#Downstream Processing Market Research
Explore tagged Tumblr posts
Text
https://carbonfacesocial.org/blogs/69163/Downstream-Processing-Market-Size-Overview-Share-and-Forecast-2031
The Downstream Processing Market in 2023 is US$ 27.35 billion, and is expected to reach US$ 65.34 billion by 2031 at a CAGR of 11.5%.
#Downstream Processing Market#Downstream Processing Market Forecast#Downstream Processing Market Research
0 notes
Link
#market research future#downstream processing market#downstream processing industry#downstream processing size#downstream market trends
0 notes
Text
3D and 4D Printing Technology Market - Strategic Developments
Business Market Insights recently announced the release of the market research titled 3D and 4D Printing Technology Market Outlook to 2031 | Share, Size, and Growth. The report is a stop solution for companies operating in the 3D and 4D Printing Technology market. The report involves details on key segments, market players, precise market revenue statistics, and a roadmap that assists companies in advancing their offerings and preparing for the upcoming decade. Listing out the opportunities in the market, this report intends to prepare businesses for the market dynamics in an estimated period.
Is Investing in the Market Research Worth It?
Some businesses are just lucky to manage their performance without opting for market research, but these incidences are rare. Having information on longer sample sizes helps companies to eliminate bias and assumptions. As a result, entrepreneurs can make better decisions from the outset. 3D and 4D Printing Technology Market report allows business to reduce their risks by offering a closer picture of consumer behavior, competition landscape, leading tactics, and risk management.
A trusted market researcher can guide you to not only avoid pitfalls but also help you devise production, marketing, and distribution tactics. With the right research methodologies, Business Market Insights is helping brands unlock revenue opportunities in the 3D and 4D Printing Technology market.
If your business falls under any of these categories – Manufacturer, Supplier, Retailer, or Distributor, this syndicated 3D and 4D Printing Technology market research has all that you need.
What are Key Offerings Under this 3D and 4D Printing Technology Market Research?
Global 3D and 4D Printing Technology market summary, current and future 3D and 4D Printing Technology market size
Market Competition in Terms of Key Market Players, their Revenue, and their Share
Economic Impact on the Industry
Production, Revenue (value), Price Trend
Cost Investigation and Consumer Insights
Industrial Chain, Raw Material Sourcing Strategy, and Downstream Buyers
Production, Revenue (Value) by Geographical Segmentation
Marketing Strategy Comprehension, Distributors and Traders
Global 3D and 4D Printing Technology Market Forecast
Study on Market Research Factors
Who are the Major Market Players in the 3D and 4D Printing Technology Market?
3D and 4D Printing Technology market is all set to accommodate more companies and is foreseen to intensify market competition in coming years. Companies focus on consistent new launches and regional expansion can be outlined as dominant tactics. 3D and 4D Printing Technology market giants have widespread reach which has favored them with a wide consumer base and subsequently increased their 3D and 4D Printing Technology market share.
Report Attributes
Details
Segmental Coverage
Technology
Stereolithography
Fused Deposition Modelling
Selective Laser Sintering
Direct Metal Laser Sintering
Polyjet/Multijet Printing
Electron Beam Melting
Digital Light Processing
Material
Plastics
Metal
Ceramics
Composites
Resins
Bio-Metals
Hybrid Metals
Application
Prototyping
Manufacturing
Research & Development
End Use Industry
Aerospace & Defence
Automotive
Healthcare
Consumer Goods
Construction
Education & Research
Electronics
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
Stratasys Ltd.
3D Systems Corporation
Materialise NV
Autodesk, Inc.
Hewlett Packard Enterprise (HP)
EOS GmbH
Desktop Metal, Inc.
Organovo Holdings, Inc.
Dassault Syst?mes SE
Nano Dimension Ltd.
Other key companies
What are Perks for Buyers?
The research will guide you in decisions and technology trends to adopt in the projected period.
Take effective 3D and 4D Printing Technology market growth decisions and stay ahead of competitors
Improve product/services and marketing strategies.
Unlock suitable market entry tactics and ways to sustain in the market
Knowing market players can help you in planning future mergers and acquisitions
Visual representation of data by our team makes it easier to interpret and present the data further to investors, and your other stakeholders.
Do We Offer Customized Insights? Yes, We Do!
The Business Market Insights offer customized insights based on the client’s requirements. The following are some customizations our clients frequently ask for:
The 3D and 4D Printing Technology market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
About Us:
Business Market Insights is a market research platform that provides subscription services for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductors, Aerospace & Defense, Automotive & Transportation, Energy & Power, Healthcare, Manufacturing & Construction, Food & Beverages, Chemicals & Materials, and Technology, Media & Telecommunications.
Contact Us:
If you have any questions about this report or would like further information, please contact us:
Contact person: Ankit Mathur
Email: [email protected]
Phone: +16467917070

#3D and 4D Printing Technology Market#3D and 4D Printing Technology Market Size#3D and 4D Printing Technology Market Trends#3D and 4D Printing Technology Market Shares#3D and 4D Printing Technology Market Growth
0 notes
Text
E-Beam Wafer Inspection System : Market Trends and Future Scope 2032
The E-Beam Wafer Inspection System Market is poised for significant growth, with its valuation reaching approximately US$ 990.32 million in 2024 and projected to expand at a remarkable CAGR of 17.10% from 2025 to 2032. As the semiconductor industry evolves to accommodate more advanced technologies like AI, IoT, and quantum computing, precision inspection tools such as E-beam wafer systems are becoming indispensable. These systems play a pivotal role in ensuring chip reliability and yield by detecting defects that traditional optical tools might overlook.
Understanding E-Beam Wafer Inspection Technology
E-Beam (electron beam) wafer inspection systems leverage finely focused beams of electrons to scan the surface of semiconductor wafers. Unlike optical inspection methods that rely on light reflection, E-beam systems offer significantly higher resolution, capable of detecting defects as small as a few nanometers. This level of precision is essential in today’s era of sub-5nm chip nodes, where any minor defect can result in a failed component or degraded device performance.
These systems operate by directing an electron beam across the wafer's surface and detecting changes in secondary electron emissions, which occur when the primary beam interacts with the wafer material. These emissions are then analyzed to identify defects such as particle contamination, pattern deviations, and electrical faults with extreme accuracy.
Market Drivers: Why Demand Is Accelerating
Shrinking Node Sizes As semiconductor manufacturers continue their pursuit of Moore’s Law, chip geometries are shrinking rapidly. The migration from 10nm to 5nm and now toward 3nm and beyond requires metrology tools capable of atomic-level resolution. E-beam inspection meets this demand by offering the only feasible method to identify ultra-small defects at such scales.
Increasing Complexity of Semiconductor Devices Advanced nodes incorporate FinFETs, 3D NAND, and chiplets, which make inspection significantly more complex. The three-dimensional structures and dense integration elevate the risk of process-induced defects, reinforcing the need for advanced inspection technologies.
Growing Adoption of AI and HPC Devices Artificial intelligence (AI) chips, graphics processing units (GPUs), and high-performance computing (HPC) applications demand flawless silicon. With their intense performance requirements, these chips must undergo rigorous inspection to ensure reliability.
Yield Optimization and Cost Reduction Identifying defects early in the semiconductor fabrication process can help prevent downstream failures, significantly reducing manufacturing costs. E-beam inspection offers a proactive quality control mechanism, enhancing production yield.
Key Market Segments
The global E-Beam Wafer Inspection System Market is segmented based on technology type, application, end-user, and geography.
By Technology Type:
Scanning Electron Microscope (SEM) based systems
Multi-beam inspection systems
By Application:
Defect inspection
Lithography verification
Process monitoring
By End-User:
Integrated Device Manufacturers (IDMs)
Foundries
Fabless companies
Asia-Pacific dominates the market owing to the presence of major semiconductor manufacturing hubs in countries like Taiwan, South Korea, Japan, and China. North America and Europe also contribute significantly due to technological innovations and research advancements.
Competitive Landscape: Key Players Driving Innovation
Several global players are instrumental in shaping the trajectory of the E-Beam Wafer Inspection System Market. These companies are heavily investing in R&D and product innovation to cater to the growing demand for high-precision inspection systems.
Hitachi Ltd: One of the pioneers in E-beam inspection technology, Hitachi’s advanced systems are widely used for critical defect review and metrology.
Applied Materials Inc.: Known for its cutting-edge semiconductor equipment, Applied Materials offers inspection tools that combine speed and sensitivity with atomic-level precision.
NXP Semiconductors N.V.: Although primarily a chip manufacturer, NXP’s reliance on inspection tools underscores the importance of defect detection in quality assurance.
Taiwan Semiconductor Manufacturing Co. Ltd. (TSMC): The world’s largest dedicated foundry, TSMC uses E-beam systems extensively in its advanced process nodes to maintain top-tier yield rates.
Renesas Electronics: A leader in automotive and industrial semiconductor solutions, Renesas emphasizes defect detection in complex system-on-chip (SoC) designs.
Challenges and Opportunities
Despite its numerous advantages, E-beam wafer inspection systems face challenges such as:
Throughput Limitations: Due to the nature of electron beam scanning, these systems generally operate slower than optical tools, affecting wafer processing time.
High Capital Investment: Advanced E-beam systems are expensive, which can deter smaller fabs or start-ups from adopting the technology.
However, ongoing innovations like multi-beam inspection systems and AI-powered defect classification are paving the way for faster and more cost-effective inspection solutions. These enhancements are expected to mitigate traditional drawbacks and further fuel market expansion.
Future Outlook
With semiconductors becoming more ingrained in everyday life—powering everything from smartphones to electric vehicles and cloud data centers—the importance of precise defect detection will only intensify. The E-Beam Wafer Inspection System Market is set to benefit tremendously from this surge in demand.
The integration of machine learning algorithms to speed up defect classification, along with the emergence of hybrid inspection platforms combining optical and electron beam technologies, will revolutionize wafer inspection methodologies in the coming years.
In conclusion, the E-Beam Wafer Inspection System Market is not just growing—it’s transforming the foundation of quality assurance in semiconductor manufacturing. As fabrication becomes more intricate and expectations for reliability increase, E-beam systems will remain a cornerstone technology, ensuring the chips that power our digital lives meet the highest standards of performance and precision.
Browse more Report:
Muscle Strengthening Devices Market
Monopolar Electrosurgery Instrument Market
Medical Styrenic Block Copolymers Market
Hard-Wired Commercial Surge Protection Devices Market
Solar Street Lighting Market
0 notes
Text
Latest Protease Manufacturing Plant Project Report by Procurement Resource

Procurement Resource, a renowned provider of procurement intelligence and market research solutions, has released its latest Protease Manufacturing Report. This comprehensive guide serves as a strategic resource for entrepreneurs and industry stakeholders aiming to establish a successful protease production unit, offering critical insights into industry trends, process design, capital investment, equipment needs, and future growth prospects.
Protease: A Vital Industrial Enzyme
Protease, also known as peptidase or proteinase, is a crucial class of enzymes that catalyze the breakdown of proteins into amino acids. These enzymes have wide-ranging applications across sectors such as food and beverages, detergents, pharmaceuticals, animal feed, and leather processing. Due to the rising demand for enzymatic solutions that are both efficient and environmentally friendly, the global protease market is witnessing consistent growth.
Exhaustive Manufacturing Plant Report for Business Success
The Procurement Resource report delivers an in-depth blueprint for setting up a protease manufacturing facility, providing practical and data-driven insights to enable informed and profitable business decisions.
Market Analysis:
In-depth analysis of market dynamics, demand-supply trends, and regional consumption patterns
Pricing trends for raw materials (such as fermentation media and substrates) and final enzyme formulations
Evaluation of global industrial demand, regulatory outlook, and sustainability factors
Check out my latest social media article and industry report:-
https://manufacturing-reports.hashnode.dev/pvd-salt-manufacturing-report
https://www.linkedin.com/pulse/latest-potassium-sulfide-manufacturing-plant-project-report-suraj-jha-yrvbc
Technical and Operational Insights:
Step-by-step production process (e.g., microbial fermentation, downstream processing, drying, and formulation)
Machinery specifications (Automatic/Semi-automatic/Manual production lines)
Infrastructure and utility requirements: fermenters, centrifuges, filtration systems, and HVAC
Labor needs, quality control systems, and compliance with GMP and ISO standards
Financial and Economic Assessment:
Breakdown of capital expenditure (CapEx) and operational expenditure (OpEx)
Detailed profit margin analysis and return on investment (ROI)
Break-even analysis, scalability forecasts, and risk assessment models
Sustainability and Market Trends
With increasing environmental awareness and the shift towards bio-based and non-toxic industrial inputs, protease enzymes are gaining traction as sustainable alternatives to synthetic chemicals. The Asia-Pacific region leads in consumption due to its robust food processing and textile industries. The adoption of green manufacturing technologies and enzyme immobilization techniques is expected to enhance the efficiency and cost-effectiveness of protease production.
Why Choose Procurement Resource?
Procurement Resource brings together a team of seasoned analysts and domain experts to provide clients with precise, up-to-date intelligence for informed procurement and strategic planning. Their services empower businesses with:
Detailed cost modeling and production cost benchmarking
Comprehensive market research tailored to industry-specific needs
Real-time data and trend analysis
Supply chain optimization and risk mitigation strategies
Request a Free Sample Report
For entrepreneurs and investors aiming to explore the opportunities in protease enzyme manufacturing, this detailed report by Procurement Resource serves as a crucial tool to facilitate effective planning, reduce setup costs, and maximize return.
👉 Request for a Free Sample Report: https://www.procurementresource.com/reports/protease-manufacturing-plant-project-report/request-sample
About Procurement Resource
Procurement Resource is committed to helping businesses excel through actionable insights, strategic intelligence, and advanced cost analysis across industries. Their services include:
Market research and feasibility studies
Procurement and sourcing strategy support
Real-time price and trend tracking
Customized cost analysis reports
Contact Information
Company Name: Procurement Resource Contact Person: Ashish Sharma (Sales Representative) Email: [email protected] Location: 30 North Gould Street, Sheridan, WY 82801, USA Phone Numbers: UK: +44 7537171117 USA: +1 307 363 1045 Asia-Pacific (APAC): +91 8850629517
#Protease#Protease Manufacturing#Protease Manufacturing Report#Protease Manufacturing Plant Project Report
1 note
·
View note
Text
HighPrep PCR Beads vs. AMPure XP: 3 Reasons to Make the Switch
When it comes to PCR cleanup in next-generation sequencing (NGS) workflows, SPRI bead-based purification remains the gold standard. For years, AMPure XP has dominated the market with its reliable but expensive solution. However, labs are now increasingly turning to a smarter alternative—MagBio Genomics' HighPrep PCR beads.
Why are more researchers switching from AMPure XP to HighPrep PCR? Because they want better value, equal (or better) performance, and true workflow flexibility. In this article, we dive into the top three reasons why HighPrep PCR has become the preferred choice for DNA purification—and why it might be time for your lab to make the switch.
Reason 1: Cost-Efficiency Without Performance Compromise
One of the biggest drivers for labs to reconsider their reagent suppliers is cost. AMPure XP is known for high recovery rates and reproducibility, but its cost per reaction adds up fast—especially for high-throughput facilities. HighPrep PCR offers a cost savings of up to 40% per sample, without compromising yield or quality.
Side-by-side tests show:
DNA recovery from 100 bp to 2 kb is nearly identical for both products.
HighPrep PCR performs equally well in removing primers, dimers, and enzymes.
Labs switching to HighPrep PCR report equivalent sequencing coverage and fidelity.
Over the course of a year, switching to HighPrep PCR can lead to tens of thousands of dollars in savings for mid-to-large-scale labs. For academic labs, that translates into more samples processed, more grant deliverables achieved, and more experiments completed on time.
By reducing per-sample purification costs, labs using HighPrep PCR can reallocate budget toward additional experiments, new equipment, or expanded sample sizes.
Reason 2: Reliable, Consistent Performance Across Applications
Whether you're doing amplicon sequencing, library preparation, or qPCR cleanup, consistency matters. HighPrep PCR beads are manufactured under strict quality controls to ensure batch-to-batch reproducibility. Unlike some generic alternatives, HighPrep PCR has gone through rigorous testing to meet the needs of sensitive molecular biology workflows.
HighPrep PCR is validated for:
Illumina and Ion Torrent library prep workflows
Post-PCR cleanup for qPCR and Sanger sequencing
Adapter dimer cleanup after ligation
Double-sided size selection protocols for fragment tuning
Automation with KingFisher, Tecan, and Hamilton platforms
In fact, one high-throughput genomics core saw a 10% improvement in recovery reproducibility after switching from AMPure XP to HighPrep PCR. The magnetic bead suspension in HighPrep remains uniform and pipetting-friendly, reducing technical variability between users and across runs.
For long-term experiments that demand reproducible input DNA, this level of consistency translates directly into higher confidence in downstream data.
Reason 3: Flexible Workflows and Automation Compatibility
HighPrep PCR is designed with user flexibility in mind. From low-input DNA to automation integration, it supports a range of protocols without sacrificing ease of use. It’s fully compatible with manual and automated workflows, making it suitable for both bench-top researchers and large core labs.
Key advantages include:
Stable bead suspension compatible with multichannel pipettes and robotic arms
Broad input range from 5 ng to several μg of DNA
Works in 96- and 384-well formats with minimal protocol adjustments
Validated for double-SPRI workflows with tunable size exclusion parameters
Automation labs save not only on cost, but also reduce hands-on time and error rates, thanks to HighPrep's compatibility with widely used platforms like KingFisher Flex and Tecan Fluent.
How HighPrep PCR Matches Up in Common Use Cases
Let’s explore how HighPrep PCR performs in real lab scenarios:
Use Case: NGS Library Prep for Illumina
Objective: Cleanup post-PCR and remove dimers before sequencing.
Outcome: HighPrep PCR shows identical Bioanalyzer traces compared to AMPure XP, with better yield retention in low-input samples.
Use Case: Size Selection for Targeted Panels
Objective: Enrich for 350–500 bp fragments.
Outcome: Dual-SPRI using 0.6x/0.8x ratio yields high purity with narrow distribution, matching performance of AMPure XP.
Use Case: High-Throughput PCR Cleanup in 384-Well Plates
Objective: Maximize speed and recovery.
Outcome: HighPrep PCR allows faster separation and easier elution, increasing daily throughput by 20%.
Performance Comparison: HighPrep PCR vs AMPure XP

Why AMPure XP Users Are Making the Switch
While AMPure XP has long set the standard, users report growing frustration with:
Rising reagent costs
Supply chain delays and backorders
Limited flexibility in size selection
Reduced support for protocol customization
MagBio Genomics recognized these pain points and engineered HighPrep PCR to offer a more responsive, transparent, and scalable alternative. Labs making the switch often report that they "should have done it sooner."
Technical Tips for Optimizing HighPrep PCR Use
Use fresh ethanol for wash steps to minimize salt retention.
Avoid overdrying the beads, which can reduce elution efficiency.
Use magnetic stands with tight separation windows for fast and clean separation.
Pre-wet pipette tips to reduce bead carryover during transfers.
Elute in a pre-warmed buffer (37°C) for maximum recovery from HMW DNA.
MagBio also provides detailed protocol templates for Illumina workflows, long-read prep, and automated systems, ensuring a smooth transition from AMPure XP.
Transition Made Easy: From AMPure XP to HighPrep PCR
MagBio offers personalized onboarding and protocol migration support. For labs making the switch, MagBio provides:
Side-by-side comparison guides
Bead ratio conversion charts
Automation scripts for major platforms
Free sample kits for initial validation
This ensures a seamless transition with minimal disruption and quick results.
Frequently Asked Questions
Q: Will I need to change my current protocol? A: In most cases, no. HighPrep PCR can be used with the same volumes and timings as AMPure XP, or optimized for specific workflows.
Q: Can I still do double-sided size selection? A: Yes, HighPrep PCR is fully compatible with SPRI-based dual-cut protocols.
Q: Is there a minimum or maximum DNA input range? A: HighPrep PCR works with inputs from 5 ng to over 5 μg of DNA.
Q: What about RNA applications? A: While this kit is designed for DNA, MagBio offers HighPrep RNA kits specifically for RNA cleanup.
Final Thoughts
Switching to HighPrep PCR from AMPure XP isn't just about saving money—it's about empowering your lab to do more, faster, and with greater flexibility. With equivalent performance, lower cost, and validated automation support, HighPrep PCR delivers everything modern genomics labs need.
Labs around the world are making the switch. From academic research centers to clinical sequencing facilities, HighPrep PCR is transforming how DNA cleanup is done—one sample at a time.
Make the switch today and join hundreds of labs improving their workflows with HighPrep PCR beads. Explore the HighPrep PCR Clean-Up Kit at MagBio Genomics Inc.
0 notes
Text
Iron & Steel Research Journal: 33 Years of Thought Leadership by SERC
In a world driven by innovation, infrastructure, and industrial sustainability, one sector continues to play a foundational role — Iron & Steel. Behind the scenes of this ever-evolving industry is a publication that has chronicled its journey for over three decades: the Iron & Steel Research Journal, known widely as Steel Scenario, published by the Spark Economy Research Centre (SERC).
From thought leadership to data-backed insights, this journal has become a must-read for professionals, researchers, and policymakers who shape the global iron and steel ecosystem.
What is Steel Scenario?
Since its inception in July 1991, Steel Scenario has been more than just a technical journal — it’s a strategic knowledge platform. Initially a quarterly publication, it evolved into a monthly issue in 2011, keeping pace with the dynamic shifts in global and Indian metallurgy, engineering, and allied sectors.
It offers comprehensive coverage of:
Iron & steel production trends
Policy and regulatory updates
Technological innovation in metallurgy
Market intelligence and trade data
Sustainability and environmental strategies
Case studies from steel plants and downstream users
Why Iron & Steel Research Still Matters
Despite the rise of digital technologies and green materials, iron and steel remain irreplaceable in infrastructure, manufacturing, energy, and transportation. The sector is now rapidly adapting to:
Hydrogen-based steelmaking
Carbon capture innovations
Digital twins and process automation
Energy-efficient furnaces
Circular economy in metals recycling
The Iron & Steel Research Journal plays a key role in documenting, analyzing, and amplifying these breakthroughs to a global audience.
Who Reads Steel Scenario?
SERC’s readership spans the full length of the value chain. Whether you’re a scientist in a metallurgical lab or a policymaker shaping the steel sector’s roadmap, this journal provides essential content.
Regular readers include:
Integrated steel plants and mini mills
Steel re-rollers and foundries
Research institutions and academic bodies
Industrial consultants and EPC firms
Global trade houses and exporters
Government departments and regulatory bodies
Associations in mining, refractories, and metals
Notably, it also reaches Central and State Ministers, and key Chambers of Commerce & Industry across India and abroad.
Featured Sections
Each monthly issue includes:
Editorial Insights — Expert commentary on current challenges and future outlook
Research Articles — Peer-reviewed papers from scientists and technologists
Sector Watch — Industry data, production forecasts, policy updates
Innovation Focus — Spotlight on startups, green steel tech, and digital transformation
Event Coverage — Conferences, exhibitions, and summits from India and overseas
Why Subscribe or Collaborate?
By subscribing to Steel Scenario, you get:
✅ Credible, long-form content curated by industry experts ✅ Monthly updates that keep you ahead of trends ✅ Visibility among thought leaders and decision-makers ✅ Opportunities to publish, advertise, and collaborate ✅ A platform that connects B2B networks across geographies
If you’re in steel, metallurgy, construction, energy, or logistics — this is where your market begins and your knowledge grows.
About the Publisher — Spark Economy Research Centre (SERC)
Based in India, SERC is a pioneering think tank and publisher at the intersection of steel, economy, and industrial research. With its second journal, Industry Scenario (launched in 2021), SERC has extended its expertise to cover verticals like automotive, infrastructure, cement, and more.
Its strength lies in its deep industry connect, credibility, and mission to bridge academia, industry, and policy.
Final Word:
As the world shifts toward a sustainable and technologically empowered industrial future, staying informed is not an option — it’s a necessity.
Explore Steel Scenario, the official Iron & Steel Research Journal by SERC, and be a part of the narrative that builds nations, strengthens economies, and shapes tomorrow’s industry.
Subscribe. Contribute. Advertise. Lead.
1 note
·
View note
Text
Bacillus Coagulans API Manufacturers In India
India has emerged as a global hub for Active Pharmaceutical Ingredients (APIs), with a robust pharmaceutical manufacturing base, regulatory infrastructure, and growing research capabilities. Among the numerous APIs produced, Bacillus coagulans is gaining attention due to its wide-ranging health benefits, particularly as a probiotic strain. With increasing demand from dietary supplement, pharmaceutical, and nutraceutical industries worldwide, India is now a key player in the manufacturing of Bacillus coagulans APIs.

What are Bacillus Coagulans?
Unlike many other probiotics, B. coagulans forms heat-resistant spores, allowing it to survive harsh conditions such as stomach acid and high temperatures during processing. This unique characteristic makes it especially suitable for inclusion in dietary supplements, functional foods, and pharmaceuticals.
Health Benefits
Research has shown that Bacillus coagulans may:
Support digestive health by improving gut flora
Reduce symptoms of irritable bowel syndrome (IBS)
Enhance immune function
Help in managing inflammation
Aid in the absorption of nutrients
Promote overall gastrointestinal balance
Given these benefits, the demand for Bacillus coagulans-based products is rapidly growing across global markets, especially in the United States, Europe, and Asia-Pacific regions.
Why India is Emerging as a Preferred Manufacturing Hub
1. Cost-Effective Production
The lower cost structure allows Indian manufacturers to produce Bacillus coagulans APIs at competitive prices, making them attractive to international buyers.
2. Skilled Workforce and Technical Expertise
The country boasts a large pool of skilled microbiologists, biotechnologists, and pharmaceutical scientists who are well-versed in fermentation technology, strain optimization, and downstream processing—key aspects in the production of probiotic APIs like Bacillus coagulans.
3. Regulatory Compliance
Many Indian API manufacturers comply with global regulatory standards, including:
Good Manufacturing Practices (GMP)
US FDA certification
EU GMP
WHO-GMP
ISO certifications
Such compliance ensures that the APIs produced are of high quality and suitable for global pharmaceutical markets.
4. Infrastructure and R&D Capabilities
The Indian pharmaceutical sector has significantly invested in state-of-the-art fermentation units, clean rooms, and quality control laboratories. Many manufacturers also operate in dedicated industrial zones with access to centralized utilities and logistics support, further enhancing efficiency.
Additionally, India's growing emphasis on research and development has led to innovations in strain development, yield optimization, and scalable fermentation techniques—making Bacillus coagulans production more efficient and sustainable.
Key Manufacturing Considerations for Bacillus Coagulans API
Producing Bacillus coagulans API is complex and requires precise control of microbial growth conditions, purification, and quality assurance. The typical steps involved include:
Strain Selection and Maintenance: Selecting high-performing, stable Bacillus coagulans strains is critical. These strains must demonstrate robust growth, spore-forming ability, and clinically proven health benefits.
Fermentation: Controlled fermentation is carried out in bioreactors under specific pH, temperature, and oxygen levels. This step is crucial to maximize spore production.
Downstream Processing: Post-fermentation, the bacterial culture is separated, purified, and stabilized. The spore form is harvested, dried (often through freeze-drying or spray drying), and standardized to a specific Colony Forming Unit (CFU) count.
Quality Control and Stability Testing: Rigorous microbiological and biochemical assays ensure the product meets pharmacopeial standards for purity, potency, identity, and safety. Long-term stability studies are also conducted to guarantee shelf life.
Packaging and Storage: Since the product is sensitive to humidity and temperature, appropriate packaging (usually in moisture-resistant, sealed containers) and storage conditions (cool and dry) are maintained.
Export Potential
India’s Bacillus coagulans API manufacturers are well-positioned to cater to global demand. With the surge in probiotic research and growing consumer preference for gut health products, the export market for this API is expanding rapidly. Indian producers are increasingly engaging in:
Contract manufacturing for international brands
Custom strain development for specific health applications
Bulk supply to pharmaceutical and nutraceutical companies
Moreover, the presence of Indian manufacturers at international expos, trade fairs, and business forums reflects the growing importance of Bacillus coagulans in the country’s API export portfolio.
Challenges and Future Outlook
While India’s manufacturing ecosystem is strong, several challenges remain:
Regulatory Hurdles: Despite local compliance, navigating varying international probiotic regulations can be complex.
Cold Chain Management: Though Bacillus coagulans is heat-stable, certain applications still require temperature-controlled logistics, especially in finished product formats.
Consumer Education: Global awareness about Bacillus coagulans is growing, but it still lags behind more popular strains like Lactobacillus and Bifidobacterium.
Looking ahead, as demand for probiotics becomes more mainstream in functional foods, medical nutrition, and personalized health, Bacillus coagulans is likely to play an increasingly prominent role. Innovations in synbiotic formulations (combining probiotics and prebiotics), strain-specific health claims, and next-gen delivery systems (like microencapsulation) are expected to drive further growth.
Conclusion
India's rise as a manufacturing powerhouse for Bacillus coagulans API is the result of a confluence of factors—scientific capability, cost-efficiency, regulatory compliance, and strong infrastructure. As global interest in probiotics continues to rise, Indian manufacturers are not only meeting the current demand but are also shaping the future of probiotic applications across diverse therapeutic and nutritional domains.
For businesses looking to source high-quality Bacillus coagulans API or explore custom probiotic solutions, India offers a compelling mix of quality, scale, and innovation.
URL: For more information, visit Vakya Lifescience : Bacillus Coagulans API Manufacturers In India
0 notes
Text
Subscription and Billing Management Market Size, Share, Trends, Demand, Growth, Challenges and Competitive Analysis
Executive Summary Subscription and Billing Management Market :
Subscription and Billing Management Market research report studies various parameters throughout the report which analyses the market status in detail. It offers key measurements, status of the manufacturers and is a major source of direction for the businesses and organizations. Such market insights can be accomplished with this comprehensive Subscription and Billing Management Market research report which takes into account all the aspects of current and future market. In addition, Subscription and Billing Management Market research report predicts the size of the market with information on key vendor revenues, development of the industry by upstream & downstream, industry progress, key companies, segment type & market application.
The report carefully studies market definition, market segmentation, competitive analysis and key developments in the market. This Subscription and Billing Management Market research report consists of latest, comprehensive and most up-to-date market information and a precious data. Subscription and Billing Management Market report gives the market insights which help to have a more precise understanding of the market landscape, issues that may impose on the industry in the future, and how to position specific brands in the best way. It also studies the market status, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, and distributors with the help of SWOT analysis and Porter's Five Forces Analysis.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Subscription and Billing Management Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-subscription-and-billing-management-market
Subscription and Billing Management Market Overview
**Segments**
- **By Component** - Software - Services - **By Deployment Mode** - Cloud - On-Premises - **By Organization Size** - Small and Medium-Sized Enterprises (SMEs) - Large Enterprises - **By Vertical** - Banking, Financial Services, and Insurance (BFSI) - Healthcare - Telecom and IT - Retail - Manufacturing - Others
The global subscription and billing management market is segmented based on various factors such as component, deployment mode, organization size, and vertical. When it comes to components, the market is divided into software and services. Within deployment mode, the market can be categorized into cloud and on-premises solutions. Organization size plays a crucial role, with segmentation into small and medium-sized enterprises (SMEs) and large enterprises. Furthermore, the market is diversified across verticals like BFSI, healthcare, telecom and IT, retail, manufacturing, and other sectors.
**Market Players**
- Zuora, Inc. - Aria Systems, Inc. - Oracle Corporation - SAP SE - Netsuite Inc. - cleverbridge AG - Salesforce.com, inc. - ServiceNow, Inc. - Gotransverse - Chargebee
Moreover, the emergence of cloud-based subscription and billing management solutions is fueling market growth, as businesses look for scalable and cost-effective alternatives to traditional on-premises systems. Cloud deployment offers flexibility, agility, and accessibility, allowing organizations to manage their subscriptions and billing processes efficiently from anywhere in the world. This trend is further supported by the rising adoption of Software as a Service (SaaS) models, which are driving the demand for cloud-based subscription and billing management solutions among SMEs and large enterprises alike.
In terms of competition, the global subscription and billing management market is witnessing intense rivalry among key players such as Zuora, Aria Systems, Oracle, SAP, and Salesforce. These industry giants are investing heavily in research and development to innovate and differentiate their offerings in the market. For instance, Zuora is focusing on subscription-based monetization strategies, while SAP is emphasizing on end-to-end billing solutions for enterprises. Meanwhile, companies like cleverbridge, ServiceNow, Gotransverse, and Chargebee are also making their mark by providing specialized subscription and billing management services tailored to specific industry verticals.
As the market continues to evolve, regulatory compliance and data security are becoming critical considerations for organizations investing in subscription and billing management solutions. With the increasing focus on data privacy and protection, market players are enhancing their platforms to ensure compliance with regulations such as GDPR and CCPA. Additionally, the integration of advanced technologies like artificial intelligence (AI) and machine learning (ML) is enabling companies to automate billing processes, personalize customer interactions, and improve revenue forecasting.
One of the key trends shaping the market is the growing emphasis on cloud-based solutions, which provide scalability, agility, and accessibility for organizations looking to optimize their subscription and billing processes. Cloud deployment offers cost-effective alternatives to traditional on-premises systems and is gaining traction among SMEs and large enterprises alike. The rising demand for Software as a Service (SaaS) models further accelerates the adoption of cloud-based subscription and billing management solutions, driving market growth and innovation.
In addition to technology advancements, regulatory compliance and data security are becoming paramount considerations for organizations investing in subscription and billing management solutions. With a focus on data privacy and protection, market players are refining their platforms to ensure compliance with global regulations like GDPR and CCPA. This increased focus on security and compliance reflects the industry's commitment to safeguarding customer data and ensuring trust in subscription and billing processes.
Furthermore, as businesses across sectors recognize the value of efficient subscription management and accurate billing processes, the market is poised for continued growth. The diverse range of players in the market offers specialized solutions tailored to specific industry verticals, catering to the unique needs of organizations in sectors such as BFSI, healthcare, telecom and IT, retail, and manufacturing. Collaboration and consolidation among market players are expected to drive further innovation and address the evolving demands of the digital economy, ensuring sustainable growth and competitiveness in the global subscription and billing management market.
The Subscription and Billing Management Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-subscription-and-billing-management-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Influence of the Subscription and Billing Management Market Report:
Comprehensive assessment of all opportunities and risk in the Subscription and Billing Management Market
Lead Subscription and Billing Management Market recent innovations and major events
Detailed study of business strategies for growth of the Subscription and Billing Management Market market-leading players
Conclusive study about the growth plot of Subscription and Billing Management Market for forthcoming years
In-depth understanding of Subscription and Billing Management Market -particular drivers, constraints and major micro markets
Favourable impression inside vital technological and Subscription and Billing Management Marketlatest trends striking the Cannabis Seeds Market
Browse More Reports:
Global Fondaparinux Market Global Folding Furniture Market Global Fluid Therapy Market Global Fluid Power Equipment Market Global File Sharing Market Global Field Effect Rectifier Dioded Market Global Feminine Care Pouch Film Market Global Feed Amino Acids Market Global Farm Equipment Market Global Ethylene Copolymers Market Global Essential Thrombocytosis Treatment Market Global Erdheim Chester Disease Market Global E-Prescribing Solutions Market Global Enterprise Robotic Process Automation Market Global Enterprise Medical Image Viewers Market Global Engineered Quartz Surface Market Global Endoscope Cleaning and Disinfecting Device Market Global Emulsifier Free Skincare Market Global Email Protection Security Market Global Email Applications Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag
Subscription and Billing Management Market Size, Subscription and Billing Management Market Share, Subscription and Billing Management Market Trend, Subscription and Billing Management Market Analysis, Subscription and Billing Management Market Report, Subscription and Billing Management Market Growth, Latest Developments in Subscription and Billing Management Market, Subscription and Billing Management Market Industry Analysis, Subscription and Billing Management Market Key Player, Subscription and Billing Management Market Demand Analysis
0 notes
Text
The Downstream Processing Market in 2023 is US$ 27.35 billion, and is expected to reach US$ 65.34 billion by 2031 at a CAGR of 11.5%.
#Downstream Processing Market#Downstream Processing Market Research#Downstream Processing Market Report
0 notes
Text
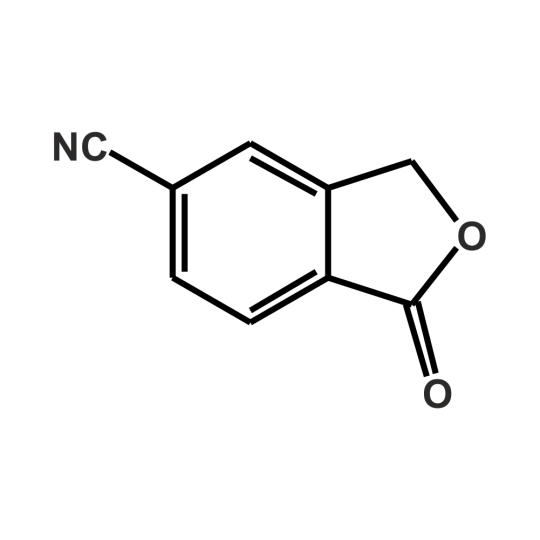
5-Cyanophthalide: Trusted Quality Backed by Jay Finechems Expertise in Fine Chemicals
In today’s rapidly advancing pharmaceutical and specialty chemical industries, the demand for high-purity intermediates has never been greater. One such compound, 5-Cyanophthalide, plays a critical role in the synthesis of key active pharmaceutical ingredients (APIs), including antidepressants like Citalopram. For manufacturers, researchers, and formulators, consistent quality, traceability, and regulatory confidence are non-negotiable. This is where Jay Finechem sets the benchmark.
As an established name in the fine chemical manufacturing space, Jay Finechem brings decades of expertise, process excellence, and unwavering commitment to purity and consistency. Our journey with 5-Cyanophthalide reflects our broader mission—delivering reliable chemical solutions that meet the complex needs of regulated industries. Whether it’s used as a core intermediate in pharmaceutical development or for custom synthesis in specialized research, our customers rely on us not just for a product—but for peace of mind.
At Jay Finechem, we understand that quality is not a checkbox; it’s a continuous process. That’s why our manufacturing of 5-Cyanophthalide adheres to stringent quality control protocols, supported by validated analytical methods and GMP-aligned practices. The result? A product with superior chemical purity, low residual solvents, and full documentation—ideal for sensitive downstream applications. We partner closely with our clients to provide complete technical support, COAs, and regulatory documentation, making their procurement process seamless and efficient.
Beyond product excellence, it’s our people and philosophy that make the real difference. Our chemists and production experts are not only skilled in synthetic chemistry but also understand the broader implications of compliance, consistency, and customer needs. We stay current with international standards and offer scalable solutions—from gram-scale R&D supply to multi-ton commercial batches.
What sets Jay Finechem apart in the market is our ability to integrate 5-Cyanophthalide manufacturing within a robust network of quality systems, customer support, and logistical agility. We cater to both domestic and global clients with equal precision, ensuring timely delivery and reliable supply chains. With facilities based in India—specifically in the chemical manufacturing hub of Vapi—we are strategically positioned to meet rising demands without compromising speed or service.
In a world where trust and traceability matter more than ever, Jay Finechem stands as a dependable partner. Our track record, regulatory preparedness, and deep product knowledge make us a top choice for companies seeking high-performance fine chemicals like 5-Cyanophthalide. As industries evolve, our focus remains the same: delivering quality you can depend on, backed by the science and service you deserve.
If you’re seeking a trusted supplier for 5-Cyanophthalide, partner with Jay Finechem—where expertise meets reliability, and every molecule counts.
https://Jay Finechem.com/product/5-cyanophthalide/
0 notes
Text
Precision Fermentation Ingredients Market Drives Sustainable Protein Growth

Precision fermentation ingredients leverage advanced biotechnology to produce high-purity proteins, enzymes and bioactive compounds that closely mimic traditional animal-derived ingredients without relying on livestock. This innovative approach offers significant advantages, including reduced environmental footprint, consistent product quality, and scalable production. Companies operating in this space employ genetically engineered microbial strains—such as yeast, fungi or bacteria—to convert simple sugars into complex molecules like dairy proteins, egg whites and heme pigments. These ingredients address growing consumer concerns about animal welfare, food safety and greenhouse gas emissions, while enabling formulators to create plant-based cheeses, vegan dairy alternatives, functional beverages and meat analogs with authentic taste and texture.
As global dietary patterns shift toward sustainable and health-focused choices, demand for clean-label, high-performance ingredient solutions is intensifying. In response, Precision Fermentation Ingredients Market offers a platform for rapid innovation, shorter time-to-market and improved supply chain resilience amid raw material volatility. Furthermore, ongoing investments in bioreactor optimization, feedstock diversification and downstream processing are driving cost reductions and enhancing market opportunities.
The precision fermentation ingredients market is estimated to be valued at USD 6.68 Bn in 2025 and is expected to reach USD 84.52 Bn by 2032. It is expected to grow at a compound annual growth rate (CAGR) of 43.7% from 2025 to 2032. Key Takeaways Key players operating in the Precision Fermentation Ingredients Market are:
-Perfect Day Inc.
-Motif Foodworks
-Impossible Foods Inc.
-The EVERY Company (Clara Foods)
-Novozymes A/S
These market companies are at the forefront of product development, leveraging proprietary strains and patent-protected processes to secure market share and intellectual property. Perfect Day Inc. has pioneered animal-free dairy proteins that replicate casein and whey, while Motif Foodworks focuses on texturizing ingredients for plant-based dairy alternatives. Impossible Foods Inc. utilizes heme proteins for meaty flavor enhancement, and The EVERY Company specializes in egg white proteins through cell-free fermentation. Novozymes A/S brings decades of enzyme expertise, supporting clients with tailored solutions for flavor, texture and shelf-life improvement. Through strategic partnerships and joint ventures, these players drive market research, expand production capacity and enhance their competitive positioning. Rising consumer awareness of health benefits and environmental impact is fueling growing demand for precision-fermented ingredients. As meat analogs and dairy substitutes gain mainstream acceptance, formulators are seeking functional proteins and enzymes that deliver consistent performance across diverse applications. Market insights indicate a strong preference for clean-label, non-GMO claims, prompting R&D investments to optimize yield and streamline regulatory approval. Moreover, as supply chains face disruptions—ranging from feedstock shortages to export controls—precision fermentation offers a localized production model that mitigates risks and ensures continuity. This surge in market demand is projected to unlock new opportunities in sports nutrition, personalized nutrition and therapeutic foods, further driving market growth and expanding industry share.
‣ Get More Insights On: Precision Fermentation Ingredients Market
‣ Get this Report in Japanese Language: 精密発酵原料市場
‣ Get this Report in Korean Language: 정밀발효성분시장
‣ Read More Related Articles- The Emerging Role of Precision Medicine in Cancer Treatment
0 notes
Text
North America Kaolin Market - Upcoming Trends
Business Market Insights recently announced the release of the market research titled North America Kaolin Market Outlook to 2028 | Share, Size, and Growth. The report is a stop solution for companies operating in the North America Kaolin market. The report involves details on key segments, market players, precise market revenue statistics, and a roadmap that assists companies in advancing their offerings and preparing for the upcoming decade. Listing out the opportunities in the market, this report intends to prepare businesses for the market dynamics in an estimated period.
Is Investing in the Market Research Worth It?
Some businesses are just lucky to manage their performance without opting for market research, but these incidences are rare. Having information on longer sample sizes helps companies to eliminate bias and assumptions. As a result, entrepreneurs can make better decisions from the outset. North America Kaolin Market report allows business to reduce their risks by offering a closer picture of consumer behavior, competition landscape, leading tactics, and risk management.
A trusted market researcher can guide you to not only avoid pitfalls but also help you devise production, marketing, and distribution tactics. With the right research methodologies, Business Market Insights is helping brands unlock revenue opportunities in the North America Kaolin market.
If your business falls under any of these categories – Manufacturer, Supplier, Retailer, or Distributor, this syndicated North America Kaolin market research has all that you need.
What are Key Offerings Under this North America Kaolin Market Research?
Global North America Kaolin market summary, current and future North America Kaolin market size
Market Competition in Terms of Key Market Players, their Revenue, and their Share
Economic Impact on the Industry
Production, Revenue (value), Price Trend
Cost Investigation and Consumer Insights
Industrial Chain, Raw Material Sourcing Strategy, and Downstream Buyers
Production, Revenue (Value) by Geographical Segmentation
Marketing Strategy Comprehension, Distributors and Traders
Global North America Kaolin Market Forecast
Study on Market Research Factors
Who are the Major Market Players in the North America Kaolin Market?
North America Kaolin market is all set to accommodate more companies and is foreseen to intensify market competition in coming years. Companies focus on consistent new launches and regional expansion can be outlined as dominant tactics. North America Kaolin market giants have widespread reach which has favored them with a wide consumer base and subsequently increased their North America Kaolin market share.
Report Attributes
Details
Segmental Coverage
Process
Water Washed
Airfloat
Calcined
Delaminated
and Others
End-Use Industry
Paper
Ceramic and Sanitaryware
Paints and Coatings
Plastic
Rubber
and Others
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
American Elements
BASF SE
Burgess Pigment Company
Imerys S.A.
Sibelco
Thiele Kaolin Company
I-Minerals Inc.
KaMin LLC
Other key companies
What are Perks for Buyers?
The research will guide you in decisions and technology trends to adopt in the projected period.
Take effective North America Kaolin market growth decisions and stay ahead of competitors
Improve product/services and marketing strategies.
Unlock suitable market entry tactics and ways to sustain in the market
Knowing market players can help you in planning future mergers and acquisitions
Visual representation of data by our team makes it easier to interpret and present the data further to investors, and your other stakeholders.
Do We Offer Customized Insights? Yes, We Do!
The Business Market Insights offer customized insights based on the client’s requirements. The following are some customizations our clients frequently ask for:
The North America Kaolin market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
About Us:
Business Market Insights is a market research platform that provides subscription services for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductors, Aerospace & Defense, Automotive & Transportation, Energy & Power, Healthcare, Manufacturing & Construction, Food & Beverages, Chemicals & Materials, and Technology, Media & Telecommunications.
Contact Us: : www.businessmarketinsights.com
#North America Kaolin Market#North America Kaolin Market Size#North America Kaolin Market Trends#North America Kaolin Market Shares#North America Kaolin Market Growth
0 notes
Text
Sample Preparation Market Anticipated to Witness High Growth Owing to Technological Advancements

The Global Sample Preparation Market is estimated to be valued at US$ 8.63 Bn in 2025 and is expected to exhibit a CAGR of 6.6 % over the forecast period 2025 to 2032.
The sample preparation market encompasses a range of products and solutions designed to isolate, purify, and concentrate analytes from diverse matrices prior to analytical testing. Instruments such as automated liquid handlers, centrifuges, and solid‐phase extraction systems streamline workflows by enhancing reproducibility and reducing manual errors. Reagents and consumables—including filtration membranes, solvent cartridges, and magnetic beads—offer high specificity and consistency, supporting downstream applications in genomics, proteomics, pharmaceutical research, and clinical diagnostics. Sample Preparation Market Insights as regulatory scrutiny intensifies and laboratories pursue higher throughput, reliable sample prep workflows become essential to maintain data integrity, accelerate time to result, and optimize research budgets. Integration of robotics and digital platforms further drives operational efficiency, enabling real-time process monitoring and predictive maintenance. Robust sample preparation not only improves sensitivity and accuracy but also supports compliance with quality standards such as GLP and ISO. With rising demand from biopharmaceutical companies, academic institutions, and diagnostic labs, this segment is increasingly seen as a cornerstone for comprehensive market insights and industry trends. Get more insights on, Sample Preparation Market
#Coherent Market Insights#Sample Preparation#Sample Preparation Market#Sample Preparation Market Insights#Instruments
0 notes
Text
Medical Power Supply Device Market 2025-2035 : Unpacking the Top Challenges Ahead
The medical power supply device market is poised for substantial growth, projected to reach approximately USD 2.87 billion by 2034, up from USD 1.89 billion in 2024, reflecting a compound annual growth rate (CAGR) of 4.2% . This expansion is driven by the increasing demand for advanced medical equipment and the integration of technologies such as the Internet of Things (IoT) in healthcare. However, the market faces several challenges that could impede its growth trajectory.
1. Stringent Regulatory Compliance
Medical power supply devices must adhere to rigorous safety and quality standards, including IEC 60601-1, to ensure patient safety. Compliance with these standards necessitates substantial investments in testing, certification, and quality assurance processes. These requirements not only escalate development costs but also prolong time-to-market, posing significant hurdles for manufacturers aiming to introduce innovative solutions swiftly .
2. Supply Chain Vulnerabilities and Geopolitical Tensions
The global medical device industry heavily relies on intricate supply chains spanning multiple countries. Recent geopolitical tensions, particularly the imposition of tariffs on imports from key manufacturing hubs like China and the European Union, have disrupted these supply chains. For instance, tariffs on medical-grade components have led to increased production costs and potential delays in device availability . Such disruptions not only affect manufacturers but also have downstream impacts on healthcare providers and patients.
3. Economic Uncertainties and Budget Constraints
Economic fluctuations and uncertainties can lead to tightened budgets for healthcare institutions, affecting their ability to invest in new medical equipment. Budget constraints may result in deferred purchases or a preference for cost-effective alternatives, thereby impacting the demand for advanced medical power supply devices. Manufacturers must navigate these economic challenges by offering flexible pricing models and demonstrating the long-term value of their products.
4. Technological Integration and Compatibility Issues
The rapid advancement of medical technologies necessitates power supply devices that can seamlessly integrate with various equipment. However, ensuring compatibility across diverse systems and maintaining performance standards is a complex task. Inadequate integration can lead to operational inefficiencies or even equipment failures, emphasizing the need for robust and adaptable power supply solutions.
5. Environmental and Energy Efficiency Concerns
As the healthcare industry moves towards sustainability, there is increasing pressure on manufacturers to develop energy-efficient and environmentally friendly power supply devices. Balancing performance with energy efficiency requires significant research and development efforts. Additionally, compliance with environmental regulations adds another layer of complexity to the manufacturing process.
6. Skilled Workforce Shortage
The development and maintenance of advanced medical power supply devices require a skilled workforce proficient in both medical and engineering disciplines. However, there is a growing shortage of such professionals, which can hinder innovation and delay product development. Investing in workforce development and training programs is essential to address this gap.
7. Cybersecurity Threats
With the increasing digitization of medical devices, cybersecurity has become a critical concern. Power supply devices that are integrated into networked systems can be vulnerable to cyberattacks, potentially compromising patient safety and data integrity. Manufacturers must prioritize cybersecurity measures in the design and development of their products to mitigate these risks.
8. Market Competition and Pricing Pressures
The medical power supply device market is highly competitive, with numerous players striving for market share. This competition can lead to pricing pressures, compelling manufacturers to reduce costs while maintaining quality. Balancing affordability with performance and compliance is a persistent challenge in this dynamic market landscape.
Conclusion
While the medical power supply device market is on a growth trajectory, it is imperative to address the multifaceted challenges that accompany this expansion. Manufacturers must navigate regulatory complexities, supply chain disruptions, economic uncertainties, and technological integration issues. By proactively tackling these challenges through innovation, strategic partnerships, and investment in workforce development, the industry can ensure the delivery of reliable, efficient, and safe power supply solutions that meet the evolving needs of the healthcare sector.
0 notes