#regulatoryaffairs
Explore tagged Tumblr posts
Text
Pharmaceutical Regulatory Affairs services are important in the successful registration of medicinal products intended for human use with different Health Authorities (HAs). The role of these services is to navigate the dynamic requirements set forth by each HA, ensuring compliance and smooth approval processes.
2 notes
·
View notes
Text
The regulatory landscape is transforming with smarter systems, connected insights, and technology-led processes.
DDReg is aligned with this evolution, ready to support innovation, accelerate submissions, and enable compliance with confidence.
Are you prepared for what’s next in regulatory?
Contact us today: https://tinyurl.com/3tujpe5p
#DDReg #RegulatoryAffairs #DigitalHealth #LifeSciences #RWD #DecentralizedTrials #AIinRegulatory
#regulatoryaffairs#regulatorycompliance#regulatoryconsulting#regulatorysolutions#regulatorystrategies#pharmaregulatoryaffairs#globalregulatoryaffairs#regulatoryservice#regulatoryconsultingfirms#pharmaceutical
0 notes
Text
#ClinicalResearch#CROIndia#ClinicalTrials#Pharmacovigilance#MedicalWriting#RegulatoryAffairs#Biosimilars
1 note
·
View note
Text
Global Regulatory Affairs Services, Regulatory Affairs Consulting

Masuu Global offers comprehensive regulatory affairs services, including white paper prep, gap analysis & dossier management for compliance
0 notes
Text

#UKRegulations#MedicalDevices2025#UKCA#CECompliance#MHRA#SushvinConsultancy#MedicalDeviceConsulting#RegulatoryAffairs
0 notes
Text

Entering the UAE pharmaceutical market requires navigating a highly regulated environment. Herms Global provides expert solutions for Pharmaceutical/medicinal product registration in UAE, helping healthcare and pharmaceutical companies comply with MOHAP and other regulatory authorities.
From dossier preparation and classification to submission and follow-up, our regulatory affairs team ensures every requirement is met to streamline your market access. We support registrations for generic drugs, branded pharmaceuticals, herbal medicines, and more.
💡 Why choose Herms Global?
Extensive knowledge of UAE and GCC pharma laws
Fast, accurate documentation
Reduced risk of compliance failure
Dedicated regulatory consultants
Expand your footprint in the UAE healthcare sector with a trusted regulatory partner by your side.
#PharmaceuticalRegistration#MedicinalProductsUAE#RegulatoryAffairs#HermsGlobal#PharmaCompliance#MOHAPUAE#HealthcareUAE#DrugRegistration#PharmaUAE#GCCHealthcare#RegulatorySupport#UAEPharmaMarket#PharmaConsulting#UAEProductApproval#GCCPharmaIndustry
0 notes
Text
Why Your Manuals Will Fail in 2025 (and How to Fix Them With 1 AI Hack You’ve Never Tried)

The Rise of Frictionless Communication
By 2025, the demand for clear, accessible technical writing will skyrocket. With AI-driven tools, immersive user guides, and a global audience hungry for simplicity, technical writers are no longer just translators of jargon — they’re architects of understanding. In fact, 82% of consumers say poorly explained technical content damages their trust in a brand (Adobe, 2024). Ready to future-proof your documentation? Let’s explore how technical writing in 2025 is breaking barriers, one plain-language sentence at a time.
Want the full breakdown? Read the full article
#TechWriting2025#AIClarity#UserCentricDesign#PlainLanguage#FutureOfWork#AIWriting#InclusiveTech#ARLearning#ComplianceMatters#TechTrends2025#DigitalTransformation#ContentCreation#SimplifiedTech#LearnSomethingNew#CareerGrowth#UXWriting#AITools#InnovationInTech#RegulatoryAffairs#ClearCommunication
0 notes
Text

Fired CPSC commissioner tells President Trump, "See you in court" Read More...
#FiredCPSCCommissioner#CPSC#Trump#SeeYouInCourt#LegalBattle#PoliticalDrama#ConsumerSafety#CourtCase#FightForJustice#FederalRegulations#PoliticalShowdown#RegulatoryAffairs#TrumpAdministration#LegalChallenges#Advocacy#ConsumerAdvocacy#NewsAlert#PoliticsInFocus#CurrentEvents
0 notes
Text
BIS CRS Certification Consultants in Delhi, India, and CRS Certificate Delhi, India
If you're a manufacturer or importer of electronics, IT products, or appliances, getting your products BIS CRS certified is mandatory before launching them in the Indian market. This article will help you understand what BIS CRS certification is, why it's essential, and how Standfill India, one of the top BIS CRS Certification Consultants in Delhi, India, can assist you.
What is BIS CRS Certification?
The Compulsory Registration Scheme (CRS) was introduced by the Bureau of Indian Standards (BIS) in 2012 under the Electronics and IT Goods (Requirement of Compulsory Registration) Order.
This scheme makes it compulsory for certain electronic and IT products to be registered with BIS before they are sold in the Indian market. The certification ensures the product is tested and approved by a BIS-recognized lab, and meets safety standards laid out by the Indian government.
This helps keep people safe from bad or unsafe products and encourages companies in India and other countries to make better quality items.
Why CRS Certificate is Mandatory in India
The Indian government made CRS certification compulsory to:
Prevent import and sale of poor-quality or unsafe electronic items.
Ensure consumer safety and product quality.
It motivates companies in India and other countries to follow the correct rules and quality standards.
Stop counterfeit and unapproved products from entering the market.
If your product falls under the CRS list and you don’t have certification, your products can be stopped at customs, removed from online marketplaces, or face penalties.
What Products Need BIS CRS Certification?
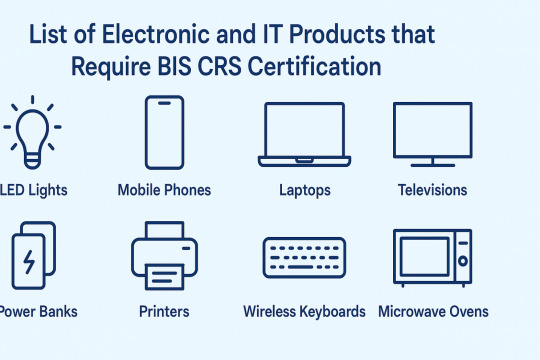
Currently, over 75 product categories fall under CRS. These include:
LED lights, bulbs, and drivers
Laptop and desktop computers
Printers and scanners
Mobile phones and power banks
Smart TVs and monitors
Microwaves, set-top boxes
Video game consoles and music systems
Even if you make a small change in design or technical specs, you may need fresh certification.
Step-by-Step CRS Certification Process in India
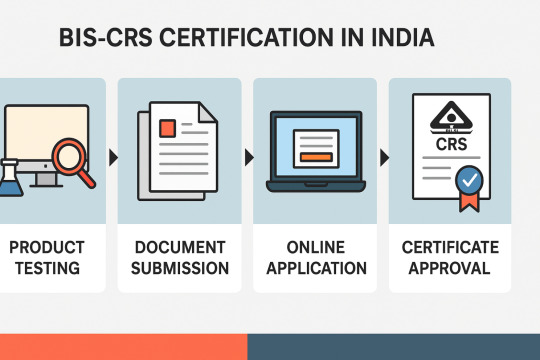
For Indian Manufacturers:
Testing the Product:
Document Submission:
Online Application:
BIS Review and Approval:
CRS Certificate Issued:
For Foreign Manufacturers:
Appoint an Authorized Indian Representative (AIR):
Send Samples to India:
Prepare Documents:
Application Filing:
Get Certified:
Documents Required for CRS Certificate Delhi, India
To get a CRS certificate, the following documents are needed:
Company registration certificate
ISO 9001 certificate (if available)
Product description and specification sheet
Internal/external photographs of product
Label and packaging design with BIS marking
Copy of test reports from BIS lab
User manual in English
Trademark registration certificate (if applicable)
Authority letter (for AIR, if foreign company)
Providing correct and clear documents is very important to avoid rejection.
Problems Faced Without a BIS CRS Consultant
Applying for BIS certification without expert help can lead to many issues:
Confusion over product category and IS standard
Incorrect lab selection for product testing
Delayed application due to document errors
No proper follow-up with BIS officials
For foreign companies, it’s hard to find a reliable Indian Representative
All these issues can cost you time, money, and market opportunity.

Why Hire BIS CRS Certification Consultants in Delhi, India
Delhi is the main city for BIS activities. Most BIS offices, labs, and officials operate from here. Local consultants offer:
Easy access to BIS labs and offices
On-ground support for documentation and sample coordination
Understanding of latest BIS rules and changes
Personalized service for foreign brands needing AIR
Consultants in Delhi help speed up the process and reduce rejections.
Real Client Story: Fast BIS Approval with Consultant Help
A company in Shenzhen, China wanted to export their phone chargers to India. They applied on their own but got rejected twice. They reached out to Standfill India, who:
Helped select the correct product category (IS standard)
Sent samples to the right lab
Corrected documentation errors
Filed the application through AIR in Delhi
The company got their BIS certificate in 27 working days. Today, they are one of the top sellers on Flipkart and Amazon India.
How Standfill India Helps You with BIS CRS Certification
Standfill India is a Delhi-based consultancy specializing in BIS CRS certification. They have years of experience helping both Indian and foreign manufacturers.

They provide:
Free initial consultation to check if your product needs certification.
Lab coordination to ensure samples are tested by approved labs.
Document assistance so everything is correct the first time.
Online application support from start to finish.
Representation for foreign brands as Indian Authorized Representative (AIR).
Post-approval services like renewals and updates.
The Standfill India Process
Step Task How Standfill India Helps
1 Free Consultation Check product eligibility and standards
2 Sample Testing Send product to the right lab
3 Document Support Prepare technical and legal documents
4 Filing Application Submit everything on BIS portal correctly
5 AIR Support Act as official representative for foreign companies
6 Follow-up Handle all BIS queries and objections
7 Certificate Delivery Deliver BIS certificate digitally and guide BIS mark usage

FAQs about BIS CRS Certification
Q1. How long does BIS CRS Certification take? It takes around 30 to 45 working days depending on product testing and document readiness.
Q2. Is BIS certification required for online sales? Yes. Major marketplaces like Amazon, Flipkart, and Reliance Digital need it.
Q3. Can foreign brands apply directly? No. BIS only accepts applications from Indian manufacturers or through an Indian representative.
Q4. What if I sell without BIS certification? You may face customs seizure, legal penalties, or e-commerce bans.
Q5. What is the validity of BIS CRS Certificate? The certificate is valid for 2 years and can be renewed.
Q6. Can I apply for multiple products in one application? Only if all products are technically identical. Otherwise, separate applications are needed.
Final Thoughts
Getting a BIS CRS certificate is not just a formality – it is a legal requirement and proof of product quality. Doing it without expert help can be risky and expensive.
Standfill India, one of the most experienced BIS CRS Certification Consultants in Delhi, India, can guide you through the process smoothly. Whether you are an Indian startup or a global brand, they make certification easier, faster, and more reliable.
#BIS#CRS#CertificationConsultants#Delhi#India#CRSCertificate#QualityCertification#BISCertification#ConsultingServices#RegulatoryCompliance#ProductCertification#DelhiBusiness#IndiaIndustry#CertificationExperts#BISConsultants#CRSExperts#QualityAssurance#ComplianceConsultants#DelhiConsultants#IndiaCertification#BISStandards#CRSStandards#CertificationServices#DelhiStartups#IndiaBusiness#QualityControl#RegulatoryAffairs#CertificationProcess#IndustryStandards#DelhiProfessionals
1 note
·
View note
Text
The Central Drugs Standard Control Organization (CDSCO) is India’s national regulatory authority responsible for the approval, oversight, and regulation of drugs, medical devices, and cosmetics. Established under the Directorate General of Health Services, CDSCO ensures that products introduced into the Indian market are safe, effective, and compliant with regulatory standards. CDSCO registration is mandatory for companies looking to import, manufacture, or distribute pharmaceutical products and medical devices in India. Without proper registration, these products cannot be legally sold in the Indian market.
#CDSCORegistration#CentralDrugsStandardControlOrganizationRegistration#PharmaCompliance#DrugRegulation#MedicaDevices#RegulatoryAffairs#PharmaRegistration#HealthcareRegulations#skmcglobal
0 notes
Text
Regulatory Pathway for In vitro diagnostics (IVD)
Registration in India: IVDs have become integral components of modern healthcare across the globe to support the diagnosis, monitoring, and personalized treatment of diseases. IVDs are also in very high demand in India, where the IVD market was worth about USD 2.64 billion in 2023 and expected to grow at a CAGR of 7.17% from 2024 until 2030 by Scania Analytics. As a result, the demand, interest, and enthusiasm of foreign IVD manufacturers to register and enter the Indian market have peaked.
However, before IVD devices can be introduced to India, manufacturers and importers must navigate a complex regulatory pathway. Moreover, understanding the registration process is crucial for entry into the Indian marketplace. This blog will help manufacturer and importers understand the IVD registration process in India, discussing the critical components necessary obtain a market launch in India. Keep reading for details.
What are IVD Devices?
IVD Devices (in vitro diagnostics) are instruments employed to analyze biological samples (e.g., blood, tissue, urine, or other bodily fluids) outside of the human body. These tests are integral in providing valuable indications for the diagnosis of disease, monitoring a patient’s condition, quantifying certain substances, and identifying infections. More familiar examples of in vitro diagnostic devices are pregnancy test kits, COVID-19 test kits, blood glucose monitoring kits, immunoassays, and human genetic testing devices.
Which Authority Regulates IVDs in India?
The authority in charge of licensing and regulating IVDS in India is the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare.
How IVDs are classified?
IVDs are classified into four classes based on risk factors:
Class A: Low risk IVDs. Eg. Clinical chemistry analyzer (not near patient testing).
Class B: Low moderate risk IVDs. Eg. Pregnancy test strips, anti-nuclear antibody testing, urine test strips.
Class C: Moderate high risk IVDs. Eg. Blood glucose self-testing system, Human leukocyte antigen (HLA) typing, prostate-specific antigen (PSA) screening.
Class D: High risk IVDs. Eg. HIV blood donor screening, ABO/Rh(D) blood grouping analyser (near patient testing).
Central Licensing Authority’s Role in Regulating IVDs
The Central Licensing Authority is responsible for:
• Providing a license for the import of all classes of IVDs.
• Regulating the manufacture of Class C and Class D (high risk) IVDs.
• Providing the approval for clinical performance evaluation studies for new IVDs before they are allowed to be marketed in India.
• Working with State Licensing Authorities to ensure that there is consistent oversight of IVDs post-marketing.
Responsibilities of State Licensing Authority in Controlling IVDs
According to the Medical Devices Rules, 2017, the responsibilities of the State Licensing Authority are:
• To grant the licenses for the manufacture, sale or distribution of Class A or Class B (low risk) In Vitro Diagnostic medical devices in their state.
• To regulate the sale, stocking, exhibition and distribution of IVDs of all classes in their states.
How to register an IVD in India?

A manufacturer, importer or distributor can register an IVD in India by following these steps:
• Identify the Class of IVD: Identify the risk class for the device/personnel E or fQA Yo can more work.
• Designate the Authorized Indian Rep(s): Authorized agent if the applicant is a foreign manufacturer.
• Complete the Application Form: along with all supporting documents, for the developer or authorized agent on the SUGAM Portal:
Manufacturer: Applies for the Manufacturing license on Form MD-3orMD-4--Class A & B apply on Form MD-3 or MD-4. Class C and D manufacturers, MD-7 or MD-8.
More information about how to obtain a device manufacturing license can be read in our article, “5 Easy Steps To Obtain Your Medical Device Manufacturing License.”
Importers: Can apply for not only the manufacture device license but the device Import license using Form MD-14.The process of obtaining a licensing device can be perused at our blog, “Medical Devices Registration for Import.”
• Application for Review: The CDSCO will review the documents and may inspect overseas manufacturing. For example, review of importers data or evidence, while for manufacturers for India, will assign a State Licensing Authority (SLA)/ CLA a full site inspection process.
• License Issuance: Subject to all requirements being fulfilled, if the CDSCO is satisfied, a manufacturing or import license will be issued that is valid for five years unless otherwise noted.
How Regulatory Solutions India Can Help You?
If you have not registered your IVDs in India yet, RSI can assist you through the whole process. Having more than ten years in the medical device regulatory process, we have completed over 450 medical device registrations in all categories.
We have worked with clients in over 20 countries and can assist with everything from submission to regulatory approval in an effort to facilitate the approval process and secure your place in the market quicker. Let RSI help with gathering your IVD registration in India, contact us today!
#regulatory solutions india#regulatory consultancy in india#IVD#InVitroDiagnostics#MedicalDevices#FDA#RegulatoryAffairs#IVDR#MedTech#Biotech#HealthcareCompliance#QualityAssurance#MedicalDeviceRegulation#ClinicalDiagnostics#FDA510k#CEMarking#HealthcareInnovation
0 notes
Text
🩺 Looking to register your Medical Devices in India? Let Freyr be your regulatory partner for CDSCO compliance and fast-track market entry!
📌 At Freyr Solutions, we offer end-to-end Medical Device Regulatory Services in India – covering everything from classification to CDSCO submissions and licensing.
🔍 Our Expertise Includes: ✅ Device classification (A, B, C, D) under MDR 2017 ✅ Import & manufacturing license support (Forms MD-14/15, MD-3/6) ✅ Indian Authorized Agent services ✅ QMS Documentation (ISO 13485) ✅ UDI, labeling & post-market vigilance ✅ CDSCO interactions and regulatory strategy
💡 Whether you’re a foreign manufacturer or an Indian distributor, we ensure your devices are compliant, registered, and ready for launch — with zero regulatory stress.
📞 Get compliant. Get to market. Get Freyr. 🔗 Learn more: https://www.freyrsolutions.in/medical-devices-regulatory-support-in-india
#MedicalDevices#CDSCO#IndiaRegulatory#MDR2017#FreyrSolutions#MedicalDeviceRegistration#RegulatoryAffairs#MedTechIndia#DeviceApproval#HealthTech
0 notes
Text

Why are pharma and clinical trial leaders doubling down on QbD, RBQM, and RBM in 2025? Because “compliance” is no longer enough, today, regulators expect quality, risk-awareness, and strategy to be embedded from the very beginning.
0 notes
Text
0 notes
Text

0 notes
Text

If you’re planning to sell non-prescription health or wellness products in the UAE, Over the Counter (OTC) Registration is a critical step—and Herms Global is here to guide you through it.
Whether you're launching supplements, cosmetics, herbal remedies, or consumer healthcare products, we help you stay compliant with MOHAP (Ministry of Health and Prevention) guidelines. Our team simplifies the process by handling classification, documentation, regulatory review, and submissions—ensuring a smooth approval process.
🔹 Avoid delays 🔹 Ensure compliance 🔹 Speed up market entry 🔹 Get expert support from start to finish
At Herms Global, we make Over the Counter Registration in the UAE seamless and stress-free.
➡️ Learn more:
#OTCRegistration#OverTheCounterUAE#HermsGlobal#MOHAPCompliance#ProductRegistration#UAEHealthcare#HealthSupplementsUAE#CosmeticRegulations#UAEPharma#RegulatoryAffairs#NonPrescriptionUAE#BusinessInUAE#GCCCompliance#MarketEntrySupport#UAEWellnessIndustry
0 notes