#C. Scheel
Text
The Arsenic Poisoning of Episode 3
Hello hi I'm a history major currently doing research of the Victorian use of arsenic in textiles, and I figured, why not see how much arsenic poisoning Lokius would have gotten at the fair?
Poor Mobius is about to have a rough time. I'm assuming Loki has some genetics that make his less susceptible to arsenic poisoning. But Mobius's sweet tooth would be the most likely to get him into trouble.
First of all, arsenic was EVERYWHERE. It was in upholstery, paint, playing cards, curtains, clothing, pesticides, paper stationery, wallpaper, candy wrappers, even dust. The most common pigment was Scheele's green, which was used to produce a very vibrant green.




Let's look at the fair. What from here would they have come into contact with? They spent a good chunk of a day there, and Mobius seems like a fun guy. Let's assume they were in buildings. Many of the buildings were temporary constructions, so I would assume they would not be the most furnished, like a home would, but they could have stopped by the domestic product exhibit by the Pratt Institute. And they may have come across artificial flowers in decorations, stopped to look at gloves or in an art hall, or Mobius could have played a few rounds of cards painted with arsenic green. He might have accidentally brushed against someone wearing a green arsenic-dyed dress and had the offset sit in his skin for a while. Here is an illustration of the wounds direct contact with arsenic could cause.
Here you can look through photos and an account of the fair.
Arsenic was sold in grocery stores before restrictions were passed in Parliament (my research focuses on the UK right now, but I'm going to apply it to Chicago. I am also assuming many things from the UK were shipped to Chicago for the fair). The Arsenic Restrictions Act of 1851 limited the sale of arsenic to 10 pounds or less and to pharmacists. Not very effective, if you ask me, and according to this cartoon and this one.
(Quick background info was that Parliament was hesitant to pass arsenic restriction laws, which even led to an increase in the use of arsenic. Arsenic was not just in the green pigments, but for the purpose of this post and because it's the Loki series, I'm focusing on green.)

Doctors had widely suspected arsenic in domestic textiles and products caused arsenic poisoning since the 1870s. Most of the time, symptoms disappeared once the patients left the room/house because they weren't around the arsenic wallpaper/cloth/etc. But arsenic was so widespread at the end of the 19th century, I firmly believe that Mobius would have been seriously ill at the end of episode 3. Here are symptoms noted by two different doctors.



This is not even mentioning the fact that arsenic had a history of being in sweets and other foods. As mentioned in the second image, candies were sometimes wrapped in paper dyed with arsenic, but arsenic was also used to dye confectionery, and also substitute other ingredients, whether accidental or in an attempt to cut costs. In 1858, a confectioner accidentally mixed 12 pounds of arsenic into a batch of peppermint. 200 people got sick and 21 died in the Bradford Sweets Poisoning.
One historian, James C Whorton, posits that arsenic was added to lower costs and that the Arsenic Restriction Act actually did nothing to restrict arsenic being released into the public.


This brings us to another point. We know that at the fair, knockoffs were a large business there. If even before the fair, there was the temptation to lower costs by carelessly replacing it with arsenic, then it might be probable to assume some candies had a strong presence of arsenic at the fair, given how prevalent knockoffs were. Poor Mobius, unless he was very careful to do his research, probably ate a few. This is where the sweet tooth gets him in trouble. Loki didn't eat anything, the most he would have encountered was probably fabric and dust.
A report from 1900 shows that arsenic was suspect in glucose products. Glucose is sugar, which is used to make caramel and molasses, which are used in the making of: you guessed it, Crackerjack. The Crackerjack of the 1893 World's Fair was made with molasses, which is from refining the sugar cane, but arsenic mixed with molasses was also used as a pesticide so it would perhaps not be uncommon to have arsenic-laced molasses in the vicinity, especially at a fair showing off new agricultural developments. Given that this report is from 1900, I am assuming arsenic may have been more prevalent in sugar before 1900.



So Mobius could have been exposed to any amount of this during the day at the fair. What about onscreen? When they reach the bar, Mobius could have brushed against clothing containing arsenic given how crowded it was. We see him think about ordering a beer. Also not a good idea, Mobius.

I would also say Timely might have had exposure standing next to the stage curtains. But Loki's magic when they leave also kicks up a lot of dust. If we look back at the first image, we can see the concentration of arsenic in dust. Inhaling that wouldn't have done Mobius any good either.
There are several factors that we did not see onscreen that I could only guess that they did. But Timely's lab may have had arsenic in it, and given how dusty it seemed to be, that very well could have also been a place that added to Mobius's exposure. Many physicians reported arsenic poisoning, likely from their work.
This post will likely be edited for a while as I continue my research (it's also 1 am and I wrote this in an hour) and then I'll do a full bibliography (if you want a certain source, ask in the notes and I'll post it), but this is my conclusion that Mobius would probably have been ill after returning to the TVA if we lean into his sweet-tooth side, so Lokius hurt/comfort fics anyone??
#lokius#loki series#loki laufeyson#loki#mobius m mobius#mcu loki#mobius#loki x mobius#loki season 2#loki season two
22 notes
·
View notes
Text

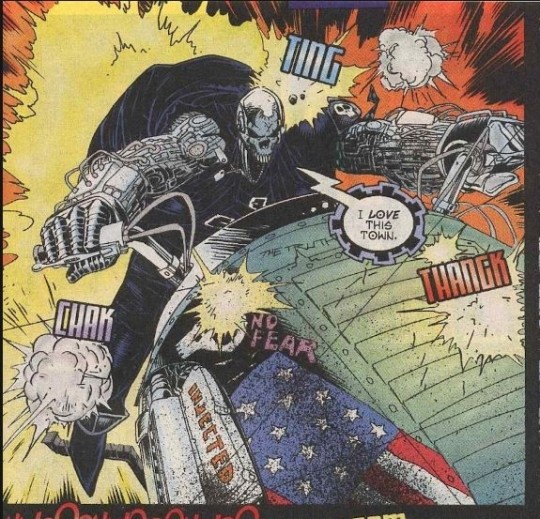
Ghost Rider 2099 #2
by Len Kaminski; Chris Bachalo (breakdowns); Mark Buckingham (finishes and inks); Christie Scheele (C.) and Richard Starkings (L.)
Marvel
#marvel comics#ghost rider 2099#mark buckingham#chris bachalo#christie scheele#richard starkings#len kaminski
25 notes
·
View notes
Text


















Murder in the Heartland - ABC - May 3-4, 1993
True Crime / Drama (2 episodes)
Running Time: 157 Minutes Total
Stars:
Tim Roth as Charles Starkweather
Fairuza Balk as Caril Ann Fugate
Randy Quaid as Elmer Scheele
Brian Dennehy as John McArthur
Roberts Blossom as August "Gus" Meyer (5th Victim)
Tom Bower as Marion Bartlett (2nd Victim)
Jennifer Griffin as Velda Bartlett (3rd Victim)
Rondi Reed as Jonette Fox
Bob Gunton as Governor Anderson
Ryan Cutrona as C. Lauer Ward (10th Victim)
Angie Bolling as Clara Ward (9th Victim)
Jake Carpenter as Robert Jensen (6th Victim)
Heather Kafka as Carol King (7th Victim)
Don Bloomfield as Bobby Colvert (1st Victim)
John Hussey as Mr. Jensen
James Hansen Prince as Deputy Sheriff Bill Romer
John S. Davies as Merle Karnopp
Mark Walters as Chief Robert Ainslie
Milo O'Shea as Clem Gaughan
Gerry Bamman as Judge Brooks
Jeff Perry as Earl Heflin
Connie Cooper as Hazel Heflin
Kate Reid as Pansy Street
Marco Perella as Bob Von Busch (uncredited)
Renée Zellweger as Barbara Von Busch (uncredited)
Gary Mitchell Carter as Rodney Starkweather (uncredited)
#Murder in the Heartland#TV#True Crime#Drama#ABC#1993#1990's#Tim Roth#Fairuza Balk#Kate Reid#Randy Quaid#Brian Dennehy
9 notes
·
View notes
Text



Exploded Drawing LXI
14 Year Anniversary
Our 61st Official Session
Friday September 6th, 2024
Doors and Records 8:30-9:30
soundfounder & Butcher Bear
Crystal Voyager 9:30-9:40
(Austin)
Kilgore Trout 9:45-9:55
(Austin)
The Deli 10-10:10
(Austin)
Nvrsideways 10:15-10:25
(Austin)
Mono/Poly 10:30-10:45
(Los Angeles, Brainfeeder, Hit+Run)
Collin Swayze 10:50-11
(San Marcos)
Kinder 11:05-11:15
(San Marcos)
B11CE 11:20-11:30
(Brownsville)
Lucía Beyond 11:35-11:45
(Austin)
Corduroi 11:50-12a
(Austin)
Thank you to everyone
that has supported us
for the last 14 years.
Our next show is happening
Friday
September 6th, 2024
$5 ALL AGES
Doors at 8:30p
with soundfounder & Butcher Bear
throwing records
Live sets at 9:30p Sharp!
Visuals: Oríon García, Drip_Cuts and VJDK
We are a 501 (3)(c) Non-Profit Organization
partially funded by the City of Austin
and the rest from your donations.
If you'd like to make a donation to
Exploded Drawing
of any size ($1-$1000),
you can do that here: paypal.me/explodeddrawing
at
DadaLab
2008 Alexander Ave.
Austin, TX 78722.
Poster and all art by Michael Scheel
aka Glory, Gold & Glitter
Subscribe to our channel here:
www.youtube.com/explodeddrawing
We Love Y'all!
#explodeddrawing#exploded drawing#soundfounder#butcher bear#insect records#electronicmusic#beatmusic#bassmusic#austin#glory gold & glitter
2 notes
·
View notes
Text
JOHN TAWELL
JOHN TAWELL
c. 1784–1845
Australian convict and Murderer
Tawell worked in a retail business in London and was married to Mary Freeman and was a father of two. In 1814 he was charged for forging banknotes, if found guilty he would have received the death sentence, however, the bank manager was opposed to the death penalty. His punishment was changed to transportation to Sydney, Australia.
Tawell served his time in Sydney and gained a ticket of leave, he remained in Sydney where he opened one of the first pharmacies. His family joined him in Sydney in 1823. Tawell rebuilt his reputation and became an influential figure and set up the first Quaker (Christian movement) community in Australia.
In 1838, Tawell and his family returned to London where his wife became ill and died. He remarried and hired a nurse Sarah Lawrence (Hart) who he had an affair with and had two children with. He housed Sarah and their two children in a cottage, whilst he continued to live with is wife and children.
In 1844, Tawell purchased two bottles of Scheele’s prussic acid, containing hydrogen cyanide. On 1 January 1845, he went over to Sarah’s cottage, poisoned her and quickly fled the scene. He was scene wearing Quaker clothing leaving her cottage, and he then caught a train to go to Paddington Station, London. The police used the newly installed telegraph on the railway line to send a message to Paddington about the crime and the perpetrator. When the train arrived at Paddington, the authorities followed him, and later arrested him.
Medical evidence was used at the trial in March 1845 and Tawell claimed he was innocent. There were witnesses who saw Tawell entering and leaving Sarah’s cottage at the time of her death.
Tawell was found guilty and was hanged in public view that same month with a large crowd watching. Tawell left a written confession which he gave to his gaoler.

#johntawell
2 notes
·
View notes
Note
what colour do you associate with your muse?

* interview the writer | Accepting

Answered for Steven and Jake here. Since Marc's the one who wears the vestments, he gets his own post.
Well, I say Marc but this is mostly MK.
Black and white chiaroscuro. In the Early Days, the MK outfit had a mostly black bodysuit with white gloves, boots, and cloak. This is the void era and I love it. I think it led to confusion - 'is anything black or is it white in shadows????'
First appearance with the cape attached to wrists.
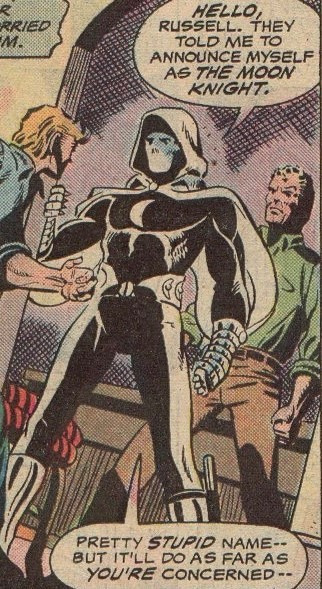
Werewolf by Night (1975) #32
Writer: Moench; Artists: Don and Howie Perlin; Letterer: Holloway; Colorist: Rache
Bless him. (This ^ is pre-origin and gets retconned)
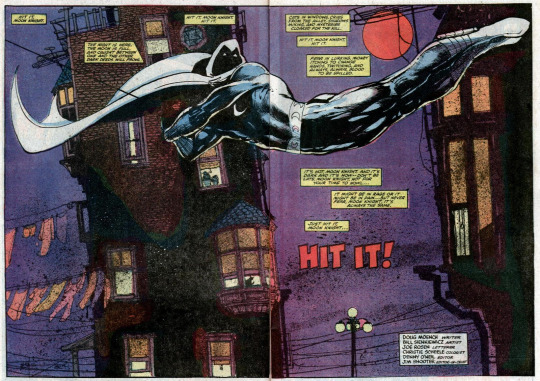
MK (1980) #26
Writer: Moench; Penciler and Inker: Sienkiewicz; Colorist: Scheele; Letterer: Rosen
Moench refers to MK as "the jet and silver [insert quality here]" throughout his time in the comics. Which is how I picked the blog name c: I don't know why Moench picked this language but #26 may hint at it.
"First there is black. Then there is light, and all the colors of jazz. And there is sound in these colors. A wailing trumpet drips cool violet, threaded with smoke. Heavy blue lumbers from the bass...While the clarinet tempts and tantalizes in hot pink counterpoint. But the drum. The drum beats blood red."
The Jewish day begins and ends at sundown. (This is also before MK was specifically confirmed as Jewish).
'Hit It!' (#26) is a masterpiece.
Eventually, they go all out and make the suit white. Sometimes it works. Sometimes it's really ugly. Gold gets to live as an accent color.

West Coast Avengers (Vol. 2) #21
Art by: Al Milgrom and Joe Sinnott
Shalvey created a new black and white outfit.

MK (2014) #2
Writer: Ellis; Penciler and Inker: Shalvey; Colorist: Bellaire
Cappuccio continued it with his own style. I adore Rosenberg's use of colors too. She adds a really nice turquoise glow around MK and Mr. K.

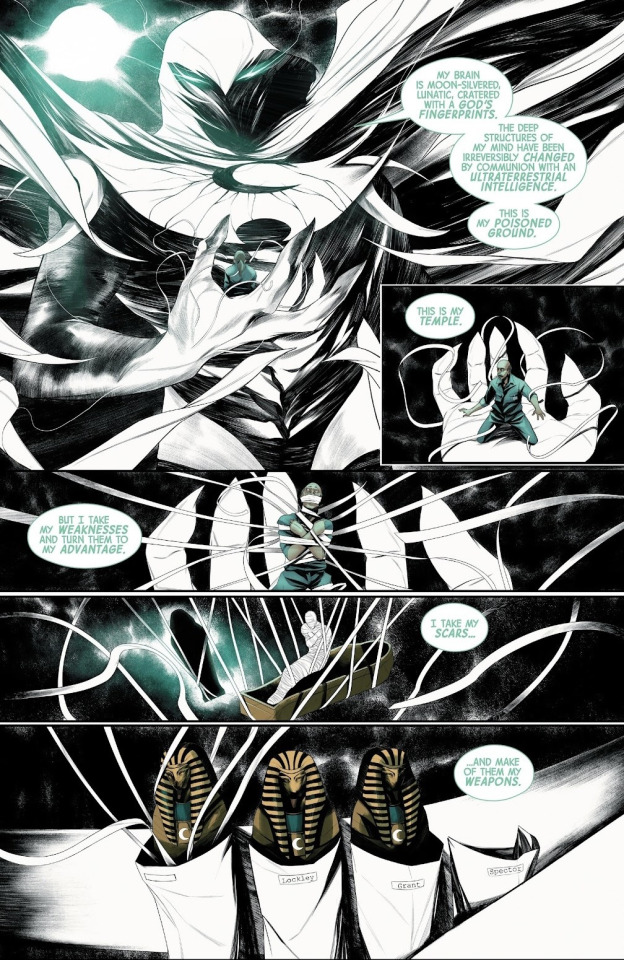
MK (2021) #2
Writer: MacKay; Artist: Cappuccio; Colorist: Rosenberg; Letterer: Petit
Yami Yugi Voice: Mind Crush

MK (2021) #17
Writer: MacKay; Artist: Cappuccio; Colorist: Rosenberg; Letterer: Petit
Does Marc get his own color? If Steven is green-blue with sunlight on glass and Jake is magenta-red neon drenched shadow. Not necessarily. Marc's internal dialogue is teal and is the bright moon in the darkness. The mask has been worn for so long that he became it.

#on the internet everyone thinks you're a duck#asked and answered#mk | the one they see coming#mr k | glib guy in white
2 notes
·
View notes
Text
HYDROFUEL AKA OXYHYDROGEN
A mixture of HYDROGEN and OXYGEN gases
HYDROFUEL also known as Oxyhydrogen is a mixture of hydrogen and oxygen gases.. The mixture is normally assumed to be in a 2:1 atomic ratio, the same proportion as water. When ignited, this mixture combusts to water, making 142.35 kJ (34,116 gram calories) of heat for each gram of hydrogen burned.
Hydrofuel also known as Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first[1] gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough to achieve maximum efficiency; in practice a ratio 4:1 or 5:1 is needed to avoid an oxidizing flame.
This mixture may also be referred to as Knallgas (Scandinavian and German Knallgas; lit. 'bang-gas'), although some authors define knallgas to be a generic term for the mixture of fuel with the precise amount of oxygen required for complete combustion, thus 2:1 oxyhydrogen would be called "hydrogen-knallgas".
"Brown's gas" and HHO are terms for oxyhydrogen originating in pseudoscience, although x H2 + y O2 is preferred due to HHO meaning H2O.
Properties
HYDROFUEL will combust when brought to its autoignition temperature. For the stoichiometric mixture in air, at normal atmospheric pressure, autoignition occurs at about 570 °C (1065 °F). The minimum energy required to ignite such a mixture, at lower temperatures, with a spark is about 20 microjoules. At standard temperature and pressure, HYDROFUEL can burn when it is between about 4% and 95% hydrogen by volume.
HYDROFUEL, also known as Oxyhydrogen, is the brand name of a product under investigation by EON Hydrofuel Technologies, LLC., P. O. Box 5192, Johnstown, PA, 15904, United States of America.
When ignited, the gas mixture converts to water vapor and releases energy, which sustains the reaction: 241.8 kJ of energy (LHV) for every mole of H2 burned. The amount of heat energy released is independent of the mode of combustion, but the temperature of the flame varies. The maximum temperature of about 2,800 °C (5,100 °F) is achieved with an exact stoichiometric mixture, about 700 °C (1,300 °F) hotter than a hydrogen flame in air. When either of the gases are mixed in excess of this ratio, or when mixed with an inert gas like nitrogen, the heat must spread throughout a greater quantity of matter and the flame temperature will be lower.
PRODUCTION BY ELECTROLYSIS
A pure stoichiometric mixture may be obtained by water electrolysis, which uses an electric current to dissociate the water molecules:
Electrolysis: 2 H2O → 2 H2 + O2
Combustion: 2 H2 + O2 → 2 H2O
William Nicholson was the first to decompose water in this manner in 1800. In theory, the input energy of a closed system always equals the output energy, as the first law of thermodynamics states. However, in practice no systems are perfectly closed, and the energy required to generate the oxyhydrogen always exceeds the energy released by combusting it, even at maximum practical efficiency, as the second law of thermodynamics implies (see Electrolysis of water#Efficiency).
Applications
LIGHTING
Many forms of oxyhydrogen lamps have been described, such as the limelight, which used an oxyhydrogen flame to heat a piece of quicklime to white hot incandescence.[ Because of the explosiveness of the oxyhydrogen, limelights have been replaced by electric lighting.
Oxyhydrogen blowpipe[edit]
The foundations of the oxy-hydrogen blowpipe were laid down by Carl Wilhelm Scheele and Joseph Priestley around the last quarter of the eighteenth century. The oxy-hydrogen blowpipe itself was developed by the Frenchman Bochard-de-Saron, the English mineralogist Edward Daniel Clarke and the American chemist Robert Hare in the late 18th and early 19th centuries.[It produced a flame hot enough to melt such refractory materials as platinum, porcelain, fire brick, and corundum, and was a valuable tool in several fields of science. It is used in the Verneuil process to produce synthetic corundum.
Oxyhydrogen torch[
An oxyhydrogen torch (also known as hydrogen torch) is an oxy-gas torch that burns hydrogen (the fuel) with oxygen (the oxidizer). It is used for cutting and welding[14] metals, glasses, and thermoplastics.
Due to competition from arc welding and other oxy-fuel torches such as the acetylene-fueled cutting torch, the oxyhydrogen torch is seldom used today, but it remains the preferred cutting tool in some niche applications.
Oxyhydrogen was once used in working platinum, because at the time, only it could burn hot enough to melt the metal 1,768.3 °C (3,214.9 °F). These techniques have been superseded by the electric arc furnace.
PREUDOSCIENTIFIC CLAIMS
Oxyhydrogen is associated with various exaggerated claims. It is often called "Brown's gas" or "HHO gas", a term popularized by fringe physicist Ruggero Santilli, who claimed that his HHO gas, produced by a special apparatus, is "a new form of water", with new properties, based on his fringe theory of "magnecules".
Many other pseudoscientific claims have been made about oxyhydrogen, like an ability to neutralize radioactive waste, help plants to germinate, and more.
Oxyhydrogen is often mentioned in conjunction with vehicles that claim to use water as a fuel. The most common and decisive counter-argument against producing this gas on board to use as a fuel or fuel additive is that more energy is always needed to split water molecules than is recouped by burning the resulting gas. Additionally, the volume of gas that can be produced for on-demand consumption through electrolysis is very small in comparison to the volume consumed by an internal combustion engine.
An article in Popular Mechanics in 2008 reported that oxyhydrogen does not increase the fuel economy in automobiles.
"Water-fueled" cars should not be confused with hydrogen-fueled cars, where the hydrogen is produced elsewhere and used as fuel or where it is used as fuel enhancement.
REFERENCE:
^ Howard Monroe Raymond (1916), "Oxy-Hydrogen Welding", Modern Shop Practice volume 1, American Technical Society, archived from the original on March 6, 2011
^ Viall, Ethan (1921). Gas Torch and Thermite Welding. McGraw-Hill. p. 10. Archived from the original on August 3, 2016.
^ W. Dittmar, "Exercises in quantitative chemical analysis", 1887, p. 189 Archived June 27, 2014, at the Wayback Machine
^ Jump up to:a b c O'Connor, Ken. "Hydrogen" (PDF). NASA Glenn Research Center Glenn Safety Manual. Archived from the original (PDF) on February 2, 2013.
^ Moyle, Morton; Morrison, Richard; Churchill, Stuart (March 1960). "Detonation Characteristics of Hydrogen Oxygen Mixtures" (PDF). AIChE Journal. 6 (1): 92–96. Bibcode:1960AIChE…6…92M. doi:10.1002/aic.690060118. hdl:2027.42/37308.
^ Jump up to:a b c Chisholm, Hugh, ed. (1911). "Oxyhydrogen Flame" . Encyclopædia Britannica. Vol. 20 (11th ed.). Cambridge University Press. p. 424.
^ Calvert, James B. (April 21, 2008). "Hydrogen". University of Denver. Archived from the original on April 18, 2009. Retrieved April 23, 2009. “An air-hydrogen torch flame reaches 2045 °C, while an oxyhydrogen flame reaches 2660 °C.”
^ "Adiabatic Flame Temperature". The Engineering Toolbox. Archived from the original on January 28, 2008. Retrieved April 23, 2009. "Oxygen as Oxidizer: 3473 K, Air as Oxidizer: 2483 K"
^ "Temperature of a Blue Flame". Archived from the original on March 16, 2008. Retrieved April 5, 2008. "Hydrogen in air: 2,400 K, Hydrogen in Oxygen: 3,080 K"
^ Jump up to:a b Tilden, William Augustus (1926). Chemical Discovery and Invention in the Twentieth Century. Adamant Media Corporation. p. 80. ISBN 978-0-543-91646-4.
^ Hofmann, A. W. (1875). "Report on the Development of the Chemical Arts During the Last Ten Years". Chemical News. Manufacturing chemists.
^ Griffin, John Joseph (1827). A Practical Treatise on the Use of the Blowpipe in Chemical and Mineral Analysis. Glasgow: R. Griffin & co.
^ "Verneuil process". Encyclopaedia Britannica. October 22, 2013. Retrieved July 11, 2018.
^ P. N. Rao (2001), "24.4 Oxyhydrogen welding", Manufacturing technology: foundry, forming and welding (2 ed.), Tata McGraw-Hill Education, pp. 373–374, ISBN 978-0-07-463180-5, archived from the original on June 27, 2014
^ "Eagle Research Institute - Brown's Gas - Myth-conceptions". Archived from the original on April 18, 2019. Retrieved July 11, 2018.
^ Jump up to:a b Ball, Philip (September 10, 2007). "Burning water and other myths". News@nature. Springer Nature. doi:10.1038/news070910-13. ISSN 1744-7933. S2CID 129704116.
^ Jump up to:a b c Ball, Philip (2006). "Nuclear waste gets star attention". News@nature. doi:10.1038/news060731-13. ISSN 1744-7933. S2CID 121246705.
^ Weimar, Carrie (May 7, 2007). "Snubbed By Mainstream, Scientist Sues". St. Petersburg Times. Retrieved February 3, 2011.
^ Schadewald, R.J. (2008). Worlds of Their Own: A Brief History of Misguided Ideas: Creationism, Flat-Earthism, Energy Scams, and the Velikovsky Affair. Xlibris US. ISBN 978-1-4628-1003-1. Retrieved July 11, 2018.
^ Simpson, Bruce (May 2008). "The proof that HHO is a scam". Aardvark Daily. Archived from the original on February 11, 2012. Retrieved February 12, 2012.
^ Water-Powered Cars: Hydrogen Electrolyzer Mod Can't Up MPGs Archived March 20, 2015, at the Wayback Machine, Mike Allen, August 7, 2008, Popularmechanics.com
0 notes
Text
Out And About
It’s that time of year again. Sure, Halloween is still 10 days off, but right now, retailers are looking beyond candy and costume sales. It’s time to focus on the holidays. And even with many online deals having hatched weeks ago now, there’s still enough emphasis on the most hallowed of shopping days—Black Friday—to keep it in the mix.
And one company is not having any of it. God bless you, REI, for having the guts several years ago to turn off the lights on the day after Thanksgiving. Better yet, thank you for announcing last year that you were done with Black Friday forever. They’re sticking to their guns, and REI will be closed on what some could argue is still a very big day for making sales.
Thanks to the democratization of hashtag creation, they made their own to commemorate the non-event: #OptOutside. It’s a good one with a lot of PR value. Basically, they’re making a big deal out of being outside, doing the things that their lifestyle-themed store sells.Brilliant.

At the macro level, REI’s move is a generous gift to all of its employees, not just retail clerks, but also office and warehouse. Everyone gets the day off, as well as the day before. They’ll be back at it on Saturday. You can still place your order online—because the internet never sleeps, you know—but it wont’t be fulfilled until Saturday.
There’s a bit of a trend these days in retail, with many—following Walmart’s big lead—closing down for the holiday. If you think you might need some more pumpkin pie filling, you better grab it on Wednesday, because you’ll be out of luck the next day. Gone are the people hanging out inside a Walmart on Thanksgiving evening waiting for the sales to drop at midnight. You’ll have to get up Friday morning like everyone else.
Allowing employees to have family time, or as in REI’s case, outdoor time, is much-needed. We as a society have gone a little too crazy with all this consumerism. It’s just not fun anymore, and for workers to have to clock in when they would much rather be with their family and friends, or in Nature, is a little much. Yeah, there will still be some folks working the holiday (I’m thinking c-stores and truck stops), but we need to start building boundaries around what is sacred.
REI is calling Walmart, and raising the bet. Good on them, because retail will wait. Besides, it’s in their best interests that I use my gear, because it means it will wear out sooner and I will be back for more. I see some new trail runners in my future, and another 10 miles on Black Friday will put me that much closer to a new pair.
The odd thing is that the traditional holiday shopping kick-off is just an artifact of the calendar and when American Thanksgiving falls. If we were in Canada, it would just be the fourth Friday of the month, a day like any other day. They had their Thanksgiving a couple of weeks ago on the second Monday of October. We were busy remembering Christopher Columbus or indigenous peoples. Take your pick.
Bold move, REI. You already had my patronage, but now I feel even better about it. I’ll leave the employees and shoppers at Dick’s, Academy, Scheel’s, and so forth to deal with the pressures of it all. As for me, I’ll be down in Palo Duro Canyon, trying not to think about any of that.
Dr “#OptingOutside” Gerlich
Audio Blog
0 notes
Text
《Lonely Warrior》Tungsten - Embracing You with Temperatures Beyond Magma
Tungsten is a rare high-melting-point metallic element with an atomic number of 74. It belongs to the VIB group in the sixth period (the second longest period) of the periodic table. Tungsten is a silvery-white metal that resembles steel, and it can appear steel gray or silver-white in color. It is known for its high hardness and melting point. Tungsten is also denoted as "W," which comes from the name given to it by the Swedish chemist C.W. Scheele. In 1781, Scheele discovered a white mineral in Sweden, and when writing his research report, he named it "tungsten" because of its high density. In Swedish, "tun" means heavy, and "sten" means stone, so it can be translated as "heavy stone."
Tungsten has very stable chemical properties and does not react with air or water at room temperature. When not heated, tungsten is unreactive with hydrochloric acid, sulfuric acid, nitric acid, hydrofluoric acid, and even aqua regia at any concentration. At temperatures ranging from 80 to 100°C, all acids, except hydrofluoric acid, have only a weak effect on tungsten. Tungsten possesses remarkable physical characteristics, especially its extremely high melting point, which is the highest among all non-alloy metals. Tungsten has a melting point of 3,410.20°C and a boiling point of 5,927°C. Lava typically ranges in temperature from approximately 700 to 1,200 degrees Celsius, which is why tungsten is referred to as a substance that cannot be melted by volcanic magma.
Tungsten makes up approximately 0.001% of the Earth's crust. There are about 20 known tungsten-bearing minerals. China is a major producer of tungsten, with tungsten reserves of 5.2 million tons, more than three times the total reserves (1.3 million tons) of 30 other tungsten-producing countries overseas. China leads the world in both tungsten production and exports.
Ultramet, with tungsten as the primary element, has developed a series of products including the Elemental Ring and the Cubic Collection. These products embody a sense of minimalistic elegance, interpreting the fearless spirit of lonely adventurers who journey through the wilderness, facing challenges with unwavering determination. The intention is to build a warm and loving connection at your fingertips, expressing gratitude for the refining process of time. Regardless of the length of the journey so far, it is only through a balanced approach, maintaining a pioneering spirit, that we can continue hand in hand with these brave souls to infinity.

Tungsten Elemental Ring - Arched Design
W ≥ 99.99%
Ring Thickness: 1.5mm
Ring Surface Width: 4mm
Customization Available

Tungsten Cube Collection
Available in 10mm, 21mm, and 25.4mm Sizes
"ULTRAMET", a brand created by a female engineer who dedicated her life to the pursuit of "the spirit of freedom, the depth of love, and the joy of creation." It is designed for all those who share the same feelings, beliefs, and frequencies. ULTRAMET has crafted multiple series of collector's-grade art pieces with the aim of selecting the rare and precious elements found on this planet. Through the exchange of souls, the collision of thoughts, and the expression of art, it seeks to capture and convey the essence deep within the human soul.
0 notes
Text
2022-23 Texas Stars Roster
Wingers
#10 Nick Caamano (Hamilton, Ontario)
#11 Riley Barber (Pittsburgh, Pennsylvania)*
#16 Curtis McKenzie (Golden, British Columbia) C
#27 Riley Tufte (Blaine, Minnesota)
#28 Matěj Blümel (Tábor, Czech Republic)**
#33 Scott Reedy (Prior Lake, Minnesota)*
#34 Marián Studenič (Holíč, Slovakia)
#39 Ben Berard (Duncan, British Columbia)**
#40 Antonio Stranges (Ann Arbor, Michigan)**
Centers
#9 Keaton Mastrodonato (Powell River, British Columbia)**
#12 Riley Damiani (Mississauga, Ontario)
#18 Fredrik Karlström (Stockholm, Sweden)
#19 Mavrik Bourque (Plessisville, Quebec)**
#20 Tanner Kero (Southfield, Michigan)
#21 Oskar Bäck (Karlstad, Sweden)
#22 Rhett Gardner (Moose Jaw, Saskatchewan)
Defensemen
#2 Ryan Shea (Milton, Massachusetts)
#4 Will Butcher (Sun Prairie, Wisconsin)*
#6 Ben Gleason (Brandon Township, Michigan)
#7 Alex Petrovic (Edmonton, Alberta) A
#8 Thomas Harley (Vaughan, Ontario)
#25 Oskari Laaksonen (Tampere, Finland)*
#26 Michael Karow (Green Bay, Wisconsin)
#38 Jerad Rosburg (Howard County, Maryland)
Goalies
#1 Rémi Poirier (Farnham, Quebec)**
#30 Dylan Wells (St. Catherines, Ontario)
#31 Adam Scheel (Lakewood, Ohio)
#32 Matt Murray (St. Albert, Alberta)**
#Sports#Hockey#Hockey Goalies#AHL#Texas Stars#Celebrities#Sweden#Pennsylvania#Canada#British Columbia#Czech Republic#Quebec#Wisconsin#Ontario#Saskatchewan#Michigan#Finland#Alberta#Minnesota#Maryland#Ohio#Massachusetts#Slovakia
0 notes
Text
Carl Wilhelm Scheele

9 December 1742 – 21 May 1786 was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydrogen, and chlorine, among others. Scheele discovered organic acids tartaric, oxalic, uric, lactic, and citric, as well as hydrofluoric, hydrocyanic, and arsenic acids. He preferred speaking German to Swedish his whole life, as German was commonly spoken among Swedish pharmacists.
Scheele was born in Stralsund, in western Pomerania, which at the time was a Swedish Dominion inside the Holy Roman Empire. Scheele's father, Joachim or Johann Christian Scheele, was a grain dealer and brewer from a respected Pomeranian family. His mother was Margaretha Eleanore Warnekros.
Friends of Scheele's parents taught him the art of reading prescriptions and the meaning of chemical and pharmaceutical signs. Then, in 1757, at the age of fourteen, Carl was sent to Gothenburg as an apprentice pharmacist to another family friend and apothecary, Martin Andreas Bauch. Scheele retained this position for eight years. During this time he ran experiments late into the night and read the works of Nicolas Lemery, Caspar Neumann, Johann von Löwenstern-Kunckel and Georg Ernst Stahl (the champion of the phlogiston theory). Much of Scheele's later theoretical speculations were based upon Stahl.
In 1765 Scheele worked under the progressive and well-informed apothecary C. M. Kjellström in Malmö, and became acquainted with Anders Jahan Retzius who was a lecturer at the University of Lund and later a professor of chemistry at Stockholm. Scheele arrived in Stockholm between 1767 and 1769 and worked as a pharmacist. During this period he discovered tartaric acid and with his friend, Retzius, studied the relation of quicklime to calcium carbonate. While in the capital, he also became acquainted with figures including Abraham Bäck, Peter Jonas Bergius, Bengt Bergius and Carl Friedreich von Schultzenheim.
In the fall of 1770 Scheele became director of the laboratory of the great pharmacy of Locke, at Uppsala, which is about 40 miles north of Stockholm. The laboratory supplied chemicals to Professor of Chemistry Torbern Bergman. A friendship developed between Scheele and Bergman after Scheele analyzed a reaction which Bergman and his assistant, Johan Gottlieb Gahn, could not resolve. The reaction was between melted saltpetre and acetic acid that produced a red vapor. TheyFurther study of this reaction later led to Scheele's discovery of oxygen (see "The theory of phlogiston" below). Based upon this friendship and respect, Scheele was given free use of Bergman's laboratory. Both men were profiting from their working relationship. In 1774 Scheele was nominated by Peter Jonas Bergius to be a member of the Royal Swedish Academy of Sciences and was elected 4 February 1775. In 1775 Scheele also managed for a short time a pharmacy in Köping. Between the end of 1776 and the beginning of 1777 Scheele established his own business there.
On 29 October 1777, Scheele took his seat for the first and only time at a meeting of the Academy of Sciences and on 11 November passed the examination as apothecary before the Royal Medical College, doing so with the highest honours. After his return to Köping he devoted himself, outside of his business, to scientific researches which resulted in a long series of important papers.
By the time he was a teenager, Scheele had learned the dominant theory of gases which in the 1770s was the phlogiston theory. Phlogiston, classified as "matter of fire", was supposed to be released from any burning material, and when it was exhausted, combustion would stop. When Scheele discovered oxygen he called it "fire air" as it supported combustion. Scheele explained oxygen using phlogistical terms because he did not believe that his discovery disproved the phlogiston theory.
Before Scheele made his discovery of oxygen, he studied air. Air was thought to be an element that made up the environment in which chemical reactions took place but did not interfere with the reactions. Scheele's investigation of air enabled him to conclude that air was a mixture of "fire air" and "foul air;" in other words, a mixture of two gases. Scheele performed numerous experiments in which he heated substances such as saltpetre (potassium nitrate), manganese dioxide, heavy metal nitrates, silver carbonateand mercuric oxide. In all of these experiments, he isolated the same gas: his "fire air," which he believed combined with phlogiston in materials to be released during heat-releasing reactions.
However, his first publication, Chemische Abhandlung von der Luft und dem Feuer, was delivered to the printer Swederus in 1775, but not published until 1777, at which time both Joseph Priestley and Antoine Lavoisier had already published their experimental data and conclusions concerning oxygen and the phlogiston theory. Carl was credited for finding oxygen with two other people, Joseph Priestley and Antoine Lavoisier. The first English edition, Chemical Observation and Experiments on Air and Fire was published in 1780, with an introduction "Chemical Treatise on Air and Fire".
Scheele achieved astonishingly prolific and important results without the expensive laboratory equipment to which his Parisian contemporary Antoine Lavoisier was accustomed. Through the studies of Lavoisier, Priestley, Scheele, and others, chemistry was made a standardized field with consistent procedures. Although Scheele was unable to grasp the significance of his discovery of the substance that Lavoisier later named oxygen, his work was essential for the abandonment of the long-held theory of phlogiston.
Scheele's study of the gas not yet named oxygen was prompted by a complaint by Torbern Olof Bergman, a professor at Uppsala University who would eventually become Scheele's friend. Bergman informed Scheele that the saltpeter he had purchased from Scheele's employer, after long heating, produced red vapors (now known to be nitrogen dioxide) when it came into contact with acetic acid. Scheele's quick explanation was that the saltpeter had absorbed phlogiston with the heat (had been reduced to nitrite, in modern terms) and gave off a new phlogisticated gas as an active principle when combined with an acid (even a weak acid).
Bergman next suggested that Scheele analyze the properties of manganese(IV) oxide. It was through his studies of manganese(IV) oxide that Scheele developed his concept of "fire air" (his name for oxygen). He ultimately obtained oxygen by heating mercuric oxide, silver carbonate, magnesium nitrate, and other nitrate salts. Scheele wrote about his findings to Lavoisier who was able to see the significance of the results. His discovery of oxygen (ca. 1771) was chronologically earlier than the corresponding work of Priestley and Lavoisier, but he did not publish this discovery until 1777, after both of his rivals had published.
Although Scheele would always believe in some form of the phlogiston theory, his work reduced phlogiston to an unusually simple form, complicated only by the fact that chemists of Scheele's day still believed that light and heat were elements and were to be found in combination with them. Thus, Scheele assumed that hydrogen was composed of phlogiston (a reducing principle lost when objects were burned) plus heat. Scheele speculated that his fire air or oxygen (which he found the active part of air, estimating it to compose one quarter of air) combined with the phlogiston in objects to produce either light or heat (light and heat were presumed to be composed of differing proportions of phlogiston and oxygen).
When other chemists later showed water is produced when burning hydrogen and that rusting of metals added weight to them and that passing water over hot iron gave hydrogen, Scheele modified his theory to suggest that oxygen was the salt (or "saline principle" of water), and that when added to iron, water was reproduced, which added weight to the iron as rust.
0 notes
Text

Longshot #4
by Ann Nocenti (W.):,Arthur Adams (P.): Whilce Portacio (I.); Christie Scheele (C.) and Joe Rosen (L.)
Marvel
#marvel comics#longshot#original art#arthur adams#whilce portacio#scott williams#Christie Scheele#Joe Rosen
8 notes
·
View notes
Text
Win This Original Tron Film Cel
End Gleam, Oct 20
0 notes
Photo

Magnolia in the Sky - Spring Impression by AdrianScheel Source: https://ift.tt/2FHJaB2
#Magnolie#adrian scheel photography#bokeh#bokehworld#macro#magnolien#spring#frühling#vintage lenses#c
5 notes
·
View notes
Text
Fokuseret opmærksomhed.
Af C. Scheel P.D. I Special pædagogik og Master i IT
Stifter af Neurological Games.
d.11/2-2019
Indhold
Intro; 1
Opmærksomhed. 1
Fokuseret opmærksomhed. 2
Vedholdendende opmærksomhed. 3
Selektiv opmærksomhed. 4
Skiftende opmærksomhed. 5
Afledning. 8
Automatisering. 9
Delt opmærksomhed. 9
Referencer 10
Intro;
Dette er skrevet med til helt almindelige mennesker, der gerne vil vide noget om opmærksomhed.
Fagfolk kan også læse artiklen; ” Falkeblik. Det faglige grundlag, klassifikation og begreber.”
Opmærksomhed.
Opmærksomhed kan overordnet opdeles i to typer af opmærksomhed.
Den ene er opmærksomhed overfor noget der sker i omgivelserne eller i en selv, uden at man har besluttet sig for at være opmærksom på det. Noget fanger vores opmærksomhed eller vi bliver gjort opmærksomme på noget.

For en sikkerheds skyld er vores alarm system næsten altid er på vagt, det opfanger ting som er uvante, uventede eller som kan være farlige.
På billedet ovenfor er en illustration af at noget er skarpt i opmærksomheden, mens andet ikke rigtigt registres. For de fleste af os er det sådan vores alarmsystem og opmærksomhed fungerer når det er blevet opmærksomt på noget der er uvant eller uventet og som kunne være farligt. Det behøver ikke kun at handle om at noget er farligt eller kunne være farligt, det kan også være at noget har fanget vores opmærksomhed. Den vigtigste forskel på denne type af opmærksomhed og fokuseret opmærksomhed er at vi her, ikke bevidst har valgt at være opmærksomme på noget bestemt.
Den anden type af opmærksomhed er den fokuserede opmærksomhed. Fokuseret opmærksomhed opstår når vi bevidst vælger at fokusere på noget, det vil sige at vi har valgt at være opmærksomme på noget bestemt.
Fokuseret opmærksomhed.
Opmærksomhed er en basal eller fundamental mental funktion.
Opmærksomhed er en forudsætning for en række forskellige processer der foregår i hjernen, derfor er den vigtig. Det er meget nemmere f.eks. at huske noget, hvis man er opmærksom på det man gerne vil huske.
Fokuseret opmærksomhed er, helt overordnet, evnen til at holde opmærksomheden på det man gerne vil. Vi taler ikke om hvor motiveret man er, men om man kan holde sin opmærksomhed rettet mod noget bestemt.
Der er nogle forskere der har inddelt fokuseret opmærksomhed i nogle underkategorier.
De fire typer af fokuseret opmærksomhed de kommer frem til er;
Vedholdende opmærksomhed.
Selektiv opmærksomhed.
Skiftende opmærksomhed.
Delt opmærksomhed.
(Sohlberg and Mateer M. M., 2001) (Jason A. Demery, 2006, s. 54)

Vedholdende opmærksomhed
Vedholdende opmærksomhed er evnen til at fastholde sin opmærksomhed på noget bestemt over længere tid adgangen. For at løse en opgave, har vi brug for at kunne holde opmærksomheden på en bestemt opgave, indtil opgaven er løst. Når man har fået en hjerneskade så kan man godt have svært ved at fastholde opmærksomheden i længere tid adgangen. Det er selve evnen til at fastholde opmærksomheden man er ramt på, uanset hvor motiveret man er.

Selektiv opmærksomhed.
Selektiv opmærksomhed, selektiv betyder at vælge noget fremfor noget andet, altså at man er opmærksom på noget og lukker noget andet ude. Vi vælger f.eks. at være opmærksomme på en bestemt ting, når vi leder efter noget. Det kunne være at lede efter en nøgle.
Når vi leder efter en nøgle, er nøglerne skarpe i opmærksomheden, mens andre typer af ting lukkes ude af opmærksomheden.

Hvis man har problemer med den selektive opmærksomhed vil det være vanskeligere at fokusere udelukkende på nøglerne og lukke alt andet ude, derfor vil andre elementer også få opmærksomhed.
Her forestiller vi os at vi leder efter nogle nøgler. I det øjeblik andre typer af elementer også får opmærksomhed, så begynder det at blive vanskeligere at holde fast i det vi leder efter, nemlig nøgler.
Skiftende opmærksomhed, handler om evnen til at skifte opmærksomhed fra en ting til noget andet og tilbage igen.
Hvis jeg er ramt på min skiftende opmærksomhed, så kan jeg have problemer med at vende tilbage til tidligere aktiviteter.
Her er et eksempel;

Det kunne være at jeg gik i gang med at male en væg.

Undervejs kommer jeg i tanke om at jeg skal have vasket tøj, så jeg sætter vaskemaskinen i gang.

Mens jeg sætter vaskemaskinen i gang får jeg øje på en fugl ude i haven, og går ud for at se på den.
Nu har jeg maling der tørrer, en vaskemaskine der kører og jeg står ude i haven og kigger på fuglerene.
Jeg går midt i en aktivitet til den næste, uden at vende tilbage til nogen af de ting jeg allerede har sat i gang.
Hvis jeg er ramt på den skiftende opmærksomhed, så kan jeg have svært ved at vende tilbage til de tidligere aktiviteter og det vil kræve en særlig indsats at gøre det.
Man kan være ramt på skiftende opmærksomhed på forskellige måder.
Skiftende opmærksomhed handler også om evnen til at skifte spor.
Hvis jeg går i gang med en opgave, så kan det godt være at det på et tidspunkt er mere hensigtsmæssigt at gøre noget andet.
Her er et eksempel;

Lad os sige at jeg går i gang med at male en væg.

Mørket falder på mens jeg maler. Hvis jeg har svært ved at skifte spor, så fortsætter jeg med at male, selvom jeg reelt ikke kan se hvad jeg laver.
Hvis jeg har problemer med skiftende opmærksomhed, så kan det betyde at jeg fortsætter ud af samme spor, ude af stand til selv at skifte eller korrigere det jeg har gang i, selvom det er uhensigtsmæssigt.
Det er en del af de overordnede styrende funktioner i hjernen.
Kompleksiteten og dermed hvor krævende opmærksomheds funktionen er, er stigende i følgende rækkefølge; vedholdende, selektiv opmærksomhed, skiftende opmærksomhed til den mest komplekse som er delt opmærksomhed.
Delt opmærksomhed. Evnen til at holde opmærksomheden rettet mod mere end en ting adgangen. (Sohlberg and Mateer M. M., 2001) (Jason A. Demery, 2006, s. 54)
Afledning.
En person kan afledes indefra af egne tanker eller udefra af sanseindtryk. Afledning er når den fokuserede opmærksomhed rettes væk fra det man er i gang med og er fokuseret på. Dvs. at det vi ser på er om man evner at fastholde opmærksomheden på en opgave trods forstyrrelser, indre såvel som ydre.
Der er forskel på om man kan, altså har evnen til at fastholde opmærksomheden, trods forstyrrelser, eller om man bliver afledt. Vi taler altså ikke om motivation, da det er evnen til at fastholde opmærksomheden, selvom man er motiveret og gerne vil fastholde opmærksomheden, men ikke kan fordi man afledes.
Afledning eller afledelighed er ikke en funktion eller en evne, men omtales ofte som en følgevirkning af en funktionsnedsættelse. Derfor optræder afledning heller ikke i teorierne som en selvstændig funktion.
Hvorfor det er sådan bliver måske endnu tydeligere når vi kigger på en dansk oversættelse af Melvin Levin, der bruger ordet distraheret. (Levine, 2005, s. 99)
Her er det et spørgsmål om i hvilken grad man evner at vælge noget ud og give det opmærksomhed, fremfor noget andet, hvilket er selektiv opmærksomhed og i flg. Mel Levins teorier en del af input kontrollen.
Opmærksomhedsstyring:
Mel Levine beskæftiger sig med input styring, altså evnen til selv at regulere/ fokusere på bestemte input, som en del af opmærksomhedssystemet.
Under styring af input har han blandt andet den selektive opmærksomhed, men også elementer som dybde og detaljeringsgrad. Dybde og detaljeringsgraden handler om at være i stand til at holde fokus på det rette niveau af detaljer eller helheder. Dette er tanken om at blive optaget af en detalje, eller komme til at fokusere for meget på en detalje, der er uvigtig i forhold til helheden.

Et eksempel på at komme til at fokusere for meget på en detalje kunne være at ville tegne et portræt og undervejs så komme til at gå i dybden med det ene øje før de overordnede linjer er på plads.
Det betyder ikke alle der kommer til at fokusere for meget på en detalje har problemer med opmærksomhedsstyring. Hvis man har problemer med opmærksomhedsstyring betyder det at man vil have meget svært ved ikke at komme til at fokusere på en enkelt detalje, med mindre man arbejder med det og er bevidst om det.
Under styring af input har han også opmærksomheds spændvidde, som på den eneside handler om at fokusere på noget over tid, altså vedholdende opmærksomhed og koncentration, men som samtidigt også inddrager evnen til at skifte fokus, som er skiftende opmærksomhed. (Levine, 2005, s. 76-100)
Afledning er når opmærksomhedsstyringen træder ud af kraft.
Automatisering.
Mange af de ting vi gør igennem et liv, bliver efterhånden automatiseret, dvs. at vi kan gøre dem uden at bruge opmærksomhed eller energi på at gøre det.
For mange os kan det være at gå, at låse en dør eller at slukke for lyset, kaffemaskinen osv.
Det er derfor vi indimellem er nødt til at gå tilbage og tjekke om vi nu fik slukket lyset, låst døren eller slukket for kaffemaskinen.
Delt opmærksomhed.
Delt opmærksomhed er den fjerde og mest krævende af de fokuserede opmærksomhedsformer.
Med delt opmærksomhed menes at man deler sin opmærksomhed mellem to aktiviteter. Det er kun først delt opmærksomhed når begge aktiviteter kræver opmærksomhed, hvis den ene aktivitet er automatiseret så er der ikke tale om delt opmærksomhed.
Delt opmærksomhed kan være at lytte til et foredrag og tage notater samtidigt.
Delt opmærksomhed kan derfor godt være at køre bil og holde øje med trafikken, hvis det er en uerfaren bilist, for hvem det at køre bil ikke er automatiseret endnu. Den erfarne bilist vil typisk kun bruge opmærksomhed på at holde øje med trafikken og derfor er der ikke tale om ikke delt opmærksomhed.
Delt opmærksomhed kunne også være at spille på et instrument og synge samtidigt.
Her er en lille aktivitet med delt opmærksomhed.
Den går ud på at tegne en genstand uden at se på papiret.
Når man tegner uden at se på papiret så deler man opmærksomheden mellem at se på genstanden og undgå at se på papiret, samtidigt med at man forsøger at forestille sig hvad man tegner på papiret og tegne det.

På billedet ovenover kan du se figuren, jeg har forsøgt at tegne uden at se på papiret.

Denne sjove tegning er resultatet.
Med venlig hilsen
www.neurologicalgames.com
Referencer
Grethe Andersen, D. D. (2016). Apopleksi, sygdom, behandling og organisation. København: Munksgaard.
Jason A. Demery, P. (17. 4 2006). Traumatic Brain Injury. Hentet fra SlidePlayer: https://slideplayer.com/slide/11600330/
Levine, M. (2005). Hjernen bag lysten til at lære - Neuropædagogik i teori og praksis. Danmark: Dansk psykologisk forlag.
Sohlberg and Mateer, M. M. (4. jan 1987). Effectiveness of an attention-training program. Journal of Clinical and Experimental Neuropsychology, volume9, 1987,issue 2., 117-130. Hentet fra www.tandfonline.com/: https://www.tandfonline.com/doi/abs/10.1080/01688638708405352
Sohlberg and Mateer, M. M. (2001). Cognitive Rehabilitation: An Integrative Neuropsychological Approach. New York: GuildFord Press.
WHO, o. S. (2005). ICF, International klassifikation af funktionsevne funktionsnedsættelse og helbredstilstand. Viborg: Munksgaard.
#opmærksomhed#fokuseretopmærksomhed#selektivopmærksomhed#deltopmærksomhed#skiftendeopmærksomhed#hjernen#neurological#neurologicalgames#C Scheel#selektiv#skiftende#neuropædagogik
1 note
·
View note
Text

Der "Tatort" im Schnellcheck Tatsächlich … Liebe?
Von Ingo Scheel 19.02.2022, 15:02 Uhr
Ein nerviger Ex-Lover, eine verschollen geglaubte Mutter, ein Serienkiller, Bönisch mit den Nerven zu Fuß, nur Faber scheint mit sich im Reinen. Dass man dem Braten nicht trauen kann, versteht sich. Nur eins ist sicher: "Liebe mich!" ist ein "Tatort" für die ewige Bestenliste.
Was passiert?
Zunächst einmal sieht alles nach einem richtig schönen Kindergeburtstag aus: Papierschlangen und Kerzen, Zuckerstangen, Popcorn und eine Torte mit einer großen 4 aus Marzipan, alles ist festlich geschmückt, es fehlen nur noch die Gäste. Schnitt.
Pawlak muss mit seiner Tochter dringend über die Mutter reden.
(Foto: WDR/Bavaria Fiction GmbH/Thomas Kost)
Auf einem Dortmunder Bestattungsgelände wird eine Frauenleiche gefunden. Nun könnte man meinen, das sei doch der adäquate Ort für die letzte Ruhe, aber hier stimmt so einiges nicht. Der Grabplatz war unter falschem Namen reserviert, die Frau wurde seit genau einem Jahr vermisst, das merkwürdige Kleid, in dem sie steckt, wirkt wie eine Verkleidung. Zudem gibt es unübersehbare Parallelen zu einem Vermisstenfall wiederum ein Jahr zuvor, und dann jährt sich das Ganze in Kürze auch noch erneut. Für Faber (Jörg Hartmann) und Bönisch (Anna Schudt) steht bald fest, dass man es hier mit einem Serienkiller zu tun hat. Pikantes Detail: die ermordeten Frauen sehen Bönisch überaus ähnlich.
All das wäre bereits genug für einen ausgelasteten Arbeitstag, aber auch drumherum ist so einiges los. Da ist der Kollege Haller (Tilmann Strauß), Bönischs Ex-Lover, der einfach nicht locker lassen will. Da ist die lange untergetauchte Mutter (Rosa Enskat) von Rosa Herzog (Stefanie Reinsperger), die plötzlich wieder auf der Bildfläche erscheint. Pawlak (Rick Okon), der seiner kleinen Tochter endlich eröffnen muss, dass die Mama im U-Haft sitzt. Und das Bestattungsinstitut, in dem einige der Spuren in diesem Fall zusammenlaufen, ist von undurchsichtigem Personal besetzt: Bestatter Thomas Ihle (Jan Krauter), seine Frau Julia (Marlina Mitterhofer), die Angestellten Nils Schmelzer (Henning Flüsloh) und Armin Röder (Björn Jung), allesamt könnten etwas auf dem Kerbholz haben. Bleibt die Frage: Was soll die Nummer mit dem Kindergeburtstag?
Worum geht es wirklich?
Zehn Jahre sind seit dem ersten Einsatz von Faber und seinem Team vergangen, von Abnutzungserscheinungen keine Spur, im Gegenteil. Regisseur Torsten C. Fischer und Autor Jürgen Werner ist hier ein hochverdichtetes Krimidrama gelungen, das lange nachhallen wird.
Wegzapp-Moment?
Gibt es nicht. Gut, für Haller, den toxischen Typen von der KTU, hätte man gern einen ganz persönlichen Aus-Schalter, davon abgesehen ist dies ein "Tatort" zum Eintauchen und Fesselnlassen. Abschalten unmöglich.
Wow-Faktor?
Mehr zum Thema
Extrem hoch. Es dauert etwas, bis man sich in dieser hochangespannten Gemengelage meint, zurechtzufinden. Dann aber entfaltet "Liebe mich!" Sogwirkung, atemnehmend bis zum Schluss.
Wie war's?
10 von 10 Punkten - kriminell verzwickt, zwischenmenschlich brisant, mörderisch konsequent, großes Kino im Zeichen des "U", das man so schnell nicht vergessen wird.
3 notes
·
View notes