#thrombectomy
Text

This drawing is quite old. It’s practically coated in dust and mold 😭😭
Holy fuck that rhymed
This drawing doesn’t have straight lines
Golly that rhymed again
I drew this in my gooning den
Jk I don’t goon
I’ll save that for the loons
Btw this is a catheter as an anime girl
Look at my apple pencil I gave it a twirl
Jk I used my finger
I’m ending this I can’t have this linger
#digital art#ihnmaims#catheter#thrombectomy#blood clots#medicine#tumblr draw#artists on tumblr#humanization
3 notes
·
View notes
Text
Neurovascular Thrombectomy Devices Market
Neurovascular thrombectomy devices are used for eliminating the blood clots that have accumulated in the neurovascular vessels during an acute ischemic stroke. These devices comprise a wide range of endovascular tools that are capable of removing thrombi from Neurovasculature in the case of patients suffering from an acute ischemic stroke. These devices are effectively used in the treatment of…

View On WordPress
2 notes
·
View notes
Text
Clot Management Devices: Clot Management for Improved Treatment of Thromboembolism

The Evolution of Clot Removal Technology
Clot formation inside blood vessels that supply the lungs, brain or other vital organs can have life-threatening consequences if not treated promptly and effectively. For decades, pharmacological interventions involving anticoagulant and thrombolytic drugs were the primary treatment approaches. However, these systemic therapies are not always fully successful in dissolving or removing clots, and carry risks of hemorrhage. In the past two decades, medical device technologies for minimally invasive mechanical removal or dissolution of clots have rapidly advanced. These clot management devices have revolutionized the treatment of pulmonary embolism and cerebral venous thrombosis.
Catheter-Based Clot Retrieval
One of the earliest and most widely used clot extraction approaches involves catheter-delivered retrievable stent designs. These devices utilize a nitinol mesh cylindrical structure that can be collapsed inside a delivery catheter and expanded upon deployment within a clot. Barbera first described use of the Tulip venous filter for retrieval of acute iliofemoral deep vein thrombosis in 1994. Building upon this, devices such as the Retrieval Inferior Vena Cava Filter and Denali Filters were developed specifically for clot capture and removal from larger veins. For pulmonary embolism, retrievable expandable stent designs like the Tulip Vena Cava Filter, Recovery Filter and OptEase Filter allowed for minimally invasive extraction of clots from the pulmonary arteries.
A major advancement was the introduction of stent retrievers optimized forremoving thromboembolic occlusions from intracranial blood vessels. The Merci Retriever was among the earliest such devices approved by the FDA in 2004 for mechanical thrombectomy in acute ischemic stroke. Second generation retrievable stents like the Solitaire and Trevo devices featured superior design and deliverability profiles enabling higher recanalization rates. Recent studies support use of stent retrievers as the first-line treatment for large vessel occlusions in the anterior cerebral circulation. Ongoing research aims to expand the utility of Clot Management Devices catether-deployed devices to more distal vessel occlusions.
Ultrasound-Accelerated Thrombolysis
While pharmacologically-assisted thrombolysis remains a mainstay for selected patients, treatment times can be prolonged. Ultrasound energy has shown promise in accelerating fibrinolysis through mechanical fracture of clot structure and increased uptake of thrombolytic drugs. Devices generating low-frequency, high-intensity ultrasound via an intravascular catheter have been evaluated. Early studies demonstrated the EKOS System's ability to rapidly dissolve pulmonary emboli when infusing alteplase, halving treatment duration versus pharmacological therapy alone.
The Sonolysis Thrombolytic Infusion Catheter was developed for treating iliofemoral deep vein thrombosis, administering ultrasound plus alteplase directly within the thrombus. More recently, the EkoSonic Endovascular System received FDA clearance for use in acute massive and submassive pulmonary embolism. Its small-profile ultrasound emitter works in tandem with front-line thrombolytic drugs to rapidly recanalize blood clots. Ongoing research also investigates combining intravascular ultrasound with novel thrombolytic drug formulations like microbubbles or plasminogen activators to potentially enhance and target fibrinolysis.
Get more insights on Clot Management Devices
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Clot Management Devices#Thrombectomy#Blood Clot Removal#Vascular Surgery#Thrombolysis#Endovascular Devices#Stroke Treatment#Deep Vein Thrombosis#Pulmonary Embolism
0 notes
Text

what can cause an AV Fistula to Fail ?
An AV fistula can fail when there is a narrowing, also called stenosis, in one of the vessels associated with the fistula. When a narrowing occurs, the volume and rate of blood flow can decrease, and you may be unable to dialyze adequately.
To Book an Appointment : +91 88665 49555
visit:https://www.hyderabadvascularcenter.com/av-fistula-treatment
#Medications#AVfistula#HyderabadVascularCenter#HVC#newfistula#Thrombectomy#AVfistulafails#avfistula#radiocephalicfistula#brachiocephalicfistula#basilicveinfistula#vascular#vascularsurgery#hemodialysis#varicosevein#varicoseveintreatment#hvc#hyderabadvascularcenter#deepveinthrombosis#dvt#stoptheclot#varicoseveins#venousthromboembolism#communityconnection
0 notes
Text
The global thrombectomy devices market is projected to reach USD 1,682.7 million in 2023, registering at a Compound Annual Growth Rate (CAGR) of 5.8% during the forecast period 2024-2030. The growth of the marketis majorly driven by the technological advancements in medical devices used for capturing blood clots
0 notes
Text
The Neurovascular Thrombectomy Devices market size was $872.5 million in 2023. Factors such as constant revision of protocol and best practice guidelines will drive the market growth at a CAGR of more than 8% from 2023 to 2033.
0 notes
Text
Thrombectomy Devices Market Size, Share, Trends, Opportunities, Key Drivers and Growth Prospectus
A Qualitative Research Study accomplished by Data Bridge Market research's database of 350 pages, titled as Global Thrombectomy Devices Market with 100+ market data Tables, Pie Charts, Graphs & Figures spread through Pages and easy to understand detailed analysis. The rapidly revolutionizing market place demands the best market and business solutions to thrive in the market.
Thrombectomy Devices Market business report can be referred efficiently by both established and new players in the industry for absolute understanding of the market. It covers various parameters that range from latest trends, market segmentation, new market entry, industry forecasting, target market analysis, future directions, opportunity identification, strategic analysis, insights to innovation. In this industry report, industry trends have been described on the macro level which makes it possible outline market landscape and probable future issues. The statistical and numerical data collected to generate this report is mostly denoted with the graphs, tables and charts as required which make this report more user-friendly.
Access Full 350 Pages PDF Report @
Data Bridge Market Research analyses that the thrombectomy devices market which was USD 1.3 billion in 2022, would rocket up to USD 2.06 billion by 2030, and is expected to undergo a CAGR of 5.9% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
The Thrombectomy Devices Market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance.
Major Points Covered in TOC:
Thrombectomy Devices Market Overview: It incorporates six sections, research scope, significant makers covered, market fragments by type, Thrombectomy Devices Market portions by application, study goals, and years considered.
Thrombectomy Devices Market Landscape: Here, the opposition in the Worldwide Thrombectomy Devices Market is dissected, by value, income, deals, and piece of the pie by organization, market rate, cutthroat circumstances Landscape, and most recent patterns, consolidation, development, obtaining, and portions of the overall industry of top organizations.
Thrombectomy Devices Profiles of Manufacturers: Here, driving players of the worldwide Thrombectomy Devices Market are considered dependent on deals region, key items, net edge, income, cost, and creation.
Thrombectomy Devices Market Status and Outlook by Region: In this segment, the report examines about net edge, deals, income, creation, portion of the overall industry, CAGR, and market size by locale. Here, the worldwide Thrombectomy Devices Market is profoundly examined based on areas and nations like North America, Europe, China, India, Japan, and the MEA.
Thrombectomy Devices Application or End User: This segment of the exploration study shows how extraordinary end-client/application sections add to the worldwide Thrombectomy Devices Market.
Thrombectomy Devices Market Forecast: Production Side: In this piece of the report, the creators have zeroed in on creation and creation esteem conjecture, key makers gauge, and creation and creation esteem estimate by type.
Keyword: Research Findings and Conclusion: This is one of the last segments of the report where the discoveries of the investigators and the finish of the exploration study are given.
What to Expect from the Report, a 7-Pointer Guide
The Thrombectomy Devices Market report dives into the holistic Strategy and Innovation for this market ecosystem
The Thrombectomy Devices Market report keenly isolates and upholds notable prominent market drivers and barriers
The Thrombectomy Devices Market report sets clarity in identifying technological standardization as well as the regulatory
framework, besides significantly assessing various implementation models besides evaluation of numerous use cases
The Thrombectomy Devices Market report is also a rich repository of crucial information across the industry, highlighting details on novel investments as well as stakeholders and relevant contributors and market participants.
A through market analytical survey and forecast references through the forecast tenure, encapsulating details on historical developments, concurrent events as well as future growth probability
Some of the major players operating in the thrombectomy devices market are:
Medtronic (Ireland)
Inari Medical, Inc (U.S.)
AngioDynamics (U.S.)
Terumo Corporation (Japan)
Stryker (U.S.)
Zimmer Biomet (U.S.)
Boston Scientific Corporation (U.S.)
BD (U.S.)
Straub Medical AG (Switzerland)
Penumbra, Inc (U.S.)
Edwards Lifesciences Corporation (U.S.)
ARGON MEDICAL. (U.S.)
Teleflex Incorporated (U.S.)
phenox GmbH (Germany)
Acandis GmbH (Germany)
Merit Medical Systems (U.S.)
Bayer AG (Germany)
Getinge AB (Sweden)
Philips Healthcare (U.S.)
Browse Trending Reports:
Percutaneous Nephroscope Market
Mobile Telepresence Robots Market
Pacs And Ris Market
Wireless Medical Device Connectivity Market
Alzheimers Disease Diagnostic Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Thrombectomy Devices Market Size#Share#Trends#Opportunities#Key Drivers and Growth Prospectus#market report#market size#market share#marketresearch#market trends#market analysis#markettrends#market research
0 notes
Text
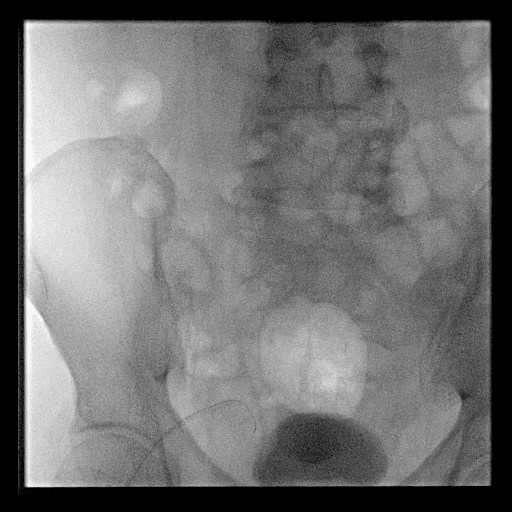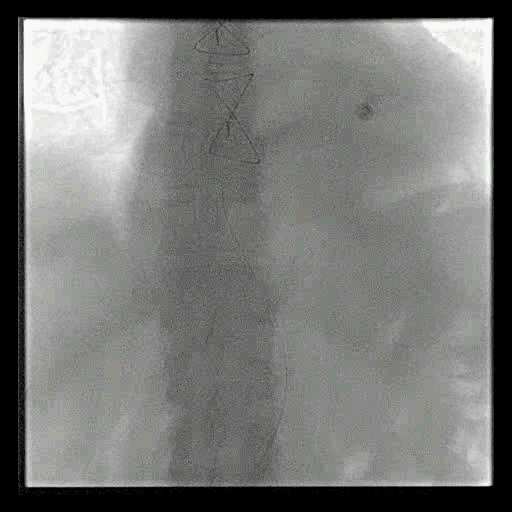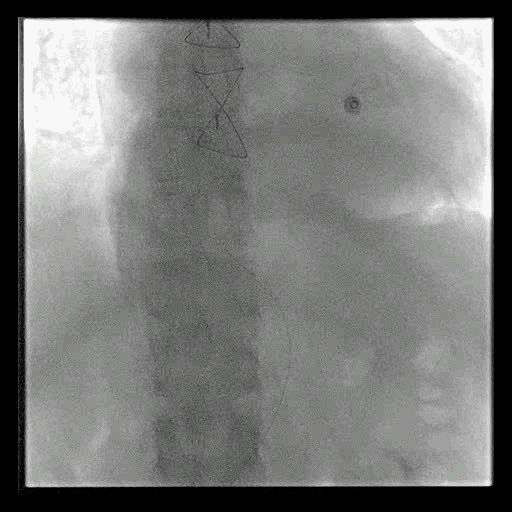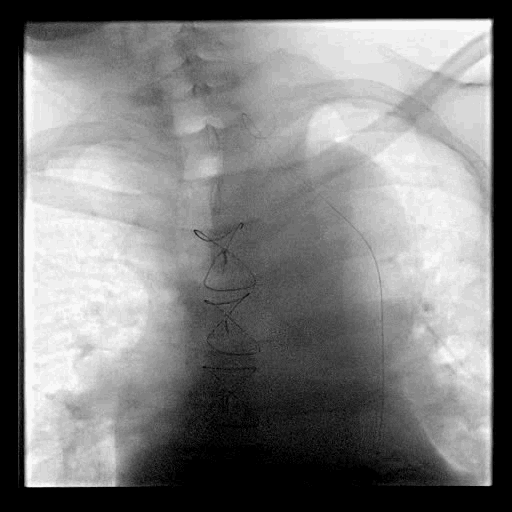

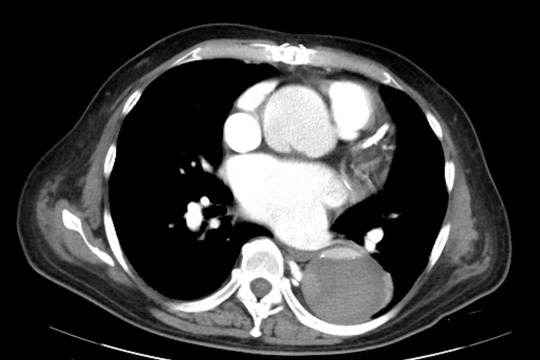
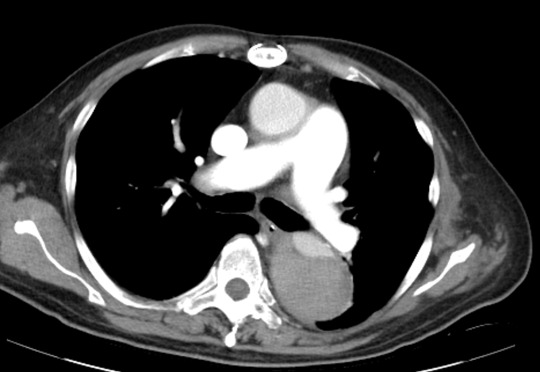
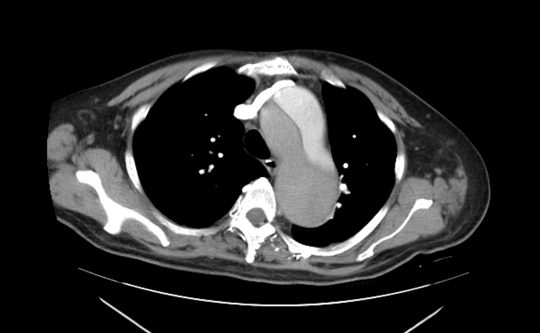
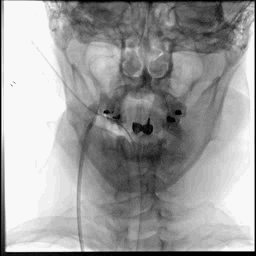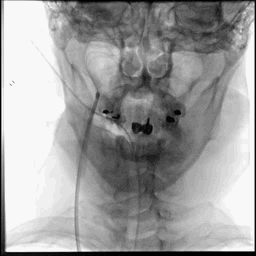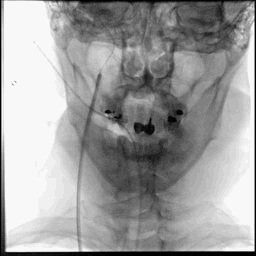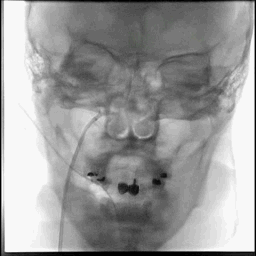
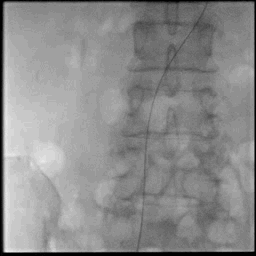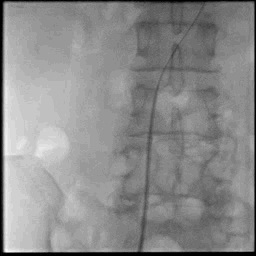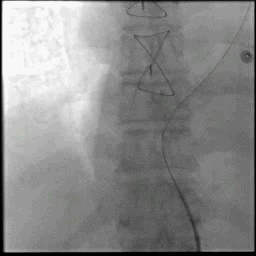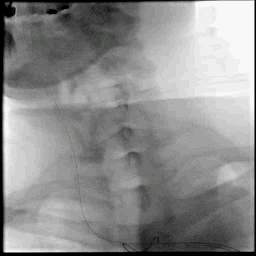
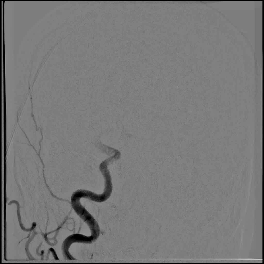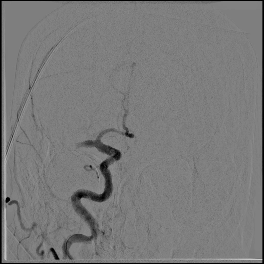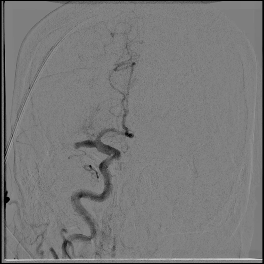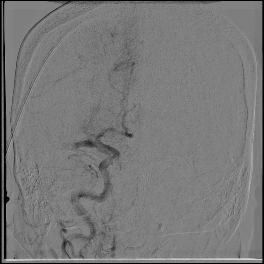
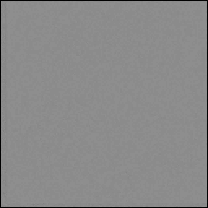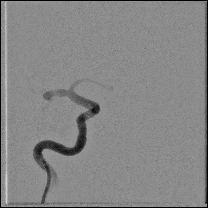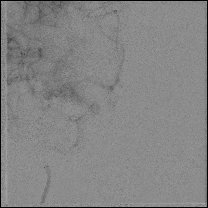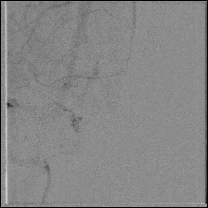
Acute stroke IA thrombectomy in a patient with established DAA.
The critical technique in this case is how to feel that the 0.035-inch Terumo GlideWire was in the true lumen?
The tactile feeling of the 0.035-inch wire was the key point.
1 note
·
View note
Text
Improving the stroke pathway in southern Sweden
By implementing a series of procedural and technological innovations, Skåne University Hospital in Lund and 13 local hospitals in the area have greatly improved the patient pathway in the stroke network of southern Sweden. Saving valuable time was a prime objective but also the identification of more patients eligible for endovascular thrombectomy.
Distance is a major challenge in Sweden’s…

View On WordPress
0 notes
Text
The Rising Global Demand for Minimally Invasive Procedures will drive the Blood Clot Retrieval Devices Market
The blood clot retrieval devices market is a niche but rapidly growing segment due to the increasing preference for minimally invasive procedures for treating conditions such as cardiovascular diseases and neurological disorders. Blood clot retrieval devices are used to remove blood clots from arteries or veins, usually during acute ischemic stroke. They help restore blood flow and limit neurological damage. Key devices available include stent retrievers, aspiration devices, vascular snares, and clot-busting drugs.
The global blood clot retrieval devices market is estimated to be valued at USD 1.62 Bn in 2024 and is expected to reach USD 4.40 Bn by 2031, exhibiting a compound annual growth rate (CAGR) of 15.3% from 2024 to 2031.
Key Takeaways
Key players operating in the blood clot retrieval devices are Stryker Corporation, Medtronic plc, Johnson & Johnson, Boston Scientific Corporation, Penumbra, Inc., Terumo Corporation, Edwards Lifesciences Corporation, Argon Medical Devices, Inc., Cook Medical Inc., Control Medical Technology, Acandis GmbH, Phenox GmbH, B. Braun Melsungen AG, Teleflex Incorporated, MicroVention, Inc., Merit Medical Systems, W. L. Gore & Associates, Inc., and Inari Medical, Inc. Stent retrievers are increasingly being used for mechanical thrombectomy to remove large blood clots from arteries during acute ischemic stroke. Aspiration catheters are also growing in popularity due to their ease of use.
The increasing global disease burden of strokes and cardiac arrests is a major driver of demand for Blood Clot Retrieval Devices Market Demand . According to WHO, over 15 million people suffer a stroke each year worldwide. Rising awareness about minimally invasive procedures and availability of advanced devices are supporting the adoption of mechanical thrombectomy for time-critical conditions. Technology advancements such as bioabsorbable polymers, self-expanding stent designs and optimized catheter structures are helping improve outcomes.
Key trends in the market include the introduction of newer generation stent retrievers and aspiration catheters with enhanced efficacy. Companies are focusing on developing innovative retrieval techniques as well as devices compatible with imaging modalities like CT and MRI. Wider access todevices aided by favorable reimbursement and regulatory approvals will further support market growth. Expanding applications from neurovascular to peripheral vascular procedures present new opportunities.
The main opportunities in the Blood Clot Retrieval Devices Market Analysis raising awareness in developing regions, launch of low-cost devices, and utilization of latest materials and automation technologies for better clot removal success rates. Emerging economies with large patient populations but low treatment rates offer scope for market penetration. With ongoing R&D, retrieval devices may replace conventional open surgeries over the long run.
Get more insights on Blood Clot Retrieval Devices Market
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)
#Coherent Market Insights#Blood Clot Retrieval Devices Market#Blood Clot Retrieval Devices#Medical Devices#Thrombectomy#Endovascular Therapy#Stroke Treatment#Neurointervention#Vascular Surgery#Clot Removal#Minimally Invasive Surgery
0 notes
Text
Global Mechanical Thrombectomy Devices Market was valued at US$ 760 million in 2021 and is projected to expand at a CAGR of 6% by 2026
A common health concern that requires immediate medical attention is blood clots. Mechanical thrombectomy devices are used in procedures used to treat arterial blockages caused by blood clots. A mechanical thrombectomy can be used to treat ischemic stroke, pulmonary embolism (PE), and deep vein thrombosis (DVT).
The Global Mechanical Thrombectomy Devices Market was valued at US$ 760 million in 2021 and is set to witness a healthy growth rate of 6% in the next 5 years. The rising cases of stroke, venous thromboembolism (deep vein thrombosis & pulmonary embolism), approval/launch of novel mechanical thrombectomy devices, favorable clinical trial outcomes with mechanical thrombectomy, and acquisitions by leading players to fortify their vascular portfolio, and treat patients with blood clots are some of the important factors driving the global mechanical thrombectomy devices market.
However, some of the crucial factors that are likely to hamper its development are inadequate specialists and limited awareness and education of both the physicians and the patients.
Technological Advancements in Mechanical Thrombectomy Devices Drives the Mechanical Thrombectomy Devices Market
Technological advancements are expected to boost the mechanical thrombectomy devices market.
For instance,
In April 2022, Penumbra announced that its Indigo Aspiration System with Lightning 7 and Lightning 12 has secured CE Mark and are now commercially available in Europe. Both technologies are part of Penumbra’s Indigo Aspiration System - now with Intelligent Aspiration for mechanical thrombectomy -and are designed for single-session arterial and venous thrombus removal, including the treatment of pulmonary embolisms
“Existing mechanical thrombectomy treatment options do not adequately treat VTE for several reasons. Most current mechanical thrombectomy devices are designed to aspirate fresher arterial clot, which is small and soft. As a result, these devices can be inadequate and ineffective for removing the larger, older clots associated with VTE.” - General Manager, Commercial-stage Medical Device Company, APAC
Explore Premium Report on Mechanical Thrombectomy Devices Market @ https://meditechinsights.com/mechanical-thrombectomy-devices-market/
Covid-19 Impact on the Mechanical Thrombectomy Devices Market
In 2020, the number of elective procedures reduced significantly due to Covid-19 which resulted in a decline in the market. There are various plausible reasons for the apparent reduction in mechanical thrombectomy performance between 2020-2021:
Delays in accessing specialized care, resulting in patients presenting outside of the therapeutic window
Reluctance of acute ischemic stroke patients to present at emergency departments
Reduction in stroke occurrence during the COVID-19 pandemic as a result of mandated social distancing
However, towards the end of 2021 fourth quarter, companies witnessed the pressure from COVID-19 gradually alleviated and businesses are now trending close to normalized run rates.
Organic and Inorganic Growth Strategies Adopted by Players to Establish Their Foothold in Mechanical Thrombectomy Devices Market
The players operating in the mechanical thrombectomy devices market adopted both organic and inorganic growth strategies such as acquisitions and new product launches to garner market share.
For instance,
In April 2022, Wallaby Medical acquired phenox GmbH. phenox has a broad product portfolio covering both ischemic and hemorrhagic stroke as well as Access & Support. The key product lines of phenox include the p64/p48 range of flow diverters for the treatment of intracranial aneurysms and the pRESET range of stent retrievers for the mechanical thrombectomy of ischemic strokes
The outlook for the mechanical thrombectomy devices market looks promising due to the increasing geriatric population and cases of stroke, venous thromboembolism, technological developments in mechanical thrombectomy devices, strong product pipeline, established coding & payments for mechanical thrombectomy in key markets such as the U.S. and growing awareness about mechanical thrombectomy due to patient advocacy groups and clinical society support.
Competitive Landscape Analysis of Mechanical Thrombectomy Devices Market
The global mechanical thrombectomy devices market is marked by the presence of players such as Medtronic, Stryker, Penumbra, Boston Scientific, AngioDynamics, Control Medical, Inari Medical, among others.
For More Detailed Insights, Contact Us @ https://meditechinsights.com/contact-us/
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
0 notes
Note
What's yer job like?
I work in a hybrid operating room at a level 1 trauma center, so I get to do a lot of cool things!
I scrub in with a surgeon to help perform operations like aortic aneurysm repair (before or after rupture, and with or without a fenestrated graft), aortic transection or dissection repair, thrombectomy, embolization, atherectomy, transcarotid artery revascularization, and more.
At my hospital my position always seems to be short staffed 😅 so I end up taking a lot of call outside of my work hours. I love my job, though! I just wish there were more hours in each day for other things
53 notes
·
View notes
Text
Neurovascular Thrombectomy Devices Market Analysis
Explore segments, trends, and forecasts for neurovascular thrombectomy.
0 notes
Text
When time, read:
[ ] double barrel wet colostomy
[ ] appendiceal cancer
[ ] malignant bowel protocol
[ ] May Thurner syndrome
[ ] Po vs IV vs rectal vs vaginal contrast
As always, so, so tired. Last "work" night of 5 weeks with a call shift tomorrow. So the night goes oncology night shift > present research @ 0930 > sleep > graduation @ 1800 > call shift.
I'm dreading the whole freaking day. My research proposal is not good. My slides are messed up. It's confusing. I think the study itself will be fine, but a 5 week night float was not the time or place to get my shit together for this. I just hope the program coordinator can find it within herself to update my slides in the morning, or else I'm fucked. And then I'm gonna get such LITTLE SLEEP before I have to go to graduation for 4 hrs and pretend to be in a good mood and socialize. At least my call shift right afterwards is normal R2 crap, antepartum/benign gynecology stuff.
To be honest covering the oncology service has been chill enough the past shift-and-half, except for when it's bad it's BAD and probably the worst service to be on. I like onc nights more than days, though, because there's not a lot of extra people and I can just sit in my little work room upstairs and be alone and read through things. It's mostly covering the OR at the end of the day shift, seeing overnight direct admits and transports, and following up vitals and miscellaneous labs, post-op checks, etc. And then sometimes people surgical emergencies, or people crump or straight up die.
So, a medicine service +/- OR time.
My first night I had two transports: a malignant large bowel obstruction iso newly diagnosed HGSOC that is being managed conservatively given its size <13 cm but also her significant neutropenia due to her neoadjuvant Avastin therapy (her prognosis.... isn't amazing), and a "frequent flier" with recurrent vulvar cancer (and the gnarliest genitourinary anatomy I've seen) s/p MULTIPLE resections, EUAs, ablations admitted with c/f sepsis in the setting of a new perirectal abscess. All things considered it went well enough but my presentations were rocky, like I'd expect them to be for a new R2, but I'm almost R3. I was flustered because they both came at the same time and the LBO made me nervous, so I felt rushed trying to get the other transport tucked in so I only had to call the attending once.
Tonight started out with the potential to be a NIGHTMARE. There were two ORs running late, an exlap followed by an EUA with one attending, and a robotic hyst with another, and then I got sign out on four (4) incoming transports, and I also had four (4) post-op checks all due around the same time. I ended up not having to go to the OR because the exlap said I don't need to scrub anyone out, the EUA was cancelled, and one of the transports came at the same time so my R3 said to see that instead of scrubbing her out.
It's a very sad case, though. I mean, all onc cases are sad, but she was with her dad which just tore at my heart. It's a woman <50 yo, no hx cancer, with 3-4 months of back pain who finally had an ultrasound and CT that showed 3 large abdominopelvic masses and widespread mets. :c She's getting omental biopsies tomorrow. We ultimately think it's appendiceal or some other gastrointestinal malignancy because her CA-125 is only about 180, which for a pre-menopausal woman is low. Another one where the prognosis is not very good.
Actually, none of these people I admitted have good prognoses. I guess that's just how gyne onc is. :-/
The other transports probably aren't coming but I'm going to try and prep them in a second. There's a pelvic fluid collection in a woman 5d s/p a hyst with a post-op course c/b May Thurner syndrome and PE s/p extensive thrombectomy now on Eliquis, another vulvar cancer patient whose left drain fell out and now there is c/f infection in addition to just general failure to thrive, and someone who probably won't come who was found to be hypokalemic apparently on outpatient labs. And then some mystery woman in her 80s that may or may not show up on the ED board with recurrent HGSOC and a GTube for gastrointestinal issues.
3 h til signout, and then I have about 2-2.5 hr to sleep and practice my presentation. I'm ready for this weekend to be overrrrrrrrrrrr
2 notes
·
View notes
