#PCRTest
Explore tagged Tumblr posts
Text
Companion Diagnostics Market Size, Share, and Growth Rate: A Look Ahead to 2032

The companion diagnostics market is a rapidly evolving segment of the healthcare industry, driven by advancements in personalized medicine and the growing need for targeted therapies. Companion diagnostics (CDx) are essential tools that help identify the appropriate patients for specific treatments, enhancing therapeutic efficacy and minimizing adverse effects. As the healthcare landscape continues to shift towards personalized medicine, the companion diagnostics market is poised for significant growth over the next decade.
As of 2024, the global companion diagnostics market was valued at approximately $7.76 billion. This market is expected to grow at a compound annual growth rate (CAGR) of around 12.5% from 2025 to 2032, potentially reaching $19.92 billion by the end of the forecast period. The increasing prevalence of chronic diseases, advancements in genomics, and the rising demand for personalized medicine are major factors driving this growth.
Request a Free Sample Copy - https://www.skyquestt.com/sample-request/companion-diagnostics-market
Key Market Segments
1. Technology Type
- Polymerase Chain Reaction (PCR)
- In Situ Hybridization (ISH)
- Next-Generation Sequencing (NGS)
- Immunohistochemistry (IHC)
2. Application
- Oncology
- Cardiovascular Diseases
- Infectious Diseases
- Neurological Disorders
3. End-User
- Pharmaceutical Companies
- Clinical Laboratories
- Research Institutions
Regional Insights
North America currently holds the largest share of the companion diagnostics market, driven by a robust healthcare infrastructure, high levels of investment in R&D, and favorable reimbursement policies.
Europe follows closely, with significant contributions from countries like Germany and the UK.
The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, fueled by increasing healthcare expenditures, rising awareness of personalized medicine, and expanding pharmaceutical sectors.
Top Player’s Company Profiles
Abbott (United States)
IDVet (France)
F. Hoffmann-La Roche Ltd. (Switzerland)
Agilent Technologies, Inc. (United States)
AniCell Biotech (United States)
Illumina, Inc. (United States)
Guardant Health (United States)
Thermo Fisher Scientific Inc. (United States)
BIOMERIEUX (France)
NEOGEN Corporation (United States)
Zoetis Inc. (United States)
Myriad Genetics, Inc. (United States)
Virbac SA (France)
Take Action Now: Secure Your Companion Diagnostics Market Today - https://www.skyquestt.com/buy-now/companion-diagnostics-market
Growth Drivers
1. Rising Demand for Personalized Medicine: The shift from traditional one-size-fits-all treatments to personalized approaches are a significant driver of the companion diagnostics market. By identifying specific biomarkers, companion diagnostics enable tailored treatment plans.
2. Advancements in Genomic Technologies: The rapid advancement of genomic technologies, particularly next-generation sequencing, has revolutionized the development of companion diagnostics, allowing for more precise patient stratification.
3. Regulatory Support: Regulatory bodies, such as the FDA, have increasingly recognized the importance of companion diagnostics, leading to more streamlined approval processes and encouraging investment in this sector.
4. Growing Oncology Market: With cancer being one of the leading causes of death globally, the demand for effective targeted therapies and companion diagnostics in oncology is a significant growth driver.
Future Outlook
The companion diagnostics market is expected to continue its upward trajectory, driven by innovation and increasing collaboration between diagnostic companies and pharmaceutical firms. The emergence of digital health technologies and artificial intelligence in diagnostics may further enhance market potential.
By 2032, the landscape of companion diagnostics will likely be characterized by:
- Greater Integration with Therapeutics: As more targeted therapies emerge; companion diagnostics will become integral to treatment protocols.
- Increased Adoption in Emerging Markets: The Asia-Pacific region will play a crucial role in the market's expansion, supported by growing healthcare investments.
- Focus on Multi-Omics Approaches: The integration of genomics, proteomics, and metabolomics in companion diagnostics will enable more comprehensive patient assessments.
Read Companion Diagnostics Market Report Today - https://www.skyquestt.com/report/companion-diagnostics-market
#CompanionDiagnostics#PrecisionMedicine#PersonalizedMedicine#CancerDiagnostics#OncologyDiagnostics#MolecularDiagnostics#GenomicTesting#Biomarkers#TargetedTherapy#NextGenSequencing#PCRTesting#Immunohistochemistry#NGSTechnology#FDAApproved#ClinicalTrials#MedTechInnovation#BiotechNews#HealthcareInnovation#DrugDevelopment#ClinicalDiagnostics#HealthTechTrends#DigitalHealth#RegulatoryAffairs#PatientCentricCare#AIinHealthcare
0 notes
Text
Lyme Disease Testing Market Trends and Analysis: Comprehensive Overview of Market Size, Share, Growth
The global lyme disease testing market size is anticipated to reach USD 18.57 billion by 2030 and is projected to grow at a CAGR of 8.2% from 2024 to 2030, according to a new report by Grand View Research, Inc. The primary factors driving this market are the increasing prevalence, supportive government policies regarding healthcare facilities, and the introduction of new diagnostic tests for Lyme disease. In addition, the growing incidence of tick-borne diseases further expands the global market. For instance, in August 2022, the U.S. Fair Health Organization reported that Lyme disease has significantly increased in prevalence over the past 15 years, becoming a growing national concern.
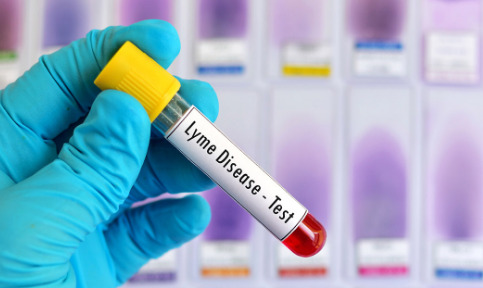
Lyme Disease Testing Market Report Highlights
Based on technology, the other segment led the market with the largest revenue share of 88.6% in 2023. The widespread adoption of these tests, considered the gold standard for testing due to their high specificity and sensitivity, significantly contributed to the growth of this segment
Based on testing, the serological test segment led the market with the largest revenue share of 53.1% in 2023. These tests dominated the market as they are widely available, affordable, established, and reliable in detecting antibodies against Borrelia burgdorferi. This makes them a trusted and accessible option for healthcare providers and patients
Based on sample, the blood segment led the market with the largest revenue share of 62.4% in 2023. This is due to the blood tests, such as ELISA and Western blot, which can effectively detect antibodies against Borrelia burgdorferi, the bacteria causing Lyme disease. Blood samples are also easy to obtain and handle, making them a practical choice for widespread diagnostic use
Based on end use, the hospital segment led the market with the largest revenue share of 58.4% in 2023. Firstly, hospitals can access advanced technology and diagnostic tools, enabling accurate & timely testing. The high hospital patient volume, including those with complex and severe symptoms, necessitates reliable diagnostic services, further driving their market dominance
In January 2024, DiaSorin announced that it submitted the LIAISON LymeDetect test to the U.S. FDA in December 2023. Developed in partnership with QIAGEN, this test detects IgG, IgM, and T-cell mediated responses using QIAGEN’s proprietary QuantiFERON technology, an Interferon-Gamma Release Assay (IGRA). This novel solution will enhance DiaSorin's LIAISON immunodiagnostic offerings in the U.S. market, representing a significant milestone in its collaboration with QIAGEN
For More Details or Sample Copy please visit link @: Lyme Disease Testing Market Report
Growing government initiatives and preventive measures significantly drive the market growth. For instance, in July 2022, an article titled "New laws seek to boost funding of Lyme disease research" highlighted that New York officials have increased funding for Lyme disease and tick-borne illnesses through a new law signed by Governor Kathy Hochul. This bipartisan measure will establish a voluntary tax check-off box to support education, research, and prevention efforts related to the disease in the region. Despite the high incidence of Lyme and tick-borne illnesses, it is believed that the actual number of cases is much higher due to inaccurate diagnostic testing.
Heightened awareness and education are further propelling market growth. Public health campaigns to educate communities about prevention strategies and symptoms have become increasingly prevalent. This growing awareness leads to more individuals seeking medical attention when they exhibit symptoms consistent with the disease, thereby driving demand for reliable testing options. Furthermore, educational initiatives targeting healthcare professionals ensure they remain informed about best practices for diagnosis and treatment.
List of major companies in the Lyme Disease Testing Market
DiaSorin S.p.A
BIOMÉRIEUX
Oxford Immunotec
Bio-Rad Laboratories, Inc.
Thermo Fisher Scientific, Inc.
T2 Biosystems
IGeneX
Gold Standard Diagnostics
ZEUS Scientific
Trinity Biotech
For Customized reports or Special Pricing please visit @: Lyme Disease Testing Market Analysis Report
We have segmented global lyme disease testing market report based on technology, testing, sample, end-use, and region.
#LymeDisease#LymeDiseaseTesting#DiagnosticTesting#InfectiousDisease#HealthDiagnostics#MedicalTesting#VectorBorneDiseases#TickBorneIllness#LymeDiseaseDiagnosis#SerologyTests#PCRTesting#LymeDiseaseAwareness#PointOfCareTesting#LaboratoryDiagnostics#HealthcareMarket#MedicalDevices#ImmunoassayTests#DiagnosticsMarket
0 notes
Text

PCR Cabinet LPCR-A11
Labtron PCR Cabinet features a sliding glass window for maximum light and visibility. HEPA filter efficiency is 99.999% at 0.3 µm, with adjustable airflow velocity from 0.3 to 0.5 m/s. Includes a 90-minute UV timer and 2 waterproof sockets on the back wall. The motorized anti-UV window enclosure with a 300 mm opening ensures user protection.
0 notes
Text

Introduction to Microbial Analysis. Microbial analysis plays a crucial role in various industries, ensuring the safety and quality of products, raw materials, and environments. From pharmaceuticals to food production and water testing, microbial analysis helps detect harmful microorganisms that could compromise health and product integrity. In this blog, we will explore the importance, methods, and applications of microbial analysis. What is Microbial Analysis?Microbial analysis refers to the process of identifying, quantifying, and evaluating microorganisms present in a given sample. These microorganisms can include bacteria, fungi, viruses, and other microbial entities. By performing microbial testing, industries can ensure compliance with safety standards and regulations. Importance of Microbial AnalysisEnsuring Product Safety: Contaminated products can lead to severe health risks. Microbial testing helps in identifying and eliminating harmful pathogens. Regulatory Compliance: Many industries must adhere to strict microbial limits as per national and international guidelines. Quality Control: Helps maintain product consistency and prevents spoilage. Environmental Monitoring: Identifies microbial contamination in air, water, and surfaces to prevent disease outbreaks. Common Methods Used in Microbial AnalysisCulture-Based Methods: Traditional methods where microorganisms are grown on specific media to identify their presence. Molecular Techniques: Techniques like PCR (Polymerase Chain Reaction) detect microbial DNA for accurate identification. Biochemical Testing: Includes tests like Gram staining, catalase test, and oxidase test to differentiate microbial species. Spectrophotometry & Flow Cytometry: Used for rapid quantification of microbial load. ATP Bioluminescence: Measures microbial contamination by detecting ATP (adenosine triphosphate) levels. Applications of Microbial Analysis Food & Beverage Industry: Ensures products are free from harmful microbes like Salmonella, E. coli, and Listeria. Pharmaceuticals: Maintains sterility in drug manufacturing to prevent contamination. Water Quality Testing: Detects microbial contamination in drinking water and wastewater. Cosmetics Industry: Prevents microbial growth in beauty and skincare products. Healthcare & Hospitals: Monitors cleanliness to prevent the spread of infections. Get Expert Microbial Analysis Services For reliable and comprehensive microbial testing services, visit Anand Biochem’s Microbial Analysis Services. Their advanced testing methods ensure quality and safety across various industries. ConclusionMicrobial analysis is essential for safeguarding public health and maintaining high product standards. Whether in food safety, pharmaceuticals, or environmental monitoring, microbial testing helps in early detection and prevention of contamination risks. If you require expert microbial testing solutions, consider professional services like Anand Biochem.
MicrobialAnalysis
Microbiology
FoodSafety
QualityControl
LabTesting
BacterialTesting
WaterTesting
PharmaceuticalTesting
EnvironmentalMonitoring
MicrobialContamination
MicrobialTesting
PCRTesting
BiochemicalTesting
ProductSafety
MicrobialLoad
0 notes
Text
"Market Dynamics 2024-2033: The Arbovirus Testing Revolution"
Arbovirus Testing Market : Arbovirus testing has become a critical tool in managing and preventing the spread of mosquito-borne diseases like Zika, dengue, and West Nile virus. With cutting-edge diagnostic methods, healthcare providers can now detect arbovirus infections faster and more accurately, helping public health agencies respond swiftly to outbreaks. These advanced tests, including PCR and serology, allow for early detection and differentiation of multiple viruses, ensuring that patients receive timely treatment and communities can take necessary precautions to minimize transmission risks.
To Request Sample Report : https://www.globalinsightservices.com/request-sample/?id=GIS26941 &utm_source=SnehaPatil&utm_medium=Article
In addition to improving patient outcomes, arbovirus testing is essential for global surveillance efforts. By identifying hotspots and tracking the spread of viruses, testing helps inform vaccination strategies, vector control initiatives, and public health policies. As climate change increases the reach of vector-borne diseases, robust and accessible arbovirus testing will play a pivotal role in safeguarding populations worldwide.
#ArbovirusTesting #PublicHealth #DiseasePrevention #ZikaVirus #DengueFever #WestNileVirus #PCRTesting #Serology #VectorBorneDiseases #InfectiousDiseaseControl #GlobalHealth #EarlyDetection #HealthTech #EpidemicResponse #MosquitoControl
0 notes
Text
"Protecting Plates: Trends in Food Pathogen Testing (2024-2033)"
Food Pathogen Testing Market : Food pathogen testing is an essential process in the food industry, aimed at identifying harmful microorganisms that can pose serious health risks to consumers. With the increasing complexity of food supply chains and the growing demand for ready-to-eat meals, ensuring food safety has never been more critical. Advanced testing methods, such as polymerase chain reaction (PCR) and next-generation sequencing, allow for rapid and accurate detection of pathogens like Salmonella, E. coli, and Listeria. By implementing stringent testing protocols, food manufacturers can mitigate the risks of contamination, protect public health, and maintain consumer trust in their products.
To Request Sample Report@https://www.globalinsightservices.com/request-sample/?id=GIS24243&utm_source=SnehaPatil-Article
As consumers become more aware of food safety issues, the demand for transparency and accountability in food production is on the rise. Food pathogen testing not only helps in compliance with regulatory standards but also plays a crucial role in preventing foodborne outbreaks. Companies that prioritize rigorous testing and quality assurance can differentiate themselves in a competitive market, showcasing their commitment to safety and quality. With innovations in testing technologies, including portable testing kits and real-time monitoring systems, the food industry is better equipped to respond swiftly to potential threats, ensuring that safe, high-quality food reaches consumers’ tables.
#FoodPathogenTesting #FoodSafety #PublicHealth #MicrobialTesting #FoodborneIllness #QualityAssurance #PCRTesting #FoodIndustry #ConsumerTrust #PathogenDetection #SafeFood #HealthAndSafety #RegulatoryCompliance #FoodQuality #NextGenTesting
0 notes
Text

We are thrilled to offer the STI-Q Comprehensive Multiplex Real-Time #PCR Kit. This cutting-edge #solution is designed to qualitatively detect and differentiate 13 causative organisms of Sexually Transmitted Infections.
🔑 Key Features:
Versatile Sample Compatibility: Detects from a range of clinical specimens including urine, tissue swabs (vagina, cervix, urethra), endocervical swabs, genital swabs, fluids, and prostatic fluids.
High Sensitivity & Specificity: Ensures accurate detection with superior performance.
Universal Compatibility: Works seamlessly with all available real-time PCR #instruments.
Explore the benefits of the STI-Q Comprehensive Multiplex Real-Time PCR Kit today!
#HealthcareInnovation #PCRTesting #STIDetection #MedicalTechnology #LaboratoryScience #testing #kits #rtpcr #clinicalpanel #ivd #sexuallytransmitted #transmitted
Contact us at [email protected] or +91 8800821778 if you need any further assistance!
Visit our website for more information https://www.genes2me.com/
#instruments#rt pcr#health care innovation#sti detection#testing#kits#medical technology#laboratory science#pcr#ivd#ngs#genes2me
0 notes
Text

One of my son heard the sounds of cicadas for the first time in this year, yesterday.
He wanted to go to a park where easier to fing&get incects today because his summer time has started the sounds he heard yesterday.
So, we went to catch summer insects there. But, it was a bit hot &windy to get them.
He've changed his mind to play with water insted of catching insects.
It was a good time to play with water at lunch time& windy day,because not much people there I thought.
It's much harder for me to choose where to go with my sons from since May 8th to untill now.
Because, free PCR tests finished & it costs more than over 2000yen for every test.
We cannot reach to PCRtest easily at any cilnic nor hospital.
We could choose PCRtest or antigen tests before.But now,
we need to get positive by a tntigen tests before we reach to PCRtest.
I think this order doesn't save health nor life nither.
In about a years ago, my son's doctor did antigen test first when he had a fever. He got negative. Then the doctor said "Just getting negative from the antigen test doesn't lead you to the truth.Therefore,let me do the PCRtest,too."
So, most of the people in Japan can't reach to test, so they cannot reach to treat.
Futheremore, many people doesn't wear masks in inside, even in the public school or hospitals.
I wanted my son to choose where he wants to study at home or school before 2019.
But the covid19 came into our life&still here, I cannot let him to choose it becuse it's dengerous.
If the ventilation was good at the publice school or any place...(no ventilation machines,no CO2 monitors, many teachers don't wear masks, no testing for covid19...)
If we can reach to the PCRtest or the antigen test with hight sensivities...
Then I could give him more options to do everyday.
However,
I just keep wearing mask when I meet people, testing whenever I think I need to do, requesting to wear masks who I need to talk, making time to no need to wear masks for my sons,keep talking to them why we live like this and asking them how they feeling now.
Thank for reading.
1 note
·
View note
Photo

Nucleic Acid MCQ 266 Link in Bio 👆 for more mcqs recommendation #pcr #covid #coronavirus #pixelcarracer #rrna #dna #laboratory #corona #pixelart #laboratorio #dopepixels #biotechnology #microbiology #medicina #biology #lab #pcrtest #policie #sequence #test #pixelcar #salud #science #virus #biologyteacher #enfermagem #genetics #pixelracer #pcgaming #genes (at Royal City Nanded) https://www.instagram.com/p/CpIF_EQPYAq/?igshid=NGJjMDIxMWI=
#pcr#covid#coronavirus#pixelcarracer#rrna#dna#laboratory#corona#pixelart#laboratorio#dopepixels#biotechnology#microbiology#medicina#biology#lab#pcrtest#policie#sequence#test#pixelcar#salud#science#virus#biologyteacher#enfermagem#genetics#pixelracer#pcgaming#genes
2 notes
·
View notes
Text
Rapid Microbiology Testing Market Outlook: Projected Growth, Key Players, Dynamics by 2032

The global Rapid Microbiology Testing Market is witnessing significant growth as healthcare and food safety industries increasingly adopt advanced technologies for faster and more accurate microbial detection. As of 2025, the market is projected to expand rapidly due to rising health concerns, the need for timely diagnosis, and increased demand for quality control across various sectors. This research provides an in-depth analysis of the rapid microbiology testing market, covering its size, share, and expected growth trends until 2032.
Microbiology testing involves the identification and analysis of microorganisms, including bacteria, fungi, viruses, and other pathogens. Traditional methods for microbial testing often require several days to produce results, which can be a limitation in sectors such as healthcare, food safety, and pharmaceuticals where rapid decision-making is critical.
Get a Free Sample Copy - https://www.skyquestt.com/sample-request/rapid-microbiology-testing-market
Rapid microbiology testing aims to provide quick and accurate results, typically within a few hours, significantly reducing the time taken for detection and enabling faster decision-making processes. These tests use advanced technologies such as molecular diagnostics, immunoassays, chromatography, and other automated systems to provide real-time results.
Market Size and Growth Forecast (2025–2032)
The global Rapid Microbiology Testing Market was valued at USD 5.27 billion in 2024 and is expected to grow at a CAGR of 10.12% from 2025 to 2032. The market is projected to reach USD 10.05 billion by 2032.
- Healthcare Segment Growth: The healthcare sector is expected to account for the largest share of the market. The demand for rapid tests in clinical diagnostics, especially for infectious diseases, will continue to rise.
- Food & Beverage Industry: The increasing demand for food safety and quality assurance will drive significant adoption of rapid microbiology testing in the food industry.
- Technological Advancements: Innovations such as multiplex PCR tests and next-generation sequencing (NGS) are expected to fuel market expansion.
Key Market Drivers
1. Increased Prevalence of Infectious Diseases: The rising incidence of infectious diseases, such as bacterial and viral infections, is one of the main drivers of the rapid microbiology testing market. The global COVID-19 pandemic highlighted the urgent need for faster diagnostic testing. With continued concerns about emerging diseases, both public and private healthcare systems are prioritizing rapid diagnostic methods.
2. Technological Advancements in Diagnostic Tools: Advancements in molecular biology, automation, and real-time PCR (Polymerase Chain Reaction) have greatly enhanced the sensitivity, accuracy, and speed of microbiology tests. These technologies enable the detection of pathogens in a matter of hours, driving the demand for rapid testing solutions across various industries.
3. Food Safety Regulations: The need for stringent food safety regulations to prevent outbreaks of foodborne illnesses has propelled the adoption of rapid microbiology testing methods in the food industry. The increasing focus on food safety is expected to drive the market as manufacturers seek to ensure compliance with health and safety standards.
4. Rising Awareness and Focus on Preventive Healthcare: With rising awareness of the importance of early diagnosis, particularly in hospitals and clinics, the demand for rapid testing methods has surged. Preventive healthcare practices, including the early detection of infections, are crucial in minimizing the spread of diseases and improving patient outcomes.
5. Growing Pharmaceutical and Biotechnology Research: The pharmaceutical and biotechnology sectors utilize microbiology testing extensively for drug development, clinical trials, and quality control processes. As these industries continue to grow, the demand for accurate, rapid testing solutions to identify microbial contamination will increase.
Make an Inquiry to Address your Specific Business Needs - https://www.skyquestt.com/speak-with-analyst/rapid-microbiology-testing-market
Market Segmentation
The rapid microbiology testing market can be segmented based on product type, application, technology, and geography.
By Product Type:
1. Instruments - PCR systems, mass spectrometers, microscopes, and other diagnostic tools.
2. Reagents & Kits - Consumables such as testing kits and reagents used in rapid microbiology testing.
3. Software - Diagnostic software solutions that integrate with testing devices to provide real-time results and data analysis.
By Application:
1. Healthcare - Hospitals, diagnostic labs, and clinics use rapid testing for pathogen detection in samples from patients.
2. Food & Beverage - Rapid testing is widely used in food production, packaging, and distribution to ensure product safety and regulatory compliance.
3. Pharmaceuticals & Biotechnology - The pharmaceutical industry uses rapid microbiology testing in quality control, drug discovery, and manufacturing.
4. Environmental Monitoring - Rapid testing is used to monitor microbial contamination in water, air, and soil.
By Technology:
1. Polymerase Chain Reaction (PCR) - PCR technology is widely used in molecular diagnostics for pathogen identification.
2. Immunoassay - Antigen-antibody-based methods for quick microbial detection.
3. Mass Spectrometry - Analytical techniques used for the identification of microorganisms in clinical and research labs.
By Region:
1. North America - North America dominates the global market, with high adoption rates of rapid testing technologies in the U.S. and Canada.
2. Europe - Europe is a significant player in the market, with several countries focusing on food safety and healthcare regulations.
3. Asia Pacific - Asia Pacific is expected to witness the fastest growth due to the increasing healthcare investments, rapid urbanization, and growing food safety concerns.
4. Rest of the World - Regions such as the Middle East and Latin America are also expected to grow, driven by investments in healthcare infrastructure and food safety measures.
Take Action Now: Secure Your Rapid Microbiology Testing Market Today - https://www.skyquestt.com/buy-now/rapid-microbiology-testing-market
Competitive Landscape
The rapid microbiology testing market is highly competitive, with key players constantly innovating to improve their product offerings. Major companies in this space include:
Biomérieux SA
Becton, Dickinson and Company
Danaher Corporation
Abbott Laboratories
Thermo Fisher Scientific Inc.
Merck KGaA
Bruker
QuidelOrtho Corporation
Sartorius AG
Charles River Laboratories
Neogen Corporation
Don Whitley Scientific Limited
Rapid Micro Biosystems, Inc.
Gradientech
PerkinElmer Inc.
RQmicro AG
Colifast AS
SeroSep Limited
BTNX Inc.
R-Biopharm A
These companies are focusing on mergers and acquisitions, partnerships, and technological innovations to strengthen their position in the market. They are also expanding their product portfolios to include rapid testing solutions for various applications such as healthcare, food safety, and environmental monitoring.
The global rapid microbiology testing market is poised for robust growth through 2032. As industries across healthcare, food safety, pharmaceuticals, and biotechnology continue to prioritize timely diagnostics, the demand for rapid testing solutions is expected to surge. Technological advancements, along with growing awareness of the need for faster, more accurate microbial detection, will continue to shape the market's trajectory in the coming years.
Read Rapid Microbiology Testing Market Report Today - https://www.skyquestt.com/report/rapid-microbiology-testing-market
Investments in research and development, combined with strategic partnerships, will play a pivotal role in accelerating market growth, leading to the widespread adoption of rapid microbiology testing across various sectors.
#RapidMicrobiologyTesting#MicrobialDetection#InfectiousDiseaseTesting#HealthcareDiagnostics#FoodSafetyTesting#MicrobiologyMarket#RapidTesting#DiagnosticsInnovation#PathogenDetection#MolecularDiagnostics#PCRTesting#Immunoassays#MedicalTechnology#Biotechnology#MicrobiologyResearch#DiagnosticSolutions#MicrobialContamination#ClinicalDiagnostics#FoodQualityControl#PharmaceuticalTesting
0 notes
Text
G, wie gesund. Aber das will keiner.
Heute traf eine Nachricht von einem Kumpel ein; er hätte endlich mal von seiner Impfung profitiert, war im Fitnessstudio. Für einen kurzen Augenblick schwebte mein Finger über dem Button "Gruppe verlassen". Ich atmete einen Moment durch, drehte mein Handy auf den Bildschirm, legte es hin und ging weg. Nein, die Gesellschaft wird mich so leicht nicht ausstoßen. Ich bin von nun an dieses 4. G, das keiner will. Ich bin ungeimpft, ungetestet, ungenesen, denn ich bin gesund. (Nach deren Logik wäre ich ja dann UUUG, wie lange und das könnte als Status ziehen?)
Kumpel Klaus weiß nichts von meinem Status. Wir haben uns in der Vergangenheit immer nur getroffen, wenn der Inzidenzwert es "offiziell" zugelassen hat oder eben in meiner Wohnung, da fiel mein fehlendes G nie auf. Kumpel Klaus kann auch nicht ahnen, wie mich seine Aussage getroffen hat. Im Grunde ist die Aussage "Yeah...von Impfung profitiert. War in Gym." doch voll ok, oder? Die hat nicht mehr Inhalt als mein "Äpfel von Oma ausm Garten und gleich mal literweise Apfelmus gemacht!" Ob sich Kumpel Klaus darüber echauffieren würde, wenn er allergisch auf Äpfel wäre? Wenn ich so überlege, weiß ich gar nicht, ob Kumpel Klaus überhaupt Äpfel mag. Ich muss ihn mal fragen.
4 notes
·
View notes
Text
Demonstration
Saturday 18th of September: World Wide Demonstration for Freedom https://worldwidedemonstration.com/ We Will All Be There. We are taking a stand for 5 important Freedoms: • Freedom of Speech. • Freedom of Movement. • Freedom of Choice. • Freedom of Assembly. • Freedom of Health. #WeWillAllBeThere We will not allow our inalienable Human Rights to be re-packaged as Human Privileges, to be conditioned upon compliance with Authoritarianism. Throughout history, Humanity has been tried and tested in difficult times, but in the end, Freedom Always Wins. We stand for the rights of all people to push back against infringements against their Freedom by joining us.
#WeWillAllBeThere#freedom#freedomofspeech#freedomofmovement#freedomofhealth#freedomofchoice#freedomofassembly#DavidWilcock#Olgasheean#OleDammegard#demonstration#worldwidedemonstration#human rights#pcrtest#PCRtests#phonegatealert petition EMFoff EMF stop5G#STOPCOVIDPasssport#stop 5g#stop5G#noWIFI#phonegate#phonegatealert
1 note
·
View note
Video
instagram
I Just Have Questions...AGAIN Question Everything. #freespeech #freethinkers It's not ILLEGAL! We all have options and are entitled to our opinions without judgment. Be Well! 🙏🏾😌💯��💓 Reposted from @griffinfyah Reposted from @drrachael #zerofucks #theupsidedown #scamdemic2020 #scamdemic2021 #ɢᴇᴛᴠᴀᴄᴄɪɴᴀᴛᴇᴅ #jabdone #maskingthetruth #Maskerade #endmaskmandates #endthelockdowns #stopfundingbigpharma #bigpharmaforthewin #firefauci #drpetermccullough #drkarymullis #drmikovits #pcrtest #billgatespopulationcontrol #exitthematrix #drchristinaparks #drparks #housebill4471 (at Worldwide) https://www.instagram.com/p/CTax8qYjYvQ/?utm_medium=tumblr
#freespeech#freethinkers#zerofucks#theupsidedown#scamdemic2020#scamdemic2021#ɢᴇᴛᴠᴀᴄᴄɪɴᴀᴛᴇᴅ#jabdone#maskingthetruth#maskerade#endmaskmandates#endthelockdowns#stopfundingbigpharma#bigpharmaforthewin#firefauci#drpetermccullough#drkarymullis#drmikovits#pcrtest#billgatespopulationcontrol#exitthematrix#drchristinaparks#drparks#housebill4471
1 note
·
View note
Video
The Truth About PCR Tests
3 notes
·
View notes
Link
Book your PCR test with us now and get your results within 90 hours, if you are not able to come into one of our test centres then we are able to mail you a testing kit and you can use it and send it back for examination. What we aim to do here is get people tested before they go for Hajj and Umrah, we provide this service in a lot of places in the Gulf and in some places in Africa and this is because we need to make sure that we can get as many people tested as we can so that we are able to get those who have tested negative to be on their way to Hajj and Umrah and those who tested positive can be medicated and quarantined. We provide tests for Mutamers, they are those who are not residents, at reasonable and competitive prices, we do understand that not a lot of people who are travelling may not be able to get the PCR test so if they able to they can get a test in their home country and make sure to carry the negative PCR test certificate with them to prove that they are COVID-19 free. We have partnered up with one of the leading laboratories called Al Borg to be able to distribute these tests and Al Borg is highly reliable as they have been named and rewarded with the fastest growing business.
1 note
·
View note