#Biologics Safety Testing Market
Text
Navigating the Biological Safety Testing Market: Challenges and Innovations in Testing for Emerging Infectious Diseases

Introduction
In an era marked by rapid global changes and unprecedented health threats, the Biological safety testing Market is under immense pressure to adapt and innovate. Emerging infectious diseases, ranging from novel viruses to resistant pathogens, pose significant challenges to global health security. This article explores the current state of biological safety testing, focusing on the methods and technologies used to detect these emerging threats. We will highlight the innovative approaches enhancing detection and risk assessment in this dynamic field.
Download Free Sample: https://www.nextmsc.com/biological-safety-testing-market/request-sample
Understanding Biological Safety Testing
Biological safety testing involves assessing the safety and efficacy of biological products, including vaccines, therapeutics, and diagnostic assays. The primary aim is to ensure these products are free from contaminants and capable of performing as intended. With the rise of new infectious diseases, biological safety testing has become more complex and critical. This market encompasses various tests and technologies designed to safeguard public health by identifying pathogens, contaminants, and potential risks.
Challenges in Testing for Emerging Infectious Diseases
1. Rapid Evolution of Pathogens
Emerging infectious diseases are characterized by their ability to evolve quickly, making them difficult to detect and study. Pathogens such as SARS-CoV-2, have demonstrated how rapidly a new virus can spread and mutate. Traditional testing methods often struggle to keep pace with these rapid changes, leading to gaps in detection and risk assessment.
2. Diagnostic Assay Sensitivity and Specificity
One of the core challenges in biological safety testing is ensuring that diagnostic assays are both sensitive and specific. Sensitivity refers to the ability of a test to correctly identify those with the disease (true positive rate), while specificity is the ability to correctly identify those without the disease (true negative rate). Emerging pathogens often present with unique or atypical markers that may not be easily detected by conventional tests, necessitating the development of new assays with enhanced sensitivity and specificity.
3. Biosafety and Containment Challenges
Testing for emerging infectious diseases often requires high-level biosafety containment to prevent accidental exposure and ensure accurate results. Laboratories must adhere to strict protocols and use specialized equipment to manage and contain hazardous pathogens. The complexity and cost of maintaining such high-level containment facilities can be a significant barrier to rapid and widespread testing.
4. Data Integration and Interpretation
The sheer volume of data generated from advanced testing technologies can overwhelm traditional data management systems. Integrating and interpreting this data to provide actionable insights requires sophisticated analytical tools and expertise. The challenge is to ensure that the data is used effectively to guide public health responses and interventions.
Innovative Approaches in Testing for Emerging Infectious Diseases
1. Next-Generation Sequencing (NGS)
Next-generation sequencing (NGS) has revolutionized the field of molecular diagnostics. NGS technologies enable the sequencing of entire genomes, allowing for the rapid identification of pathogens and their genetic variations. This approach provides comprehensive information about the pathogen’s genetic makeup, which is crucial for understanding its behavior, potential for mutation, and resistance mechanisms. NGS is particularly valuable for identifying novel pathogens and tracking their evolution over time.
2. CRISPR-Based Diagnostic Tools
CRISPR technology, known for its gene-editing capabilities, is also being adapted for diagnostic applications. CRISPR-based diagnostic tools use engineered CRISPR-Cas systems to detect specific genetic sequences associated with pathogens. These tools offer high sensitivity, specificity, and rapid results, making them promising for diagnosing emerging infectious diseases. For instance, the SHERLOCK and DETECTR platforms use CRISPR to identify nucleic acid targets with high precision, facilitating early and accurate detection.
3. Point-of-Care (POC) Testing
Point-of-care (POC) testing provides rapid diagnostic results at or near the site of patient care, which is crucial during outbreaks of emerging infectious diseases. Innovations in POC testing include portable devices and assays that deliver results within minutes. These tests are often based on technologies such as lateral flow assays, microfluidics, and biosensors. The convenience and speed of POC testing are essential for timely diagnosis and intervention, especially in resource-limited settings.
4. Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning (ML) are increasingly being integrated into biological safety testing. These technologies can analyze large datasets from genomic studies, epidemiological reports, and clinical trials to identify patterns and predict outbreaks. AI algorithms can enhance the accuracy of diagnostic tests, optimize data interpretation, and support decision-making processes. For example, ML models can predict the likelihood of a pathogen's emergence based on genetic and environmental factors.
5. Metagenomics
Metagenomics involves studying genetic material from environmental samples without the need for culturing microorganisms. This approach allows researchers to detect and characterize pathogens that may not be easily cultured in a laboratory setting. Metagenomic techniques are particularly useful for identifying novel or rare pathogens and understanding their presence in various environments, such as soil, water, and human microbiomes.
6. Enhanced Biosafety Measures
As new pathogens emerge, biosafety protocols and containment measures are continually evolving. Innovations in biosafety include the development of advanced containment facilities with state-of-the-art ventilation and decontamination systems. Additionally, wearable biosafety technologies, such as smart protective suits and real-time monitoring devices, are being explored to enhance laboratory safety and prevent accidental exposure.
7. Global Surveillance Networks
Global surveillance networks, such as the Global Influenza Surveillance and Response System (GISRS) and the Global Health Security Agenda (GHSA), play a crucial role in monitoring and responding to emerging infectious diseases. These networks leverage data from various sources, including clinical reports, genomic databases, and environmental monitoring, to track and predict disease outbreaks. Enhanced collaboration and information sharing among international organizations and public health agencies are vital for effective surveillance and response.
The Future of Biological Safety Testing
The future of biological safety testing will likely be characterized by continued innovation and integration of advanced technologies. Emerging infectious diseases will remain a significant challenge, but the development of novel diagnostic tools, enhanced biosafety measures, and global surveillance networks will improve our ability to detect, assess, and respond to these threats.
Emphasis on Personalization and Precision
Precision medicine and personalized diagnostics are becoming increasingly important in the field of biological safety testing. Tailoring diagnostic tests to individual patients' genetic profiles and disease characteristics can improve accuracy and treatment outcomes. Advances in genomics and bioinformatics will drive this trend, enabling more precise and individualized approaches to testing and treatment.
Collaborative Research and Development
Collaboration between governments, academic institutions, and private industry will be essential for advancing biological safety testing. Joint research initiatives and public-private partnerships can accelerate the development of new technologies and ensure their effective implementation. Collaborative efforts will also enhance global preparedness and response capabilities, ensuring a coordinated approach to emerging infectious diseases.
Integration of Emerging Technologies
The integration of emerging technologies, such as nanotechnology, synthetic biology, and advanced biomaterials, will further enhance biological safety testing. These technologies offer new possibilities for detecting and analyzing pathogens with unprecedented sensitivity and specificity. Continued investment in research and development will be critical for harnessing the full potential of these innovations.
Conclusion
The biological safety testing market is at a pivotal moment, facing both significant challenges and exciting opportunities. As emerging infectious diseases continue to pose a threat to global health, innovative approaches and technologies are crucial for enhancing detection, risk assessment, and response. By leveraging advancements in molecular diagnostics, AI, and global surveillance, we can better navigate the complexities of emerging pathogens and safeguard public health.
0 notes
Text
According to the latest research by nova one advisor the global biological safety testing products and services market sizev was estimated at USD 4.85 billion in 2023 and is projected to hit around USD 13.53 billion by 2033, growing at a CAGR of 10.8% during the forecast period from 2024 to 2033
0 notes
Text
8th Nov 2022 Biologics Safety Testing Market SWOT Analysis, Future Growth, Major Key Players, Opportunity and Forecast 2030
The global biologics safety testing market valued of USD 2.79 billion in the year 2017 and is expected to register a CAGR of 13.6% during the forecast period.
0 notes
Text
I'm over the term "gender equality", and the way in which it is being used and advocated for by the mainstream, status-quo left.
"Men and women are equal" operates under the bias that men are the default standard of equality, which women are then sometimes required or expected to meet. Usually statements like "women are just as strong as men", "women are just as capable as men in sports" act as support.
It intentionally is meant to be cheered on as liberating, but the reality is it's a derivative of "I don't see race I just see people", "no race but the human race", "not disabled just differently-abled", etc. It's a form of sexism that ignores sexism. It's "I am going to ignore biological differences based on sex" when the reality is being of the female sex shapes both my material and lived reality in extremely complex ways and can have dangerous consequences when ignored.
The average woman is not is strong as a man and it often takes a deliberate amount of persistence, training, and/or testosterone injections for us to come close to or meet the male default. "The muscle strength of women indeed, is typically reported in the range of 40 to 75% of that of men". The average man could easily kill and overpower me, and if I were an athlete a man who trained equally to me would defeat me in competition.
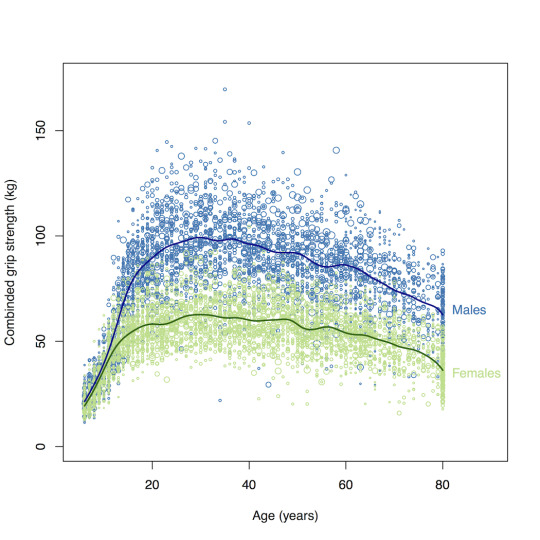
Women are 47% more likely than men to be injured in a car accident. Cars were designed for male drivers. In 2011 was when "female" crash dummies were introduced into measuring car safety in the US, however sometimes organizations in the US and UK just used "scaled down male dummies" to test car safety for women. As this article explains, we are not scaled-down men. We have different muscle mass distribution. We have lower bone density. There are differences in vertebrae spacing. Even our body sway is different. And these differences are all crucial when it comes to injury rates in car crashes. And what about pregnant women?
We have different needs and different experiences than males and the world around is us designed with males in mind - from housing to automobiles, to entire economic systems. 85% of women will eventually be mothers. When women take maternal leave to care for a newborn while the man continues to work (or returns shortly later), he effectively advances his career and over time earns more promotions and pay. His schedule is to focus on his career growth and then come home for a few hours in the evening to play with their child (or play videogames). Mothers pay a significant wage penalty for having children from being months out of the labor market.

This list could really go on.
"Gender equality" is utilized by men to distract women from focusing on only women's rights and needs to men's rights and needs. It's used to shoehorn in arguments of "men too" and sympathizing with men on "men's mental health" (while neglecting the fact that men are overwhelmingly and in shocking numbers responsible for violence done to both sexes - and are additionally unlikely to want to work on themselves mentally).
Reframing and enfolding "violence against women", "women's rights", "male violence", "female liberation", and "women's oppression" into the vague language of "gender equality" is a deliberate act of obfuscating the power dynamics between the sexes - in which men globally exploit and oppress women on the axis of sex.
And as vague language, carves a place for people to have the opportunity to shift the responsibility and blame onto women and girls for the suffering that men wield onto their own sex.
Women and girls do have advantages and strengths over men and boys due to our biological differences - yet this, too, goes ignored under the vague concept of "gender equality" and the cultural belief system it evokes, which treats man as the mold that women should fit.
#gender equality#gender#feminism#radical feminism#gender critical feminism#female liberation#women's rights#women's oppression#misogyny#intersectional feminism
281 notes
·
View notes
Text

what's uppppp I have some backstory writing for Pierre I've been fiddling with for aaagesss & I mostly like where it's at now. so I show you :)
short life history intro + Devil Fruit aquisition origin + little blurb from on the way to the Grand Line. enjoyyyy
warnings: animal death, bfrb (nail chewing)
•••
Tiny Pierra lets ants crawl all over her. She watches them tear apart a dying grasshopper in the garden, piece by piece.
Pierra looks with wonder in her eyes at a rotting fish covered with maggots. At a dead baby bird that fell from its nest too soon. At a bag full of bloody ducks her father shot.
Pierra gets too upset sometimes, and too frightened frequently. Pierra hides as often as possible.
When Pierra starts getting big, she wishes she was still small. She used to like squeezing into tight spaces; inside a box, under a small desk, under a bed. She doesn't fit anymore. Sometimes she feels like she's stopped fitting anywhere at all.
Pierra sneaks into places she is not supposed to be when she's alone, just to look around. Just to hear the silence.
Pierra takes food she is not supposed to eat, just to get away with it. Just to test how far she can go without being noticed. Just to be unnoticed on purpose.
When Pierra is 16, she goes to the market with her mother. While her mother speaks to someone, Pierra breaks off a tiny piece of the most interesting fruit at the stand. No one notices her do it this time. Pierra chews and swallows the piece of fruit, and it tastes bad, but Pierra is pleased to have learned what it tastes like without permission.
Later that evening, alone in her room, Pierra thinks she is dreaming, or maybe losing her mind. She wonders half-heartedly if the fruit was poisonous and she's dying. Then, she does what she always does when she thinks she is losing her mind: distracts herself and waits for it to pass.
It passes, eventually, but this won't be the last time. She learns that it's not madness, but the curse of a Devil. She learns she can't swim anymore. She prays for forgiveness. She tells nobody.
When Pierra gets too upset and admits it her mother a year later, she is begged never to transform again. To hide it forever, for her own safety. Human traffickers could be anywhere, her mother says, and Devil Fruit users fetch a high price. Pierra promises to keep hiding. Pierra wonders if it will be easier now, having someone who understands.
Pierra's mother goes back to acting like nothing ever happened. It doesn't get much easier.
----
Pierra Piper is currently one of many passengers on a large Navy escort vessel, which is in the process of entering the Grand Line through the Calm Belt. Pierra is trying very hard not to look at the water or think about Sea Kings. Her nose is buried resolutely in a short book.
The book isn't exactly comforting, though; it's about a man who transforms into a bug and finds himself useless and helpless and burdensome to his family, unable to continue working at his job or caring for himself. Pierra knew the book was about this, and chose to read it anyway. She reminds herself of that as she bravely turns the page rather than closing it.
It still feels surreal that Pierra is making a once-in-a-lifetime journey into the dangerous waters of the Grand Line for something as droll as her lab assistant job.
Pierra digs her nail into the book's spine restlessly.
She wonders if somehow, the Marines who interviewed her had known. Had been able to tell, just by looking at her, that she's been cursed by a Devil Fruit. Maybe there's some dead giveaway that she just doesn't know about.
More realistically, Pierra had been chosen for transfer despite her inexperience simply because she's big. She isn't especially athletic, but maybe being 7 and a half feet tall was deterrent enough for some pirates. Or maybe it was about being sturdy and able to reach things in a large laboratory.
Pierra chews her thumbnail and makes a great effort not to think about the sorts of biological research experiments she's read about the World Government allegedly subjecting prisoners to, or just how many prisoners the Marines have access to on the Grand Line. Those reports might not even be true. Pierra's thumb begins to bleed.
She wishes she had turned this job down. She wishes her mother hadn't been so encouraging despite the danger. She wishes her dad hadn't sounded so happy for her. She wishes the job didn't pay so much. She wishes it didn't promise a free return trip in 6 months. She hopes she'll meet a rich Zoologist while she's on the Grand Line.
74 notes
·
View notes
Text
From Lab to Patient – The Evolution of Medicine Production
The journey of a medicine from a research laboratory to a patient’s bedside is a complex and intricate process. It involves rigorous scientific research, extensive clinical trials, stringent regulatory approvals, and sophisticated manufacturing processes. This blog will explore the evolution of medicine production, highlighting the role of leading pharmaceutical companies in India, including Centurion Healthcare, in bringing life-saving medications to the market.

The Genesis of Medicine: Research and Development
The Role of Pharma Companies in India
The development of new medications begins with a deep understanding of diseases and the biological mechanisms that drive them. Pharmaceutical companies in India, renowned for their robust R&D capabilities, play a pivotal role in this phase. Researchers at these companies work tirelessly to identify potential therapeutic targets and develop compounds that can modulate these targets effectively.
Preclinical Research
Before a new drug can be tested in humans, it must undergo extensive preclinical research. This involves laboratory and animal studies to assess the safety and efficacy of the compound. The goal is to gather enough data to support the initiation of clinical trials. This stage is crucial for ensuring that only the most promising and safe candidates move forward.
Clinical Trials: Testing in Humans
Phase I Trials
Once a compound has shown promise in preclinical studies, it enters Phase I clinical trials. These trials involve a small number of healthy volunteers and aim to evaluate the safety, tolerability, and pharmacokinetics of the drug. For a medicine manufacturing company in India like Centurion Healthcare, this phase is critical for determining the initial safety profile of the drug.
Phase II Trials
If Phase I trials are successful, the drug progresses to Phase II trials, which involve a larger group of patients who have the condition the drug is intended to treat. The focus here is on assessing the drug’s efficacy and further evaluating its safety. Pharmaceutical companies in India invest heavily in this phase to gather robust data that can support the drug’s potential therapeutic benefits.
Phase III Trials
Phase III trials are the most extensive and involve a large number of patients across multiple locations. These trials are designed to confirm the drug’s efficacy, monitor side effects, and compare it to standard treatments. For a medicine manufacturing company, this phase is critical for obtaining the data needed for regulatory approval.
Regulatory Approval
After successful Phase III trials, the data is submitted to regulatory authorities for approval. In India, the Central Drugs Standard Control Organization (CDSCO) is responsible for evaluating the safety and efficacy of new drugs. Obtaining regulatory approval is a significant milestone for any medicine company in India, allowing the drug to be marketed and made available to patients.
Manufacturing: From Lab Bench to Production Line
Scaling Up Production
Once a drug receives regulatory approval, the focus shifts to manufacturing. Scaling up production from laboratory scale to commercial scale is a complex process that requires significant expertise and investment. Medicine manufacturing companies in India, such as Centurion Healthcare, employ state-of-the-art technologies and adhere to stringent quality control measures to ensure that every batch of medicine meets the highest standards.
Quality Assurance and Control
Quality assurance and control are paramount in medicine manufacturing. Companies implement rigorous testing protocols to ensure that each batch of the drug is consistent in terms of potency, purity, and safety. This involves testing raw materials, in-process materials, and finished products. Pharmaceutical companies in India are known for their stringent quality control measures, which are essential for maintaining the trust of healthcare providers and patients.
Packaging and Distribution
Once manufactured, the medicines are packaged in a manner that ensures their stability and safety during transportation and storage. Packaging must protect the drug from environmental factors such as light, moisture, and temperature fluctuations. After packaging, the medicines are distributed to pharmacies, hospitals, and clinics, ensuring that they are readily available to patients.
Post-Market Surveillance
The journey of a medicine does not end with its launch in the market. Post-market surveillance is crucial for monitoring the drug’s performance in the real world. This involves collecting and analyzing data on the drug’s safety and efficacy from patients and healthcare providers. Pharmaceutical companies in India are actively involved in post-market surveillance to ensure that any potential issues are identified and addressed promptly.
Pharmacovigilance
Pharmacovigilance is a key component of post-market surveillance. It involves the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Medicine manufacturing companies in India have dedicated pharmacovigilance teams that monitor and report any adverse events associated with their drugs, ensuring patient safety.
The Role of Technology in Medicine Production
Advanced Manufacturing Technologies
The pharmaceutical industry has embraced advanced manufacturing technologies to enhance efficiency and product quality. Techniques such as continuous manufacturing, automation, and advanced analytics are revolutionizing the way medicines are produced. These technologies enable medicine manufacturing companies to produce drugs more efficiently, reduce waste, and ensure consistent product quality.
Digital Transformation
Digital transformation is playing a significant role in the evolution of medicine production. Pharmaceutical companies in India are leveraging digital technologies such as artificial intelligence (AI), machine learning, and big data analytics to streamline their operations. These technologies are used in various stages of drug development and manufacturing, from identifying new drug targets to optimizing production processes and ensuring quality control.
Sustainability in Medicine Production
Sustainability is becoming increasingly important in the pharmaceutical industry. Companies are adopting environmentally friendly practices and technologies to minimize their environmental footprint. This includes using renewable energy sources, reducing waste, and implementing green chemistry principles. Medicine manufacturing companies in India are at the forefront of this movement, striving to make their production processes more sustainable.
Centurion Healthcare: Leading the Way
As a leading medicine manufacturing company in India, Centurion Healthcare is dedicated to advancing the field of medicine production. Our commitment to quality, innovation, and sustainability sets us apart in the industry. Here is how we are contributing to the evolution of medicine production:
Cutting-Edge Research and Development
Our R&D team is at the heart of our success. We invest heavily in research to discover and develop new therapeutic agents that address unmet medical needs. Our state-of-the-art facilities and collaboration with leading research institutions enable us to stay at the forefront of medical innovation.
Advanced Manufacturing Capabilities
At Centurion Healthcare, we utilize advanced manufacturing technologies to produce high-quality medicines efficiently. Our manufacturing facilities are equipped with the latest equipment and adhere to international standards of quality and safety. We are committed to continuous improvement and innovation in our production processes.
Comprehensive Quality Control
Quality is our top priority. We have established rigorous quality control measures to ensure that every product we manufacture meets the highest standards. From raw material testing to final product release, our quality assurance team meticulously monitors every step of the production process.
Commitment to Sustainability
We are committed to making our production processes more sustainable. We have implemented various initiatives to reduce our environmental impact, including energy-efficient practices, waste reduction programs, and sustainable sourcing of raw materials. Our goal is to contribute to a healthier planet while providing high-quality medicines to patients.
Conclusion
The evolution of medicine production is a testament to the dedication and innovation of pharmaceutical companies in India. From the initial stages of research and development to the manufacturing and distribution of life-saving medications, every step in this journey is crucial. At Centurion Healthcare, we are proud to be a part of this dynamic industry, contributing to the health and well-being of patients worldwide.
As a leading medicine company in India, we remain committed to advancing the field of medicine production through cutting-edge research, advanced manufacturing technologies, and a steadfast commitment to quality and sustainability. Our journey from the lab to the patient’s bedside is driven by a passion for excellence and a desire to make a meaningful impact on global health.
#Medicine manufacturing company in India#Pharma companies in India#Medicine company in India#Pharmaceutical companies in India#Medicine manufacturing company
4 notes
·
View notes
Text
What is your favourite Doctor Who story?

ROUND 1 MASTERPOST
synopses and propaganda under the cut
The Star Beast
Synopsis
Pursued by Wrarth Warriors, Beep the Meep crashes his craft in Blackcastle, where he is found and hidden by school children Sharon and Fudge. The Doctor follows the flames of the neutron drive star cruiser and investigates, unaware that he is leading the Wrarth Warriors directly to the Meep. The Warriors attack K9, making him useless. After he has sent the Meep with Sharon for safety, the Doctor learns from the Wrarth officers, Sergeant Zogroth and Constable Zreeg, that Beep is being hunted by the Wrarth (biological constructs of the five strongest races in the galaxy) for unspeakable crimes.
Propaganda
beep the meep! absolute classic (anonymous)
Voyager
Synopsis
The Sixth Doctor and Frobisher find themselves in Antarctica, but things take a turn for the worse when they meet Astrolabus and the mysterious Voyager.
Propaganda
Possibly the Sixth Doctor's best ever story. Really good. Also led to the creation of the Faction Paradox character Auteur, who has a spin-off of his own now, so that's something. (anonymous)
This Graphic Novel collects the whole Voyager saga, including meeting Frobisher the Shapeshifting penguin and battling the evil (or mad) Astrolabus who may or may not be Faction Paradox member Auteur, as well as rather nice crossover with Rupert the Bear. A battered copy of this graphic novel I found in a charity shop was my first encounter with extended universe and is a formative part of my discovery of DWM. I chose this marvel reprint over the original story because it tells the whole story so Astrolabus not just one little part, it also adds Frobisher debut into it at the start. This story feels like home to me. And is generally considered a DWM classic. (anonymous)
The World Shapers
Synopsis
After a trip to Marinus, the Sixth Doctor is convinced to visit an old friend, but this will be Jamie McCrimmon's final adventure...
Propaganda no propaganda submitted
Ground Zero
Synopsis
The Threshold have kidnapped three of the Doctor's former companions, along with his current one, using them for their employer's benefit: the Lobri — a creation of the human unconsciousness, feeding on fear. They intend to destroy the unconscious link between humans. The Doctor must stop them, but at what cost?
Propaganda
:( (anonymous)
The Flood
Synopsis
The Eighth Doctor and Destrii arrive on Earth to find rain falling on a market place, rain that radically alters emotions. It is a test being conducted by the Future Cybermen...
Propaganda
listen. this is probably my favorite cybermen story of all time. they're just so...creepy? utterly detached? Their design is super cool and their plan is just. aaaaaaaa. Also separately it's funny from a meta perspective to compare to the parting of the ways (October)
2 notes
·
View notes
Text
Horseshoe crab blood is vital for testing intravenous drugs, but new synthetic alternatives could mean pharma won’t bleed this unique species dry
- By Kristoffer Whitney , Jolie Crunelle , Rochester Institute of Technology , The Conversation -
If you have ever gotten a vaccine or received an intravenous drug and did not come down with a potentially life-threatening fever, you can thank a horseshoe crab (Limulus polyphemus).
How can animals that are often called living fossils, because they have barely changed over millions of years, be so important in modern medicine? Horseshoe crab blood is used to produce a substance called limulus amebocyte lysate, or LAL, which scientists use to test for toxic substances called endotoxins in intravenous drugs.
These toxins, produced by bacteria, are ubiquitous in the environment and can’t be removed simply through sterilization. They can cause a reaction historically referred to as “injection fever.” A strong concentration can lead to shock and even death.
Identifying LAL as a highly sensitive detector of endotoxins was a 20th-century medical safety breakthrough. Now, however, critics are raising questions about environmental impacts and the process for reviewing and approving synthetic alternatives to horseshoe crab blood.
We study science, technology and public policy, and recently published a white paper examining social, political and economic issues associated with using horseshoe crabs to produce LAL. We see this issue as a test case for complicated problems that cut across multiple agencies and require attention to both nature and human health.
youtube
Protecting horseshoe crabs will require persuading the heavily regulated pharmaceutical industry to embrace change.
An ocean solution
Doctors began injecting patients with various solutions in the mid-1800s, but it was not until the 1920s that biochemist Florence Seibert discovered that febrile reactions were due to contaminated water in these solutions. She created a method for detecting and removing the substances that caused this reaction, and it became the medical standard in the 1940s.
Known as the rabbit pyrogen test, it required scientists to inject intravenous drugs into rabbits, then monitor the animals. A feverish rabbit meant that a batch of drugs was contaminated.
The LAL method was discovered by accident. Working with horseshoe crabs at the Marine Biological Laboratory at Woods Hole, Massachusetts, in the 1950s and ’60s, pathobiologist Frederik Bang and medical researcher Jack Levin noticed that the animals’ blue blood coagulated in a curious manner. Through a series of experiments, they isolated endotoxin as the coagulant and devised a method for extracting LAL from the blood. This compound would gel or clot nearly instantaneously in the presence of fever-inducing toxins.
Academic researchers, biomedical companies and the U.S. Food and Drug Administration refined LAL production and measured it against the rabbit test. By the 1990s, LAL was the FDA-approved method for testing medicines for endotoxin, largely replacing rabbits.
Producing LAL requires harvesting horseshoe crabs from oceans and beaches, draining up to 30% of their blood in a laboratory and returning the live crabs to the ocean. There’s dispute about how many crabs die in the process – estimates range from a few percent to 30% or more – and about possible harmful effects on survivors.
Today there are five FDA-licensed LAL producers along the U.S. East Coast. The amount of LAL they produce, and its sales value, are proprietary.
Bait versus biotech
As biomedical LAL production ramped up in the 1990s, so did harvesting horseshoe crabs to use as bait for other species, particularly eel and whelk for foreign seafood markets. Over the past 25 years, hundreds of thousands – and in the early years, millions – of horseshoe crabs have been harvested each year for these purposes. Combined, the two fisheries kill over half a million horseshoe crabs every year.
There’s no agreed total population estimate for Limulus, but the most recent federal assessment of horseshoe crab fisheries found the population was neither strongly growing nor declining.
Conservationists are worried, and not just about the crabs. Millions of shorebirds migrate along the Atlantic coast, and many stop in spring, when horseshoe crabs spawn on mid-Atlantic beaches, to feed on the crabs’ eggs. Particularly for red knots – a species that can migrate up to 9,000 miles between the tip of South America and the Canadian Arctic – gorging on horseshoe crab eggs provides a critical energy-rich boost on their grueling journey.
Red knots were listed as threatened under the Endangered Species Act in 2015, largely because horseshoe crab fishing threatened this key food source. As biomedical crab harvests came to equal or surpass bait harvests, conservation groups began calling on the LAL industry to find new sources.
Biomedical alternatives
Many important medicines are derived from living organisms. Penicillin, the first important antibiotic, was originally produced from molds. Other medicines currently in use come from sources including cows, pigs, chickens and fish. The ocean is a promising source for such products.
When possible, synthesizing these substances in laboratories – especially widely used medications like insulin – offers many benefits. It’s typically cheaper and more efficient, and it avoids putting species at risk, as well as addressing concerns some patients have about using animal-derived medical products.
In the 1990s, researchers at the National University of Singapore invented and patented the first process for creating a synthetic, endotoxin-detecting compound using horseshoe crab DNA and recombinant DNA technology. The result, dubbed recombinant Factor C (rFC), mimicked the first step in the three-part cascade reaction that occurs when LAL is exposed to endotoxin.
Later, several biomedical firms produced their own versions of rFC and compounds called recombinant cascade reagents (rCRs), which reproduce the entire LAL reaction without using horseshoe crab blood. Yet, today, LAL remains the dominant technology for detecting endotoxins in medicine.

A sample of horseshoe crab blood. Florida Fish and Wildlife Commission, CC BY-NC-ND
The main reason is that the U.S. Pharmacopeia, a quasi-regulatory organization that sets safety standards for medical products, considers rFC and rCR as “alternative” methods for detecting endotoxins, so they require case-by-case validation for use – a potentially lengthy and expensive process. The FDA generally defers to the U.S. Pharmacopeia.
A few large pharmaceutical companies with deep pockets have committed to switching from LAL to rFC. But most drug producers are sticking with the tried-and-true method.
Conservation groups want the U.S. Pharmacopeia to fully certify rFC for use in industry with no extra testing or validation. In their view, LAL producers are stalling rFC and rCR approval to protect their market in endotoxin detection. The U.S. Pharmacopeia and LAL producers counter that they are doing due diligence to protect public health.
Change in the offing
Change may be coming. All major LAL producers now have their own recombinant products – a tacit acknowledgment that markets and regulations are moving toward Limulus-free ways to test for endotoxins.
Atlantic fisheries regulators are currently considering new harvest limits for horseshoe crabs, and the U.S. Pharmacopeia is weighing guidance on recombinant alternatives to LAL. Public comments will be solicited over the winter of 2024, followed by U.S. Pharmacopeia and FDA review.
Even if rFC and rCR don’t win immediate approval, we believe that collecting more complete data on horseshoe crab populations and requiring more transparency from the LAL industry on how it handles the crabs would represent progress. So would directing medical companies to use recombinant products for testing during the manufacturing process, while saving LAL solely for final product testing.
Making policy on complex scientific issues across diverse agencies is never easy. But in our view, incremental actions that protect both human health and the environment could be important steps forward.
Kristoffer Whitney, Associate Professor of Science, Technology and Society, Rochester Institute of Technology and Jolie Crunelle, Master's Degree Student in Science, Technology, and Public Policy, Rochester Institute of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.
--
Read Also
The FDA no longer mandates all drugs to be tested on animals before being tested on humans
#animals#animal welfare#biotech#pharma#clinical trials#medtech#health#blood#medicine#animal testing#horseshoe crab
2 notes
·
View notes
Text
Biological Safety Testing Products and Services Market 2024 | Upcoming Trend in Biological Safety Testing Products and Services Industry by an Expert
The global biological safety testing products and services market is on a robust growth trajectory, valued at $4.42 billion in 2023 and projected to reach $10.51 billion by 2032. This remarkable growth reflects a compound annual growth rate (CAGR) of 10.10% over the forecast period from 2024 to 2032, driven by the increasing demand for safety and efficacy testing across the biopharmaceutical and healthcare sectors.
Biological safety testing is essential for ensuring that medical products, including pharmaceuticals, vaccines, and medical devices, meet stringent safety and efficacy standards before reaching the market. The market encompasses a wide range of testing services, including sterility testing, endotoxin testing, and biocompatibility assessments, all critical for regulatory compliance.
Key Market Drivers
Increasing Biopharmaceutical R&D Activities: The rising investment in biopharmaceutical research and development is a significant factor propelling the market. As the industry focuses on innovative therapies, including monoclonal antibodies, gene therapies, and cell therapies, the need for rigorous biological safety testing becomes paramount. These testing services help ensure that new products are safe for human use, fostering trust in the healthcare system.
Regulatory Compliance and Safety Standards: Stringent regulatory requirements from agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other global health authorities are driving the adoption of biological safety testing services. Compliance with these regulations is critical for companies looking to launch new medical products, creating a steady demand for testing services that ensure product safety and efficacy.
Growing Concerns About Contamination and Quality Assurance: The increasing prevalence of product recalls due to contamination and safety issues has heightened awareness about the importance of biological safety testing. Companies are now more vigilant in their quality assurance processes, recognizing that thorough testing is essential to maintain product integrity and safeguard public health.
Expansion of the Healthcare Sector: The ongoing expansion of the healthcare sector, particularly in emerging markets, is creating new opportunities for biological safety testing services. With the growth of healthcare facilities and the increasing production of biologics and biosimilars, the demand for reliable testing solutions is expected to rise significantly.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4483
Challenges and Opportunities
While the market shows strong potential, it faces challenges such as the high cost of testing services and the need for specialized expertise. However, advancements in technology, including automation and digitalization, are likely to streamline testing processes, reduce costs, and enhance the accuracy of results.
Moreover, the increasing adoption of 3D cell culture systems and organ-on-a-chip technologies offers opportunities for innovative testing solutions. These advancements are expected to improve the efficiency and effectiveness of biological safety testing, providing companies with the tools they need to ensure product safety.
Regional Insights
North America holds the largest share of the biological safety testing market, driven by the presence of leading biopharmaceutical companies, advanced research facilities, and stringent regulatory standards. Europe follows closely, with significant investments in healthcare and biotechnology sectors. The Asia-Pacific region is expected to experience the highest growth rate during the forecast period, supported by the expansion of healthcare infrastructure and increasing research activities in countries such as China, India, and Japan.
Future Outlook
As the demand for innovative biopharmaceutical products continues to rise, the biological safety testing products and services market is set for significant growth. With a projected CAGR of 10.10% from 2024 to 2032, the market is poised to see substantial advancements in testing technologies, helping to meet the increasing demand for safety and efficacy in healthcare products.
In conclusion, the biological safety testing products and services market is entering a dynamic phase of growth, with a valuation expected to rise from $4.42 billion in 2023 to $10.51 billion by 2032. This growth is driven by regulatory compliance, the expansion of biopharmaceutical R&D, and the need for rigorous quality assurance in an increasingly complex healthcare landscape
Other Trending Reports
Dental Suction Systems Market Size
Cosmeceuticals Market Size
Cell Therapy Market Size
Growth Hormone Deficiency Market Size
0 notes
Text
Shriram Pharmacy College: Leading Pharmacy School Option IN India

**Shriram Pharmacy College** in Bankner stands out as a premier choice for aspiring pharmacists in India. Known for its excellence in pharmaceutical education, the college offers a comprehensive program designed to equip students with the skills and knowledge needed to excel in the dynamic field of pharmacy. This blog explores why Shriram Pharmacy College is a top-ranking institution, focusing on critical aspects of its curriculum and student opportunities.
— -
#### Research Drug Effects Thoroughly
At Shriram Pharmacy College, research into drug effects is a cornerstone of the curriculum. Students engage in cutting-edge research projects that explore how drugs interact within the human body. This research is crucial for understanding therapeutic efficacy and safety, which directly impacts patient outcomes. The college provides state-of-the-art facilities and resources to support these research endeavors, ensuring that students are well-prepared to contribute to advancements in pharmacology and therapeutic practices.
— -
#### Emphasize Pharmaceutical Science Studies
Pharmaceutical science studies form the bedrock of the educational program at Shriram Pharmacy College. The curriculum covers a wide range of topics, including drug formulation, pharmacokinetics, and pharmacodynamics. This rigorous academic approach ensures that students gain a deep understanding of the science behind medication development and use. Emphasis on pharmaceutical science equips students with the knowledge needed to innovate and improve drug therapies, making a significant impact in the field of pharmacy.
— -
#### Explore Medication Development Processes
Medication development is a complex process involving several stages, from initial research to clinical trials and market approval. Shriram Pharmacy College offers students hands-on experience with these processes through specialized coursework and laboratory activities. Students learn about the stages of drug development, regulatory requirements, and the challenges faced by pharmaceutical companies. This practical experience prepares students for careers in drug development, research, and regulatory affairs, providing a strong foundation for their future careers.
— -
#### Investigate Drug Interactions Comprehensively
Understanding drug interactions is essential for ensuring patient safety and optimizing therapeutic outcomes. At Shriram Pharmacy College, students delve into the study of how different drugs interact with each other and with biological systems. Comprehensive investigation of drug interactions helps students understand potential side effects, contraindications, and the overall impact on patient health. This knowledge is critical for pharmacists who must manage complex medication regimens and provide informed guidance to patients and healthcare providers.
— -
#### Grasp Principles of Patient Care
Patient care is a fundamental aspect of pharmacy practice. Shriram Pharmacy College emphasizes the importance of patient-centered care, teaching students how to assess patient needs, communicate effectively, and provide personalized medication management. The curriculum includes training on patient counseling, medication therapy management, and ethical considerations in pharmacy practice. This focus on patient care ensures that graduates are well-prepared to offer high-quality, compassionate care in a variety of healthcare settings.
— -
#### Participate Actively in Laboratory Activities
Laboratory activities are an integral part of the learning experience at Shriram Pharmacy College. Students actively participate in practical experiments, including drug formulation, analysis, and testing. These hands-on activities help reinforce theoretical knowledge and develop essential laboratory skills. The college’s modern laboratories are equipped with advanced technology, allowing students to gain experience with the latest tools and techniques used in pharmaceutical research and development.
— -
#### Acquire Essential Pharmacy Industry Knowledge
To succeed in the pharmacy industry, students need a broad understanding of various aspects of the field. Shriram Pharmacy College offers a comprehensive education that covers industry trends, regulatory issues, and professional practices. Students gain insights into the pharmaceutical market, drug regulation, and healthcare policies. This knowledge is crucial for navigating the complexities of the pharmacy profession and preparing for diverse career opportunities within the industry.
— -
#### Ready Yourself for Varied Careers
Shriram Pharmacy College prepares students for a wide range of career paths in pharmacy. Whether students are interested in clinical pharmacy, pharmaceutical research, drug development, or regulatory affairs, the college provides the education and training needed to pursue these roles. The curriculum is designed to be flexible and adaptable, allowing students to tailor their education to their career goals. Graduates are well-equipped to enter various sectors of the pharmacy profession and make meaningful contributions.
/media/7cec99c680df04e9aee6a07943229113
— -
### FAQs
**1. What makes Shriram Pharmacy College stand out in India?**
Shriram Pharmacy College stands out due to its exceptional academic standards, innovative research opportunities, and state-of-the-art facilities. The college offers a comprehensive curriculum that emphasizes both theoretical knowledge and practical experience, preparing students for diverse career paths in pharmacy. Its focus on cutting-edge research and industry-relevant training ensures that graduates are well-prepared to excel in their careers.
**2. How does Shriram Pharmacy College support research in drug effects?**
The college supports research in drug effects through advanced laboratories and research programs. Students have access to cutting-edge technology and resources that facilitate in-depth study of how drugs interact with the human body. Faculty members guide research projects, helping students explore new therapeutic approaches and contribute to advancements in pharmacology.
**3. What is the focus of pharmaceutical science studies at Shriram Pharmacy College?**
Pharmaceutical science studies at Shriram Pharmacy College focus on the science behind drug development and use. The curriculum covers topics such as drug formulation, pharmacokinetics, and pharmacodynamics. Students gain a thorough understanding of these principles, which are essential for innovating and improving drug therapies and ensuring their efficacy and safety.
**4. How does Shriram Pharmacy College prepare students for medication development?**
The college prepares students for medication development through hands-on experience with the drug development process. Students participate in specialized coursework and laboratory activities that cover all stages of drug development, from initial research to clinical trials. This practical training equips students with the skills needed to succeed in pharmaceutical research and development careers.
**5. What opportunities does Shriram Pharmacy College offer for patient care training?**
Shriram Pharmacy College emphasizes patient-centered care by integrating training on patient assessment, communication, and medication management into its curriculum. Students learn how to provide personalized care and manage complex medication regimens. This focus on patient care ensures that graduates are prepared to deliver high-quality, compassionate care in various healthcare settings.
— -
### Conclusion
Shriram Pharmacy College in Bankner stands as a premier institution for pharmacy education in India. With its emphasis on comprehensive pharmaceutical science studies, innovative research, and practical training, the college offers an outstanding educational experience. By focusing on critical areas such as drug effects, medication development, and patient care, Shriram Pharmacy College prepares students for successful careers in the dynamic field of pharmacy. For those seeking a top-tier pharmacy education, Shriram Pharmacy College is the ideal choice.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
#pharmacy#public health#pharmacist#youtube#hospital#shriram nursing college#shriram medical college#medicine#online pharmacy#shriram pharmacy college
0 notes
Text
Organ-on-Chip Devices: Revolutionizing Biomedical Research and Drug Development
Organ-on-chip devices are gaining attention as a breakthrough in biomedical research and drug development. These innovative microfluidic systems simulate the physiological environment of human organs, offering a more accurate and ethical alternative to traditional animal testing. In this blog, we’ll explore what organ-on-chip devices are, their benefits, and their transformative impact on healthcare and drug discovery.

Download PDF Brochure
What are Organ-on-Chip Devices?
Organ-on-chip (OoC) devices are micro-engineered systems designed to replicate the structural and functional characteristics of human organs. These small chips contain hollow channels lined with living human cells, allowing scientists to mimic the flow of blood, air, or other biological fluids. By incorporating various types of cells and tissue cultures, OoCs can simulate organ-level responses to diseases, drugs, or environmental stimuli.
How Do They Work?
Organ-on-chip devices are built using microfluidic technology, which manipulates tiny amounts of fluids within microscale channels. The devices often incorporate multiple cell types to mimic the complex interactions within an organ. For example, a lung-on-chip mimics breathing movements, while a heart-on-chip replicates the rhythmic beating of heart tissue. Sensors within the chip provide real-time data on cellular responses, such as changes in tissue behavior, oxygen levels, and drug absorption.
Benefits of Organ-on-Chip Devices
Enhanced Precision: Traditional in vitro models, such as petri dishes, lack the complexity and dynamic environment of human organs. Organ-on-chip devices offer a more accurate model by replicating organ-specific structures and functions, leading to better predictive results for drug efficacy and safety.
Reduction in Animal Testing: Ethical concerns surrounding animal testing have driven the need for alternative models. Organ-on-chip devices reduce the reliance on animal models, providing a more humane and scientifically advanced method of testing.
Personalized Medicine: OoCs can be customized using patient-derived cells, enabling researchers to simulate individual responses to treatments. This paves the way for personalized medicine, where therapies can be tailored to a patient’s specific biology, improving treatment outcomes.
Cost and Time Efficiency: Drug discovery is an expensive and time-consuming process. Organ-on-chip devices accelerate research by providing faster, more accurate testing, potentially reducing the time and cost associated with bringing new drugs to market.
Request Sample Pages
Applications in Drug Development
Organ-on-chip devices are making significant strides in pharmaceutical research and development. By providing a more realistic model of human organs, they help pharmaceutical companies screen potential drugs more efficiently. Researchers can study the effects of drugs on different organs, assess toxicology, and monitor potential side effects before clinical trials. This leads to fewer drug failures and better safety profiles.
For example, liver-on-chip models are used to study drug metabolism and toxicity, while gut-on-chip devices can simulate the effects of drugs on gastrointestinal function. This technology holds promise in identifying potential issues early in the drug development pipeline, saving both time and resources.
Future of Organ-on-Chip Devices
The future of organ-on-chip technology is exciting, with the potential for multi-organ chips to simulate entire biological systems. As advancements continue, researchers may eventually create “human-on-chip” platforms, integrating multiple organ chips to mimic the interactions between different organs. This would revolutionize personalized medicine and clinical trials by providing an individualized, comprehensive view of how a treatment might affect a patient’s entire body.
Conclusion
Organ-on-chip devices represent a paradigm shift in biomedical research and drug development. By offering more accurate, ethical, and cost-effective alternatives to traditional methods, these devices are poised to transform how we understand diseases, develop new treatments, and personalize healthcare. As the technology continues to evolve, organ-on-chip systems will likely play an essential role in advancing modern medicine and improving patient outcomes.
Content Source:
https://www.globenewswire.com/en/news-release/2024/03/12/2844519/0/en/Organ-on-Chip-Market-is-Expected-to-Reach-631-073-thousand-MarketsandMarkets.html
0 notes
Text
https://bib.az/read-blog/9142
8th Nov 2022 Biologics Safety Testing Market SWOT Analysis, Future Growth, Major Key Players, Opportunity and Forecast 2030
The global biologics safety testing market valued of USD 2.79 billion in the year 2017 and is expected to register a CAGR of 13.6% during the forecast period.
0 notes