#Reprocessed Medical Devices Market Research
Explore tagged Tumblr posts
Text
#Reprocessed Medical Devices Market#Reprocessed Medical Devices Market Trends#Reprocessed Medical Devices Market Growth#Reprocessed Medical Devices Market Industry#Reprocessed Medical Devices Market Research#Reprocessed Medical Devices Market Report
0 notes
Text
Sterilization Pouches Market is Driven by Stringent Regulatory Standards

Sterilization pouches are medical-grade packaging products designed to maintain sterility of surgical instruments, dental tools, and other healthcare devices until the point of use. Made from breathable, puncture-resistant materials such as medical-grade paper and transparent polymer films, these pouches allow for efficient steam, ethylene oxide, or hydrogen peroxide sterilization processes. Their clear windows enable visual inspection of instrument integrity and chemical indicators, ensuring patient safety and compliance with infection control protocols.
Advantages include barrier protection against microbial contamination, ease of handling, and compatibility with colorimetric sterilization indicators. As healthcare facilities worldwide prioritize patient safety and infection prevention, the demand for reliable Sterilization Pouches Market solutions has surged. Rising awareness of healthcare-associated infections, coupled with stringent regulations from bodies like the FDA and EMA, has underscored the need for standardized sterilization practices. In addition to hospitals and clinics, dental practices and outpatient surgical centers are expanding their use of sterilization pouches to streamline instrument reprocessing workflows and reduce turnaround times.
The sterilization pouches market is estimated to be valued at USD 53.80 Bn in 2025 and is expected to reach USD 88.10 Bn by 2032, growing at a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032. Key Takeaways
Key players operating in the Sterilization Pouches Market are:
-Amcor plc
-Mondi Group
-Berry Global
-3M Company
-Dynarex Corporation
These market companies leverage extensive R&D capabilities and robust distribution networks to innovate pouch materials and expand product portfolios. Amcor plc focuses on sustainable packaging solutions, while Mondi Group emphasizes advanced barrier films that enhance instrument protection. Berry Global and 3M Company invest in market research to optimize pouch design and performance, and Dynarex Corporation targets niche segments with cost-effective, single-use sterilization wraps. Through strategic alliances and mergers, these key players aim to consolidate market share and capitalize on market growth opportunities.
‣ Get More Insights On: Sterilization Pouches Market
‣ Get this Report in Japanese Language: プレハブ建築市場
‣ Get this Report in Korean Language: 살균파우치시장
0 notes
Text
The Clean Sweep: A Report on the Japan Medical Device Cleaning Market
Japan Medical Device Cleaning Market Growth & Trends
The Japan Medical Device Cleaning Market size is anticipated to reach USD 1,169.49 million by 2030 and is projected to grow at a CAGR of 10.83% over the forecast period, according to a new report by Grand View Research, Inc. This growth can be attributed to the increasing competition among the market players and the growing efforts to reduce hospital-acquired infections.
Several studies are being published focusing on the increasing number of infections acquired in hospitals and the measures that can prevent them. For instance, a study published by the National Library of Medicine in May 2020 found that nosocomial infections, also known as healthcare-acquired infections, are a significant burden on hospitalized patients in Japan. This study analyzed the Japanese claims database and found that out of 73,962,409 inpatients registered in the database, 9.7% had community-acquired infections (CAI), and 4.7% had nosocomial infections (NI). As a result, the growing burden of hospital-acquired infections is expected to increase the demand for medical device cleaning products in Japan.
Moreover, the growing use of single-use devices and the rising manufacturing of medical devices are anticipated to propel the Japanese medical device cleaning market. Industry stakeholders are focusing on increasing the development of medical devices in the country. For instance, in May 2023, Terumo Corporation, a medical device company, invested around USD 360 million to construct a new manufacturing facility for the Medical Care Solutions Company in Japan.
In addition, in January 2022, Kaneka Corporation invested around USD 69 million to build a new medical device plant in the Tomatoh Industrial Area in the northern area of Japan. Companies are expanding their manufacturing facilities. These expanding manufacturing facilities will require medical device cleaning solutions for sterilization, reprocessing, and cleaning in the future. Thus, the rise in investments in medical device manufacturing and development in Japan is projected to increase the demand for medical device cleaning products in the coming years.
Curious about the Japan Medical Device Cleaning Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Japan Medical Device Cleaning Market Report Highlights
Based on device type, the semi-critical segment dominated the market in 2023 and accounted for 46.02% of revenue share. However, the critical segment is anticipated to grow fastest over the forecast period due to the increasing infection control awareness and growing aging population.
Based on technique, the disinfection segment dominated the market in 2023 and accounted for 49.53% of the revenue share. However, the sterilization segment is anticipated to grow fastest from 2024 to 2030. Advancements in sterilization technologies are expected to boost segment growth in the coming years.
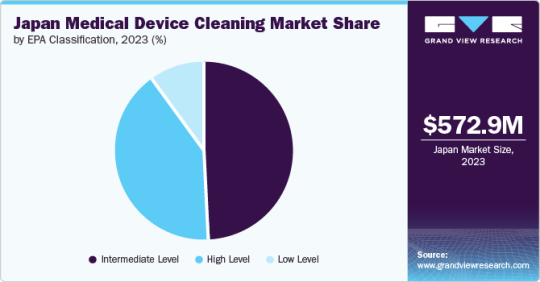
Based on EPA classification, the intermediate-level segment dominated the Japan market and accounted for the largest revenue share, 48.84%, in 2023. In contrast, the high-level segment is expected to grow fastest, with the fastest CAGR over the forecast period.
Japan Medical Device Cleaning Market Segmentation
Grand View Research has segmented the Japan Medical device cleaning market based on the device type, technique, and EPA classification:
Japan Medical Device Cleaning Device Type Outlook (Revenue, USD Million, 2018 - 2030)
Non-Critical
Semi-Critical
Critical
Japan Medical Device Cleaning Technique Outlook (Revenue, USD Million, 2018 - 2030)
Cleaning
Detergents
Buffers
Chelators
Enzymes
Others
Disinfection
Chemical
Alcohol
Chlorine & Chorine Compounds
Aldehydes
Others
Metal
Ultraviolet
Others
Sterilization
Heat Sterilization
Ethylene Dioxide (ETO) Sterilization
Radiation Sterilization
Japan Medical Device Cleaning EPA Classification Outlook (Revenue, USD Million, 2018 - 2030)
High Level
Intermediate Level
Low Level
Download your FREE sample PDF copy of the Japan Medical Device Cleaning Market today and explore key data and trends.
0 notes
Text
#Medical Device Reprocessing Market#Medical Device Reprocessing Market Share#Medical Device Reprocessing Market Size#Medical Device Reprocessing Market Trends
0 notes
Text
#Medical Device Reprocessing Market#Medical Device Reprocessing Market Size#Medical Device Reprocessing Market Report#Medical Device Reprocessing Market growth#Medical Device Reprocessing Market Share
0 notes
Text
Ortho Phthalaldehyde Market Size, Share, and Competitive Landscape
Rising Demand for High-Efficiency Disinfectants Drives Growth in the Ortho Phthalaldehyde Market

The Ortho Phthalaldehyde Market size was valued at USD 5.83 billion in 2023. It is expected to grow to USD 9.25 billion by 2032 and grow at a CAGR of 5.26% over the forecast period of 2024-2032.
The Ortho Phthalaldehyde (OPA) Market is driven by increasing demand for high-performance disinfectants in healthcare, pharmaceuticals, and industrial applications. OPA is widely recognized for its superior antimicrobial properties, making it a preferred alternative to traditional disinfectants like glutaraldehyde. The rising need for effective sterilization solutions, coupled with stringent hygiene regulations in hospitals and medical facilities, is fueling the market expansion. Additionally, its applications in chemical synthesis and water treatment contribute to the growing global demand.
Key Players in the Market
The Ortho Phthalaldehyde market features a competitive landscape, with key industry players focusing on product innovation, regulatory compliance, and sustainability. Leading companies in the sector include:
AK Scientific Inc.
Alfa Aesar
MP Biomedicals
DPX Fine Chemicals
Virox
Thermo Fisher Scientific Inc.
Parchem Fine & Specialty Chemicals
TCI America
Merck Millipore Corporation
Sigma-Aldrich Co. LLC
These companies are investing in advanced formulations and expanding their production capacities to cater to the growing demand for OPA-based disinfectants.
Future Scope and Emerging Trends
The future of the Ortho Phthalaldehyde Market looks promising as healthcare facilities worldwide prioritize infection control and patient safety. The shift toward non-toxic and biodegradable disinfectants is driving research into environmentally friendly OPA formulations. Moreover, the rising adoption of automated disinfection systems in hospitals and laboratories is increasing the use of OPA-based solutions. Additionally, innovations in high-purity OPA for pharmaceutical and biotechnology applications are expanding its market potential. With growing concerns over hospital-acquired infections (HAIs), the demand for OPA in sterilization and medical device reprocessing is expected to surge.
Key Market Points:
✅ Rising Healthcare Demand: Increased usage of OPA-based disinfectants in medical facilities. ✅ Superior Antimicrobial Properties: Preferred over glutaraldehyde for sterilization due to enhanced safety and efficacy. ✅ Growth in Water Treatment Applications: Expanding use in industrial and municipal water treatment processes. ✅ Regulatory Compliance: Companies focusing on meeting stringent safety and environmental standards. ✅ Advancements in Chemical Research: Ongoing R&D to develop safer and more sustainable OPA formulations.
Conclusion
The Ortho Phthalaldehyde Market is set for continued growth, driven by increasing hygiene awareness, advancements in disinfection technologies, and expanding industrial applications. As industries and healthcare providers seek safer and more effective sterilization solutions, OPA is emerging as a key component in infection control. Companies investing in sustainable production methods and innovative applications will be well-positioned to capitalize on this growing market.
Read Full Report: https://www.snsinsider.com/reports/ortho-phthalaldehyde-market-3861
Contact Us:
Jagney Dave — Vice President of Client Engagement
Phone: +1–315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Ortho Phthalaldehyde Market#Ortho Phthalaldehyde Market Size#Ortho Phthalaldehyde Market Share#Ortho Phthalaldehyde Market Report#Ortho Phthalaldehyde Market Forecast
0 notes
Text
The global demand for Single Use Flexible Endoscope was valued at USD 1214.8 Million in 2022 and is expected to reach USD 7012.2 Million in 2030, growing at a CAGR of 24.50% between 2023 and 2030.In the rapidly evolving medical device landscape, single-use flexible endoscopes have emerged as a pivotal innovation. These devices offer significant benefits over reusable counterparts, particularly in terms of infection control, cost-efficiency, and operational convenience. The market for single-use flexible endoscopes has been expanding, driven by technological advancements and an increasing emphasis on patient safety and hygiene. This article delves into the current state of the single-use flexible endoscope market, its growth drivers, challenges, and future outlook.
Browse the full report at https://www.credenceresearch.com/report/single-use-flexible-endoscope-market
Market Overview
Single-use flexible endoscopes are designed for one-time use and then disposed of, eliminating the need for reprocessing and sterilization. This model addresses key issues associated with reusable endoscopes, including cross-contamination and the high costs of maintenance and sterilization. The market for these endoscopes has seen substantial growth due to their inherent advantages, such as reduced risk of infection, convenience, and enhanced performance consistency.
Growth Drivers
1. Infection Control and Patient Safety: One of the primary drivers for the adoption of single-use flexible endoscopes is the heightened focus on infection control. Reusable endoscopes have been associated with outbreaks of healthcare-associated infections (HAIs) due to difficulties in achieving complete sterilization. Single-use endoscopes mitigate this risk by eliminating the need for complex reprocessing.
2. Technological Advancements: Advances in endoscopic technology have led to the development of high-quality single-use endoscopes that offer comparable performance to reusable models. Innovations in materials and design have improved image quality, flexibility, and overall functionality, making these devices suitable for a wide range of medical applications.
3. Cost Efficiency: While the upfront cost of single-use endoscopes may be higher than that of reusable devices, they offer long-term cost savings. The elimination of reprocessing costs, repair expenses, and the need for sterilization facilities contribute to a more cost-effective solution in the long run.
4. Regulatory Support: The increasing regulatory support for single-use medical devices has further fueled market growth. Regulatory agencies are recognizing the benefits of single-use endoscopes in enhancing patient safety, which has led to faster approvals and greater acceptance in the healthcare community.
Challenges
Despite their advantages, single-use flexible endoscopes face several challenges:
1. Environmental Concerns: The disposal of single-use endoscopes contributes to medical waste, raising environmental concerns. Efforts are being made to develop more eco-friendly materials and recycling programs to mitigate the environmental impact.
2. Cost of Innovation: The development and production of high-quality single-use endoscopes require significant investment in research and development. This can lead to higher costs for manufacturers, which may be passed on to healthcare providers and patients.
3. Market Penetration: In some regions, particularly where reusable endoscopes are deeply entrenched, the adoption of single-use endoscopes may face resistance due to established practices and cost concerns. Education and awareness are crucial in overcoming these barriers.
Future Outlook
The single-use flexible endoscope market is poised for continued growth, driven by ongoing advancements in technology, increasing emphasis on patient safety, and the need for cost-effective medical solutions. Manufacturers are likely to focus on innovation to address environmental concerns and enhance the functionality of these devices. As healthcare systems globally evolve, the adoption of single-use endoscopes is expected to become more widespread, offering enhanced safety and efficiency in medical procedures.
Key Players
Ambu A/S
Olympus America
Boston Scientific Corporation
Fujifilm Holdings Corporation
Neoscope Inc.
Coloplast Corp
Laborie
Shenzhen Tianlang Medical
Intesurgical
Teleflex Incorporated
Segmentation
By Specialty:
Gastroenterology
Urology
Pulmonology
Ear, Nose, and Throat (ENT)
Gynecology
Orthopedics
By Procedure Type:
Diagnostic
Therapeutic
Screening
By Endoscope Type:
Colonoscopes
Gastroscopes
Bronchoscopes
Cystoscopes
Hysteroscopes
Nasopharyngoscopes
Others
By End User:
Hospitals
Ambulatory Surgery Centers (ASCs)
Clinics
By Material:
Disposable Fiber Optics
Video Endoscopes
Others
By Region
North America
US
Canada
Mexico
Europe
Germany
France
UK.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/single-use-flexible-endoscope-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Key Factors Driving Market Size in Medical Device Reprocessing

The Medical Device Reprocessing Market size was estimated at USD 2.69 billion in 2023 and is expected to reach USD 9.44 billion by 2031 at a CAGR of 17% during the forecast period of 2024-2031.The Medical Device Reprocessing Market is witnessing significant growth, driven by increasing healthcare cost containment measures and a growing focus on sustainability within the healthcare sector. Reprocessing involves the cleaning, disinfection, sterilization, and testing of medical devices to ensure they are safe for reuse, offering a cost-effective alternative to single-use devices.
Technological advancements in reprocessing techniques, stringent regulatory guidelines, and rising awareness about the environmental impact of medical waste are key factors propelling this market. As hospitals and healthcare providers seek to optimize resources while maintaining high standards of patient care, the demand for reprocessed medical devices is expected to surge, fostering innovation and expansion within this crucial segment of the medical industry.
An in-depth research of the market environment for the anticipated time period is included in the Medical Device Reprocessing Market research report along with strategy analysis, trend and scenario analysis for micro and macro markets, pricing analysis, and market position analysis. The information is then gathered and reviewed utilizing various market projections and data validation techniques. We also make use of an internal data prediction engine to project market growth. The study goes into great detail about new market trends, market drivers, development opportunities, and market restraints that may have an impact on the market dynamics of the sector.
Get Sample of This Report @ https://www.snsinsider.com/sample-request/3485
Market Segmentation
By Type
Reprocessing Support & Services
Reprocessed medical devices
By Device Category
Critical Devices
Semi- Critical Devices
Non- Critical Devices
By Application
Cardiology
Gastroenterology
Gynecology
Arthroscopy & Orthopedic Surgery
General Surgery and Anesthesia
Other Device Categories (Urology, non-invasive surgeries, patient monitoring)
Regional Overview
The Medical Device Reprocessing Market may be segmented into five major geographical regions based on the results of the regional analysis: North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa. The research covers a wide range of topics, including import and export, market size and share, production and consumption ratios, and infrastructure development.
COVID-19 Impact Analysis
Undoubtedly, when COVID-19 spreads around the globe, the socioeconomic environment will shift. The difficulty lies in managing the realities of those advancements while balancing corporate and societal goals for stakeholders and customers. The market impact on the entire world will depend on how quickly the international community can stop the virus from spreading and restart their economies. Businesses in the travel, tourist, retail, and hospitality industries will take longer to recover. But in certain businesses, the opposite is true. The market research report carefully examines the impact of COVID-19 on the Medical Device Reprocessing Market .
Key Reasons to Purchase Medical Device Reprocessing Market Report
The analysis contains crucial information, such as market trends and future outlooks.
Regional, sub regional, and national statistics encompass market influence, demand, and supply dynamics.
A wide array of significant companies, cutting-edge technology, and strategies are present in the competitive landscape.
Detailed product companies, significant financial data, newsworthy events, SWOT analyses, and business plans of key players.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in re framing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Oncology Drugs Market Forecast
Osteoporosis Treatment Market Forecast
Peripheral Neuropathy Market Forecast
Active Pharmaceutical Ingredient Market Forecast
Blood Group Typing Market Forecast
0 notes
Text
Bone Punch Market to Scale New Heights as Market Players Focus on Innovations 2024 – 2030
Bone punch is a tool usually used for the removal of bone, cartilage and tissue in various surgical disciplines. It reduces the physical effort required, very good cutting properties, disassembly enables efficient reprocessing and a two-level safety mechanism. The factors such as the Increased Number of Hospitals and Clinics and Growing Healthcare Infrastructure in Developing Regions are driving the global bone punch market.
Free Sample Report + All Related Graphs & Charts @: https://www.advancemarketanalytics.com/sample-report/44331-global-bone-punch-market?utm_source=Organic&utm_medium=Vinay
Latest released the research study on Global Bone Punch Market, offers a detailed overview of the factors influencing the global business scope. Bone Punch Market research report shows the latest market insights, current situation analysis with upcoming trends and breakdown of the products and services. The report provides key statistics on the market status, size, share, growth factors of the Bone Punch The study covers emerging player’s data, including: competitive landscape, sales, revenue and global market share of top manufacturers are J&J Medical Devices (United States), B.Braun (Germany), Arthrex (United States), KLS Martin Group (Germany), A. Eberle GmbH & Co. KG (Germany), Acclarent, Inc. (United States), USTOMED INSTRUMENTS Ulrich Storz GmbH & Co. KG (Germany), Millennium Surgical Corp. (United States), Aesculap, Inc. (United States), Johns Hopkins Medicine (United States)
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Southeast Asia.
Contact Us:
Craig Francis (PR & Marketing Manager)
AMA Research & Media LLP
Unit No. 429, Parsonage Road Edison, NJ
New Jersey USA – 08837
0 notes
Text
0 notes
Text
The Rise of Disposable Endoscopes in Modern Medicine
Disposable endoscopes are revolutionizing the field of endoscopy by offering a safer, more efficient, and cost-effective alternative to traditional reusable endoscopes.
These single-use devices eliminate the risk of cross-contamination and infection transmission, which can be a significant concern with reusable instruments despite rigorous sterilization processes. The convenience of disposable endoscopes lies in their immediate availability and the elimination of the need for time-consuming cleaning and reprocessing, thereby enhancing workflow efficiency in busy medical settings. They are particularly advantageous in emergency situations and for patients with infectious diseases, ensuring that every procedure is conducted with a fresh, sterile instrument. Additionally, the advancement in the design and technology of disposable endoscopes has led to high-definition imaging and improved diagnostic accuracy, matching the performance of their reusable counterparts. As healthcare facilities increasingly adopt these innovative devices, they are witnessing improved patient safety, reduced costs associated with sterilization and maintenance, and enhanced overall patient care. The shift towards disposable endoscopes reflects a broader trend in medical practice towards minimizing infection risks and optimizing operational efficiency.#DisposableEndoscopes #MedicalInnovation #PatientSafety #InfectionControl #HealthcareEfficiency #Endoscopy #MedicalTechnology #SafeHealthcare #ModernMedicine #TechInMedicine #PatientCare #SterileInstruments #HealthcareTrends #CostEffectiveCare #MedicalAdvancements
0 notes
Text
Endoscopy Devices Market Competitive Analysis and Forecast 2023-2033
Market Definition
An endoscopy is a medical procedure that involves inserting a thin, flexible tube called an endoscope into the body. The endoscope is equipped with a light and a camera, which allows the doctor to see inside the body. Endoscopy is used to diagnose and treat a variety of conditions.
Market Outlook
There are several key trends in endoscopy devices technology.
One is the miniaturization of endoscopes. This has led to the development of new, smaller devices that can be used for a variety of procedures.
Another trend is the use of new imaging technologies, such as 3D imaging, that provide more detailed images of the inside of the body.
There is also a trend towards the use of more sophisticated devices that can be controlled remotely, which allows for more precise procedures.
The main drivers of the endoscopy devices market are the increasing prevalence of gastrointestinal diseases, the growing geriatric population, the rising number of minimally invasive surgical procedures, and the increasing adoption of endoscopy devices.
The rising prevalence of gastrointestinal diseases is one of the key drivers of the endoscopy devices market. The rising prevalence of gastrointestinal diseases is attributable to the growing geriatric population, the changing lifestyle, and the increasing incidence of obesity.
The growing geriatric population is another key driver of the endoscopy devices market. The elderly population is more susceptible to gastrointestinal diseases.
The rising number of minimally invasive surgical procedures is another key driver of the endoscopy devices market. Minimally invasive surgery is associated with shorter hospital stays, less pain, and faster recovery times.
The increasing adoption of endoscopy devices is another key driver of the endoscopy devices market. Endoscopy is a minimally invasive procedure that allows the visualization of the digestive tract.
To Know More: https://www.globalinsightservices.com/reports/endoscopy-devices-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21225/
Market Segments
The Endoscopy Devices Market is segmented by product, hygiene, application, end user, and region. On the basis of product, the market is categorized into endoscope, mechanical endoscopic equipment, accessories, and other endoscopy equipment. On the basis of hygiene, the market is segmented into single-use, reprocessing, and sterilization. By application, it is classified into bronchoscopy, arthroscopy, laparoscopy, and others. By end user, it is segmented into hospitals, ambulatory surgery centers & clinics, and others. Region-wise the market is divided into North America, Europe, Asia-Pacific, and the Rest of the World.
Request Customization: https://www.globalinsightservices.com/request-customization/GIS21225/
Key Players
The Endoscopy Devices Market includes players such as HOYA Corporation, Olympus Corporation, Stryker Corporation, Boston Scientific Corporation, Fujifilm Holdings Corporation, CONMED Corporation, Medtronic Plc, Karl Storz GmbH & Co. KG, Johnson & Johnson, and Medrobotics Corporation.
Request Discounted Pricing: https://www.globalinsightservices.com/request-special-pricing/GIS21225/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
0 notes
Text
Driving Forces and Future Outlook: Navigating the Competitive Landscape of the Disposable Endoscopes Market
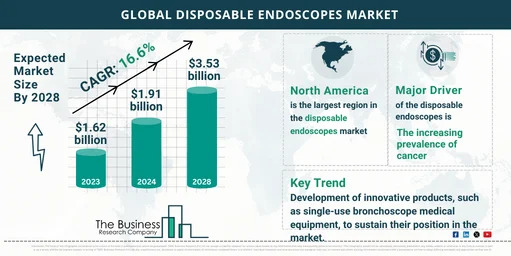
Overview and Scope Disposable endoscopes refer to medical devices designed used on a patient for a single procedure and discarded after use. It is used by physicians to access, visualize and perform procedures in the urinary system and kidney to visualize and examine internal organs, tissues and structures. Sizing and Forecast The disposable endoscopes market size has grown rapidly in recent years. It will grow from $1.62 billion in 2023 to $1.91 billion in 2024 at a compound annual growth rate (CAGR) of 18.1%. The disposable endoscopes market size is expected to see rapid growth in the next few years. It will grow to $3.53 billion in 2028 at a compound annual growth rate (CAGR) of 16.6%. To access more details regarding this report, visit the link: https://www.thebusinessresearchcompany.com/report/disposable-endoscopes-global-market-report Segmentation & Regional Insights The disposable endoscopes market covered in this report is segmented – 1) By Product: Gastroscopes; Bronchoscopes; Duodenoscopes; Laryngoscopes; Colonoscopes; Ureteroscopes; Other Endoscopes 2) By Patient Type: Adult; Pediatric 3) By End User: Hospitals; Ambulatory Surgical Centers; Diagnostic Centers North America was the largest region in the disposable endoscope market in 2023. The regions covered in disposable endoscopes market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. Intrigued to explore the contents? Secure your hands-on sample copy of the report: https://www.thebusinessresearchcompany.com/sample.aspx?id=13009&type=smp Major Driver Impacting Market Growth The increasing prevalence of cancer is expected to propel the growth of the disposable endoscopes market. Cancer is a complex group of diseases characterized by the uncontrolled growth and division of abnormal cells. Disposable endoscopes can provide high-quality imaging capabilities, allowing for more precise observations during endoscopic examinations. This medical equipment aids in detecting cancerous lesions or abnormalities within the gastrointestinal tract. Key Industry Players Major players in the disposable endoscopes market are Medtronic PLC, Fujifilm Holdings Corporation, Baxter International Inc., Boston Scientific Corporation, Olympus Corporation, Steris Corporation, Karl Storz SE & Co. KG, PENTAX Medical, Ambu A/S, Cooper Surgical Inc. The disposable endoscopes market report table of contents includes: 1. Executive Summary 2. Market Characteristics 3. Market Trends And Strategies 4. Impact Of COVID-19 5. Market Size And Growth 6. Segmentation 7. Regional And Country Analysis . . . 27. Competitive Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis Explore the trending research reports from TBRC:
Endoscopy Devices And Equipment Global Market Report 2024 Endoscope Reprocessing Global Market Report 2024
Dermatology Endoscopy Devices Global Market Report 2024 Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected] Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
0 notes
Text
Navigating Regulatory Landscapes: Compliance and Safety in Reprocessed Medical Devices

Reprocessed Medical Devices: An eco-friendly alternative to single-use devices The healthcare industry has made tremendous progress in recent decades, allowing physicians to treat an increasing number of conditions and offer advanced care to patients. However, this progress comes at a cost—both financially and environmentally. One area that poses unique challenges is the sustainable use and disposal of medical devices. With single-use devices being the norm, millions of tons of medical waste are generated each year across the world. This article explores reprocessed medical devices as an eco-friendly and cost-effective alternative that can help address this issue. What are reprocessed medical devices? A reprocessed medical device refers to a previously used single-use device that has been retrieved after use, cleaned, disinfected, sterilized, inspected, and prepared for reuse on additional patients. Reprocessing involves comprehensive cleaning, testing, replacement of worn components, and repackaging to help ensure patient safety. While single-use devices are disposed of after one use, reprocessed devices allow multiple safe uses thus reducing medical waste. Common reprocessed devices Some common medical devices that are frequently reprocessed include arthroscopy shavers, biopsy forceps, dilation and curettage sets, endoscopy equipment and tubing sets. Reprocessing is also done for devices used in procedures like colonoscopies, laparoscopies and hysteroscopies. Rigorous cleaning, testing and restoration methods enable safe reuse of these precision instruments. Benefits of reprocessed medical devices Reprocessing helps address several industry challenges in a sustainable manner. Cost savings: Reprocessed devices can help lower the costs of care for hospitals and patients. Studies show price savings of 40-60% compared to original single-use devices. This can free up funds to expand access to care. Environmental impact: With millions of devices be reprocessed instead of disposed of, significant medical waste and carbon emissions are prevented every year. This protects the environment for future generations. Supply chain resilience: Reprocessing reduces reliance on imported single-use devices. It ensures availability of essential devices even during supply constraints seen during pandemics. Patient access: Cost savings are often passed on to patients through lower out-of-pocket costs for procedures. This improves affordability and access to quality healthcare. Rigorous quality standards While cost-effective and eco-friendly, reprocessing raises valid concerns regarding quality and safety standards. However, FDA-compliant reprocessors have robust processes that effectively mitigate risks: Post-market surveillance: Continuous monitoring through user feedback helps detect any quality issues and institute corrective/preventive actions. When followed diligently, these quality practices help allay concerns regarding reprocessing and ensure patient safety remains the top priority. Leading certification bodies also conduct periodic third-party audits of reprocessors. Market adoption and outlook Currently an estimated 10-15% of eligible devices are reprocessed in the U.S. given regulatory approvals and proven safety record. Key adopters include the VA, Kaiser Permanente and Catholic Health Initiatives. Reprocessing is much more prevalent in countries like Japan where up to 50% devices are reprocessed to curb spiraling healthcare costs. In summary, reprocessing presents a win-win solution to curb medical waste and device costs without compromising quality. With standardized best practices scaled across the industry, it has immense potential to help healthcare organizations address the economic, social and environmental challenges of our times. Ongoing research and innovations will also further bolster the safety, efficacy and market success of this eco-friendly alternative to single-use devices.
0 notes
Text
Quality Assurance and Innovation: Shaping the Future of Reprocessed Medical Devices Market
Introduction
The reprocessed medical devices market has emerged as a sustainable and economically viable solution in the healthcare industry. As healthcare costs continue to rise and environmental concerns gain prominence, reprocessing medical devices offers a win-win situation by reducing expenses for healthcare facilities while minimizing medical waste. In this article, we will explore the dynamics, growth factors, and challenges of the reprocessed medical devices market.
Market Overview
Reprocessed medical devices refer to medical equipment and instruments that undergo a thorough cleaning, disinfection, and sterilization process to meet the same quality and safety standards as new devices. These devices are typically single-use and are often discarded after one use, contributing to the growing problem of medical waste. Reprocessing helps extend the lifespan of these devices, reducing the need for constant replacement and saving hospitals and healthcare facilities a significant amount of money.
Market Growth Factors
1. Cost Savings: The primary driver of the reprocessed medical devices market is cost savings. Hospitals and healthcare facilities can reduce their expenses by opting for reprocessed devices instead of buying new ones. This cost-effectiveness is particularly crucial in the face of increasing healthcare costs and budget constraints.
2. Environmental Benefits: Reprocessing medical devices also aligns with the growing emphasis on sustainability and reducing healthcare-related waste. By reusing medical equipment, less medical waste ends up in landfills, reducing the environmental impact of the healthcare industry.
3. Regulatory Approvals: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) have stringent guidelines for reprocessing medical devices. Increased regulatory approvals have boosted confidence in the safety and efficacy of reprocessed devices, encouraging healthcare providers to adopt these practices.
4. Technological Advancements: Advances in reprocessing technologies have made it possible to maintain the quality and safety of reprocessed devices, ensuring they meet or exceed the standards set for new devices. This has further accelerated market growth.
5. Growing Healthcare Industry: The healthcare industry continues to expand, creating a growing demand for medical devices. Reprocessing offers a cost-effective solution for healthcare providers to meet this demand without stretching their budgets.
Market Challenges
1. Perception and Resistance: Some healthcare professionals and institutions still have reservations about using reprocessed medical devices due to concerns about safety and efficacy. Overcoming these perceptions and resistance remains a challenge for the market.
2. Regulatory Compliance: Ensuring strict compliance with regulatory standards and keeping up with evolving regulations can be challenging for reprocessing companies. Any failure in compliance can have severe consequences.
3. Competition: The reprocessed medical devices market is becoming increasingly competitive as more companies enter the space. This competition can drive innovation but also poses challenges in terms of market share and differentiation.
4. Quality Control: Maintaining the highest quality standards is essential in the reprocessing industry. Any lapses in quality control can result in patient safety issues and damage the reputation of reprocessed devices.
Market Future
The reprocessed medical devices market is expected to continue its growth trajectory in the coming years. As healthcare costs remain a pressing concern, and sustainability becomes a top priority, reprocessed devices offer a compelling solution. Moreover, ongoing research and development efforts are likely to further enhance the safety and efficacy of reprocessed devices, addressing some of the remaining concerns in the market.
Conclusion
The reprocessed medical devices market represents a promising solution for healthcare facilities seeking to reduce costs and minimize their environmental footprint. With advancements in technology and increased regulatory approvals, reprocessed devices are gaining acceptance in the healthcare industry. However, challenges related to perception, regulatory compliance, and competition must be overcome for the market to reach its full potential. As the healthcare industry continues to evolve, reprocessed medical devices will play a crucial role in providing sustainable and cost-effective healthcare solutions.
0 notes