGrowth#CAGR#HealthcareTrends#JohnsonAndJohnson#EliLilly#Merck#Pfizer#Cipla#SunPharma#iRestore#HairCareInnovation#Biopharma#MarketForecast#Dermatology#LifestyleImpact#MedicalResearch
0 notes
Text

🧠 Driving Innovation in Alzheimer's Research with Real-World Imaging Data The significance of Alzheimer’s disease research cannot be overstated due to its widespread impact and complex nature. By harnessing imaging datasets, we can drive meaningful advancements in Alzheimer’s research as it aids in biomarker discovery and patient identification for treatment. At Segmed, we provide high-quality fit-for-purpose regulatory grade real-world imaging datasets (RWiD) and multimodal longitudinal datasets that empower pharmaceutical and biotech companies to develop life-saving treatments in neurology, oncology, and cardiology. To know more about how our data sets and solutions have supported real-world evidence generation and identification of suitable patient cohorts for treatment, visit our site: segmed.ai/solutions
#oncology#Neurology#Cardiology#Biopharma#Alzheimer’s disease#Segmed#RealWorldData#RWE#RealWorldEvidence
0 notes
Text

Track 31: INTELLECTUAL PROPERTY IN PHARMACEUTICALS CALL FOR ABSTRACTS – From Ideas to Impact: Present Your Work! Be a part of the 15th Digital Pharmaceutical Innovations Exhibition & Congress! 📍 Join us May 14-16, 2025, in San Francisco or virtually! 🔗 Submit your abstract here: https://pharmacy.utilitarianconferences.com/submit-abstract 📅 Submission Deadline: January 31, 2025 #PharmaInnovation #IPinPharma #DrugDevelopment #BiotechResearch #PharmaTech #PatentLaw #PharmaIP #HealthcareInnovation #ClinicalResearch #RegulatoryScience #PharmaceuticalPatents #LifeSciences #MedTech #DrugDiscovery #Biopharma #HealthcarePolicy
#PharmaInnovation#IPinPharma#DrugDevelopment#BiotechResearch#PharmaTech#PatentLaw#PharmaIP#HealthcareInnovation#ClinicalResearch#RegulatoryScience#PharmaceuticalPatents#LifeSciences#MedTech#DrugDiscovery#Biopharma#HealthcarePolicy
0 notes
Text
🌟 𝐓𝐫𝐚𝐧𝐬𝐟𝐨𝐫𝐦𝐢𝐧𝐠 𝐂𝐚𝐧𝐜𝐞𝐫 𝐓𝐫𝐞𝐚𝐭𝐦𝐞𝐧𝐭: 𝐓𝐡𝐞 𝐑𝐢𝐬𝐞 𝐨𝐟 𝐀𝐧𝐭𝐢𝐛𝐨𝐝𝐲 𝐃𝐫𝐮𝐠 𝐂𝐨𝐧𝐣𝐮𝐠𝐚𝐭𝐞𝐬 (𝐀𝐃𝐂𝐬) 🌟-IndustryARC™
The Antibody Drug Conjugate Market size is estimated to reach $15275 million by 2030, growing at a CAGR of 14.20% during the forecast period 2024-2030.
👉 𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞
𝐇𝐞𝐫𝐞 𝐚𝐫𝐞 𝐬𝐨𝐦𝐞 𝐤𝐞𝐲 𝐟𝐢𝐧𝐝𝐢𝐧𝐠𝐬 𝐟𝐫𝐨𝐦 𝐭𝐡𝐞 𝐫𝐞𝐩𝐨𝐫𝐭
𝐀𝐝𝐯𝐚𝐧𝐜𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐓𝐚𝐫𝐠𝐞𝐭𝐞𝐝 𝐓𝐡𝐞𝐫𝐚𝐩𝐲: ADCs represent a targeted approach to cancer therapy, allowing for selective delivery of cytotoxic drugs to cancer cells, reducing damage to healthy cells and enhancing treatment effectiveness. This shift towards targeted therapies is a major trend in oncology.
𝐆𝐫𝐨𝐰𝐢𝐧𝐠 𝐏𝐢𝐩𝐞𝐥𝐢𝐧𝐞 𝐨𝐟 𝐀𝐃𝐂𝐬: Pharmaceutical companies are actively developing ADCs, with a growing number of ADC candidates in clinical trials. Increased R&D investments and strategic collaborations are fueling the expansion of ADC pipelines, particularly for solid tumors and hematologic cancers.
𝐈𝐧𝐜𝐫𝐞𝐚𝐬𝐢𝐧𝐠 𝐅𝐃𝐀 𝐀𝐩𝐩𝐫𝐨𝐯𝐚𝐥𝐬 𝐚𝐧𝐝 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐒𝐮����𝐩𝐨𝐫𝐭: Regulatory bodies are accelerating approvals for ADCs due to their effectiveness and safety profile in cancer treatment. Recent approvals of ADCs, such as those targeting breast and bladder cancers, have driven further interest and investment.
𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐢𝐜𝐚𝐥 𝐈𝐦𝐩𝐫𝐨𝐯𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐋𝐢𝐧𝐤𝐞𝐫 𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐲: Innovations in linker technology, which attaches the antibody to the drug payload, are enhancing ADC stability and precision. Advanced linkers improve the therapeutic index, enabling more controlled drug release and minimizing off-target effects.
𝐅𝐨𝐜𝐮𝐬 𝐨𝐧 𝐍𝐨𝐯𝐞𝐥 𝐏𝐚𝐲𝐥𝐨𝐚𝐝𝐬: ADCs are moving beyond traditional cytotoxic agents, incorporating novel payloads such as immune modulators and DNA-damaging agents. These novel payloads expand ADC applications and offer enhanced potency against resistant cancer cells.
𝐄𝐱𝐩𝐚𝐧𝐬𝐢𝐨𝐧 𝐢𝐧𝐭𝐨 𝐍𝐨𝐧-𝐎𝐧𝐜𝐨𝐥𝐨𝐠𝐲 𝐀𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧𝐬: While oncology remains the primary focus, ADCs are increasingly being explored for autoimmune and infectious diseases. This diversification presents new opportunities and broadens the market’s scope beyond cancer.
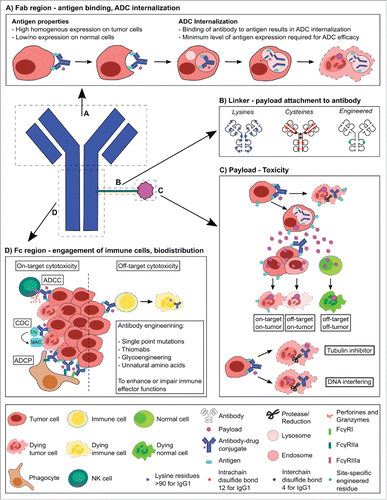
#antibodydrugconjugates#oncology#cancertherapy#targetedtherapy#precisionmedicine#cancerresearch#biotechnology#drugdevelopment#pharma#cancerimmunotherapy#biopharma#noveltherapeutics#tumortargeting#personalizedmedicine
0 notes
Text
BIOCHINA 2025 we are Suzhou from 13th to 15th March and waiting for you. Booth No. SC1-005

1 note
·
View note
Text
The Next Wave of Inflammatory Bowel Disease Treatments in U.S.: Biologics & Beyond
The U.S. inflammatory bowel disease treatment market size is anticipated to reach USD 14.1 billion by 2030 and is projected to grow at a CAGR of 2.5% from 2024 to 2030, according to a new report by Grand View Research, Inc. The rising prevalence of ulcerative colitis and Crohn’s disease across the U.S. is anticipated to drive market growth in the forecast period. A combination of genetic predisposition, immune system dysfunction, environmental factors, gut microbiota imbalance, and lifestyle factors likely influence the development of inflammatory bowel disease (IBD). According to an article published in 2023, inflammatory bowel disease is a relatively common chronic condition affecting >0.7% of Americans and is most prevalent in the Northeastern region. This growing patient population necessitates advanced treatment options, driving the market growth.

U.S. Inflammatory Bowel Disease Treatment Market Report Highlights
Based on type, the Crohn’s disease segment dominated the market in 2023 with the largest revenue share. This large share is due to the requirement for more treatment options and advancements due to the chronic inflammatory nature of Crohn’s disease, which affects the gastrointestinal tract and causes symptoms such as abdominal pain, diarrhea, weight loss, and fatigue.
On the other hand, the ulcerative colitis segment is expected to register the fastest CAGR during the forecast period, owing to its increasing incidence rate.
Based on drug class, the TNF inhibitors segment accounted for the largest market revenue share in 2023 due to their effectiveness in managing the symptoms and reducing inflammation.
Based on route of administration, the injectable segment held the largest market share in 2023. Injectable medications, such as biologics and biosimilars, are designed to target specific proteins or cells involved in the inflammatory process of inflammatory bowel disease.
For More Details or Sample Copy please visit link @: U.S. Inflammatory Bowel Disease Treatment Market Report
The Crohn's & Colitis Foundation works toward enhancing product development to improve the quality of life of patients suffering from inflammatory bowel illnesses. The foundation has launched these ventures, a dedicated funding system to support product-oriented research and development.
In September 2023, Takeda announced the FDA's acceptance of the biologics license application (BLA) for the subcutaneous administration of ENTYVIO (vedolizumab) to treat Crohn’s disease. Regulatory approvals pave the way for new medications, biologics, and devices to enter the market. This can lead to a broader range of treatment options for patients with IBD, potentially improving treatment outcomes and quality of life.
List of Key Companies in U.S. Inflammatory Bowel Disease (IBD) Treatment Market
Biogen
Novartis AG
Eli Lilly and Company
UCB S.A.
Celltrion Inc.
Merck & Co., Inc.
Johnson & Johnson Services, Inc
#InflammatoryBowelDisease#IBDTreatment#CrohnsDisease#UlcerativeColitis#Gastroenterology#ChronicDisease#IBDCare#IBDMarket#USHealthcare#PharmaTrends#MedicalInnovation#HealthcareIndustry#MarketAnalysis#Biopharma#LifeSciences
0 notes
Text
New Drug Modalities – Asrar Qureshi’s Blog Post 1047
#Antibodies#Asrar Qureshi#Biopharma#Biotechnology#Drug Development#Gene Therapy#mAbs#New Modalities#Pharma Veterans#Recombinant#Semaglutide
0 notes