#Pharma Testing Laboratory
Text
Innovations in Legionella Water Testing: The Future of Risk Management

Legionella bacteria can cause a severe form of pneumonia called Legionnaires' disease, which can be fatal in some cases. The bacteria can grow in water systems such as cooling towers, hot tubs, and decorative fountains. As a result, Legionella testing has become an essential part of risk management in various industries, including healthcare, hospitality, and manufacturing.
The Global Legionella Water Testing Market is expected to grow at a significant rate in the coming years. The industry for legionella water testing was estimated to be USD 220.9 billion in 2019 and is predicted to reach USD 509.9 billion by 2030 with a CAGR of 7.9% from 2020- 2030, according to a report by Next Move Strategy Consulting.
One of the primary drivers of the Legionella water testing market is the increasing awareness about Legionnaires' disease and the need for regular testing of water systems to prevent outbreaks. Governments and regulatory bodies are mandating Legionella testing in various industries, including healthcare, hospitality, and manufacturing, which is further driving market growth.
Avail a free sample pdf: https://www.nextmsc.com/Legionella-Water-Testing-Market/request-sample
In addition, the Legionella water testing market is experiencing a wave of technological innovation, with new technologies transforming the way Legionella bacteria are detected, monitored, and managed. The traditional culture-based methods of Legionella testing are being challenged by new and advanced technologies, such as molecular diagnostics, next-generation sequencing (NGS), digital polymerase chain reaction (dPCR), and flow cytometry.
One of the significant market disruptors in the Legionella water testing market is the adoption of NGS technology. NGS is a high-throughput technology that enables the sequencing of millions of DNA fragments simultaneously, providing a more in-depth and accurate understanding of microbial diversity and identification. In Legionella testing, NGS has the potential to detect multiple Legionella species in a single test, making it a valuable tool in outbreak investigations and risk assessments.
Digital PCR is another disruptive technology in the Legionella water testing market, which provides a high level of sensitivity and specificity in detecting Legionella bacteria. Digital PCR is a quantitative PCR method that uses microfluidic chips to partition samples into thousands of reaction chambers, allowing the detection of low levels of Legionella bacteria in a sample. This technology is particularly useful in monitoring the effectiveness of Legionella control measures, such as disinfection and water treatment.
Flow cytometry is another technology that is transforming Legionella water testing. Flow cytometry is a cell analysis technique that can rapidly detect and identify microbial cells based on their size, shape, and fluorescence properties. Flow cytometry has the potential to detect and quantify Legionella bacteria in water samples, making it an ideal tool for routine monitoring of water systems.
The emergence of these new technologies is challenging the traditional culture-based methods of Legionella testing, which can be time-consuming, labour-intensive, and prone to false-negative results. The new technologies are providing more rapid, accurate, and cost-effective methods of Legionella testing, which is transforming the way Legionella risk is managed in various industries.
In addition to the technological advancements, the Legionella water testing market is also witnessing the development of new diagnostic tests for Legionella bacteria. Rapid diagnostic tests (RDTs) for Legionella bacteria are being developed, which can provide results in a matter of minutes, making them ideal for outbreak situations. These RDTs can be used for screening, surveillance, and outbreak investigations, and are expected to improve the speed and accuracy of Legionella testing.
The emergence of new technologies such as NGS, dPCR, and flow cytometry is challenging the traditional culture-based methods of Legionella testing, providing more rapid, accurate, and cost-effective methods of detection and identification. The market disruptors are transforming the way Legionella risk is managed in various industries and are expected to drive the growth of the Legionella water testing market in the coming years.
However, the market is not without challenges. The high cost of Legionella testing is a major factor hindering market growth, particularly for smaller businesses. The lack of awareness about Legionnaires' disease and the importance of Legionella testing in some regions is also a challenge for market growth.
The Legionella water testing market is highly competitive, with several major players, including; Albagaia Ltd., Becton, Dickinson & Company, BioMérieux SA, Aquacert Ltd., Idexx Laboratories Inc., Merck KGaA, Merck Millipore (Merck KGaA), Pall Corporation (Danaher Corporation), Pacific Water Technology, Phigenics Llc, Qiagen N.V., and Thermo Fisher Scientific Inc., amongst others. These companies are investing in research and development to develop new and innovative testing methods to stay ahead of the competition.
In recent years, companies have also been focusing on expanding their geographical reach through mergers and acquisitions. For instance, in 2019, Eurofins Scientific acquired Beacon Environmental, a Canadian company specializing in Legionella testing, to expand its water testing services in North America.
North America holds the major share in the legionella water testing market, owing to the presence of many leading pharmaceutical and biotechnological players, increasing prevalence of waterborne diseases and huge government support In conclusion, the Legionella water testing market is expected to continue growing in the coming years, driven by the increasing demand for Legionella testing and the development of new and innovative testing methods.
However, the market also faces challenges, such as the high cost of testing and the lack of awareness about Legionnaires' disease in some regions. Companies must continue to invest in research and development and expand their geographical reach to stay ahead of the competition in this rapidly growing market.
#legionella#water testing#pharma#healthcare#testing#laboratory#clinics#hospitals#biotechnology#diagnostics#opportunity#growth
0 notes
Text
Scientists are sounding the alarm after laboratory testing found that Covid mRNA vaccines “accelerate cancer” in all those who received the shots.
Lioness of Judah Ministry
Sep 19, 2024
By Hunter Fielding September 18, 2024
Scientists are sounding the alarm after laboratory testing found that Covid mRNA vaccines “accelerate cancer” in all those who received the shots.
Back in February, U.S. Republican Senator Ron Johnson from Wisconsin held a roundtable discussion titled ‘Federal Health Agencies and the COVID Cartel: What Are They Hiding?’.
In attendance at that forum was an internal medicine specialist and medical doctor named Sabine Hazan, who conducts and supervises clinical trials for cutting-edge medical research, including that based on gastroenterology.
Dr. Hazan investigates Big Pharma companies for corruption, a very daring and courageous effort to help the world realize how much fraud and insidious planning Western Medicine engages in, especially the Vaccine Industrial Complex.
The key component that makes up 90% of our biological seat of immunity for fighting diseases is KILLED OFF by spike proteins from mRNA jabs that travel to the gut. Anyone who got Covid vaccinated could be catapulting cancer, IBS, autism, dementia, and catching Covid or the next pandemic of Bird Flu, Monkeypox, or whatever other gain-of-function lab-made virus Big Pharma releases into the wild.
Gastroenterologist Dr. Sabine Hazan tested the microbiomes of doctors who volunteered to be tested BEFORE and AFTER getting Covid vaccinated with spike protein prion-creating mRNA jabs and discovered that their most important gut bacteria were wiped out within 30 days to almost non-existent.
13 notes
·
View notes
Text
Chemzin Biotox - Innovative Research Laboratory in India
Chemzin Biotox is a research lab in India that does testing for a wide range of industries like Preclinical, In-Vivo Toxicology, Pharma, & cell testing. Call Now!
2 notes
·
View notes
Text
From Lab to Patient – The Evolution of Medicine Production
The journey of a medicine from a research laboratory to a patient’s bedside is a complex and intricate process. It involves rigorous scientific research, extensive clinical trials, stringent regulatory approvals, and sophisticated manufacturing processes. This blog will explore the evolution of medicine production, highlighting the role of leading pharmaceutical companies in India, including Centurion Healthcare, in bringing life-saving medications to the market.

The Genesis of Medicine: Research and Development
The Role of Pharma Companies in India
The development of new medications begins with a deep understanding of diseases and the biological mechanisms that drive them. Pharmaceutical companies in India, renowned for their robust R&D capabilities, play a pivotal role in this phase. Researchers at these companies work tirelessly to identify potential therapeutic targets and develop compounds that can modulate these targets effectively.
Preclinical Research
Before a new drug can be tested in humans, it must undergo extensive preclinical research. This involves laboratory and animal studies to assess the safety and efficacy of the compound. The goal is to gather enough data to support the initiation of clinical trials. This stage is crucial for ensuring that only the most promising and safe candidates move forward.
Clinical Trials: Testing in Humans
Phase I Trials
Once a compound has shown promise in preclinical studies, it enters Phase I clinical trials. These trials involve a small number of healthy volunteers and aim to evaluate the safety, tolerability, and pharmacokinetics of the drug. For a medicine manufacturing company in India like Centurion Healthcare, this phase is critical for determining the initial safety profile of the drug.
Phase II Trials
If Phase I trials are successful, the drug progresses to Phase II trials, which involve a larger group of patients who have the condition the drug is intended to treat. The focus here is on assessing the drug’s efficacy and further evaluating its safety. Pharmaceutical companies in India invest heavily in this phase to gather robust data that can support the drug’s potential therapeutic benefits.
Phase III Trials
Phase III trials are the most extensive and involve a large number of patients across multiple locations. These trials are designed to confirm the drug’s efficacy, monitor side effects, and compare it to standard treatments. For a medicine manufacturing company, this phase is critical for obtaining the data needed for regulatory approval.
Regulatory Approval
After successful Phase III trials, the data is submitted to regulatory authorities for approval. In India, the Central Drugs Standard Control Organization (CDSCO) is responsible for evaluating the safety and efficacy of new drugs. Obtaining regulatory approval is a significant milestone for any medicine company in India, allowing the drug to be marketed and made available to patients.
Manufacturing: From Lab Bench to Production Line
Scaling Up Production
Once a drug receives regulatory approval, the focus shifts to manufacturing. Scaling up production from laboratory scale to commercial scale is a complex process that requires significant expertise and investment. Medicine manufacturing companies in India, such as Centurion Healthcare, employ state-of-the-art technologies and adhere to stringent quality control measures to ensure that every batch of medicine meets the highest standards.
Quality Assurance and Control
Quality assurance and control are paramount in medicine manufacturing. Companies implement rigorous testing protocols to ensure that each batch of the drug is consistent in terms of potency, purity, and safety. This involves testing raw materials, in-process materials, and finished products. Pharmaceutical companies in India are known for their stringent quality control measures, which are essential for maintaining the trust of healthcare providers and patients.
Packaging and Distribution
Once manufactured, the medicines are packaged in a manner that ensures their stability and safety during transportation and storage. Packaging must protect the drug from environmental factors such as light, moisture, and temperature fluctuations. After packaging, the medicines are distributed to pharmacies, hospitals, and clinics, ensuring that they are readily available to patients.
Post-Market Surveillance
The journey of a medicine does not end with its launch in the market. Post-market surveillance is crucial for monitoring the drug’s performance in the real world. This involves collecting and analyzing data on the drug’s safety and efficacy from patients and healthcare providers. Pharmaceutical companies in India are actively involved in post-market surveillance to ensure that any potential issues are identified and addressed promptly.
Pharmacovigilance
Pharmacovigilance is a key component of post-market surveillance. It involves the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Medicine manufacturing companies in India have dedicated pharmacovigilance teams that monitor and report any adverse events associated with their drugs, ensuring patient safety.
The Role of Technology in Medicine Production
Advanced Manufacturing Technologies
The pharmaceutical industry has embraced advanced manufacturing technologies to enhance efficiency and product quality. Techniques such as continuous manufacturing, automation, and advanced analytics are revolutionizing the way medicines are produced. These technologies enable medicine manufacturing companies to produce drugs more efficiently, reduce waste, and ensure consistent product quality.
Digital Transformation
Digital transformation is playing a significant role in the evolution of medicine production. Pharmaceutical companies in India are leveraging digital technologies such as artificial intelligence (AI), machine learning, and big data analytics to streamline their operations. These technologies are used in various stages of drug development and manufacturing, from identifying new drug targets to optimizing production processes and ensuring quality control.
Sustainability in Medicine Production
Sustainability is becoming increasingly important in the pharmaceutical industry. Companies are adopting environmentally friendly practices and technologies to minimize their environmental footprint. This includes using renewable energy sources, reducing waste, and implementing green chemistry principles. Medicine manufacturing companies in India are at the forefront of this movement, striving to make their production processes more sustainable.
Centurion Healthcare: Leading the Way
As a leading medicine manufacturing company in India, Centurion Healthcare is dedicated to advancing the field of medicine production. Our commitment to quality, innovation, and sustainability sets us apart in the industry. Here is how we are contributing to the evolution of medicine production:
Cutting-Edge Research and Development
Our R&D team is at the heart of our success. We invest heavily in research to discover and develop new therapeutic agents that address unmet medical needs. Our state-of-the-art facilities and collaboration with leading research institutions enable us to stay at the forefront of medical innovation.
Advanced Manufacturing Capabilities
At Centurion Healthcare, we utilize advanced manufacturing technologies to produce high-quality medicines efficiently. Our manufacturing facilities are equipped with the latest equipment and adhere to international standards of quality and safety. We are committed to continuous improvement and innovation in our production processes.
Comprehensive Quality Control
Quality is our top priority. We have established rigorous quality control measures to ensure that every product we manufacture meets the highest standards. From raw material testing to final product release, our quality assurance team meticulously monitors every step of the production process.
Commitment to Sustainability
We are committed to making our production processes more sustainable. We have implemented various initiatives to reduce our environmental impact, including energy-efficient practices, waste reduction programs, and sustainable sourcing of raw materials. Our goal is to contribute to a healthier planet while providing high-quality medicines to patients.
Conclusion
The evolution of medicine production is a testament to the dedication and innovation of pharmaceutical companies in India. From the initial stages of research and development to the manufacturing and distribution of life-saving medications, every step in this journey is crucial. At Centurion Healthcare, we are proud to be a part of this dynamic industry, contributing to the health and well-being of patients worldwide.
As a leading medicine company in India, we remain committed to advancing the field of medicine production through cutting-edge research, advanced manufacturing technologies, and a steadfast commitment to quality and sustainability. Our journey from the lab to the patient’s bedside is driven by a passion for excellence and a desire to make a meaningful impact on global health.
#Medicine manufacturing company in India#Pharma companies in India#Medicine company in India#Pharmaceutical companies in India#Medicine manufacturing company
4 notes
·
View notes
Text
Horseshoe crab blood is vital for testing intravenous drugs, but new synthetic alternatives could mean pharma won’t bleed this unique species dry
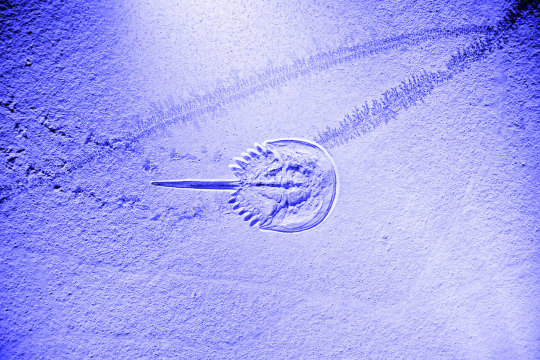
- By Kristoffer Whitney , Jolie Crunelle , Rochester Institute of Technology , The Conversation -
If you have ever gotten a vaccine or received an intravenous drug and did not come down with a potentially life-threatening fever, you can thank a horseshoe crab (Limulus polyphemus).
How can animals that are often called living fossils, because they have barely changed over millions of years, be so important in modern medicine? Horseshoe crab blood is used to produce a substance called limulus amebocyte lysate, or LAL, which scientists use to test for toxic substances called endotoxins in intravenous drugs.
These toxins, produced by bacteria, are ubiquitous in the environment and can’t be removed simply through sterilization. They can cause a reaction historically referred to as “injection fever.” A strong concentration can lead to shock and even death.
Identifying LAL as a highly sensitive detector of endotoxins was a 20th-century medical safety breakthrough. Now, however, critics are raising questions about environmental impacts and the process for reviewing and approving synthetic alternatives to horseshoe crab blood.
We study science, technology and public policy, and recently published a white paper examining social, political and economic issues associated with using horseshoe crabs to produce LAL. We see this issue as a test case for complicated problems that cut across multiple agencies and require attention to both nature and human health.
youtube
Protecting horseshoe crabs will require persuading the heavily regulated pharmaceutical industry to embrace change.
An ocean solution
Doctors began injecting patients with various solutions in the mid-1800s, but it was not until the 1920s that biochemist Florence Seibert discovered that febrile reactions were due to contaminated water in these solutions. She created a method for detecting and removing the substances that caused this reaction, and it became the medical standard in the 1940s.
Known as the rabbit pyrogen test, it required scientists to inject intravenous drugs into rabbits, then monitor the animals. A feverish rabbit meant that a batch of drugs was contaminated.
The LAL method was discovered by accident. Working with horseshoe crabs at the Marine Biological Laboratory at Woods Hole, Massachusetts, in the 1950s and ’60s, pathobiologist Frederik Bang and medical researcher Jack Levin noticed that the animals’ blue blood coagulated in a curious manner. Through a series of experiments, they isolated endotoxin as the coagulant and devised a method for extracting LAL from the blood. This compound would gel or clot nearly instantaneously in the presence of fever-inducing toxins.
Academic researchers, biomedical companies and the U.S. Food and Drug Administration refined LAL production and measured it against the rabbit test. By the 1990s, LAL was the FDA-approved method for testing medicines for endotoxin, largely replacing rabbits.
Producing LAL requires harvesting horseshoe crabs from oceans and beaches, draining up to 30% of their blood in a laboratory and returning the live crabs to the ocean. There’s dispute about how many crabs die in the process – estimates range from a few percent to 30% or more – and about possible harmful effects on survivors.
Today there are five FDA-licensed LAL producers along the U.S. East Coast. The amount of LAL they produce, and its sales value, are proprietary.
Bait versus biotech
As biomedical LAL production ramped up in the 1990s, so did harvesting horseshoe crabs to use as bait for other species, particularly eel and whelk for foreign seafood markets. Over the past 25 years, hundreds of thousands – and in the early years, millions – of horseshoe crabs have been harvested each year for these purposes. Combined, the two fisheries kill over half a million horseshoe crabs every year.
There’s no agreed total population estimate for Limulus, but the most recent federal assessment of horseshoe crab fisheries found the population was neither strongly growing nor declining.
Conservationists are worried, and not just about the crabs. Millions of shorebirds migrate along the Atlantic coast, and many stop in spring, when horseshoe crabs spawn on mid-Atlantic beaches, to feed on the crabs’ eggs. Particularly for red knots – a species that can migrate up to 9,000 miles between the tip of South America and the Canadian Arctic – gorging on horseshoe crab eggs provides a critical energy-rich boost on their grueling journey.
Red knots were listed as threatened under the Endangered Species Act in 2015, largely because horseshoe crab fishing threatened this key food source. As biomedical crab harvests came to equal or surpass bait harvests, conservation groups began calling on the LAL industry to find new sources.
Biomedical alternatives
Many important medicines are derived from living organisms. Penicillin, the first important antibiotic, was originally produced from molds. Other medicines currently in use come from sources including cows, pigs, chickens and fish. The ocean is a promising source for such products.
When possible, synthesizing these substances in laboratories – especially widely used medications like insulin – offers many benefits. It’s typically cheaper and more efficient, and it avoids putting species at risk, as well as addressing concerns some patients have about using animal-derived medical products.
In the 1990s, researchers at the National University of Singapore invented and patented the first process for creating a synthetic, endotoxin-detecting compound using horseshoe crab DNA and recombinant DNA technology. The result, dubbed recombinant Factor C (rFC), mimicked the first step in the three-part cascade reaction that occurs when LAL is exposed to endotoxin.
Later, several biomedical firms produced their own versions of rFC and compounds called recombinant cascade reagents (rCRs), which reproduce the entire LAL reaction without using horseshoe crab blood. Yet, today, LAL remains the dominant technology for detecting endotoxins in medicine.

A sample of horseshoe crab blood. Florida Fish and Wildlife Commission, CC BY-NC-ND
The main reason is that the U.S. Pharmacopeia, a quasi-regulatory organization that sets safety standards for medical products, considers rFC and rCR as “alternative” methods for detecting endotoxins, so they require case-by-case validation for use – a potentially lengthy and expensive process. The FDA generally defers to the U.S. Pharmacopeia.
A few large pharmaceutical companies with deep pockets have committed to switching from LAL to rFC. But most drug producers are sticking with the tried-and-true method.
Conservation groups want the U.S. Pharmacopeia to fully certify rFC for use in industry with no extra testing or validation. In their view, LAL producers are stalling rFC and rCR approval to protect their market in endotoxin detection. The U.S. Pharmacopeia and LAL producers counter that they are doing due diligence to protect public health.
Change in the offing
Change may be coming. All major LAL producers now have their own recombinant products – a tacit acknowledgment that markets and regulations are moving toward Limulus-free ways to test for endotoxins.
Atlantic fisheries regulators are currently considering new harvest limits for horseshoe crabs, and the U.S. Pharmacopeia is weighing guidance on recombinant alternatives to LAL. Public comments will be solicited over the winter of 2024, followed by U.S. Pharmacopeia and FDA review.
Even if rFC and rCR don’t win immediate approval, we believe that collecting more complete data on horseshoe crab populations and requiring more transparency from the LAL industry on how it handles the crabs would represent progress. So would directing medical companies to use recombinant products for testing during the manufacturing process, while saving LAL solely for final product testing.
Making policy on complex scientific issues across diverse agencies is never easy. But in our view, incremental actions that protect both human health and the environment could be important steps forward.
Kristoffer Whitney, Associate Professor of Science, Technology and Society, Rochester Institute of Technology and Jolie Crunelle, Master's Degree Student in Science, Technology, and Public Policy, Rochester Institute of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.
--
Read Also
The FDA no longer mandates all drugs to be tested on animals before being tested on humans
#animals#animal welfare#biotech#pharma#clinical trials#medtech#health#blood#medicine#animal testing#horseshoe crab
3 notes
·
View notes
Text
If you're worrying about Hazard in this whole sparkling ordeal which you weren't until I just mentioned it and made your heart drop to your knees, don't worry!
During/after the events of the Grabbening, they will be extra dissuaded from experimenting on test subjects that haven't been cleared by high command to experiment on. Because not only did they threaten the vehicons' favorite human, but also because of the unlucky vehicon escaping and Soundwave being severely not happy about Hazard nearly causing another incident with lackadaisical laboratory care and lack of scientific ethics.
So by the time the sparkling comes around Hazard's already stubbornly decided they aren't going to leave their lab and are going to sulk for a year or three. They'll be relevant to dealing with Tarn, the Fallen, and Pharma in future events, but for now Soundwave is pretty okay with said sulking because as I've mentioned Soundwave dislikes Hazard (not enough to kill them quite yet, but enough to enjoy their pissed in the punchbowl attitude)
#tfp au#transformers#oc talk#by the time they actually do see the sparkling it's gonna be quite a while in the future— actually it'll be at the surprise weddings party#though at that point they would've been through a mindnumbing amount of lessons on lab care and ethics lessons#and some level of ''oh if i do [thing] there will be consequences i can't get away with so I won't do [thing]''#yes Soundwave will end up saving Fowler from Hazard and the vehicon will escape that lab#he deserves a name beyond his association with hazard lol#silverlight#hazard
5 notes
·
View notes
Text
Indian health authorities have halted all production at the main factory of Maiden Pharmaceuticals near New Delhi after its cough syrups were linked to the deaths of dozens of children in Gambia.
Anil Vij, the home minister of India's northern Haryana state, said authorities inspected the Maiden facility four times this month and found 12 violations of good practices.
Maiden Pharma sells its products domestically as well as exports to countries in Asia, Africa and Latin America.
"Keeping this in view, the entire production of the company has been banned and notice has also been issued," Vij said.
Maiden Pharma has two other manufacturing plants in Haryana state.
What is the drug company accused of?
The World Health Organization (WHO) released a report last week saying that cold and cough syrups manufactured by Maiden Pharma may be linked to the deaths of at least 66 children in Gambia.
The WHO said that laboratory analysis of four Maiden products showed they had "unacceptable" amounts of potentially life-threatening contaminants that can seriously damage kidneys.
The Gambian health minister, Ahmadou Lamin Samateh, said over the weekend that the number of children who died from severe kidney damage had risen to 69.
The WHO also issued a medical product alert last week, asking regulators to remove the Indian company's goods from the market.
The drug company's director has denied the accusation to the Indian press.
The Gambian police, in a preliminary investigation report on Tuesday, said that the children's deaths from kidney damage were linked to cough syrups made in India, without naming the company.
Police added that the medicinal syrup was imported via a US-based company.
India's Health Ministry said last week it was informed about the WHO findings last month and it was waiting on results of tests of all four Maiden products from a federal laboratory.
India was not licensed to sell the drug domestically, but was only allowed to export them to Gambia, the Indian Health Ministry added.
3 notes
·
View notes
Text
Shriram Pharmacy College: Best Pharmacy College In India

Pharmacy is a dynamic field with diverse career opportunities, and Shriram Pharmacy College, Bankner, is a distinguished name in pharmacy education in India. With comprehensive programs and a focus on both theoretical and practical aspects, the college prepares students for a multitude of career paths. From community pharmacists to academic professionals, Shriram Pharmacy College offers a broad range of specializations to help students excel in their chosen careers. Let’s explore some of the top career opportunities available to graduates of Shriram Pharmacy College.
### 1. Serve as a Community Pharmacist
Community pharmacists play a crucial role in healthcare by providing medications, advice, and support to patients. Graduates from Shriram Pharmacy College are trained to excel in this role, mastering the art of patient counseling, understanding drug interactions, and managing prescriptions. The college’s curriculum emphasizes practical skills, patient communication, and pharmaceutical ethics, ensuring graduates are well-equipped to work in community settings. By becoming a community pharmacist, one contributes significantly to public health, helping people manage their health conditions and medications effectively.
### 2. Work as a Hospital Pharmacist
Hospital pharmacists are integral to the healthcare team, responsible for the safe and effective use of medications in hospitals. At Shriram Pharmacy College, students receive in-depth training in pharmacotherapy, patient care, and clinical practices. The college’s comprehensive practical lab sessions and clinical exposure prepare students to handle complex medication regimens and collaborate with healthcare professionals. Graduates can work in both government and private hospitals, ensuring the proper dispensing of medicines, educating healthcare staff, and contributing to patient care.
### 3. Become a Clinical Research Associate
Clinical research is a growing field that focuses on developing new drugs and therapies. Shriram Pharmacy College offers a strong foundation in research methodologies, clinical trials, and regulatory guidelines. The college equips students with the knowledge to become Clinical Research Associates (CRAs), professionals who oversee clinical trials and ensure compliance with regulatory standards. CRAs play a critical role in advancing pharmaceutical science by ensuring that clinical trials are conducted ethically and effectively, ultimately leading to the development of new, life-saving medications.
### 4. Specialize in Regulatory Affairs
The field of regulatory affairs involves ensuring that pharmaceutical products meet all legal and scientific requirements. Shriram Pharmacy College provides specialized courses in pharmaceutical regulations, documentation, and compliance. Students learn about national and international regulatory frameworks, preparing them to work with companies to register and license products in various markets. Graduates can pursue careers in regulatory affairs, ensuring that medications are safe, effective, and accessible to the public while navigating complex regulatory landscapes.
### 5. Analyze as Quality Control Specialist
Quality control (QC) is essential in the pharmaceutical industry to maintain the safety and efficacy of medicines. Shriram Pharmacy College’s curriculum includes rigorous training in quality assurance, analytical techniques, and laboratory practices. Graduates can pursue careers as Quality Control Specialists, responsible for testing raw materials, in-process samples, and finished products. They ensure that all pharmaceutical products meet the required quality standards, contributing to the industry’s reputation and consumer safety.
### 6. Sell as a Pharma Representative
Pharmaceutical representatives are crucial in promoting new drugs and therapies to healthcare providers. Shriram Pharmacy College prepares students with excellent communication skills, a deep understanding of pharmacology, and sales techniques. Graduates can pursue careers as Pharma Representatives, engaging with doctors, hospitals, and clinics to promote the latest medications and treatments. This role offers a unique blend of science and business, providing excellent growth opportunities in the pharmaceutical industry.
### 7. Teach as an Academic Professional
Pharmacy education is essential for the development of future pharmacists and researchers. Shriram Pharmacy College, with its focus on research and teaching, prepares its students to become academic professionals. Graduates can pursue careers as lecturers, professors, or researchers in pharmacy colleges and universities. This career path allows them to contribute to the academic world by educating the next generation of pharmacists, conducting research, and publishing scientific papers in pharmaceutical sciences.
### 8. Pursue a Pharmaceutical Sales Career
A career in pharmaceutical sales can be highly rewarding for those with a knack for marketing and a passion for healthcare. At Shriram Pharmacy College, students learn about pharmaceutical business dynamics, sales strategies, and marketing ethics. This knowledge helps graduates become successful in pharmaceutical sales, where they promote products to healthcare professionals, negotiate contracts, and drive sales growth for pharmaceutical companies. A pharmaceutical sales career provides a dynamic work environment, excellent earning potential, and opportunities for rapid advancement.
/media/1299d54961bb485b84a1f6e74f71c195
### **FAQs about Shriram Pharmacy College and Pharmacy Careers**
**1. What makes Shriram Pharmacy College the best choice for pharmacy education?**
Shriram Pharmacy College is renowned for its comprehensive curriculum, experienced faculty, and state-of-the-art facilities. The college focuses on both theoretical knowledge and practical skills, ensuring students are well-prepared for the dynamic field of pharmacy. Offering a variety of specializations and career guidance, the college supports students in achieving their professional goals. With a strong emphasis on research, industry partnerships, and clinical exposure, Shriram Pharmacy College provides an unparalleled education that fosters innovation and leadership in the pharmaceutical sector.
**2. How does Shriram Pharmacy College prepare students for a career as a Community Pharmacist?**
Shriram Pharmacy College prepares students for a career as a Community Pharmacist by offering a curriculum that covers pharmacology, patient counseling, and drug management. Practical lab sessions, internships, and community engagement are integral parts of the program, allowing students to gain real-world experience. Emphasizing communication skills and ethical practices, the college ensures graduates can effectively manage community pharmacies, offer patient care, and provide accurate drug information, thus making a significant impact on public health and patient outcomes.
**3. What opportunities does Shriram Pharmacy College offer for aspiring Clinical Research Associates?**
For aspiring Clinical Research Associates, Shriram Pharmacy College offers specialized training in research methodologies, clinical trial management, and regulatory compliance. The college provides exposure to real-world clinical research through collaborations with hospitals and research institutions. Students learn to design, monitor, and analyze clinical trials, ensuring they meet ethical standards. Graduates are well-equipped to work in pharmaceutical companies, contract research organizations, or academic institutions, contributing to the development of new drugs and medical therapies.
**4. What is the scope for graduates in Regulatory Affairs from Shriram Pharmacy College?**
Graduates specializing in Regulatory Affairs from Shriram Pharmacy College have a vast scope in pharmaceutical companies, regulatory agencies, and consultancies. The college provides a deep understanding of global regulatory frameworks, drug approval processes, and documentation standards. This training enables graduates to ensure that pharmaceutical products comply with regulatory standards in different countries. Career roles include Regulatory Affairs Specialists, Compliance Officers, and Regulatory Consultants, who play crucial roles in guiding companies through the complex process of bringing new drugs to market.
**5. How does Shriram Pharmacy College support students interested in Pharmaceutical Sales?**
Shriram Pharmacy College supports students interested in Pharmaceutical Sales by providing courses in pharmaceutical marketing, sales strategies, and product management. The curriculum combines scientific knowledge with business acumen, preparing students to effectively communicate with healthcare professionals. The college also offers training in negotiation and presentation skills, essential for a successful career in sales. Graduates can work as Medical Representatives, Sales Managers, or Product Managers, promoting pharmaceutical products and achieving business growth in a competitive market.
### **Conclusion**
Shriram Pharmacy College, Bankner, stands out as a premier institution for pharmacy education in India, offering diverse career paths ranging from community pharmacists to pharmaceutical sales professionals. With its emphasis on practical training, research, and industry engagement, the college ensures that its graduates are well-prepared to meet the challenges of the pharmaceutical industry. Whether you’re interested in research, quality control, regulatory affairs, or sales, Shriram Pharmacy College provides the knowledge and skills needed for a successful and rewarding career in pharmacy.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
Pharmacy is a dynamic field with diverse career opportunities, and Shriram Pharmacy College, Bankner, is a distinguished name in pharmacy education in India. With comprehensive programs and a focus on both theoretical and practical aspects, the college prepares students for a multitude of career paths. From community pharmacists to academic professionals, Shriram Pharmacy College offers a broad range of specializations to help students excel in their chosen careers. Let’s explore some of the top career opportunities available to graduates of Shriram Pharmacy College.
### 1. Serve as a Community Pharmacist
Community pharmacists play a crucial role in healthcare by providing medications, advice, and support to patients. Graduates from Shriram Pharmacy College are trained to excel in this role, mastering the art of patient counseling, understanding drug interactions, and managing prescriptions. The college’s curriculum emphasizes practical skills, patient communication, and pharmaceutical ethics, ensuring graduates are well-equipped to work in community settings. By becoming a community pharmacist, one contributes significantly to public health, helping people manage their health conditions and medications effectively.
### 2. Work as a Hospital Pharmacist
Hospital pharmacists are integral to the healthcare team, responsible for the safe and effective use of medications in hospitals. At Shriram Pharmacy College, students receive in-depth training in pharmacotherapy, patient care, and clinical practices. The college’s comprehensive practical lab sessions and clinical exposure prepare students to handle complex medication regimens and collaborate with healthcare professionals. Graduates can work in both government and private hospitals, ensuring the proper dispensing of medicines, educating healthcare staff, and contributing to patient care.
### 3. Become a Clinical Research Associate
Clinical research is a growing field that focuses on developing new drugs and therapies. Shriram Pharmacy College offers a strong foundation in research methodologies, clinical trials, and regulatory guidelines. The college equips students with the knowledge to become Clinical Research Associates (CRAs), professionals who oversee clinical trials and ensure compliance with regulatory standards. CRAs play a critical role in advancing pharmaceutical science by ensuring that clinical trials are conducted ethically and effectively, ultimately leading to the development of new, life-saving medications.
### 4. Specialize in Regulatory Affairs
The field of regulatory affairs involves ensuring that pharmaceutical products meet all legal and scientific requirements. Shriram Pharmacy College provides specialized courses in pharmaceutical regulations, documentation, and compliance. Students learn about national and international regulatory frameworks, preparing them to work with companies to register and license products in various markets. Graduates can pursue careers in regulatory affairs, ensuring that medications are safe, effective, and accessible to the public while navigating complex regulatory landscapes.
### 5. Analyze as Quality Control Specialist
Quality control (QC) is essential in the pharmaceutical industry to maintain the safety and efficacy of medicines. Shriram Pharmacy College’s curriculum includes rigorous training in quality assurance, analytical techniques, and laboratory practices. Graduates can pursue careers as Quality Control Specialists, responsible for testing raw materials, in-process samples, and finished products. They ensure that all pharmaceutical products meet the required quality standards, contributing to the industry’s reputation and consumer safety.
### 6. Sell as a Pharma Representative
Pharmaceutical representatives are crucial in promoting new drugs and therapies to healthcare providers. Shriram Pharmacy College prepares students with excellent communication skills, a deep understanding of pharmacology, and sales techniques. Graduates can pursue careers as Pharma Representatives, engaging with doctors, hospitals, and clinics to promote the latest medications and treatments. This role offers a unique blend of science and business, providing excellent growth opportunities in the pharmaceutical industry.
### 7. Teach as an Academic Professional
Pharmacy education is essential for the development of future pharmacists and researchers. Shriram Pharmacy College, with its focus on research and teaching, prepares its students to become academic professionals. Graduates can pursue careers as lecturers, professors, or researchers in pharmacy colleges and universities. This career path allows them to contribute to the academic world by educating the next generation of pharmacists, conducting research, and publishing scientific papers in pharmaceutical sciences.
### 8. Pursue a Pharmaceutical Sales Career
A career in pharmaceutical sales can be highly rewarding for those with a knack for marketing and a passion for healthcare. At Shriram Pharmacy College, students learn about pharmaceutical business dynamics, sales strategies, and marketing ethics. This knowledge helps graduates become successful in pharmaceutical sales, where they promote products to healthcare professionals, negotiate contracts, and drive sales growth for pharmaceutical companies. A pharmaceutical sales career provides a dynamic work environment, excellent earning potential, and opportunities for rapid advancement.
/media/1299d54961bb485b84a1f6e74f71c195
### **FAQs about Shriram Pharmacy College and Pharmacy Careers**
**1. What makes Shriram Pharmacy College the best choice for pharmacy education?**
Shriram Pharmacy College is renowned for its comprehensive curriculum, experienced faculty, and state-of-the-art facilities. The college focuses on both theoretical knowledge and practical skills, ensuring students are well-prepared for the dynamic field of pharmacy. Offering a variety of specializations and career guidance, the college supports students in achieving their professional goals. With a strong emphasis on research, industry partnerships, and clinical exposure, Shriram Pharmacy College provides an unparalleled education that fosters innovation and leadership in the pharmaceutical sector.
**2. How does Shriram Pharmacy College prepare students for a career as a Community Pharmacist?**
Shriram Pharmacy College prepares students for a career as a Community Pharmacist by offering a curriculum that covers pharmacology, patient counseling, and drug management. Practical lab sessions, internships, and community engagement are integral parts of the program, allowing students to gain real-world experience. Emphasizing communication skills and ethical practices, the college ensures graduates can effectively manage community pharmacies, offer patient care, and provide accurate drug information, thus making a significant impact on public health and patient outcomes.
**3. What opportunities does Shriram Pharmacy College offer for aspiring Clinical Research Associates?**
For aspiring Clinical Research Associates, Shriram Pharmacy College offers specialized training in research methodologies, clinical trial management, and regulatory compliance. The college provides exposure to real-world clinical research through collaborations with hospitals and research institutions. Students learn to design, monitor, and analyze clinical trials, ensuring they meet ethical standards. Graduates are well-equipped to work in pharmaceutical companies, contract research organizations, or academic institutions, contributing to the development of new drugs and medical therapies.
**4. What is the scope for graduates in Regulatory Affairs from Shriram Pharmacy College?**
Graduates specializing in Regulatory Affairs from Shriram Pharmacy College have a vast scope in pharmaceutical companies, regulatory agencies, and consultancies. The college provides a deep understanding of global regulatory frameworks, drug approval processes, and documentation standards. This training enables graduates to ensure that pharmaceutical products comply with regulatory standards in different countries. Career roles include Regulatory Affairs Specialists, Compliance Officers, and Regulatory Consultants, who play crucial roles in guiding companies through the complex process of bringing new drugs to market.
**5. How does Shriram Pharmacy College support students interested in Pharmaceutical Sales?**
Shriram Pharmacy College supports students interested in Pharmaceutical Sales by providing courses in pharmaceutical marketing, sales strategies, and product management. The curriculum combines scientific knowledge with business acumen, preparing students to effectively communicate with healthcare professionals. The college also offers training in negotiation and presentation skills, essential for a successful career in sales. Graduates can work as Medical Representatives, Sales Managers, or Product Managers, promoting pharmaceutical products and achieving business growth in a competitive market.
### **Conclusion**
Shriram Pharmacy College, Bankner, stands out as a premier institution for pharmacy education in India, offering diverse career paths ranging from community pharmacists to pharmaceutical sales professionals. With its emphasis on practical training, research, and industry engagement, the college ensures that its graduates are well-prepared to meet the challenges of the pharmaceutical industry. Whether you’re interested in research, quality control, regulatory affairs, or sales, Shriram Pharmacy College provides the knowledge and skills needed for a successful and rewarding career in pharmacy.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
#pharmacy#public health#pharmacist#hospital#youtube#shriram nursing college#shriram medical college#medicine#online pharmacy#shriram pharmacy college
0 notes
Text
Oncology Market Rising Trends, Demand and Future Scope 2024 to 2031
Leading market research firm SkyQuest Technology Group recently released a study titled 'Oncology Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Oncology report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Oncology Market extensively demonstrates existing and upcoming opportunities, profitability, revenue growth rates, pricing, and scenarios for recent industry analysis.
The research analysis on the global Oncology Market report 2024 offers a close watch on top industry rivals along with briefings on their company profiles, strategical surveys, micro as well as macro industry trends, futuristic scenarios, analysis of pricing structure, and an all-encompassing overview of the Oncology Market circumstances in the forecast period between 2024 and 2031. The global Oncology Market is a dynamic and rapidly evolving sector, encompassing the development, production, and distribution. This market is essential for improving global market and driving economic growth through innovation and industry advancements.
Market Growth
The Oncology Market has experienced robust growth over the past decade and is projected to continue expanding. Oncology Market size was valued at USD 286.04 billion in 2022 and is poised to grow from USD 309.3 billion in 2023 to USD 581.25 billion by 2031, growing at a CAGR of 8.2% in the forecast period (2024-2031). This growth is driven by several factors, including an aging global population, increasing prevalence of advancements in technology, and rising global expenditure.
Chance to get a free sample @ https://www.skyquestt.com/sample-request/oncology-market
Detailed Segmentation and Classification of the report (Market Size and Forecast - 2031, Y-o-Y growth rate, and CAGR):
The Oncology Market can be segmented based on several factors, including product type, application, end-user, and distribution channel. Understanding these segments is crucial for companies looking to target specific markets and tailor their offerings to meet consumer needs.
Cancer Diagnostics & Treatment
Cancer Diagnostics {Tumor Biomarker Test, Imaging, Biopsy, Liquid Biopsy, Immunohistochemistry, In Situ Hybridization}, Cancer Treatment {Chemotherapy, Targeted Therapy, Immunotherapy, Hormonal Therapy}
Cancer Type
Lung Cancer, Prostate Cancer, Colon & Rectal Cancer, Gastric Cancer, Esophageal Cancer, Liver Cancer, Breast Cancer
End-Use
Hospitals, Diagnostic Laboratories, Diagnostic Imaging Centers, Academia, Specialty Clinics
Get your customized report @ https://www.skyquestt.com/speak-with-analyst/oncology-market
Following are the players analyzed in the report:
Roche Holding AG
Novartis International AG
Bristol-Myers Squibb Company
Pfizer Inc.
Merck & Co., Inc.
AstraZeneca PLC
Johnson & Johnson
Sanofi S.A.
Takeda Pharmaceutical Company Limited
Amgen Inc.
AbbVie Inc.
Eli Lilly and Company
Gilead Sciences, Inc.
Celgene Corporation (a subsidiary of Bristol-Myers Squibb Company)
Bayer AG
Biogen Inc.
Daiichi Sankyo Company, Limited
Astellas Pharma Inc.
Eisai Co., Ltd.
Teva Pharmaceutical Industries Ltd.
Regional Analysis
1. North America:
- The United States and Canada dominate the North American Oncology Market. The U.S. is the largest market globally, driven by advanced global infrastructure, high R&D investments, and significant Oncology consumption.
2. Europe:
- Europe is a significant player, with major Oncology Markets in Germany, France, and the United Kingdom. The region benefits from strong regulatory frameworks, high industry standards, and a robust R&D sector.
3. Asia-Pacific:
- This region is experiencing rapid growth, with countries like China and India leading the charge. Factors such as increasing industry access, growing middle-class populations, and expanding Oncology manufacturing capabilities contribute to this growth.
4. Latin America:
- Brazil and Mexico are key markets in Latin America. Growth in this region is driven by rising industry needs, increasing investments in industry infrastructure, and a growing demand for affordable medications.
5. Middle East and Africa:
- The Oncology Market in this region is expanding due to rising market spending, increased prevalence of diseases, and improvements in Market infrastructure, although the market is relatively smaller compared to other regions.
Future Outlook
The Oncology Market is poised for continued growth driven by technological advancements, expanding global market access, and increasing global industry needs. As the industry adapts to evolving challenges and seizes emerging opportunities, it is likely to see ongoing innovation and expansion, contributing significantly to global health and economic development.
Buy your full report: https://www.skyquestt.com/buy-now/oncology-market
0 notes
Text
Antibiotics Market: Combating Bacterial Infections in a New Era of Medicine
The Antibiotics market plays a crucial role in global healthcare by providing treatments that combat bacterial infections. As antibiotic resistance rises, the market is witnessing both challenges and innovations, making it essential for healthcare providers and decision-makers to stay informed. This article explores the latest trends, market segmentation, key growth drivers, and leading companies in the antibiotics industry.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/antibiotics-market
Market Overview
According to SkyQuest’s Antibiotics Market report, the global antibiotics market is valued at USD 41.4 billion in 2023 and is projected to grow at a CAGR of 2.5% during the forecast period. This growth is propelled by the increasing incidence of infectious diseases, the rising global population, and advancements in antibiotic development.
Market Segmentation
By Product Type:
Beta-Lactam and Beta-Lactamase Inhibitors: Widely used for treating a variety of bacterial infections, including respiratory and urinary tract infections.
Quinolones: Effective against a broad spectrum of bacterial pathogens, commonly used for respiratory infections.
Macrolides: Used primarily for respiratory infections and soft tissue infections.
Aminoglycosides: Target severe bacterial infections, especially in hospital settings.
Tetracyclines: Commonly used for skin infections, respiratory infections, and sexually transmitted diseases.
Others: Includes specialized antibiotics for niche infections or resistant bacterial strains.
By Spectrum of Activity:
Broad-Spectrum Antibiotics: Effective against a wide variety of bacteria, frequently prescribed for undiagnosed infections.
Narrow-Spectrum Antibiotics: Target specific bacteria, reducing the risk of resistance and minimizing side effects.
By Route of Administration:
Oral Antibiotics: Convenient for outpatient care and commonly prescribed for less severe infections.
Injectable Antibiotics: Typically used in hospitals for serious infections or when rapid treatment is needed.
Topical Antibiotics: Applied directly to the skin to treat local infections or prevent wound infections.
By End-User:
Hospitals & Clinics: Major users of antibiotics, especially for severe infections requiring immediate attention.
Pharmacies: Provide antibiotics for outpatient care, over-the-counter purchases, or prescription-based sales.
Research Laboratories: Focus on the development of new antibiotics and testing their efficacy against resistant bacteria.
Get more info at: — https://www.skyquestt.com/report/antibiotics-market
Key Growth Drivers
Rising Prevalence of Infectious Diseases: The continuous rise in bacterial infections worldwide, especially in developing regions, drives the demand for antibiotics.
Antibiotic Resistance: The emergence of drug-resistant bacteria necessitates the development of new antibiotics, boosting market growth.
Government Support and Initiatives: Various global health organizations and governments are actively funding research to combat antibiotic resistance.
Technological Advancements: Progress in biotechnology and genetic research is aiding the development of novel antibiotics.
Leading Companies in the Market
SkyQuest’s report highlights several key players in the Antibiotics market, including:
Merck & Co., Inc., Allergan plc (AbbVie), GlaxoSmithKline plc., Pfizer Inc., Novartis AG, Sanofi S.A., AstraZeneca plc, Johnson & Johnson, Teva Pharmaceutical Industries Ltd., Mylan N.V., Eli Lilly and Company, Bayer AG, Bristol-Myers Squibb Company, AbbVie Inc., Astellas Pharma Inc., Boehringer Ingelheim GmbH, Daiichi Sankyo Company, Limited, Roche Holding AG, Sun Pharmaceutical Industries Ltd., Takeda Pharmaceutical Company Limited.
Challenges and Opportunities
The antibiotics market faces significant challenges, including antibiotic resistance and stringent regulatory approval processes. However, these challenges also create opportunities for innovation in developing next-generation antibiotics and alternative therapies.
Future Outlook
The future of the antibiotics market is shaped by ongoing efforts to combat antibiotic resistance and the development of advanced, targeted therapies. As companies invest in research and innovation, the market is expected to see steady growth. For more detailed insights and strategic recommendations, refer to SkyQuest’s in-depth Antibiotics Market report.
The Antibiotics market remains a cornerstone of global healthcare, with ongoing advancements aimed at tackling bacterial infections and resistance. Decision-makers who focus on innovation and the development of new antibiotics will stay at the forefront of this critical healthcare sector. For more in-depth insights and emerging trends, consult SkyQuest's Antibiotics Market report.
0 notes
Text
The Bioassay Services Market Is Anticipated To Witness High Growth Owing To Increasing R&D Investment In Pharmaceutical And Biotechnology Industries

The Bioassay Services Market facilitates testing of biomolecule interactions and activities through contracted or outsourced research organizations. Bioassays help evaluate the potency and purity of biotherapeutics, biosimilars, and other biological products in a cost-effective manner. They provide data to support drug development, clinical trials, quality control, and regulatory submissions. As bioassay testing requires specialized expertise and infrastructure, many pharmaceutical and biotech companies prefer outsourcing such services to Contract Research Organizations (CROs).
Bioassays play a vital role in the development and manufacture of biologics such as antibodies, proteins, vaccines, and cell and gene therapies. They aid in determining the biological activity, target specificity, and pharmacological effects of these complex molecules. Through cell-based or receptor-binding assays, bioassays quantify the biological potency of biotherapeutics. This helps ensure their efficacy and safety. They also support characterization, comparability assessment, stability monitoring, and release testing of biologics. As more biologics receive regulatory approvals and enter the market, demand for bioassay services is surging. Advancements in cell-based assays further facilitate high-throughput screening of biotherapeutics.
The Bioassay Services Market is estimated to be valued at US$ 340 Mn in 2024 and is expected to exhibit a CAGR of 30.% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the bioassay services are CCRM,Nexelis,Pacific BioLabs,PPD Laboratories,WuXi Advanced Therapies. They provide a range of bioassay testing and support services to pharmaceutical and biotech clients.
Growing demand for biologics and biosimilars is driving the need for extensive bioassay testing during drug development and manufacturing. As biologics development increases in complexity, requirements from regulatory agencies are also becoming stringent necessitating optimized bioassay data packages.
Advancements in cell-based assays and automation have enabled development of more predictive and high-throughput bioassays systems. 3D cell culture-based assays now better mimic human physiology aiding in selection of safer and more efficacious biotherapeutic candidates.
Market Trends
Increased outsourcing of bioassay testing- Pharma companies prefer outsourcing bioassay activities to specialized CROs to leverage their expertise and infrastructure and focus on core drug development functions. This is expected to boost demand for outsourced bioassay services.
Adoption of non-animal testing methods- Restrictions on animal testing and emphasis on alternative methods by regulators will promote use of human cells and tissue-based bioassay platforms over animal models where applicable. This supports the 3Rs (Replacement, Reduction, and Refinement) principle in drug testing.
Market Opportunities
Emerging biotherapeutic modalities - Cell and gene therapies, RNA/DNA based medicines, biologics for chronic diseases offer significant opportunities as they require extensive biocharacterization and testing during development and lot release.
Partnerships for specialized testing- Demand for niche bioassays for toxins, viruses, vaccines etc. presents opportunities for expert CROs to partner with pharma innovators working in such complex domains.
Impact Of COVID-19 On Bioassay Services Market Growth
The ongoing COVID-19 pandemic has significantly impacted the Bioassay Services Market. During the initial outbreak, the restrictions imposed by governments worldwide led to temporary closures of contract research organization facilities and delays in clinical trial activities. This affected the demand for bioassay services from pharmaceutical and biotechnology companies engaged in drug development. However, as the pandemic progressed, bioassay services emerged as an essential part of vaccine and therapeutic development efforts against COVID-19. Many CROs ramped up their bioassay capabilities to support the surge in COVID-19 related research. This provided some buffer to the market from the adverse impact witnessed initially. Going forward, as vaccine development and therapeutic discovery continues at an accelerated pace, the demand for bioassay services is expected to steadily rise over the forecast period.
The Asia Pacific region currently accounts for the largest share of the Bioassay Services Market in terms of value. Several factors have contributed to the concentration of market in Asia Pacific, primarily the presence of a large pool of clinical trial participants, availability of low-cost and high-quality bioassay services, and growing pharmaceutical outsourcing to countries such as China and India. Within the region, China and India are the top locations for outsourcing of drug discovery and clinical research activities, bolstering service provider revenue. Moreover, investments by governments to promote life sciences R&D have spurred market growth. Going forward, emerging Asian countries with improving regulatory compliance may attract higher outsourcing, sustaining APAC's leading position.
At the same time, the North American region has emerged as the fastest growing market for bioassay services globally. Pre-COVID, growth drivers included rising R&D expenditure of biopharma players based in the US and Canada, well-established pharmaceutical infrastructure, and increasing adoption of outsourcing models. However, the pandemic has significantly accelerated regional market expansion. Heightened research focus on COVID-19 therapeutics and vaccines has boosted service demand. Moreover, the region is home to prominent global CROs with expanded pandemic-relevant testing capabilities. Considering these factors, North America is anticipated to continue outpacing other regions in terms of Bioassay Services Market growth over the forecast period.
Get more insights on this topic: https://www.pressreleasebulletin.com/bioassay-services-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-cell-based-assay-techniques/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
What Are The Key Data Covered In This Bioassay Services Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Bioassay Services Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Bioassay Services Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Bioassay Services Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Bioassay Services Market vendors
FAQ’s
Q.1 What are the main factors influencing the Bioassay Services Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Bioassay Services Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Bioassay Services Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Bioassay Services Market Trend#Bioassay Services Market Size#Bioassay Services Market Information#Bioassay Services Market Analysis#Bioassay Services Market Demand
0 notes
Text
Industrial Adhesives & Coatings | chemline India Limited
CHEMLINE is certified as an International Organization of Standardization (ISO Certified) Company for “Quality Management System” standards “ISO 9001:2015”, “Environment Management System” standards “ISO 14001:2015” and “Occupational Health & Safety Management System” standards “OHSAS 45001:2018” & scope of supply detailed below.
CHEMLINE possesses a very modern and green manufacturing installation near Delhi with ‘State of the Art’ Facilities and Equipments. The emphasis of the company is on ‘Innovation’, and therefore the core of the company is its Research and Development Wing. It has a fully equipped modern laboratory staffed by dedicated, highly qualified and experienced scientists. This has been the major reason for development of such a wide range of products. Equally advanced, separate laboratories are there for Quality Control, Product Applications and their Testing.
Chemline India Limited — manufactures a complete range of world class Adhesives, Coatings for Paper, Packaging and Converting Industry, Hygiene Industry, Labelling Adhesives for Breweries, Distilleries and Pharma Industries & specialized Hot Melt Adhesives for vast range of applications, along with various downstream products like Self Adhesive, Label Stocks, Metallized Paper/ Paper Boards, Coated Papers, Industrial Tapes
Apart from being in the forefront of developing innovative technologies, Chemline is supporting the social causes to transform the lives of the poor and needy. To achieve this end, Chemline has established “Chemline Charitable Foundation” for regular and sustainable support.
0 notes
Text
What is a Steam Sterilizer used for?
A Steam Sterilizer, also known as an autoclave, is a crucial piece of equipment used in various industries to achieve sterilization, particularly in pharmaceutical manufacturing, healthcare, and laboratories. INSTECH PHARMA offers advanced steam sterilizers designed to ensure the highest standards of hygiene and safety.

Key Uses of a Steam Sterilizer:
1. Sterilization of Equipment and Instruments: Steam sterilizers are primarily used to sterilize medical instruments, laboratory equipment, surgical tools, and glassware. The high-pressure steam effectively kills bacteria, viruses, fungi, and spores, ensuring that the items are free from any harmful microorganisms.
2. Sterilization of Pharmaceutical Products: In the pharmaceutical industry, steam sterilizers are used to sterilize products like injectable solutions, surgical dressings, and certain medications. This ensures that the products are safe for patient use and meet stringent regulatory standards.
3. Preparation of Culture Media: In laboratories, steam sterilizers are used to sterilize culture media, which is crucial for microbiological testing and research. Sterilizing the media prevents contamination and ensures accurate results in experiments.
4. Waste Sterilization: Steam sterilizers are also used to sterilize medical and laboratory waste before disposal. This reduces the risk of spreading infections and ensures that waste is safely handled and disposed of.
INSTECH PHARMA's steam sterilizers are designed for efficiency, reliability, and safety, making them an essential tool for maintaining sterile environments and ensuring product integrity in the pharmaceutical and healthcare sectors.
For more details, please contact us!
Website :- https://www.pharmaceutech.com/
Contact No. :- +91–9873069138, +91–8896456000
Email :- http://cssd.tecnik.de@http://gmail.com, [email protected]
0 notes
Text
Precision Medicine Market Forecast to Grow at 11.4% CAGR from 2024 to 2031 | SkyQuest Technology

SkyQuest projects that the global Precision Medicine Market will attain a value of USD 75.30 billion by 2031, with a CAGR of 11.4% during the forecast period (2024-2031). The precision medicine market will grow exponentially through rapid genomic research, biotechnology, and data analytics. With growing incidences of chronic diseases like cancer and cardiovascular conditions, there is an impending requirement for personalized treatment plans that precision medicine offers. The basis of precision medicine is, therefore, a unique concept where two individuals infected by the same disease do not necessarily have to respond to the sickness in the same way physically.
Download a detailed overview:https://www.skyquestt.com/sample-request/precision-medicine-market
Hospitals & Clinics Dominate the Market Share by Offering Advanced and Improved Diagnostic Treatments
The hospitals and clinics segment forms the dominant end-user landscape within the precision medicine market, as hospitals are the very places that provide acute care to patients requiring advanced diagnostics and personalized treatments. Hospitals are better equipped and skilfully adept at adopting complex genome-based tests and molecular diagnostics. Substantial investments by healthcare systems and government initiatives in integrating precision medicine into standard clinical practices are also predicted to dominate the segment by improving patient outcomes and raising healthcare efficiency.
Home Care Settings to be the Fastest Growing as Individual Patient Care Becomes More Prevalent
Home care settings are one of the most rapidly growing areas in precision medicine. Technologies that mean tests can be conducted away from hospitals have been the driver of this growth, where care is then given to patients as an individual—something which has not been possible other than in hospitals. The drivers like portable diagnostic instruments and companion diagnostics, together with remote monitoring systems and telemedicine services are driving the growth of this market.
North America Dominates the Global Market Owing to the Rising Number of Cancer Patients
North American precision medicine market is expected to hold major market share in this forecasted period. Cancer being the leading cause of death in this region, have underscored the need for more enhanced treatment modalities such as precision medicine. The region has established key facilities for genomic studies and individualized treatment. The degree of awareness regarding precision medicine is very high, and the emphasis on the ever-increasing need to provide patients with individualized cancer treatments favours the growth of the market.
Precision Medicine Market Insights:
Drivers
Rising Incidence of Chronic and Genetic Diseases
Advancements in Genomic Technologies
Patient-Centric Approach and Preventive Medicine
Restraints
High Cost of Precision Medicine Technologies
Data Privacy and Security Concerns
Interpretation Difficulties and Clinical Relevance
Prominent Players in Precision Medicine Market
Illumina, Inc.
Thermo Fisher Scientific Inc.
Novartis AG
Abbott Laboratories
Agilent Technologies
Almac Group
Amgen Inc.
Astellas Pharma Inc.
AstraZeneca PLC
Bio-Rad Laboratories, Inc.
Key Questions Answered in Precision Medicine Market Report
What is Precision Medicine?
How big is the Precision Medicine Market?
At what value will the Precision Medicine Market grow during the forecasted period?
This report provides the following insights:
Analysis of key drivers (growing demand for better data security, rising adoption of Blockchain and IoT technologies, increasing use of decentralized systems in different industry verticals), restraints (interoperability issues with legacy systems, complexities in integration with existing infrastructure, concerns regarding scalability of gas cleaning technologies systems), and opportunities (growing demand for data monetization, rising demand for transparent yet trusted transactions), influencing the growth of precision medicine market.
Market Penetration: All-inclusive analysis of product portfolio of different market players and status of new product launches.
Product Development/Innovation: Elaborate assessment of R&D activities, new product development, and upcoming trends of the precision medicine market.
Market Development: Detailed analysis of potential regions where the market has potential to grow.
Market Diversification: Comprehensive assessment of new product launches, recent developments, and emerging regional markets.
Competitive Landscape: Detailed analysis of growth strategies, revenue analysis, and product innovation by new and established market players.
About Us:
SkyQuest is an IP focused Research and Investment Bank and Accelerator of Technology and assets. We provide access to technologies, markets and finance across sectors viz. Life Sciences, CleanTech, AgriTech, NanoTech and Information & Communication Technology.
We work closely with innovators, inventors, innovation seekers, entrepreneurs, companies and investors alike in leveraging external sources of R&D. Moreover, we help them in optimizing the economic potential of their intellectual assets. Our experiences with innovation management and commercialization have expanded our reach across North America, Europe, ASEAN and Asia Pacific.
Contact:
Mr. Jagraj Singh
Skyquest Technology
1 Apache Way,
Westford,
Massachusetts 01886
USA (+1) 351-333-4748
Email: [email protected]
Visit Our Website: https://www.skyquestt.com/
#Precision Medicine Market Size#Precision Medicine Market Share#Precision Medicine Market Trends#Precision Medicine Market Forecast#Precision Medicine Market News#Precision Medicine Market Analysis
0 notes
Text
Certified Clinical Research Professional and Certified Pharma Engineering Professional: Advancing Healthcare Through Expertise
In the ever-evolving landscape of healthcare and pharmaceutical industries, the roles of Certified Clinical Research Professionals and Certified Pharma Engineering Professionals have become increasingly crucial. These highly skilled individuals play pivotal roles in advancing medical research, drug development, and ensuring the highest standards of patient care. In this comprehensive article, we will explore the significance of these certifications, their impact on the industry, and why they are essential for professionals seeking to excel in their careers.
The Importance of Certification in Clinical ResearchClinical research forms the backbone of medical advancements, paving the way for groundbreaking treatments and therapies. As the field continues to grow in complexity, the demand for certified professionals has skyrocketed. A Certified Clinical Research Professional (CCRP) possesses the knowledge and skills necessary to navigate the intricate world of clinical trials, regulatory compliance, and patient safety.
Key Responsibilities of a CCRP
CCRPs are entrusted with a wide array of responsibilities that directly impact the success and integrity of clinical studies. These include:
1. Protocol Development: Crafting comprehensive research protocols that adhere to ethical guidelines and regulatory requirements.
2. Study Coordination: Overseeing the day-to-day operations of clinical trials, including patient recruitment and data collection.
3. Regulatory Compliance: Ensuring all research activities comply with local, national, and international regulations.
4. Data Management: Implementing robust systems for data collection, analysis, and reporting.
5. Ethical Considerations: Safeguarding the rights and well-being of study participants throughout the research process.
By obtaining CCRP certification, professionals demonstrate their commitment to excellence and their ability to uphold the highest standards in clinical research.
The Role of Certified Pharma Engineering Professionals
While clinical research focuses on the development and testing of new treatments, pharmaceutical engineering is concerned with the design, production, and distribution of pharmaceutical products. A Certified Pharma Engineering Professional (CPEP) plays a critical role in ensuring the quality, safety, and efficacy of medications that reach patients worldwide.

Key Areas of Expertise for CPEPs
Certified Pharma Engineering Professionals are well-versed in various aspects of pharmaceutical manufacturing and quality control. Their expertise encompasses:
1. Process Design: Developing efficient and scalable manufacturing processes for pharmaceutical products.
2. Quality Assurance: Implementing rigorous quality control measures to ensure product safety and consistency.
3. Facility Design: Creating state-of-the-art pharmaceutical manufacturing facilities that meet regulatory standards.
4. Technology Integration: Incorporating cutting-edge technologies to enhance production efficiency and product quality.
5. Regulatory Compliance: Ensuring all manufacturing processes adhere to Good Manufacturing Practices (GMP) and other regulatory requirements.
The CPEP certification validates a professional's proficiency in these critical areas, making them invaluable assets to pharmaceutical companies and research institutions.
The Synergy Between Clinical Research and Pharmaceutical Engineering
While CCRPs and CPEPs operate in distinct domains, their roles are intrinsically linked in the grand scheme of healthcare advancement. The collaboration between these two specialties is essential for bringing new treatments from the laboratory to the patient's bedside.
Bridging the Gap: From Research to Production
Clinical research professionals provide invaluable insights into the efficacy and safety of new drugs through rigorous clinical trials. This data is then utilized by pharmaceutical engineers to develop scalable production processes that maintain the drug's integrity and effectiveness. This seamless integration of expertise ensures that promising treatments can be efficiently manufactured and distributed to those in need.
The Future of Healthcare: Driving Innovation Through Expertise
As we look to the future, the roles of Certified Clinical Research Professionals and Certified Pharma Engineering Professionals will continue to evolve and expand. The integration of advanced technologies, such as artificial intelligence and big data analytics, will reshape the landscape of drug discovery, clinical trials, and pharmaceutical manufacturing.
Emerging Trends and Opportunities
1. Personalized Medicine: CCRPs and CPEPs will play crucial roles in developing and manufacturing tailored treatments based on individual patient profiles.
2. Digital Health Integration: The incorporation of wearable devices and remote monitoring technologies in clinical trials will require new skillsets from certified professionals.
3. Sustainable Practices: Pharmaceutical engineers will focus on developing eco-friendly manufacturing processes and reducing the industry's environmental impact.
4. Global Collaboration: Increased international cooperation in clinical research and drug development will necessitate a deep understanding of diverse regulatory environments.
By staying at the forefront of these developments, certified professionals will continue to drive innovation and improve patient outcomes worldwide.
Conclusion: Empowering the Future of Healthcare
The certifications of Clinical Research Professional and Pharma Engineering Professional represent more than just academic achievements. They embody a commitment to excellence, patient safety, and the advancement of medical science. As the healthcare industry continues to evolve, these certified professionals will remain at the forefront, shaping the future of medicine and improving lives around the globe.
0 notes