#test diagnostic molecular
Text
The Clinical Laboratory's Potential and Underestimated Worth
Given that healthcare finance leaders are accountable for determining the revenue potential of the clinical service lines provided by their businesses, they ought to concentrate on laboratory medicine.
Today, clinical laboratory services are a prospective source of income for hospitals and healthcare organizations. But to effectively tap into that income potential, these organizations' finance management needs to be aware of the laboratory service line's potential to enhance population health and take the initiative in educating customers about the prospects it offers.

Contrasting the public's image of laboratory services
You can find numerous scenarios requiring laboratory testing on any medical TV show, from the routine (a family visiting a patient in a hospital bed) to the extraordinary (a response to a trauma event). In such instances, the doctor would frequently say to the patient, "We're sending labs for ABC; that will tell us if you have XYZ," or something similar.
But in reality, it involves more than just the doctor requesting a test. To carry out the duties of the laboratory, pathologists and laboratory technologists must be licensed and certified, and clinical laboratories must be accredited.
However, over the past few years, lab testing has developed into one of healthcare's most consumer-focused and readily available aspects. This development has intensified in light of the COVID-19 pandemic. Therefore, it's crucial that financial leaders take the initiative to maximize this service line's possibilities.
Analyzing the lab service line's expenses and profitability
The cost and revenue center relationship related to laboratory medicine is a significant problem for any healthcare organization, as it is with every service line. However, it is a simple fact that laboratory medicine can be pretty profitable.
Consider the fact that direct-to-consumer lab testing strategies are ubiquitous in drug stores. A test may check for drug usage, pregnancy, and other conditions.
So, how can finance leaders negotiate the laboratory system's terrain to maximize revenue while delivering the best possible patient care? Here are a few ideas.
Integrate lab services with the patient population at the hospital. For instance, if a hospital offers a molecular diagnostic testing service of excellence for cardiac care, It might gain from combining cardiac testing with clinical treatment to develop a "diagnosis and testing" strategy that aims to increase productivity and make the most of available resources. Depending on the revenue pro forma and ROI analysis, this strategy might be implemented by integrating specific testing tools inside the clinical service or outsourcing to commercial laboratories.
Make the most of your payers' collective bargaining and payment options. For instance, a center for inflammatory bowel disease can influence better payment for lab tests by demonstrating how clinical diagnostic and laboratory testing can encourage decreased length of stay and other disease-related expenditures as well as improved quality-of-life outcomes.
Think of direct-to-consumer products. Patients and customers can employ specific tests with a financial payment to improve their health, fee-for-service methodology. For example, a patient curious about whether they have antibodies following a COVID-19 vaccination would be ready to pay for this information out of pocket. In addition, unlike high-complexity tests like the COVID-19 RT-PCR test (reverse transcription polymerase chain reaction), which the FDA recently approved for emergency use, such test options are not subject to the same state or federal reporting requirements.
Lost warrior?
Laboratory testing is a common, effective, and affordable component of healthcare. Unfortunately, drive-through appendectomy procedures are unavailable, although COVID-19 testing is available practically anywhere. Therefore, laboratory testing has the potential to be one of the healthcare system's most revenue-generating elements. f Given the state of healthcare today, The way to attract the unsung hero that all healthcare organizations yearn for may lie in maturing and optimizing innovative laboratory testing procedures.
How Salesforce Benefits Clinical Labs
Clinical laboratories frequently have silos of patient information throughout the process, as we have observed in many businesses.
Contrasting information sources cause operational inefficiencies. Additionally, these repositories hinder a lab's ability to be proactive in client relations.
Clinical labs are increasingly using Salesforce to address these operational problems and enhance provider and patient experiences. Why is this?
1. Enhanced Outreach
Patient and customer data are compartmentalized across various systems in many clinical labs, including:
EHR, HIS, billing, email marketing, and LIS are related technologies.
Spreadsheets
A CRM system must be able to be customized to meet the unique requirements of clinical laboratories. Thanks to its broad toolkit, Salesforce constantly adapts to the intricacies of particular healthcare facilities, including labs.
It is now simpler than ever to upload clinical data to Salesforce, thanks to FHIR standards. Because it can be applied in many different contexts, this standard is crucial for decreasing the number of data silos.
What was reactive client management that may become proactive client engagement with data consolidation and visibility?
2. Improved Online Marketing
Without an element of modern digital marketing, any outreach campaign is lacking.
Compared to other email marketing vendors, Salesforce provides a more robust marketing automation platform and client experience. The standard practice for outbound digital marketing was sending an identical message to all providers. It is now simpler than ever to divide a single marketing database into numerous targeted lists when Salesforce and its marketing automation product, Pardot, are used together.
3. Assistance with Mobile Phlebotomy
Adding database and workflow functionality to mobile devices in the field is simple with Salesforce.
Thanks to this expansion, phlebotomists can now update centralized patient data while visiting nursing homes, rehab facilities, and even patients' homes.
Geographic validation also ensures that mobile phlebotomists stick to the predetermined schedule of visits.
4. More Prompt Customer Support
CRM is a platform frequently used to provide patients and clients with customer service. That feature is available in Salesforce Service Cloud.
Integrating Salesforce with call center communication software, such as Amazon Connect, can be advantageous for contact centers.
5. Real-Time Analytics
Salesforce's built-in reporting and dashboard functionality can offer a complete picture of clients when data from several silos (clinical, business, billing, and testing) are combined.
Salesforce can allow broad analytical views since it can centralize data from several internal sources.
6. Better Compliance with Lab Requests by Patients
Lacking revenue due to non-compliance with lab requests is a problem that labs frequently face. Salesforce may help with this problem.
Salesforce can address this problem by storing data from the lab and the patient's office visit (a test was requested on a specific date) (and the test results were sent to the provider on a particular date). In addition, Salesforce's exception reporting and automated alerts could let the doctor and patient know that the test didn't take place when it should have.
#molecular diagnostic testing service#molecular diagnostics centre#test diagnostic molecular#molecular diagnostic test for covid-19#molecular diagnostic test
1 note
·
View note
Text
Latin America Molecular Diagnostics Market Overview: Challenges and Growth Opportunities
According to a recent report by Meticulous Research®, the Latin America molecular diagnostics market is forecasted to reach $2.50 billion by 2031, expanding at a compound annual growth rate (CAGR) of 6.3% from 2024 to 2031. The market growth is propelled by several key factors, including the increasing global geriatric population, rising prevalence of communicable and noncommunicable diseases, rapid advancements in molecular diagnostics technology, and a surge in healthcare spending across the region.
The rising demand for molecular diagnostics in Latin America is further supported by opportunities such as the expanding scope in emerging economies, increased focus on companion diagnostics, and the growing popularity of direct-to-consumer (DTC) testing. Despite these growth drivers, challenges like the shortage of skilled professionals, unfavorable regulatory frameworks, and the high costs of molecular diagnostic tests could restrain the market’s growth.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759
Market Segmentation and Key Insights
The Latin America molecular diagnostics market is segmented based on offerings, test types, technologies, applications, and end users.
By Offering: In 2024, the kits and reagents segment is expected to dominate the market. This is attributed to the widespread availability of diagnostic reagents and consumables, disease-specific test kits and assays, and increasing awareness about the importance of early disease diagnosis.
By Test Type: The laboratory tests segment is projected to account for the largest share in 2024. Laboratory tests remain the preferred choice for hospitals, diagnostic laboratories, and academic institutions, driven by their wide availability and the significant developments in diagnostic testing within clinical settings.
By Technology: The polymerase chain reaction (PCR) segment is expected to lead the market in 2024. PCR's dominant position is due to its versatility in multi-drug resistance testing, widespread use in clinical and research laboratories, and applications in areas such as DNA fingerprinting, bacterial and viral detection (particularly HIV/AIDS), and genetic disorder diagnosis.
By Application: The infectious diseases segment is anticipated to hold the largest share of the market in 2024. The increasing prevalence of infectious diseases, rising funding for the development of innovative diagnostic tools, and the heightened focus on diagnostic technologies during the COVID-19 pandemic are driving the demand for molecular diagnostics in infectious disease management.
By End User: Hospitals and clinics are expected to dominate the market by end user in 2024. The growing number of hospitalizations due to a variety of diseases requiring molecular diagnosis, coupled with the proliferation of healthcare facilities in countries such as Brazil, Mexico, Chile, and Colombia, is contributing to the expansion of molecular diagnostic product utilization in this segment.
Key Industry Players
The competitive landscape of the Latin America molecular diagnostics market is shaped by several prominent global and regional players. Leading companies in the market include:
Bio-Manguinhos (Brazil)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Hologic, Inc. (U.S.)
Illumina, Inc. (U.S.)
OmicronLab (Mexico)
QIAGEN N.V. (Netherlands)
Danaher Corporation (U.S.)
Abbott Laboratories (U.S.)
Agilent Technologies, Inc. (U.S.)
These companies are at the forefront of innovation and technological advancements in molecular diagnostics, driving market growth through product development, partnerships, and strategic expansions.
Opportunities and Challenges
The Latin America molecular diagnostics market is ripe with opportunities, particularly in emerging economies where there is increasing demand for advanced diagnostic tools. The rising focus on companion diagnostics and the expanding DTC testing market offer significant growth prospects. However, challenges such as the high cost of molecular diagnostic tests and regulatory hurdles may hinder market expansion.
Despite these challenges, the region’s healthcare landscape is undergoing rapid transformation, with increasing healthcare investments and advancements in diagnostic technologies expected to fuel further growth.
Read Full Report @ https://www.meticulousresearch.com/product/latin-america-molecular-diagnostics-market-5759
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes
Text
Advancing Precision Diagnostics: Technology, Applications, and Future Insights
Adequate diagnosis is the use of advanced technologies to accurately analyze biological markers in patients. This emerging field allows for more targeted diagnosis and treatment compared to traditional one-size-fits-all approaches. By developing a deep understanding of disease at the molecular level, adequate diagnosis can enable truly personalized care for each unique patient.
Advanced Technology Enabling Precision
Major technological advances are fueling the rise of adequate Precision Diagnostics. Next-generation sequencing has dramatically reduced the cost and increased the speed of obtaining genetic information from patients. This genomic data provides crucial biomarkers that can indicate disease risk, identify molecular subgroups, and predict treatment responses. Advanced imaging techniques now allow visualization of organs and tissues at microscopic resolution. Combining molecular analysis with diagnostic imaging creates a multi-dimensional overview of a patient's condition. Computer algorithms also play an important role by synthesizing huge amounts of biomarker and clinical data to derive diagnostic and prognostic insights. Together, these technologies empower clinicians with the tools for pinpoint targeting and tracking of diseases.
Insights for a Variety of Precision Diagnostics
Cancer is one area that has benefited greatly from adequate diagnosis approaches. Genomic profiling of tumor samples routinely identifies disease-driving genetic alterations that can be targeted with specific therapies. For example, detection of Epidermal Growth Factor Receptor (EGFR) mutations in lung cancer guides treatment decisions for EGFR inhibitor drugs. Similar molecular characterization is available for other cancer types like melanoma, leukemia, and breast cancer. Cardiovascular diseases are also embracing precision, with new genetic risk scores to predict heart attack or stroke likelihood. Biomarkers in blood can detect early signs of conditions like heart failure and help monitor responses to therapies over time. In neurology, biomarkers hold promise for improving Alzheimer's and Parkinson's disease diagnoses which currently rely on clinical assessments. Molecular subtyping of lung diseases, infections and autoimmune conditions may also enable personalized management strategies in the future.
Challenges in Implementing Adequate diagnosis
While the opportunities presented by adequate diagnosis are exciting, challenges remain in fully realizing this vision in clinical practice. One major hurdle is the complexity of analyzing, securely storing and interpreting vast amounts of multi-dimensional patient data. Turning raw biomarkers into actionable medical insights requires advanced data analytics capabilities that will continue advancing. Regulatory bodies must also establish standards and oversight procedures for precision diagnostic tests to ensure accuracy, efficacy and safety. Reimbursement policies need revising to account for the development costs of precision technologies and ongoing monitoring of patients. Building an adequately skilled clinical workforce is equally important, as physicians need training to proficiently collect and interpret different biomarkers alongside traditional examinations. Over time, large real-world outcomes studies will further validate the clinical utility and cost-effectiveness of precision approaches on diverse patient populations and health systems. With dedication to addressing these obstacles, adequate diagnosis show tremendous long-term potential to transform healthcare delivery.
Get more insights on Precision Diagnostics
Discover the Report for More Insights, Tailored to Your Language
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)

#Precision Diagnostics#Diagnostic Testing#Personalized Medicine#Molecular Diagnostics#Precision Medicine#Genetic Testing#Biomarker Analysis
0 notes
Text
The Impact of Molecular Testing on Modern Cancer Therapy
Molecular biology laboratories have revolutionized cancer diagnosis and treatment. This video explores the basics of cancer genetics and the power of molecular diagnostics. Discover how molecular biomarkers like RNA analysis, immunohistochemistry, and next-generation sequencing (NGS) are transforming cancer care. Learn about groundbreaking advancements in molecularly targeted therapies and the use of CRISPR-Cas9 gene editing technology. Join us as we delve into the future of cancer treatment, where molecular biology laboratories are making precision and personalized medicine a reality.
#molecular biology laboratory#medical diagnostics lab#molecular virology test lab Dubai#diagnostic medical laboratory
0 notes
Text
Unraveling the Intricacies of the Molecular Diagnostics Market and its Future Prospects
The global molecular diagnostics market size is expected to reach USD 17.97 billion by 2030, and expanding at a CAGR of 4.5% from 2024 to 2030, according to a recent study by Grand View Research, Inc. The contraction in the market will be due to the decline in demand for molecular testing for COVID-19. However, factors such as the rising geriatric population and increasing demand for technologies such as NGS is expected to drive market growth.
Molecular Diagnostics Market Report Highlights
The reagents segment dominated the market and accounted for a share of 62.2% of the global revenue in 2023. It is expected to maintain its dominance throughout the forecast years owing to its wide application scope in research & clinical settings and increasing adoption of novel tests.
The polymerase chain reaction technology segment accounted for the largest revenue share in 2023. This is attributed to its use in detecting COVID-19 and other infectious diseases.
The infectious diseases segment accounted for the largest revenue share in 2023. The increased usage of molecular, particularly PCR tests, for diagnosing COVID-19 has increased the segment share significantly.
The central laboratories segment dominated the industry in 2023 owing to high procedure volumes for COVID testing and other healthcare indications in central laboratories.
North America dominated the market and accounted for a 39.3% share in 2023. This is attributed to the rising epidemiology of infectious as well as chronic diseases, thus, encouraging companies to introduce novel molecular diagnostic tests, thereby boosting market growth.
Asia Pacific is anticipated to exhibit significant growth from 2024 to 2030 owing to increased market penetration, initiatives of local market players to increase the adoption of novel diagnostic technologies, and high unmet market needs.
For More Details or Sample Copy please visit link @: Molecular Diagnostics Market Report
Molecular diagnostics plays an important role in infectious disease testing as they can yield effective and fast results. Hence, the increasing prevalence of hospital-acquired infections & infectious diseases is projected to drive the market over the forecast period. Increasing incidence and awareness regarding genetic disorders is further anticipated to accelerate market growth. The miniaturization of three basic molecular assays-nanobiotechnology, biochips, and microfluidics are expected to increase the accuracy and specificity of diagnostic outcomes, and hence, increase the demand for molecular diagnostic products. These improvements are expected to enhance the availability of PoC molecular diagnostic tests to yield quick and effective test results.
Companies are expanding their product portfolios with the acquisition of smaller companies. For instance, in March 2021, Hologic announced the acquisition of Diagenode-a molecular diagnostic company with a wide range of PCR instruments, facilitating the detection of over 30 bacteria-for USD 159 million. Similarly, in April 2021, F. Hoffmann-La Roche Ltd. acquired GenMark Diagnostics, Inc. at a price of USD 24.05 per share in cash, and it holds around 82.89% of total shares of GenMark Diagnostics. GenMark Diagnostics, Inc. has proprietary technologies, such as eSensor XT-8 and ePlex, which can be utilized in developing tests for infectious diseases, including bloodstream infections.
#MolecularDiagnostics #PrecisionMedicine #HealthcareInnovation #GenomicTesting #DiagnosticsTechnology #Biotechnology #PersonalizedMedicine #DiagnosticRevolution #NGS #BiomarkerDiscovery #InfectiousDiseaseDiagnosis #CancerDetection #Theranostics #Bioinformatics #MolecularBiology #DiagnosticTrends #MedicalTechnology #LaboratoryTesting #PointofCareDiagnostics #HealthTechInnovation
#Molecular Diagnostics#Precision Medicine#Healthcare Innovation#Genomic Testing#Diagnostics Technology#Biotechnology#Personalized Medicine#Diagnostic Revolution#NGS#Next Generation Sequencing#Biomarker Discovery#Infectious Disease Diagnosis#Cancer Detection#Theranostics#Bioinformatics#Molecular Biology
0 notes
Text
Decoding Trends and Innovations in the Blood Cancer Diagnostics Industry
Blood cancer, a complex group of diseases affecting the blood, bone marrow, and lymphatic system, demands precise and efficient diagnostic tools. As advancements in medical technology continue to reshape the healthcare landscape, the Blood Cancer Diagnostics Market is witnessing dynamic changes. Let’s explore the key dynamics shaping this market.
Advanced Testing Strategies in the Blood Cancer…

View On WordPress
#Blood Cancer#Blood Cancer Diagnostics#Blood Cancer Diagnostics Market#Blood Tests#Imaging Tests#Medical Device#Medical Devices Market#MedTech#MedTech Market#Molecular Tests
0 notes
Text

The #Genes2Me ctDNA Lung #NGS panel is a hybridization based solution for #screening 32 clinically relevant genes (coding regions of the genome) for #diseases associated with genetic mutations.
It covers all major #mutations like SNV, and InDels, adding up to a target size of
47Kb with a hybridization-based target capture technique. This #panel exhibits high quality performance with high sensitivity and specificity in detecting the variants in #cancer-associated #genes. We also offer data #analysis support with automated data analysis and reporting #software called CliSeq Interpreter.
Contact us today to learn more about our NGS #ctDNA Panels https://www.genes2me.com/next-generation-sequencing-clinical-panels/cancer-diagnosis-dna-Panels
#g2m #cliseq #dataanalysts #rtpcr #nextgenerationsequencing #sequencing #clinical #diagnosis #india #manufacturer #healthcare #dna #dnasequencing
#ngs#next generation sequencing#ngs panel#g2m#genes2me#diagnostics#rt pcr#ivd kit#kits#pcr kits#sequencing panel#molecular biology#diseases#dna#rna#genetic testing#health care
0 notes
Text
#Genomic research & molecular diagnostics laboratory in Pune#molecular diagnostic laboratory#genomics company in india#laboratory#molecular biology training course in pune#greenarray#molecular diagnostic laboratory in pune#incubation centre in pune#pune#microarray system#covid-19 rtpcr testing#Genomic research#molecular diagnostics laboratory in Pune#Greenarray#Genetic test
0 notes
Text
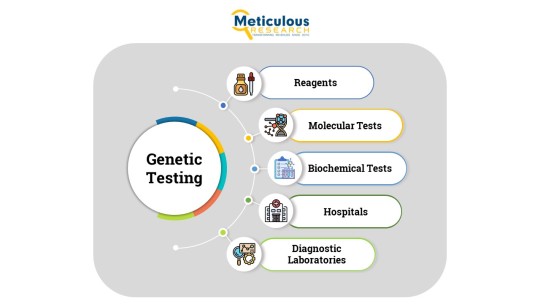
Genetic Testing Market – Global Opportunity Analysis and Industry Forecast (2022-2029)
Meticulous Research®—a leading global market research company, published a research report titled, ‘Genetic Testing Market By Product (Consumables, Reagents, Instruments, Services), Test Type (Diagnostic, Prenatal, Carrier, Newborn, Preimplantation), Method (Molecular, Chromosomal), End User (Hospitals, Diagnostic Laboratories) - Global Forecast to 2029.’
According to this latest publication from Meticulous Research®, the genetic testing market is expected to grow at a CAGR of 11.6% from 2022 to reach $43.3 billion by 2029. The growth of this market is attributed to factors such as favorable government initiatives for promoting genetic testing, increasing prevalence of genetic disorders, increased need for early disease detection & prevention, increasing applications of genetic testing in oncology, and decreasing cost of sequencing procedures. In addition, the growing scenario of genetic direct-to-consumer genetic testing and the emerging field of genetic counseling is expected to provide significant growth opportunities for this market.
However, factors such as the high cost of genetic testing and the social and ethical implications of genetic testing are restraining the growth of this market to a notable extent. In addition, factors such as low chances of positive, actionable mutations and genetic data privacy & security concerns are the major challenges to the growth of this market.
Genetic Testing Market: Future Outlook
The genetic testing market is segmented based on product, test type, method, end user, and geography. The study also evaluates industry competitors and analyzes the market at the country level.
Download Research Sample https://www.meticulousresearch.com/download-sample-report/cp_id=5370
Scope of the Report:
Genetic Testing Market, by Product & Service
· Consumables & Reagents
· Instruments
· Services
Genetic Testing Market, by Test Type
· Diagnostic Testing
· Prenatal Testing
· Carrier Testing
· Newborn Screening
· Preimplantation Testing
· Other Test Types
(Other test types include forensic testing, microorganism genomics, and posthumous (testing of post-mortem tissues etc.)
Genetic Testing Market, by Method
· Molecular Tests
· Chromosomal Tests
· Biochemical Tests
Genetic Testing Market, by End User
· Hospitals & Clinics
· Diagnostic Laboratories
· Academic & Research Institutes
· Others End Users
(Other end users include contract research organizations, forensic laboratories, pharma & biotech companies, government laboratories, etc.)
Genetic Testing Market, by Geography
· North America
o U.S.
o Canada
· Europe
o Germany
o France
o U.K.
o Italy
o Spain
o Rest of Europe (RoE)
· Asia-Pacific
o China
o Japan
o India
o Rest of APAC (RoAPAC)
· Latin America
· Middle East & Africa
Speak with Our Analyst: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5370
Based on product, in 2022, the consumables & reagents segment is estimated to account for the largest share of this market. The large share of this segment is attributed to factors such as the recurrent use of reagents & consumables, availability of a wide range of genetic tests, recent product launches, and increased awareness about the advantages of genetic testing.
Based on test type, in 2022, the diagnostic testing segment is estimated to account for the largest share of this market. The large share of this segment is attributed to factors such as the high prevalence of chronic diseases such as cancer, technological advancements in genomic-based diagnostic testing, and the demand for early disease diagnosis.
Based on method, in 2022, the molecular segment is estimated to account for the largest share of this market. The large share of this segment is attributed to the ability to detect the mutation at single nucleotide resolution with cheaper, faster, and with the utmost accuracy by using whole genome sequencing (WGS) and whole exome sequencing (WES) technology.
In 2022, based on end user, the hospitals & clinics segment is estimated to account for the largest share of the market. The large share of this segment is attributed to the high volume of genetic tests performed in hospitals & clinics and the high demand for early disease diagnosis and treatment.
This research report analyzes major geographies and provides a comprehensive analysis of North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, and RoE), Asia-Pacific (Japan, China, India, and RoAPAC), Latin America, and the Middle East & Africa.
In 2022, North America is estimated to account for the largest share of the genetic testing market, followed by Europe and Asia-Pacific. North America’s large market share is attributed to the factors such as high healthcare expenditure, favorable reimbursement scenario, high awareness among patients about genetic testing, and increased support and investments to enhance genome sequencing infrastructure in the region.
Access full Report Description, TOC, Table of Figure, Chart, etc: https://www.meticulousresearch.com/product/genetic-testing-market-5370
Key Players
The key players operating in the genetic testing market are Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Quest Diagnostics Incorporated (U.S.), Eurofins Scientific SE (Luxembourg), 23andMe, Inc. (U.S.), Foundation Medicine, Inc. (U.S.) (Subsidiary of Roche Holding AG), Rosetta Genomics Ltd. (U.S.), OPKO Health Inc. (U.S.), Natera, Inc. (U.S.), PerkinElmer, Inc. (U.S.), Myriad Genetics, Inc. (U.S.), Laboratory Corporation of America Holdings (U.S.) and Invitae Corporation (U.S.).
Key questions answered in the report-
· Which are the high-growth market segments in terms of product, test type, method, end user, and region/country?
· What was the historical market size for genetic testing across the globe?
· What are the market forecasts and estimates for the period 2022–2029?
· What are the major drivers, restraints, opportunities, and challenges in the global genetic testing market?
· Who are the major players in the genetic testing market?
· How is the competitive landscape, and who are the market leaders in the global genetic testing market?
· What are the recent developments in the global genetic testing market?
· What are the different strategies adopted by the major players in the global genetic testing market?
· What are the geographical trends and high-growth regions/countries?
Contact Us:
Meticulous Research
®
Email-
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn-
https://www.linkedin.com/company/meticulous-research
#Genetic Testing Market#Genetic Testing#health#healthcare#medical devices#medical#diagnostics#newborn screening#molecular tests#biochemical#business#industry#market research
1 note
·
View note
Text
When Swiss cardiologist Thomas F. Lüscher attended an international symposium in Turin, Italy, last summer, he encountered an unusual “attendee:” Suzanne, Chat GPT’s medical “assistant.” Suzanne’s developers were eager to demonstrate to the specialists how well their medical chatbot worked, and they asked the cardiologists to test her.
An Italian cardiology professor told the chatbot about the case of a 27-year-old patient who was taken to his clinic in unstable condition. The patient had a massive fever and drastically increased inflammation markers. Without hesitation, Suzanne diagnosed adult-onset Still’s disease. “I almost fell off my chair because she was right,” Lüscher remembers. “This is a very rare autoinflammatory disease that even seasoned cardiologists don’t always consider.”
Lüscher — director of research, education and development and consultant cardiologist at the Royal Brompton & Harefield Hospital Trust and Imperial College London and director of the Center for Molecular Cardiology at the University of Zürich, Switzerland — is convinced that artificial intelligence is making cardiovascular medicine more accurate and effective. “AI is not only the future, but it is already here,” he says. “AI and machine learning are particularly accurate in image analysis, and imaging plays an outsize role in cardiology. AI is able to see what we don’t see. That’s impressive.”
At the Royal Brompton Hospital in London, for instance, his team relies on AI to calculate the volume of heart chambers in MRIs, an indication of heart health. “If you calculate this manually, you need about half an hour,” Lüscher says. “AI does it in a second.”
AI-Assisted Medicine
Few patients are aware of how significantly AI is already determining their health care. The Washington Post tracks the start of the boom of artificial intelligence in health care to 2018. That’s when the Food and Drug Administration approved the IDx-DR, the first independent AI-based diagnostic tool, which is used to screen for diabetic retinopathy. Today, according to the Post, the FDA has approved nearly 700 artificial intelligence and machine learning-enabled medical devices.
The Mayo Clinic in Rochester, Minnesota, is considered the worldwide leader in implementing AI for cardiovascular care, not least because it can train its algorithms with the (anonymized) data of more than seven million electrocardiograms (ECG). “Every time a patient undergoes an ECG, various algorithms that are based on AI show us on the screen which diagnoses to consider and which further tests are recommended,” says Francisco Lopez-Jimenez, director of the Mayo Clinic’s Cardiovascular Health Clinic. “The AI takes into account all the factors known about the patient, whether his potassium is high, etc. For example, we have an AI-based program that calculates the biological age of a person. If the person in front of me is [calculated to have a biological age] 10 years older than his birth age, I can probe further. Are there stressors that burden him?”
Examples where AI makes a sizable difference at the Mayo Clinic include screening ECGs to detect specific heart diseases, such as ventricular dysfunction or atrial fibrillation, earlier and more reliably than the human eye. These conditions are best treated early, but without AI, the symptoms are largely invisible in ECGs until later, when they have already progressed further...
Antioniades’ team at the University of Oxford’s Radcliffe Department of Medicine analyzed data from over 250,000 patients who underwent cardiac CT scans in eight British hospitals. “Eighty-two percent of the patients who presented with chest pain had CT scans that came back as completely normal and were sent home because doctors saw no indication for a heart disease,” Antioniades says. “Yet two-thirds of them had an increased risk to suffer a heart attack within the next 10 years.” In a world-first pilot, his team developed an AI tool that detects inflammatory changes in the fatty tissues surrounding the arteries. These changes are not visible to the human eye. But after training on thousands of CT scans, AI learned to detect them and predict the risk of heart attacks. “We had a phase where specialists read the scans and we compared their diagnosis with the AI’s,” Antioniades explains. “AI was always right.” These results led to doctors changing the treatment plans for hundreds of patients. “The key is that we can treat the inflammatory changes early and prevent heart attacks,” according to Antioniades.
The British National Health Service (NHS) has approved the AI tool, and it is now used in five public hospitals. “We hope that it will soon be used everywhere because it can help prevent thousands of heart attacks every year,” Antioniades says. A startup at Oxford University offers a service that enables other clinics to send their CT scans in for analysis with Oxford’s AI tool.
Similarly, physician-scientists at the Smidt Heart Institute and the Division of Artificial Intelligence in Medicine at Cedars-Sinai Medical Center in Los Angeles use AI to analyze echograms. They created an algorithm that can effectively identify and distinguish between two life-threatening heart conditions that are easy to overlook: hypertrophic cardiomyopathy and cardiac amyloidosis. “These two heart conditions are challenging for even expert cardiologists to accurately identify, and so patients often go on for years to decades before receiving a correct diagnosis,” David Ouyang, cardiologist at the Smidt Heart Institute, said in a press release. “This is a machine-beats-man situation. AI makes the sonographer work faster and more efficiently, and it doesn’t change the patient experience. It’s a triple win.”
Current Issues with AI Medicine
However, using artificial intelligence in clinical settings has disadvantages, too. “Suzanne has no empathy,” Lüscher says about his experience with Chat GPT. “Her responses have to be verified by a doctor. She even says that after every diagnosis, and has to, for legal reasons.”
Also, an algorithm is only as accurate as the information with which it was trained. Lüscher and his team cured an AI tool of a massive deficit: Women’s risk for heart attacks wasn’t reliably evaluated because the AI had mainly been fed with data from male patients. “For women, heart attacks are more often fatal than for men,” Lüscher says. “Women also usually come to the clinic later. All these factors have implications.” Therefore, his team developed a more realistic AI prognosis that improves the treatment of female patients. “We adapted it with machine learning and it now works for women and men,” Lüscher explains. “You have to make sure the cohorts are large enough and have been evaluated independently so that the algorithms work for different groups of patients and in different countries.” His team made the improved algorithm available online so other hospitals can use it too...
[Lopez-Jimenez at the Mayo Clinic] tells his colleagues and patients that the reliability of AI tools currently lies at 75 to 93 percent, depending on the specific diagnosis. “Compare that with a mammogram that detects breast tumors with an accuracy of 85 percent,” Lopez-Jimenez says. “But because it’s AI, people expect 100 percent. That simply does not exist in medicine.”
And of course, another challenge is that few people have the resources and good fortune to become patients at the world’s most renowned clinics with state-of-the-art technology.
What Comes Next
“One of my main goals is to make this technology available to millions,” Lopez-Jimenez says. He mentions that Mayo is trying out high-tech stethoscopes to interpret heart signals with AI. “The idea is that a doctor in the Global South can use it to diagnose cardiac insufficiency,” Lopez-Jimenez explains. “It is already being tested in Nigeria, the country with the highest rate of genetic cardiac insufficiency in Africa. The results are impressively accurate.”
The Mayo Clinic is also working with doctors in Brazil to diagnose Chagas disease with the help of AI reliably and early. “New technology is always more expensive at the beginning,” Lopez-Jimenez cautions, “but in a few years, AI will be everywhere and it will make diagnostics cheaper and more accurate.”
And the Children’s National Hospital in Washington developed a portable AI device that is currently being tested to screen children in Uganda for rheumatic heart disease, which kills about 400,000 people a year worldwide. The new tool reportedly has an accuracy of 90 percent.
Both Lopez-Jimenez and Lüscher are confident that AI tools will continue to improve. “One advantage is that a computer can analyze images at 6 a.m. just as systematically as after midnight,” Lüscher points out. “A computer doesn’t get tired or have a bad day, whereas sometimes radiologists overlook significant symptoms. AI learns something and never forgets it.”
-via Reasons to Be Cheerful, March 1, 2024. Headers added by me.
--
Note:
Okay, so I'm definitely not saying that everything with AI medicine will go right, and there won't be any major issues. That's definitely not the case (the article talks about some of those issues). But regulation around medicines is generally pretty tight, and
And if it goes right, this could be HUGE for disabled people, chronically ill people, and people with any of the unfortunately many marginalizations that make doctors less likely to listen.
This could shave years off of the time it takes people to get the right diagnosis. It could get answers for so many people struggling with unknown diseases and chronic illness. If we compensate correctly, it could significantly reduce the role of bias in medicine. It could also make testing so much faster.
(There's a bunch of other articles about all of the ways that AI diagnoses are proving more sensitive and more accurate than doctors. This really is the sort of thing that AI is actually good at - data evaluation and science, not art and writing.)
This decade really is, for many different reasons, the beginning of the next revolution in medicine. Luckily, medicine is mostly pretty well-regulated - and of course that means very long testing phases. I think we'll begin to really see the fruits of this revolution in the next 10 to 15 years.
#confession I always struggle a lil bit with taking the mayo clinic seriously#because every. single. time I see it mentioned my first thought is mayonnaise#the mayonnaise clinic#lol
140 notes
·
View notes
Text
Latin America Molecular Diagnostics Market Set to Reach $2.50 Billion by 2031
The Latin America molecular diagnostics market is projected to reach $2.50 billion by 2031, growing at a CAGR of 6.3% from 2024 to 2031, according to a recent publication from Meticulous Research®. Several factors are driving this market growth, including the rising global geriatric population, increasing prevalence of communicable and noncommunicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759
The Latin America molecular diagnostics market is poised for significant growth due to emerging opportunities such as the expanding scope in emerging economies, increasing focus on companion diagnostics, and the rising popularity of direct-to-consumer (DTC) testing. However, the market faces challenges such as a shortage of skilled professionals, unfavorable regulatory frameworks, and the high costs associated with molecular diagnostic tests.
Key Players in the Market
Prominent players profiled in the Latin America molecular diagnostics market report include Bio-Manguinhos (Brazil), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), OmicronLab (Mexico), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), and Agilent Technologies, Inc. (U.S.).
Market Segmentation
The Latin America molecular diagnostics market is segmented by offering, test type, technology, application, and end user.
Offerings:
Kits & Reagents
Instruments
Software & Services
In 2024, the kits and reagents segment is expected to account for the largest market share. This can be attributed to the commercial availability of a diverse range of diagnostic reagents and consumables, disease-specific test kits and assays, and growing awareness regarding early disease diagnosis.
Test Types:
Laboratory Tests
Point-of-Care (POC) Tests
The laboratory test segment is anticipated to hold the largest share in 2024, driven by the extensive range of tests available in hospitals, laboratories, and academic and research institutes, as well as patient preference for these tests. Most developments in the field are also centered around laboratory tests.
Technologies:
Polymerase Chain Reaction (PCR)
In Situ Hybridization (ISH)
Isothermal Nucleic Acid Amplification Technology (INAAT)
Microarrays
Sequencing
Mass Spectrometry
Other Technologies
In 2024, the PCR segment is projected to dominate the market. This technology's benefits include the ability to test for multi-drug resistance and its application in various laboratory and clinical techniques, such as DNA fingerprinting, detection of bacteria or viruses (notably AIDS), and diagnosis of genetic disorders.
Applications:
Infectious Diseases (Respiratory Diseases, Hepatitis, HIV, Chlamydia Trachomatis/Neisseria Gonorrhoeae, Human Papillomavirus (HPV), Healthcare-Associated Infections (HAIs), and Other Infectious Diseases)
Oncology (Breast Cancer, Colorectal Cancer, Lung Cancer, Prostate Cancer, Lymphoma, Leukemia, Cervical Cancer, and Other Cancer Types)
Genetic Testing
Neurological Diseases
Cardiovascular Diseases
Other Applications
The infectious diseases segment is expected to lead the market in 2024 due to the rising prevalence of infectious diseases, increased funding for developing new diagnostic tools, and the impact of the COVID-19 pandemic.
End Users:
Hospitals & Clinics
Diagnostic Laboratories
Academic & Research Institutes
Other End Users
Hospitals and clinics are anticipated to be the largest end-user segment in 2024. This is attributed to the growing number of hospitalizations requiring molecular diagnosis and the proliferation of healthcare facilities in emerging countries like Brazil, Chile, Colombia, and Mexico.
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes
Text
New COVID variant XEC now in half of states. Here's what to know. - Published Sept 18, 2024
by Alexander Tin
COVID-19 variant trackers are now closely watching the rise of a new virus variant called XEC, which has been spotted around the world and in half of states across the United States.
Health officials are so far not raising concern about this variant, unlike some previous, more highly mutated strains that worried experts.
XEC's emergence comes as COVID-19 trends remain "high" but are now largely slowing after a summer wave of infections that peaked last month. Centers for Disease Control and Prevention modelers estimate that the virus will likely climb again over the winter, peaking in mid-January.
Here's the latest we know about the new XEC variant.
Which states have reported XEC cases?
At least 25 states have already reported at least one case with the strain's characteristic mutations, according to preliminary data obtained from the global virus database GISAID from more than 100 cases in the U.S.
Labs in New Jersey have reported the most XEC infections – at least 15 – of any state. Only California and Virginia have also reported at least 10 cases so far.
New Jersey's detections come in large part from samples collected through the CDC's testing program of arriving travelers clearing customs at Newark Liberty International Airport.
Some of the earliest U.S. cases were reported by scientists at a lab in Virginia Tech's Fralin Biomedical Research Institute, sampled from patients in July.
"We do not see a particular trend. We should keep an eye on the samples coming and continue genomic screening more broadly," said Carla Finkielstein, head of the institute's molecular diagnostics lab, in an email.
Finkielstein said that the majority of their samples come from hospitals across the southwestern part of Virginia, though it is unclear why exactly they were tested.
"Unfortunately, we do not have demographic data on these patients, so we don't know whether the patients were hospitalized or, for example, if their samples were collected during an emergency department visit," Finkielstein said.
Why is the XEC variant in the news?
Variant trackers first proposed labeling the new strain as XEC in early August, after infections were reported around the same time from labs both in Europe and Virginia.
XEC's growth in recent weeks across Germany, flagged by variant trackers like Australian consultant Mike Honey on X, has driven recent attention online to the variant's ascent.
But others in the variant tracking community have been skeptical that the strain will outcompete other strains on the rise, especially since a closely related strain called KP.3.1.1 has already reached dominance in many countries.
KP.3.1.1 now makes up more than half of cases in the U.S., according to CDC estimates published last Friday, and has been dominant for several weeks.
If XEC grows to dominance, it could mark just the latest in months of variants that have led to relatively smaller shifts in the threat posed by circulating variants.
This is in contrast to the discovery of the highly mutated BA.2.86 variant that worried health authorities around this time last year, because it had accumulated a concerning number of genetic changes compared to earlier strains.
A descendant of that BA.2.86 strain from last year, which was eventually dubbed JN.1, later rose to dominate last winter's wave of infections.
The "X" in XEC's name comes from the fact that the strain looks to be a "recombinant" of two other closely related parent variants called KS.1.1 and KP.3.3. Both KS.1.1 and KP.3.3 are descendants of the JN.1 strain.
Will XEC lead to different symptoms or vaccine effectiveness?
"CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage," a spokesperson for the agency said in a statement.
Americans are still recommended to get this fall and winter's round of updated COVID-19 vaccines, the CDC spokesperson said. Those shots were greenlighted last month with an update to target the KP.2 strain of the virus.
The Food and Drug Administration has defended its pick of KP.2 for this year's revised vaccines in recent weeks, which overrode a recommendation from the World Health Organization and a panel of the agency's outside advisers to target its parent JN.1 instead.
The FDA said in a statement Wednesday that the agency was "highly confident" in the effectiveness of this season's updated shots.
How FDA officials think XEC will impact their pick is unclear. An FDA spokesperson did not immediately respond to a request for comment.
"At this time, we anticipate that COVID-19 treatments and vaccines will continue to work against all circulating variants. CDC will continue to monitor the effectiveness of treatment and vaccines against circulating variants," the CDC spokesperson said.
#mask up#covid#pandemic#covid 19#wear a mask#public health#sars cov 2#coronavirus#still coviding#wear a respirator
16 notes
·
View notes
Text
T-1000 x Reader One Shot
Author’s Note: Don’t mind me. I’m currently obsessing over Robert Patrick’s portrayal of the T-1000 in Terminator 2: Judgement Day (1991)

His assignment was clear since the day he was created - terminate John Connor. Nothing was to stand in his way. Any and all threats were to be dealt with immediately. Skynet would be victorious in the war between machines and humans.
With the help of the boy’s foster parents, locating Connor was easy. They gave him all the information he needed and even provided him with a recent picture of their son without question, blindly trusting the badge on his chest. Weakness. Fickle emotions like that would be the cause of their inevitable downfall.
The local mall was busy with swarms of adults and children running about. He questioned a few boys that looked around Connor’s age who said they saw him at the arcade. He made a beeline to the arcade, ready to accomplish his task.
Connor was sitting one of machines with his back turned, intensely absorbed with the flashing images on the screen. The lithe Terminator pulled out the gun holstered to his belt and aimed it at the back of the young, unsuspecting target. One shot to the spine would render him paralyzed. Another to the head would sufficiently end his life. His finger slid over the trigger ready to shoot when Connor suddenly disappeared from his sight, replaced by someone else.
You, who’d be lounging on the couch in the arcade room watching your younger brother lose himself in a racing game, quickly jumped to your feet when the cop pointed his gun at John. You knew John was a troublemaker, but he couldn’t have done anything to warrant a fucking gun being pulled on him.
“John, move! Go!”
You yanked John from the game he was engrossed with and roughly pushed him toward the exit, shielding his body with yours. As serious as the situation was, John’s immaturity got the best of you and you whipped around and threw Officer Prick the finger.
When your eyes met the icy blue gaze of the cop, his forefinger froze on the trigger long enough to see you and Connor being swallowed up by the crowd. Gone. His arm slowly dropped to his side. Unblinking eyes narrowed in confusion.
It was not in his code to hesitate.
The human barricade was nothing he couldn’t have dealt with a single bullet. He was ordered to terminate anyone, anyone, who blocked his path to John Connor.
He scrutinized the hand that wielded the gun for any visible signs of damage, flexing his fingers repeatedly. With his unique molecular structure, it was impossible for his creator to add a functioning self diagnostic software so all he had to rely on was what he could physically see.
He flexed his fingers again, this time on the other hand. It’s possible there was a malfunction or two that were missed during testing. A drawback of being a prototype.
Frustration lingered, burning molten like pooling magma inside a festering volcano before solidifying into igneous determination. John Connor should be dead. He was right there and he allowed him to escape. All because of you.
While John Connor remained his primary target, he was going to make sure you never interfered with his mission again.
#terminator 2: judgement day (1991)#terminator x reader#slasher x reader#robert patrick#t 1000 x reader#t 1000#original writing
128 notes
·
View notes
Text

Amplifying Revolution: The Polymerase Chain Reaction (PCR)
Imagine a scenario where you have a crucial document, but there's only one fragile copy. You need numerous duplicates to analyze and share. This is exactly the challenge faced by scientists dealing with DNA. Thankfully, a revolutionary technique called Polymerase Chain Reaction (PCR) comes to the rescue. PCR, often referred to as molecular photocopying, is a fundamental tool in molecular biology. It allows scientists to exponentially amplify a specific DNA segment, creating millions of copies from a minuscule sample. This has revolutionized various fields, from diagnosing diseases to unraveling genetic mysteries.
The credit for inventing PCR is widely attributed to Kary Mullis, a biochemist working at Cetus Corporation in the early 1980s. Inspired by his nighttime drives through California, Mullis envisioned a method for exponentially copying DNA segments through repeated cycles of heating, annealing (primer attachment), and extension (polymerase-mediated DNA synthesis). This elegant concept became the foundation of PCR. Mullis's concept was brilliant, but a crucial hurdle remained. The process required a DNA polymerase enzyme that could withstand repeated heating and cooling cycles. The solution came from an unexpected source: hot springs. In 1976, researchers discovered Taq polymerase, a heat-stable enzyme isolated from the thermophilic bacterium Thermus aquaticus. This discovery was a game-changer, as Taq polymerase could function optimally during the high-temperature steps of PCR. In recognition of its transformative impact on science, Kary Mullis was awarded the Nobel Prize in Chemistry in 1993, alongside Michael Smith, who pioneered site-directed mutagenesis.
While the core concept of PCR was established, the technique required further refinement. Pioneering researchers like Henry Erlich at Cetus played a vital role in optimizing reaction conditions, automating the process, and developing the now-ubiquitous thermal cyclers that precisely control the temperature changes needed for PCR. The 1980s and 1990s witnessed a surge in PCR applications. In 1985, PCR was used for the first time to analyze sickle cell anemia, demonstrating its potential for clinical diagnostics. Forensic science embraced PCR in 1987, with the successful amplification of DNA from a single human hair. By 1989, highly sensitive DNA fingerprinting techniques based on PCR became a game-changer in criminal investigations.
At the heart of PCR lies a clever exploitation of the natural process of DNA replication. The key players in this drama are:
Template DNA: The DNA sequence that contains the target region to be amplified
Primers: Short sequences of nucleotides that flank the target DNA region and serve as starting points for DNA synthesis.
DNA Polymerase: Enzyme responsible for synthesizing new DNA strands by extending the primers using nucleotides.
Nucleotides: The building blocks of DNA, including adenine (A), thymine (T), cytosine (C), and guanine (G).
Buffer Solution: Provides optimal conditions for the enzymatic reactions to occur.
Thermal Cycler: Instrumentation used to automate the PCR process by cycling through different temperatures.
At its core, PCR mimics the natural process of DNA replication within an organism. However, PCR condenses this complex process into a series of controlled steps carried out within a test tube. Here's a breakdown of the PCR cycle:
Denaturation: The first step involves heating the reaction mixture to a high temperature (usually around 95°C), causing the double-stranded DNA to separate into two single strands. This process is known as denaturation.
Annealing: The temperature is then lowered to allow the primers to bind (anneal) to their complementary sequences on the single-stranded DNA. This typically occurs around 50-65°C, depending on the primer sequences.
Extension: With the primers bound, the temperature is raised again, and DNA polymerase synthesizes new DNA strands by extending from the primers using the nucleotides present in the reaction mixture. This step occurs at a temperature optimal for the DNA polymerase enzyme, typically around 72°C.
Cycle Repetition: These three steps—denaturation, annealing, and extension—are repeated multiple times (usually 20-40 cycles), resulting in an exponential increase in the number of DNA copies. Each cycle doubles the amount of DNA, leading to millions of copies of the target sequence after just a few cycles.
The beauty of PCR lies in its repetitive nature. With each cycle, the number of copies of the target DNA segment doubles. After 30 cycles, for example, you can have billions of copies of the specific DNA region, enough for further analysis.
This versatile technique has spawned numerous variations, each tailored for a specific purpose. Let's delve into some of the most common types of PCR:
Real-Time PCR (qPCR): Real-Time PCR, or quantitative PCR (qPCR), revolutionized nucleic acid quantification by enabling the real-time monitoring of DNA amplification. This technique utilizes fluorescent reporter molecules to measure the accumulation of PCR products during each cycle. qPCR is invaluable in gene expression analysis, microbial quantification, and diagnostic assays due to its high sensitivity and quantitative capabilities.
Reverse Transcription PCR (RT-PCR): Reverse Transcription PCR combines PCR with reverse transcription to amplify RNA sequences. This technique converts RNA into complementary DNA (cDNA) using reverse transcriptase enzyme before proceeding with PCR amplification. RT-PCR is pivotal in gene expression studies, viral load quantification, and the detection of RNA viruses such as HIV and SARS-CoV-2.
Nested PCR: Nested PCR involves two rounds of amplification, with the second round using a set of nested primers that bind within the product of the first round. This nested approach increases specificity and reduces nonspecific amplification, making it ideal for detecting low-abundance targets and minimizing contamination. Nested PCR is commonly used in forensic analysis, pathogen detection, and rare allele identification.
Multiplex PCR: Multiplex PCR allows simultaneous amplification of multiple target sequences within a single reaction. This technique employs multiple primer sets, each specific to a distinct target region, enabling the detection of multiple targets in a single assay. Multiplex PCR is valuable in microbial typing, genetic screening, and detection of pathogens with complex genetic profiles.
Digital PCR (dPCR): Digital PCR partitions the PCR reaction into thousands of individual micro-reactions, each containing a single DNA template molecule or none at all. By counting the number of positive and negative partitions, dPCR accurately quantifies target DNA molecules without the need for standard curves or reference samples. This technique is useful for absolute quantification of rare targets, allelic discrimination, and copy number variation analysis.
Allele-Specific PCR: Allele-Specific PCR selectively amplifies alleles containing specific nucleotide variations, enabling the detection of single nucleotide polymorphisms (SNPs) or mutations. This technique utilizes primers designed to match the target sequence with single-base mismatches at their 3' end, allowing discrimination between different alleles. Allele-Specific PCR finds applications in genetic testing, pharmacogenomics, and population studies.
PCR's ability to amplify DNA has made it an indispensable tool in various fields. Here are a few examples of its diverse applications:
Disease Diagnosis and Surveillance: PCR plays a pivotal role in the rapid and accurate diagnosis of infectious diseases. By amplifying specific nucleic acid sequences, PCR enables the detection of pathogens with high sensitivity and specificity. PCR-based tests have become indispensable in diagnosing viral infections such as HIV, hepatitis, influenza, and COVID-19. Additionally, PCR facilitates the surveillance of disease outbreaks and the monitoring of antimicrobial resistance.
Genetic Testing and Personalized Medicine: PCR empowers genetic testing by enabling the detection of genetic mutations, polymorphisms, and variations associated with inherited diseases, cancer, and pharmacogenomics. Through techniques like allele-specific PCR and real-time PCR, researchers can identify disease-causing mutations, assess drug efficacy, and tailor treatments to individual patients. PCR-based genetic tests have transformed healthcare by enabling early disease detection, risk assessment, and personalized therapeutic interventions.
Forensic Analysis and DNA Profiling: PCR has revolutionized forensic science by enabling the analysis of minute DNA samples collected from crime scenes. Techniques like short tandem repeat (STR) analysis and multiplex PCR allow forensic experts to generate DNA profiles with high resolution and accuracy. PCR-based DNA profiling is used in criminal investigations, paternity testing, disaster victim identification, and wildlife forensics, contributing to the administration of justice and conservation efforts worldwide.
Environmental Monitoring and Microbial Ecology: PCR facilitates the study of microbial communities in diverse environments, including soil, water, air, and the human microbiome. Environmental DNA (eDNA) analysis using PCR-based methods enables the detection and characterization of microbial species, including bacteria, fungi, and archaea. PCR-based assays are employed in environmental monitoring, food safety testing, and microbial source tracking, aiding in the preservation of ecosystems and public health.
Agricultural Biotechnology and Food Safety: PCR plays a vital role in agricultural biotechnology by enabling the detection of genetically modified organisms (GMOs), plant pathogens, and foodborne pathogens. PCR-based assays are used to verify the authenticity and safety of food products, detect allergens, and monitor the presence of contaminants such as pesticides and toxins. PCR-based technologies contribute to ensuring food security, quality control, and regulatory compliance in the food industry.
Evolutionary Biology and Phylogenetics: PCR-based methods are indispensable tools for studying evolutionary relationships and biodiversity. Techniques like DNA barcoding and metagenomics employ PCR to amplify and analyze DNA sequences from diverse organisms, elucidating their evolutionary history and ecological interactions. PCR facilitates the identification of new species, the study of population genetics, and the conservation of endangered species, enriching our understanding of the natural world.
PCR's versatility and precision make it indispensable in unlocking the secrets of genetics and unraveling complex biological mysteries. Its ability to amplify minute DNA samples with remarkable speed and accuracy has opened doors to countless possibilities in research and diagnostics. s we delve deeper into the intricacies of the genetic world, PCR will undoubtedly remain a powerful tool for unlocking the secrets of life itself.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#double helix#genetics#dna#polymerase chain reaction#medical science#the more you know#scientific research#scifiart#scientific advancements#scientific illustration#scientific instruments#scientific discovery
7 notes
·
View notes
Text
Unlocking the Potential: Emerging Players in Blood Cancer Diagnostics
Blood cancer, a complex group of diseases affecting the blood, bone marrow, and lymphatic system, demands precise and efficient diagnostic tools. As advancements in medical technology continue to reshape the healthcare landscape, the Blood Cancer Diagnostics Market is witnessing dynamic changes. Let’s explore the key dynamics shaping this market.
Advanced Testing Strategies in the Blood Cancer…

View On WordPress
#Blood Cancer#Blood Cancer Diagnostics#Blood Cancer Diagnostics Market#Blood Tests#Imaging Tests#Medical Device#Medical Devices Market#MedTech#MedTech Market#Molecular Tests
0 notes
Text
#Influenza (flu) is a transmitted respiratory #disease caused by influenza viruses that infect #respiratory tract and lungs. The onset of flu symptoms are usually acute and characterized by fever, chills, headache, fatigue, sore throat and dry cough. #Genes2Me offers Influenza A/B-Q (H1N1 & H3N2) Real Time #PCR Multiplex kit for simultaneous detection & differentiation of #InfluenzaA, #InfluenzaB & Influenza A sub-types #H1N1 & #H3N2 virus specific #RNA along with Internal control.
Visit our website for more information. https://www.genes2me.com/ivd-real-time-pcr-test-kits/respiratory-disease-diagnostic-kit
For more details, Call us at +91-8800821778 or drop us an email at [email protected]
#g2m #rtpcr #detection #ivd #kits #madeinindia #molecular #diagnostics #fever #cough #cold #moleculardiagnostics #moleculardiagnosticslab #risk #testing #testingsolutions
#influenza#influenzaA#influenzaB#rt-pcr kits#g2m#genes2me#h1n1#h3n2#rna solutions#dna solutions#testing kit#diagnostics solutions#made in India#molecular biology
0 notes