#API and Pharmaceutical Intermediates
Explore tagged Tumblr posts
Text
Delve into the world of active pharmaceutical intermediates (APIs) and explore their pivotal role in drug development. Gain insights on how APIs differ from finished pharmaceutical products. Stay updated with Saurav Chemicals' informative blog on pharmaceutical industry advancements.
0 notes
Text

Vidgastech – Ruxolitinib Intermediates Manufacturer
Vidgastech is a leading manufacturer and global supplier of Ruxolitinib Intermediates. We specialize in high-purity pharmaceutical intermediates for API production, supporting the global pharma industry with quality, consistency, and innovation.
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#Ruxolitinib Raw Material#API Supplier#Vidgastech#GMP Manufacturer#Bulk Drug Intermediates#Custom Synthesis
0 notes
Text

In line with our expanding presence, we continue to collaborate with trusted clients and partners who share our long-term vision. Our consistent focus on precision, quality, and performance shapes every aspect of our service. We cater to sectors like Pharmaceuticals, Agrochemicals, and Specialty Chemicals, backed by robust CRO and CDMO support.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
What are Pharmaceutical Intermediates? A Complete companion to Their part in Drug Manufacturing
In the vast and complex world of medicines, the trip from raw accoutrements to life- saving drugs is long and intricate. One of the most critical yet frequently overlooked way in this trip is the use of pharmaceutical intermediates. These chemical composites serve as the structure blocks for active pharmaceutical constituents( APIs), which are, in turn, the essential factors of every drug we consume. But what exactly are pharmaceutical intermediates, and why are they so important in the medicine manufacturing process?

Understanding Pharmaceutical intermediates
Pharmaceutical intermediates are chemical substances that are produced during the conflation of an active pharmaceutical component( API). They are not the final medicine itself but play a crucial transitional part during the multi-step process that eventually results in the conformation of a finished pharmaceutical product.
In simpler terms, intermediates are like incompletely completed products in an assembly line. They suffer farther chemical responses and variations to come an API, which is the biologically active part of a drug responsible for its remedial effect.
Types of Pharmaceutical intermediates
Pharmaceutical intermediates can be astronomically classified into three main types
Starting Accoutrements
These are the original composites that suffer chemical changes to ultimately form the API. They're the raw accoutrements of the conflation process.
Intermediate composites
These are the substances formed between the starting material and the final API. They can go through several stages and chemical responses before reaching the final form.
Final intermediates
These are the last substances produced before the API is synthesized. They're chemically close to the API and frequently bear only minor variations to come the final active component.
Significance of Pharmaceutical intermediates
Pharmaceutical intermediates play a vital part in icing the quality, safety, and efficacity of the final medicine product. Then is why they count
Quality Control Monitoring the quality of intermediates ensures that the final API is free from contaminations and meets nonsupervisory norms.
Efficiency By producing intermediates in bulk, pharmaceutical companies can streamline the API product process, perfecting effectiveness and reducing costs.
Customization Different intermediates can be synthesized to develop new or advanced APIs, opening the door to innovative medicine development.
How intermediates Are Used in Drug Manufacturing
The process of medicine manufacturing generally involves the following way conflation of intermediates Chemical responses are performed on raw accoutrements to produce intermediates.
Conversion to API The intermediates suffer farther chemical processes to transfigure into the active pharmaceutical component.
Formulation The API is also combined with excipients( inactive substances) to form the final medicine product — similar as tablets, capsules, or injections.
Each of these stages must misbehave with strict regulations from authorities like the FDA( U.S. Food and Drug Administration), EMA( European Medicines Agency), and other global bodies to insure public safety.
Regulatory Aspects and Quality norms
The manufacture and use of pharmaceutical intermediates are rigorously regulated to maintain the integrity of the medicine force chain. Companies producing intermediates must cleave to Good Manufacturing Practices( GMP), which set the standard for product quality, hygiene, record- keeping, and traceability.
In addition to GMP, intermediates may also be subordinated to checkups and examinations by health authorities, especially if they're exported to countries with strict nonsupervisory conditions. Compliance with guidelines similar as ICH Q7( Good Manufacturing Practice for Active Pharmaceutical constituents) is essential.
Global Trade and Sourcing
India and China are among the leading directors of pharmaceutical intermediates, supplying these vital factors to global requests. numerous pharmaceutical companies in the U.S., Europe, and away calculate on Indian manufacturers for high- quality intermediates due to their cost- effectiveness and compliance with transnational norms.
Companies like Chemox Pharma, for case, specialize in manufacturing and exporting crucial intermediates used in the product of APIs like Mirabegron, Tadalafil, Atorvastatin, and numerous others. Their part is pivotal in maintaining the steady force of essential drugs worldwide.
Challenges and openings
Despite their significance, the pharmaceutical intermediates assiduity faces several challenges force Chain dislocations Events like afflictions, geopolitical pressures, or nonsupervisory shifts can impact the global inflow of intermediates.
Quality Assurance icing thickness and chastity at every step requires robust quality systems.
Environmental Impact The product of intermediates frequently involves dangerous chemicals and must be managed responsibly.
Still, there are also growing openings, especially with rising demand for general medicines, biologics, and substantiated drug. Investing in invention, sustainability, and nonsupervisory compliance will help manufacturers of pharmaceutical intermediates thrive in the long term.
Conclusion
Pharmaceutical intermediates may not be as well- known as the medicines they help produce, but their part is absolutely essential in the healthcare assiduity. They form the core of medicine manufacturing processes, icing that the drugs we calculate on are safe, effective, and constantly available. As the global medicinal geography evolves, the demand for dependable, high- quality intermediates will only continue to grow — making them a foundation of ultramodern drug.
0 notes
Text

Monday CRM offers customizable and automated workflows that reduce manual tasks and improve sales tracking. Its flexible boards and automation rules help teams align sales activities with strategic goals and adapt quickly to market changes.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#it#technology#it jobs#tech#crm benefits#crm services#sierra consulting#current events#technews#crm#crm strategy#sales crm#crm platform#crm integration#crm software#crm solutions#businesssolutions#business growth
0 notes
Text
API Intermediates Manufacturers in India
Chempro is a trusted manufacturer and supplier of high-quality API intermediates in India. A list of API (Active Pharmaceutical Ingredients) Intermediates Manufacturers in Mumbai, India, including intermediate names, CAS numbers, and corresponding API names.
Visit Us: https://www.chemprogroup.com/pharma/api-intermediate.html
0 notes
Text
Opportunities For API Manufacturing in Pharma Industry in 2024
APIs are the main components responsible for the therapeutic effects of drugs. These substances undergo several processes, transforming raw materials into APIs and pharmaceutical intermediates, which are critical steps in drug manufacturing. Pharmaceutical intermediates serve as building blocks that are essential in synthesizing APIs with desired therapeutic properties.
1 note
·
View note
Text
api intermediates manufacturers
Book drug, the capital of India, produces a wide range of intermediate products exporters. We produce high quality intermediate products at affordable prices. A leading international supplier of active pharmaceutical ingredients with the industry's most extensive portfolio of specialized international manufacturing locations. Synthetic and natural are further categorized into innovative and generic. The services produced and sold there can also be considered intermediate goods if they are used as inputs in the production process of other goods. Salt is an intermediate product, and companies incorporate it into many food and non-food final products. Wheat is an intermediate product because companies process it as part of another product, usually a food or grocery product.
2 notes
·
View notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
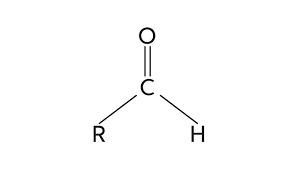
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
Impor Bahan Baku Farmasi
Impor Legal Bahan Baku Farmasi Keenam International melayani jasa impor resmi bahan baku farmasi dari berbagai negara, sesuai standar BPOM dan regulasi Kementerian Kesehatan RI. Barang yang biasa kami tangani mencakup: Active Pharmaceutical Ingredients (API) Bahan pelarut & eksipien Zat aktif & aditif Enzim & senyawa biologis Bahan intermediate untuk produksi obat 🌍 Negara Asal Utama 🇨🇳…
#API Import Indonesia#Customs BPOM#customs clearance farmasi#Freight Forwarder Obat#impor API#impor bahan baku farmasi#Impor Farmasi Indonesia#jasa freight forwarder farmasi#jasa impor bahan aktif obat#jasa impor resmi
0 notes
Text
Pharmaceutical Intermediates Manufacturers | Saurav Chemicals
Explore Saurav Chemicals for top-notch pharmaceutical intermediates, ensuring reliability, a broad spectrum of options, and adherence to GMP standards to enhance the success of your pharmaceutical synthesis endeavors. Rely on our trusted quality for optimal results in your pharmaceutical projects.
#Pharma Intermediates#API Intermediates Manufacturers#Pharma Intermediates manufacturers#Pharmaceutical Intermediates manufacturers
1 note
·
View note
Text
Ruxolitinib Intermediates: Enabling Targeted Therapy with Quality Manufacturing from Vidgastech
In the rapidly evolving world of pharmaceutical research and precision medicine, Ruxolitinib has gained significant attention as a JAK1/JAK2 inhibitor used to treat conditions like myelofibrosis, polycythemia vera, and other rare blood cancers. To ensure effective and scalable drug development, the demand for high-quality Ruxolitinib intermediates has surged — and that’s where Vidgastech leads the way.
🔬 What Are Ruxolitinib Intermediates?
Ruxolitinib intermediates are the essential chemical compounds used during the synthesis of the final active pharmaceutical ingredient (API), Ruxolitinib. These intermediates must be manufactured with strict quality standards to ensure:
Purity and consistency
Scalability for formulation
Compliance with global pharmacopeia
🏭 Vidgastech: A Trusted Source for Pharma Intermediates
Vidgastech has established itself as a reputable manufacturer and exporter of pharmaceutical intermediates in India, specializing in cutting-edge molecules like Ruxolitinib intermediates. With modern production facilities, a skilled R&D team, and commitment to regulatory standards, Vidgastech ensures:
GMP-compliant manufacturing
Customized synthesis on request
Prompt global delivery
💡 Why Choose Vidgastech for Ruxolitinib Intermediates?
High Purity Compounds: Ensuring effectiveness in the final API.
Regulatory Compliance: Following stringent quality checks.
Custom Solutions: Tailored synthesis as per client specifications.
Reliable Supply Chain: On-time delivery across the globe.
🌍 Applications in Oncology
Ruxolitinib is a vital component in the treatment of:
Myelofibrosis
Polycythemia Vera
Graft-Versus-Host Disease (GVHD)
Ongoing trials for autoimmune conditions
With the growth of targeted therapies, Ruxolitinib intermediates play a foundational role in pharmaceutical innovation.
🔗 Connect with Vidgastech
If you're a pharmaceutical manufacturer, researcher, or procurement manager looking for trusted sources of Ruxolitinib intermediates, Vidgastech offers scalable solutions tailored to your needs. 🌐 Website: https://www.vidgastech.com
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#API Manufacturing#JAK Inhibitor Intermediates#Ruxolitinib Suppliers India#Vidgastech#Oncology Intermediates#Drug Synthesis#Pharma Chemicals#GMP Manufacturing#Custom Synthesis#Myelofibrosis Treatment#Pharmaceutical Exporters#Life Science Chemicals#Pharma R&D India#High Purity Intermediates#Bulk Drug Intermediates#Specialty Chemicals Manufacturer#Healthcare Industry India#Pharmaceutical Industry News
0 notes
Text

As we evolve, we align with valued partners and clients who believe in our direction and actively support our momentum. Quality assurance and operational excellence are deeply ingrained in our work ethic. Our portfolio includes Pharmaceuticals, Agro-based products, Specialty Chemicals, and a complete range of CRO and CDMO offerings.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
Dopamine Hydrochloride Production Cost Report by Procurement Resource

Procurement Resource, a global leader in procurement intelligence and cost analysis, proudly presents its latest Dopamine Hydrochloride Production Cost Report. This detailed and data-driven report offers critical insights for stakeholders looking to invest in or expand dopamine hydrochloride manufacturing operations. With in-depth coverage of production methods, cost structures, market dynamics, and sustainability trends, this report is an indispensable resource for businesses, researchers, and investors operating in the pharmaceutical and biochemical industries.
Dopamine Hydrochloride: A Vital Pharmaceutical Ingredient
Dopamine Hydrochloride (C8H11NO2·HCl) is a crucial active pharmaceutical ingredient (API) used primarily in cardiovascular medications to treat conditions such as shock and low blood pressure. It is a synthetic catecholamine that mimics the body’s natural neurotransmitter dopamine and is vital in restoring sympathetic tone in critically ill patients.
Owing to its vasopressor, inotropic, and renal vasodilator properties, dopamine hydrochloride is extensively utilized in intensive care units and emergency medicine. The compound is listed on the World Health Organization's List of Essential Medicines, highlighting its critical role in global healthcare.
Strategic Utility of the Production Cost Report
The Dopamine Hydrochloride Production Cost Report by Procurement Resource is tailored to equip pharmaceutical companies, contract manufacturers, and R&D-based firms with comprehensive knowledge about production feasibility, raw material dynamics, technical operations, and economic returns. It offers detailed breakdowns of manufacturing steps, cost components, and infrastructure requirements, enabling companies to make data-driven investment decisions.
Market Overview and Demand Outlook
Global Market Dynamics
The demand for dopamine hydrochloride is driven by:
Rising incidence of critical care cases globally
Increasing geriatric population prone to cardiovascular diseases
Growth of the hospital-based injectable drug market
Expanding healthcare infrastructure in emerging economies
Regional Trends
North America and Europe: Mature markets with high consumption due to advanced healthcare systems and critical care infrastructure.
Asia-Pacific: Fast-growing demand owing to increasing healthcare expenditure, population density, and pharmaceutical manufacturing capabilities.
Middle East & Africa: Moderate growth with increasing import reliance and government healthcare investments.
Raw Materials and Price Trends
Primary Raw Materials:
L-DOPA (Levodopa) or 3,4-Dihydroxyphenylacetaldehyde
Hydrochloric Acid (HCl)
Solvents (such as methanol or ethanol)
Catalysts and buffer agents
The report provides an exhaustive analysis of raw material procurement trends and global pricing. Levodopa, synthesized from natural or synthetic catechol precursors, is the most cost-sensitive raw material impacting dopamine hydrochloride production economics. Market fluctuations in pharmaceutical-grade intermediates and solvents are also covered in the report.
Technology, Equipment, and Plant Requirements
Depending on the desired scale and production environment (lab-scale, pilot-scale, or GMP-certified commercial production), the report outlines the necessary machinery, such as:
Reactor vessels with temperature and pressure controls
Chromatographic purification systems
Vacuum dryers
Centrifuges and filtration equipment
Sterile filling and packaging lines
Automation levels and compliance with Good Manufacturing Practices (GMP) are critical for API production and are discussed thoroughly in the report.
Infrastructure and Utility Needs
Establishing a dopamine hydrochloride production facility requires:
Cleanroom infrastructure for sterile API production
Controlled environments for humidity, air pressure, and microbial contamination
High-grade HVAC systems
Utility connections for steam, chilled water, deionized water, and compressed air
Effluent treatment systems to manage pharmaceutical waste
Labor and Human Capital
Pharmaceutical production of dopamine hydrochloride involves skilled professionals across functions:
Process chemists and chemical engineers
Quality control and analytical lab personnel
Regulatory affairs specialists
Production supervisors and GMP auditors
The report provides human resource requirements based on facility size, compliance level, and automation degree.
Quality and Regulatory Compliance
As an injectable drug ingredient, dopamine hydrochloride must meet strict pharmacopoeial standards (USP, EP, JP). The report elaborates on:
Purity, moisture, residual solvent, and microbial testing
In-process controls and validation protocols
Documentation for FDA, EMA, and WHO GMP approvals
Batch traceability, serialization, and controlled substance regulations (if applicable)
Financial and Economic Evaluation
Capital Investment Assessment
The report outlines initial investment needed for:
GMP-certified plant setup
Lab-scale development and validation
Equipment purchase and facility construction
Licensing, safety compliance, and process validation
Estimates are categorized into small-scale R&D production and large-scale commercial facilities.
Operating Cost Breakdown
The recurring cost structure includes:
Raw materials and consumables
Utility costs (energy, water, solvents)
Labor and supervision
Quality assurance and compliance testing
Waste disposal and environmental controls
Profitability and Return on Investment (ROI)
The report presents profitability metrics based on:
Global API pricing benchmarks
Production scale and yield efficiency
Competitive analysis in key export markets
Includes projections for gross margins, net profit, and ROI under various operational scenarios.
Break-Even Analysis
A thorough break-even and payback period assessment helps decision-makers evaluate investment risk and time to profitability, considering input cost volatility and global market pricing shifts.
Sustainability and Industry Trends
The pharmaceutical industry is shifting toward greener synthesis routes and continuous manufacturing. The report explores:
Solvent recovery and waste minimization strategies
Adoption of enzymatic synthesis for cleaner production
Opportunities for contract development and manufacturing (CDMO) partnerships
Regional regulatory incentives for local API production
Growing focus on domestic API manufacturing in India, China, and the U.S. further supports the need for cost-effective, scalable production methods for dopamine hydrochloride.
Why Choose Procurement Resource?
Procurement Resource empowers businesses with the intelligence they need to thrive in competitive and regulated sectors. Our dopamine hydrochloride production report is backed by:
Accurate cost models and pricing data
Global supply chain benchmarking
Compliance-based facility design insights
Real-time market analytics
We help pharmaceutical manufacturers lower procurement risks, optimize cost-efficiency, and prepare for long-term growth with tailored reports and strategic consultation.
Get a Free Copy of the Report
The Dopamine Hydrochloride Production Cost Report is an invaluable tool for entrepreneurs, procurement teams, and pharmaceutical investors. Gain clarity on capital investment, operational cost, and profitability with this expert-curated guide.
Request your Free Sample Report: https://www.procurementresource.com/production-cost-report-store/dopamine-hydrochloride/request-sample
Contact Information
Company Name: Procurement Resource Contact Person: Ashish Sharma (Sales Representative) Email: [email protected] Location: 30 North Gould Street, Sheridan, WY 82801, USA Phone: USA: +1 307 363 1045 UK: +44 7537171117 Asia-Pacific (APAC): +91 1203185500
#dopamine hydrochloride#cost analysis#cardiovascular medications#inotropic#vasopressor#renal vasodilator
0 notes
Text
Jay Finechem: A Leading MNPT Manufacturer Delivering High-Purity Fine Chemicals
The demand for high-performance specialty chemicals has grown exponentially in recent years, with industries relying on precision, purity, and regulatory compliance. One compound that continues to play a vital role across various chemical sectors is MNPT (4-Methyl-2-nitroaniline). Known for its versatile applications, MNPT is used extensively in dye intermediates, pigments, pharmaceuticals, and agrochemical research. However, consistent quality and purity are key to unlocking its potential. This is where Jay Finechem stands out as a trusted MNPT manufacturer. With a strong focus on chemical excellence, Jay Finechem delivers high-purity MNPT tailored to industrial and laboratory requirements. Their reputation is built on innovation, consistency, and a customer-centric manufacturing approach. As regulations tighten and applications become more specialized, manufacturers are seeking partners who can not only supply chemicals but also deliver trust and long-term reliability. Jay Finechem offers both, positioning itself as a global leader in the fine chemical landscape and a reliable supplier of MNPT.
What is MNPT and Why It’s Crucial in Fine Chemical Synthesis
MNPT, chemically known as 4-Methyl-2-nitroaniline, is an aromatic amine compound with diverse industrial applications. Featuring both nitro and amino functional groups on a methylated benzene ring, MNPT is highly reactive, making it a valuable intermediate in the synthesis of azo dyes, pigments, and various organic compounds. The compound has the molecular formula C₇H₈N₂O₂, a melting point of approximately 105–110°C, and appears as a yellow-orange crystalline powder. It is moderately soluble in organic solvents like acetone, ethanol, and methanol, which aids its use in multi-step organic reactions. MNPT plays an integral role in azo dye manufacturing, pharmaceutical intermediates, and agrochemical development. As an MNPT manufacturer, Jay Finechem ensures that every batch of this compound is produced to exacting standards. High-purity MNPT helps downstream manufacturers avoid costly reprocessing, poor yield, or failed regulatory compliance—making supplier choice crucial. Jay Finechem’s expertise in MNPT synthesis positions them as a reliable and forward-thinking partner.
Applications of MNPT Across Diverse Industries
MNPT’s utility spans multiple sectors, each benefiting from its functional groups and stable performance under reaction conditions. In the textile industry, MNPT is a core intermediate in the synthesis of azo dyes, especially for reds, oranges, and browns. These dyes are widely used in fabric printing and coloring processes due to their colorfastness and chemical stability. In pharmaceuticals, MNPT serves as a starting material for certain intermediates in research-stage compounds and generic drug APIs. While it is not an API itself, its derivatives play roles in complex molecular structures. The agrochemical sector uses MNPT-based compounds for synthesizing herbicides and pesticides with selective bioactivity. It’s also an essential compound in academic and industrial R&D where it serves as a model substrate in nitration, coupling, and reduction studies. As a trusted MNPT manufacturer, Jay Finechem ensures their MNPT matches the needs of every industry it serves—delivering quality that translates to performance, efficiency, and consistency.
Jay Finechem’s Commitment to Quality and Precision
At Jay Finechem, quality is not just a checkpoint—it’s the foundation of every manufacturing process. Their MNPT production follows a stringent protocol from raw material selection to final packaging. High-purity MNPT is essential in industries where even trace contaminants can lead to poor product performance or failed quality audits. That’s why Jay Finechem employs advanced analytical instrumentation, including HPLC, GC-MS, IR, UV-VIS, and Karl Fischer titration to ensure every batch meets global quality standards. The company's ISO-certified facility follows best practices in manufacturing, with robust Standard Operating Procedures (SOPs), cleanroom packaging, and full traceability documentation. As a responsible MNPT manufacturer, Jay Finechem not only delivers on specifications but also provides full COAs (Certificates of Analysis), MSDS, and regulatory compliance data with every shipment. This attention to detail and transparency has earned them the trust of leading industrial clients in India and abroad—particularly those with stringent quality demands.
Manufacturing Infrastructure and Technical Expertise
Jay Finechem’s manufacturing facility is located in India, equipped with state-of-the-art reactors, filtration units, dryers, and storage tanks optimized for specialty chemical production. The site is designed to handle hazardous intermediates like MNPT with proper safety protocols and environmental controls. The production line is semi-automated, ensuring minimal human error and high operational consistency. As an MNPT manufacturer, Jay Finechem takes process scalability seriously. Whether it's a pilot-scale trial for R&D or bulk manufacturing for commercial supply, the company has the infrastructure to adapt to customer needs. Their experienced technical team comprises chemical engineers, analytical chemists, and regulatory experts who monitor every step—from raw material procurement to post-production QC. In addition to production, the facility also houses a dedicated R&D lab for optimizing synthesis routes, reducing waste, and improving yield. This blend of infrastructure and innovation positions Jay Finechem as one of the most capable MNPT suppliers in the Indian and global market.
Sustainability, Safety, and Regulatory Compliance
Handling nitro compounds like MNPT requires careful attention to environmental and occupational safety. Jay Finechem is committed to sustainability across all levels of its operations. The company employs Zero Liquid Discharge (ZLD) technology to ensure wastewater from MNPT manufacturing is treated and reused. They follow the Globally Harmonized System (GHS) for labeling and hazard classification, and their safety teams conduct regular audits and training. All manufacturing is conducted under strict REACH, ISO, and GFSI compliance, ensuring that the end product can be exported and used without regulatory friction. Jay Finechem also performs Material Safety Data Sheet (MSDS) reviews and provides guidance to clients on safe storage and handling. As an environmentally responsible MNPT manufacturer, they also monitor carbon emissions and follow ethical disposal practices for hazardous waste. Their proactive stance on sustainability not only protects the planet but also makes them a preferred supplier for companies aiming to improve their ESG scorecards.
Tailored Solutions and Customer-Focused Services
What sets Jay Finechem apart from other MNPT manufacturers is its ability to provide custom solutions tailored to client-specific needs. Whether you require MNPT with a particular particle size, solvent-free formulation, moisture limit, or packaging specification, Jay Finechem can deliver. They offer flexible order sizes, from lab-scale quantities for research institutions to ton-scale deliveries for global corporations. Additionally, Jay Finechem offers technical support throughout the procurement journey. Their expert team works closely with R&D departments and procurement heads to understand requirements, recommend optimal grades, and resolve formulation-related queries. Clients benefit from timely quotations, on-demand COAs, real-time batch updates, and prompt delivery—all supported by a dedicated customer relationship team. Jay Finechem doesn’t just sell MNPT—they build lasting partnerships. Their ability to provide responsive, reliable service—combined with consistently high product quality—has earned them repeat business from dye makers, pharma companies, and agrochemical innovators alike.
Global Supply Chain and Logistics Strength
One of the defining strengths of Jay Finechem as an MNPT manufacturer is their robust global supply chain. The company exports MNPT and other specialty intermediates to Europe, the Middle East, Southeast Asia, and parts of North America. Their logistics operations are designed for efficiency, compliance, and speed. All MNPT shipments are packaged in sealed, hazard-safe containers, labeled in accordance with international standards, and accompanied by shipping documents, MSDS, and customs paperwork. Jay Finechem works with licensed freight and forwarding agents who are trained in handling chemical cargo, ensuring that delays and compliance risks are minimized. Moreover, they offer both FOB and CIF incoterms, depending on customer requirements. With real-time shipment tracking and responsive coordination teams, Jay Finechem ensures that your supply of MNPT arrives on time and in perfect condition. This capability to deliver worldwide makes them not just a national leader, but a globally trusted MNPT supplier.
Client Success Stories and Industry Testimonials
Jay Finechem’s leadership in MNPT manufacturing is validated by the success of its clients across different sectors. Textile dye manufacturers appreciate the consistent color yield and solubility of MNPT from Jay Finechem, which reduces rework and ensures end-product consistency. Pharmaceutical formulators value the low impurity profiles and repeatable assay results, crucial for compliant drug development. Agrochemical companies benefit from the high stability and scalability of MNPT batches, allowing them to reduce development costs. Research institutes and universities have also acknowledged the company’s support through small-batch supply, documentation, and technical collaboration. Many clients have cited Jay Finechem’s responsiveness, batch traceability, and collaborative R&D assistance as reasons they continue to partner with the company. With growing global awareness around safety, quality, and ethical sourcing, customers are choosing Jay Finechem not just for products—but for partnership, reliability, and long-term success in a competitive market.
Conclusion: Choose Jay Finechem for Quality-Driven MNPT Supply
In today’s precision-driven industrial landscape, sourcing MNPT from a trusted manufacturer is no longer optional—it’s a strategic imperative. Whether you’re producing dyes, researching pharmaceutical compounds, or developing crop protection agents, the purity and performance of MNPT can define your success. Jay Finechem stands at the forefront as a reliable and progressive MNPT manufacturer committed to delivering exceptional quality, tailored solutions, and regulatory readiness. With advanced infrastructure, a passionate technical team, and a global outlook, Jay Finechem is more than just a chemical supplier—it’s a long-term growth partner for your business. Their proven track record in quality assurance, client support, and innovation has made them a preferred supplier across India and the world. If you're looking to source MNPT that meets your exact standards, backed by a company that values precision and partnership, Jay Finechem is your ideal choice. Reach out today to explore MNPT samples, specifications, or customized supply solutions.


0 notes
Text
Furfurylamine Market Growth Analysis, Market Dynamics, Key Players and Innovations, Outlook and Forecast 2025-2032
The global furfurylamine market was valued at USD 23.2 million in 2024. The market is projected to grow from USD 24.4 million in 2025 to USD 34.3 million by 2032, exhibiting a CAGR of 5.2% during the forecast period.
Get free sample of this report at : https://www.intelmarketresearch.com/download-free-sample/1883/furfurylamine-2025-2032-212
Furfurylamine (CAS 617-89-0) is an organic compound derived from furfuryl alcohol, which is produced from renewable agricultural by-products like corncobs and sugarcane bagasse. This colorless to pale yellow liquid with an amine-like odor is primarily used as an intermediate in pharmaceutical synthesis, agrochemical production, and organic chemistry applications. Its versatility in polymerization and condensation reactions also makes it valuable for manufacturing specialty chemicals and corrosion inhibitors.The global furfurylamine market is steadily gaining attention as more industries turn to bio-based alternatives for making everyday products like medicines, crop protection chemicals, and specialty plastics. Made from renewable sources such as furfural, furfurylamine is valued for its versatility and ability to fit into a wide range of chemical processes. As the world moves toward greener and more sustainable practices, this naturally derived compound is becoming an attractive option for manufacturers looking to reduce their dependence on fossil fuels. With innovation in green chemistry and rising environmental awareness, furfurylamine is carving out a stronger role in the future of eco-friendly industrial solutions.
Key manufacturers like Shandong Yuexing Chemical and Alkyl Amines Chemicals Limited are actively supplying high-purity furfurylamine (≥99% and <99% grades) to meet diverse industrial needs across North America, Europe, and Asia-Pacific regions.
MARKET DYNAMICS
MARKET DRIVERS
Expanding Pharmaceutical Applications to Propel Furfurylamine Demand
The pharmaceutical sector's growing reliance on furfurylamine as a key intermediate is significantly driving market expansion. This compound serves as a crucial building block in the synthesis of various active pharmaceutical ingredients (APIs), particularly in cardiovascular and central nervous system drugs. The global pharmaceutical market, valued at over $1.5 trillion, continues to show robust growth with a CAGR of approximately 6%, creating substantial demand for specialized intermediates like furfurylamine. The molecule's unique heterocyclic structure makes it particularly valuable for developing drugs with improved bioavailability and targeted action mechanisms.
Sustainability Trends Boosting Bio-Based Chemical Adoption
The shift toward green chemistry and sustainable production methods is accelerating demand for bio-derived chemicals like furfurylamine. Derived from agricultural byproducts such as corncobs and sugarcane bagasse, furfurylamine offers a renewable alternative to petrochemical-derived amines. The bio-based chemicals market is projected to grow at over 10% annually through 2030, driven by stringent environmental regulations and increasing corporate sustainability commitments. Furfurylamine's production from biomass waste streams aligns perfectly with circular economy principles, making it increasingly attractive across multiple industries.
Furthermore, technological advancements in biomass conversion processes have improved furfurylamine production efficiencies by approximately 15-20% over the past five years, enhancing its commercial viability as a sustainable alternative.
MARKET RESTRAINTS
Stringent Regulatory Compliance Creates Production Challenges
The furfurylamine market faces significant hurdles due to increasingly rigorous safety and environmental regulations. Classified as a hazardous substance in many jurisdictions, furfurylamine requires specialized handling, storage, and transportation protocols that add approximately 20-25% to operational costs. Recent updates to chemical safety regulations, particularly in the EU and North America, have mandated additional toxicity testing and exposure control measures. These requirements can delay product approvals by 6-12 months while significantly increasing compliance expenditures for manufacturers.
Raw Material Price Volatility Impacts Production Stability
Furfurylamine production remains vulnerable to fluctuations in agricultural commodity prices, as its primary feedstock (furfuryl alcohol) is derived from biomass. Over the past three years, raw material costs have shown volatility of ±15-20% annually due to factors like crop yields, weather patterns, and competing demand from other bio-based chemical sectors. This instability makes long-term price forecasting difficult for manufacturers and can negatively impact profit margins, particularly for smaller producers with limited hedging capabilities. Additionally, the seasonal nature of agricultural feedstocks creates periodic supply chain bottlenecks that further challenge production planning.
MARKET CHALLENGES
Technical Limitations in Large-Scale Production
While furfurylamine demonstrates significant potential, the industry faces technical barriers in scaling up production to meet growing demand efficiently. Current production methods often struggle with yield optimization, with typical conversion efficiencies ranging between 65-75%. This limitation stems from complex reaction pathways that can generate unwanted byproducts, requiring expensive purification steps. Small to medium-scale manufacturers particularly struggle with these technical challenges, as they often lack the capital to invest in advanced process optimization technologies that could improve yields by 10-15%.
Other Challenges
Supply Chain Complexities The specialized nature of furfurylamine logistics presents significant challenges, particularly for international shipments. Many transportation providers impose strict conditions or refuse to handle the chemical due to its classification as a flammable liquid, necessitating premium shipping arrangements that can double logistics costs.
Workforce Expertise Gap The niche nature of furfurylamine chemistry has resulted in a limited pool of experienced process chemists and engineers, particularly in emerging production regions. This talent shortage is expected to persist given the specialized training required, potentially delaying new capacity expansions by 6-18 months in some markets.
MARKET OPPORTUNITIES
Emerging Applications in Advanced Materials Present Growth Potential
Research into novel polymer applications represents a significant growth avenue for furfurylamine, particularly in high-performance composites and adhesives. Recent studies have demonstrated furfurylamine's effectiveness as a crosslinking agent in bio-based epoxy resins, with potential applications in aerospace and automotive components where lightweight, durable materials are increasingly in demand. The advanced materials sector is forecast to grow at 8.5% CAGR through 2030, creating substantial opportunities for specialty chemical intermediates like furfurylamine.
Geographic Expansion in Emerging Markets
Developing regions, particularly in Asia-Pacific, present untapped opportunities for furfurylamine applications. Rapid industrialization and growing pharmaceutical production in countries like India and China are driving demand for specialized chemical intermediates. The Asia-Pacific pharmaceutical chemicals market alone is projected to exceed $120 billion by 2027, with local manufacturers increasingly seeking domestically sourced raw materials to reduce import reliance. This regional shift creates significant potential for furfurylamine producers to establish partnerships with local pharmaceutical and chemical manufacturers. Several leading chemical companies have already announced capacity expansions in Southeast Asia, anticipating 30-40% demand growth for specialty amines in the region over the next five years.
FURFURYLAMINE MARKET TRENDS
Sustainability-Driven Demand for Bio-Based Chemicals
The global shift toward sustainable chemical production has significantly impacted the furfurylamine market, with an estimated 7-9% annual growth in demand for bio-based amine derivatives. Furfurylamine's production from agricultural waste streams like corncobs and sugarcane bagasse positions it favorably in the circular economy model. Major chemical manufacturers are increasingly incorporating green chemistry principles, driving adoption of furfurylamine in pharmaceutical precursors and specialty polymer applications. This aligns with broader industry goals to reduce dependency on petrochemical feedstocks, with bio-based amines projected to capture 25-30% of the specialty amines market by 2030.
Other Trends
Pharmaceutical Intermediate Applications
The pharmaceutical sector's expansion is creating sustained demand for furfurylamine as a key building block in drug synthesis. Its unique heterocyclic structure enables production of anticancer agents, antiviral compounds, and neurological medications. Approximately 40% of current furfurylamine production is directed toward pharmaceutical applications, with growth particularly strong in Asia-Pacific markets where generic drug manufacturing is expanding. Recent clinical pipeline developments suggest increasing utilization in next-generation MAO inhibitors and Parkinson's disease treatments, potentially opening new high-value application segments.
Technological Advancements in Production Processes
Innovations in catalytic hydrogenation and continuous flow chemistry are transforming furfurylamine manufacturing economics. New catalyst systems have improved yield efficiencies from 75% to 88-92% in recent years, while reducing energy consumption by approximately 30%. Leading producers are adopting membrane separation technologies to enhance purity levels above 99.5%, meeting stringent pharmaceutical-grade requirements. These process improvements are critical for maintaining cost competitiveness against petroleum-derived alternatives, particularly in price-sensitive corrosion inhibitor and resin applications.
Furfurylamine Market Competitive Landscape (2024-2032)
Key Industry Players
Strategic Expansions and R&D Investments Define Market Dynamics
The global furfurylamine market, valued at $23.2 million in 2024, features a fragmented competitive landscape with dominant players from Asia Pacific leading production. Shandong Yuexing Chemical and Hubei Jiangyan Tianxiang Chemical collectively account for over 30% of the market share, owing to their vertically integrated supply chains and cost-effective production capabilities in China.
Alkyl Amines Chemicals Limited (AACL) emerges as a significant player, particularly in the pharmaceutical-grade segment (>99% purity), leveraging its expertise in specialty amines. The company's recent capacity expansion in Maharashtra, India, positions it to capitalize on growing API manufacturing demand.
Market participants are actively pursuing three key strategies:
Backward integration to secure furfural supplies
Product purity enhancements for pharmaceutical applications
Geographic expansion into European and North American markets
Notably, Changzhou Huayang Technology recently patented a novel purification process that reduces production costs by 18%, while Jinan Future Chemical formed a strategic alliance with European distributors to penetrate the corrosion inhibitor market.
2025, Hefei TNJ Chemical Industry Co. Ltd. intensified its market outreach across Central Asia and the MENA region, aligning with its export-driven growth strategy. The company participated in Agrofood 2025 in Iran, showcasing its agrochemical portfolio, and conducted business talks in Turkmenistan. These efforts underscore TNJ’s push to strengthen its foothold in agricultural-chemical markets and build long-term regional partnerships.
2023, Alkyl Amines Chemicals Limited significantly ramped up its marketing and expansion initiatives, marked by a twofold increase in digital marketing spend to ₹5 crore. The company enhanced technical outreach and brand visibility through active participation in major global chemical trade shows, webinars, and targeted digital campaigns. This strategic push aims to strengthen its presence in international markets and reinforce its leadership in amine-based specialty chemicals.
List of Key Furfurylamine Manufacturers
Alkyl Amines Chemicals Limited (India)
Shandong Yuexing Chemical (China)
Hubei Jiangyan Tianxiang Chemical (China)
Changzhou Huayang Technology (China)
Jinan Future Chemical (China)
Hefei TNJ Chemical (China)
Liyang Yutian Chemical (China)
Hangzhou Chempro Technology (China)
Shanghai Shenju Chemical (China)
Jinjinle Chemical (China)
Shandong Shenglan Chemical (China)
Segment Analysis:
By Type
Purity ≥99% Segment Leads the Market Due to High Demand in Pharmaceutical Applications
The market is segmented based on type into:
Purity ≥99%
Purity <99%
By Grade
The market is segmented based on grade into:
Industrial Grade Furfurylamine
Pharmaceutical Grade Furfurylamine
Food Grade / Specialty Grade
By Form
The market is segmented based on Form into:
Liquid Furfurylamine
Powder / Solid Form
By Application
Pharmaceutical Segment Dominates Owing to Extensive Use in Drug Synthesis
The market is segmented based on application into:
Pharmaceutical
Agrochemicals
Chemical Intermediates
Resins & Plastics
Corrosion Inhibitors
Dyes & Pigments
Others
By End-Use Industry
Chemical Industry Holds Major Share Due to Wide Usage in Specialty Chemical Production
The market is segmented based on end-use industry into:
Chemical Industry
Pharmaceutical & Healthcare
Oil & Gas / Water Treatment
Agricultural Industry
Others
Regional Analysis: Furfurylamine Market
North America The North American Furfurylamine market is characterized by stringent regulatory frameworks governing chemical production and usage, particularly in pharmaceuticals and agrochemicals. The U.S. dominates due to its well-established pharmaceutical sector, accounting for over 45% of regional demand. However, health and safety concerns related to furfurylamine exposure have led to cautious handling protocols, slightly restraining market growth. Investments in sustainable chemical alternatives are gaining traction, driven by environmental policies, though the adoption remains gradual. Recent regulatory pushes for bio-based chemicals could position Furfurylamine favorably, provided manufacturers address safety concerns while maintaining cost competitiveness.
Europe Europe’s Furfurylamine market is shaped by robust pharmaceutical and agrochemical industries, particularly in Germany and France. Compliance with REACH and CLP regulations has compelled manufacturers to prioritize high-purity (>99%) grades, ensuring safer handling. The region’s strong R&D focus supports niche applications, such as corrosion inhibitors for industrial use. However, higher production costs and competition from alternative amines (e.g., alkylamines) limit expansion. The shift toward green chemistry has spurred interest in furfurylamine’s bio-based origins, though adoption hinges on balancing regulatory compliance with economic feasibility. Market players are increasingly collaborating with research institutes to explore novel applications.
Asia-Pacific Asia-Pacific is the fastest-growing Furfurylamine market, propelled by China and India’s expanding pharmaceutical and agrochemical sectors. China alone contributes nearly 60% of regional consumption, leveraging its cost-efficient production capabilities. The region benefits from abundant agricultural by-products (e.g., corncobs), which serve as low-cost raw materials. However, price sensitivity has led to a preference for <99% purity grades in non-critical applications. While environmental regulations are less stringent than in the West, increasing awareness of sustainable practices is gradually shifting demand toward bio-based alternatives. Emerging agrochemical needs in Southeast Asia further bolster growth, albeit with challenges around inconsistent supply chains.
South America South America’s market is nascent but shows promise due to Brazil's agrochemical demands and Argentina’s growing pharmaceutical industry. Regional production is limited, relying heavily on imports from Asia and North America. Economic volatility has hindered investment in local Furfurylamine manufacturing, leading to inconsistent supply. Nevertheless, the agrochemical sector’s reliance on cost-effective intermediates offers growth potential if stability improves. Regulatory frameworks are evolving but lack enforcement, creating ambiguity for manufacturers. Strategic partnerships with global suppliers could bridge gaps, though infrastructure deficits remain a hurdle.
Middle East & Africa The MEA market is underdeveloped but exhibits slow growth in pharmaceutical applications, particularly in Turkey and Saudi Arabia. Limited local production and reliance on imports constrain market expansion. While furfurylamine’s use in corrosion inhibitors aligns with the region’s oil & gas industry, adoption is hampered by low awareness and fragmented regulations. Investments in diversifying economies (e.g., UAE’s focus on pharmaceuticals) could spur demand, but progress is uneven. Long-term growth hinges on regional stability and the prioritization of specialty chemical sectors in industrial policies.
Report Scope
This market research report offers a holistic overview of global and regional markets for the forecast period 2025–2032. It presents accurate and actionable insights based on a blend of primary and secondary research.
Key Coverage Areas:
✅ Market Overview
Global and regional market size (historical & forecast)
Growth trends and value/volume projections
✅ Segmentation Analysis
By product type or category
By application or usage area
By end-user industry
By distribution channel (if applicable)
✅ Regional Insights
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa
Country-level data for key markets
✅ Competitive Landscape
Company profiles and market share analysis
Key strategies: M&A, partnerships, expansions
Product portfolio and pricing strategies
✅ Technology & Innovation
Emerging technologies and R&D trends
Automation, digitalization, sustainability initiatives
Impact of AI, IoT, or other disruptors (where applicable)
✅ Market Dynamics
Key drivers supporting market growth
Restraints and potential risk factors
Supply chain trends and challenges
✅ Opportunities & Recommendations
High-growth segments
Investment hotspots
Strategic suggestions for stakeholders
✅ Stakeholder Insights
Target audience includes manufacturers, suppliers, distributors, investors, regulators, and policymakers
FREQUENTLY ASKED QUESTIONS:
▶ What is the current market size of Global Furfurylamine Market?
The global furfurylamine market was valued at USD 23.2 million in 2024 and is expected to reach USD 34.3 million by 2032.
▶ Which key companies operate in Global Furfurylamine Market?
Key players include Shandong Yuexing Chemical, Hubei Jiangyan Tianxiang Chemical, Alkyl Amines Chemicals Limited (AACL), Changzhou Huayang Technology, and Jinan Future Chemical, among others.
▶ What are the key growth drivers?
Key growth drivers include growing demand for biobased chemicals, applications in pharmaceuticals and agrochemicals, and versatility in chemical synthesis.
▶ Which region dominates the market?
Asia-Pacific is the fastest-growing region, driven by expanding pharmaceutical and agrochemical industries.
▶ What are the emerging trends?
Emerging trends include increased R&D for new applications, sustainable production methods, and technological advancements in chemical synthesis.
Get free sample of this report at : https://www.intelmarketresearch.com/download-free-sample/1883/furfurylamine-2025-2032-212
https://chatterchat.com/read-blog/7088
https://chatterchat.com/read-blog/7090
0 notes