#PostApproval
Explore tagged Tumblr posts
Text
Brief on "Psychedelic-Assisted Group Therapy: A Systematic Review"
Paper
Trope, Alexander, Brian T. Anderson, Andrew R. Hooker, Giancarlo Glick, Christopher Stauffer, and Joshua D. Woolley. “Psychedelic-Assisted Group Therapy: A Systematic Review.” Journal of Psychoactive Drugs 51, no. 2 (March 15, 2019): 174–88. https://doi.org/10.1080/02791072.2019.1593559.
Abstract
Contemporary research with classic psychedelic drugs (e.g., lysergic acid diethylamide (LSD) and psilocybin) is indebted to the twentieth-century researchers and clinicians who generated valuable clinical knowledge of these substances through experimentation. Several recent reviews that highlight the contributions of this early literature have focused on psychedelic-assisted individual psychotherapy modalities. None have attempted to systematically identify and compile experimental studies of psychedelic-assisted group therapy. In therapeutic settings, psychedelics were often used to enhance group therapy for a variety of populations and clinical indications. We report on the results of a systematic review of the published literature in English and Spanish on psychedelic-assisted group therapies. Publications are characterized by their clinical approach, experimental method, and clinical outcomes. Given the renewed interest in the clinical use of psychedelic medicines, this review aims to stimulate hypotheses to be tested in future research on psychedelic-assisted psychotherapy, group process, and interpersonal functioning.
Table
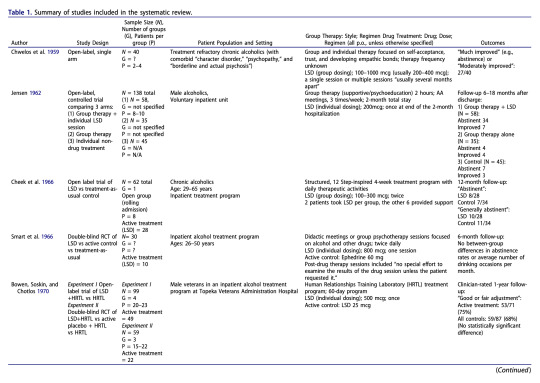
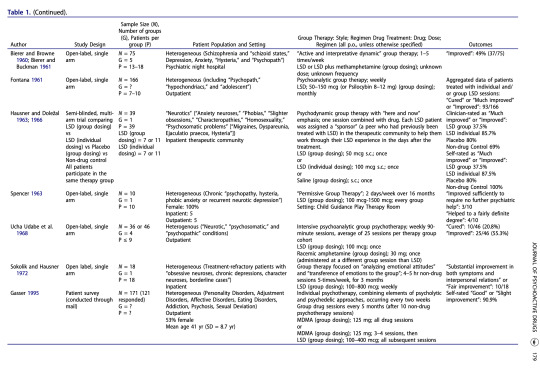
Annotations
“Group psychedelic use in nonclinical contexts (e.g., ayahuasca or peyote rituals) is beyond the scope of this review” (Trope et al., 2019, p. 174)
“As psychedelic medicines enter pivotal trials in the United States and Europe, the prospect of postapproval clinical innovation with different administration modalities, including group therapy, arises.” (Trope et al., 2019, p. 175)
“12 studies met inclusion criteria, which required that the article contain some description of group methods, demographic and diagnostic information, and quantified outcome data.” (Trope et al., 2019, p. 175)
“It is notable, however, that the two positive studies —as well as the single uncontrolled study for alcoholism (Chwelos et al. 1959)—used either a 12-Step model or group therapy specifically adapted for psychedelic administration, while the studies with null findings did not use these elements.” (Trope et al., 2019, p. 185)
“The AmericanGroup Psychotherapy Association has made available evi-dence-based clinical practice guidelines and validated mea-sures to assess interpersonal functioning and groupcohesion (Bernard et al. 2008; Krogel et al. 2013; Strauss,Burlingame, and Bormann 2008)” (Trope et al., 2019, p. 185)
“The range of clinical approaches used in these reviewed studies illustrates the complexity involved in designing future trials of psychedelic-assisted group therapy. The optimal number of group members, drug dose, sequencing and number of group sessions, and type of group therapy are among the variables to consider. As opposed to the group administration of psychedelics that was common in early research, researchers today will likely, at least at first, use groups solely for the preparation and integration of individual psychedelic administration sessions.” (Trope et al., 2019, p. 185)
“Because preparatory and integration sessions entail the majority of total therapy hours a patient receives in modern protocols, delivering these sessions in a group format may improve the cost-benefit and time efficiency of research and clinical operations (Villapiano 1998).” (Trope et al., 2019, p. 185)
Personal Memo
Although I have heard that 1960s research was quite wild, it is still shocking to read the specific descriptive lines directly: “Individual drug sessions took place in a single room with patients physically restrained to a bed with a Posey belt during the duration of peak drug effect” (Smart et al., 1966). “Pilot study of 75 patients receiving LSD or LSD plus methamphetamine in a group setting at a psychiatric night hospital that served as a partial hospitalization program” (Bierer and Browne, 1960).
In my opinion, the efficacy of psychedelic group therapy lies in the comparison to individual psychedelic therapy, but there were no studies addressing this comparison in the reviewed paper. Moreover, as the authors stated, there can be various ways group therapies can be conducted (“The range of clinical approaches used in these reviewed studies illustrates the complexity involved in designing future trials of psychedelic-assisted group therapy”). The optimal design for psychedelic group therapy would be definitely challenging but interesting.
There have been some psychedelic group therapy sessions, including ayahuasca experiments conducted since 2018, which are, of course, excluded in this paper since it was published in 2018. A new review paper summarizing such recent research would be welcomed.
0 notes
Link
Postapproval pregnancy safety studies (or studies that focus on medication use after approval) can help guide clinical practice and provide useful information for product labeling. #BioTech #science
0 notes
Text
they have names like [redacted]_3.2.P.8.2 Postapproval Stability Protocol
looooooove when someone sends me an email telling me to send an email to someone else that we both already have regular contact with to ask them a question that they could easily ask themself.
12 notes
·
View notes
Text
Post Approval Monitoring IACUC | Key Solutions Inc

Key Solutions offers Post-Approval Monitoring which facilitates regular inspection of protocols and enables immediate correction if required.
After the approval of a protocol, an organization needs to continuously monitor its activities to confirm that they are in line with the compliance application. If there are any discrepancies, the inspection team should either prescribe corrective action or, in extreme cases, suspend the research protocol. Our PAM module streamlines this entire process.
For more information please visit here: https://www.keyusa.com/post-approval-monitoring.html
0 notes
Text
CardioMEMS Postapproval Study Linked to Reduction in Heart Failure Hospitalizations at 1 Year - TCTMD
CardioMEMS Postapproval Study Linked to Reduction in Heart Failure Hospitalizations at 1 Year – TCTMD
The device is best used in patients already on OMT who are comfortable submitting their data and communicating with their doctors, researcher says.
NEW ORLEANS, LA—The performance of the CardioMEMS HF System (St. Jude Medical/Abbott) in the after-market setting is associated with decreased heart-failure hospitalizations across all patient sex, race, and ejection fraction categories with few…
View On WordPress
0 notes
Text
Belimumab and Postmarketing Experience
Belimumab and Postmarketing Experience
In this article, we will discuss Belimumab and Postmarketing Experience. So, let’s get started. Belimumab and Postmarketing ExperienceThe following adverse reactions have been identified during postapproval use of Belimumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal…
View On WordPress
0 notes
Text
The long-term outcomes of breast implants studied
The biggest investigation of bosom inserts to date furnishes ladies with some significant data in regards to uncommon yet genuine unfavorable results.A bosom embed is a prosthesis used to change the size or state of a lady's bosom.A few ladies use bosom inserts to feel more good in their bodies, while others pick bosom remaking to reproduce a characteristic looking bosom later a mastectomy Breast Enlargement Injections in Islamabad
A specialist plays out a mastectomy to eliminate bosom malignant growth cancers.The two generally famous and Food and Drug Administration (FDA)- approvedTrusted Source bosom inserts (characterized by their filler material) are the saline arrangement and the silicone gel.
The saline embed utilizes a silicone shell loaded up with a clean saline arrangement during medical procedure, while the silicone embed utilizes a silicone shell prefilled with thick silicone gel.
The administrative history of bosom inserts: The FDA have not forever been agreeable to silicone bosom inserts. In 1992, the FDA concludedTrusted Source that the information accessible at the time were not adequate to help endorsement.
The choice didn't affect gel-filled bosom inserts for patients going through bosom reproduction; individuals believed these inserts to be investigational clinical gadgets, to be additionally examined and clinically considered.In the mid 2000s, the FDA supported saline-filled bosom inserts for expansion in ladies who are matured 18 and more seasoned and for reproduction in ladies of all ages.
The endorsement for silicone gel-filled bosom inserts, all things being equal, was legitimate for a set number of increase, reproduction, and amendment patients at a predetermined number of destinations.
Then, at that point, in 2006, the FDA endorsed silicone gel-filled inserts from two producers. Interestingly, silicone gel-filled bosom inserts were accessible for increase, just as recreation and modification. Producers should direct postapproval studies to check security and adequacy.
Revealing antagonistic results: Later the endorsement of silicone bosom embeds, the conversation into security stayed open. The FDA directed a few huge postapproval studies to follow various individuals with bosom inserts, yet up to this point, no one had completely dissected the data set.
"We are sharing basic data on difficulty rates and uncommon relationship with foundational hurts. This information gives ladies significant security data about silicone bosom inserts to have genuine assumptions and to assist them with picking common decency for them," clarifies Dr. Mark W. Clemens and partners, of the University of Texas MD Anderson Cancer Center in Houston.
#Breast Enlargement Injections#Breast Enlargement Injections treatment#Breast Enlargement Injections in Islamabad
0 notes
Text
Pain O Soma | Buy pain O Soma 350, 500 mg
What is Soma and how could it be utilized?
Pain O Soma is a physician recommended medication used to treat the side effects of musculoskeletal torment. Soma might be utilized alone or with different meds.
Soma has a place with a class of medications called Skeletal Muscle Relaxants.
It isn't known whether Soma is protected and viable in kids more youthful than 16 years of age. Soma isn't suggested for geriatric patients.
What are the conceivable symptoms of Soma?
Soma might cause genuine incidental effects including:
seizure (spasms),
fomentation,
pipedreams,
fever,
perspiring,
shuddering,
quick pulse,
muscle solidness,
jerking,
loss of coordination,
sickness,
regurgitating, and
looseness of the bowels
Move clinical assistance immediately, in the event that you have any of the side effects recorded previously.
The most well-known symptoms of Soma include:
sleepiness,
dazedness, and
cerebral pain
Tell the specialist in the event that you have any incidental effect that pesters you or that doesn't disappear.
These are not every one of the conceivable results of Soma. For more data, ask your PCP or drug specialist.
Portrayal
Pain O Soma 350 (carisoprodol) Tablets are accessible as 250 mg and 350 mg round, white tablets. Carisoprodol is a white, glasslike powder, having a gentle, trademark scent and a harsh taste. It is marginally dissolvable in water; unreservedly solvent in liquor, in chloroform, and in CH3)2CO; and its dissolvability is essentially free of pH. Carisoprodol is available as a racemic combination. Artificially, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3propanediol dicarbamate and the atomic equation is C12H24N2O4, with a sub-atomic load of 260.33. The primary recipe is:
SOMA (carisoprodol) Structural Formula Illustration
Different fixings in the SOMA drug item incorporate alginic corrosive, magnesium stearate, potassium sorbate, starch, and tribasic calcium phosphate.
Signs and Dosage
Signs
Pain O Soma 500 is shown for the help of inconvenience related with intense, excruciating musculoskeletal conditions in grown-ups.
Constraint Of Use
SOMA ought to just be utilized for brief periods (up to half a month) in light of the fact that sufficient proof of adequacy for more delayed use has not been set up and on the grounds that intense, difficult musculoskeletal conditions are for the most part of brief span. [see DOSAGE AND ADMINISTRATION].
Dose AND ADMINISTRATION
The suggested portion of SOMA is 250 mg to 350 mg three times each day and at sleep time. The suggested most extreme span of SOMA use is up to half a month.
HOW SUPPLIED
Measurement Forms And Strengths
250 mg Tablets: round, curved, white tablets, recorded with SOMA 250
350 mg Tablets: round, curved, white tablets, recorded with SOMA 350
Capacity And Handling
250 mg Tablets: round, curved, white tablets, recorded with SOMA 250; accessible in containers of 100 (NDC 0037-2250-10) and jugs of 30 (NDC 0037-2250-30).
350 mg Tablets: round, curved, white tablets, recorded with SOMA 350; accessible in containers of 100 (NDC 0037-2001-01).
Capacity
Store at controlled room temperature 20° - 25°C (68° - 77°F).
Meda Pharmaceuticals Inc. , Somerset, New Jersey 08873-4120, IN-90H2-X1 Revised: Apr 2019
SLIDESHOW
Back Pain: 16 Back Pain Truths and Myths
See Slideshow
Incidental effects
Incidental effects
Clinical Studies Experience
Since clinical investigations are led under generally fluctuating conditions, antagonistic response rates saw in clinical investigations of a medication can't be straightforwardly contrasted with rates in the clinical investigations of one more medication and may not reflect rates saw practically speaking.
The information depicted beneath depend on 1387 patients pooled from two twofold visually impaired, randomized, multicenter, fake treatment controlled, one-week preliminaries in grown-up patients with intense, mechanical, lower back torment [see Clinical Studies]. In these examinations, patients were treated with 250 mg of SOMA, 350 mg of SOMA, or fake treatment three times each day and at sleep time for seven days. The mean age was around 41 years of age with 54% females and 46% guys and 74 % Caucasian, 16 % Black, 9% Asian, and 2% other.
There were no passings and there were no genuine unfriendly responses in these two preliminaries. In these two examinations, 2.7%, 2%, and 5.4%, of patients treated with fake treatment, 250 mg of SOMA, and 350 mg of SOMA, individually, suspended because of unfriendly occasions; and 0.5%, 0.5%, and 1.8% of patients treated with fake treatment, 250 mg of SOMA, and 350 mg of SOMA, separately, ended because of focal sensory system unfavorable responses.
Post-advertising Experience
The accompanying occasions have been accounted for during postapproval utilization of SOMA. Since these responses are accounted for willfully from a populace of unsure size, it isn't generally conceivable to dependably gauge their recurrence or build up a causal relationship to medicate openness.
Cardiovascular
Tachycardia, postural hypotension, and facial flushing [see OVERDOSAGE].
Focal Nervous System
Sleepiness, unsteadiness, dizziness, ataxia, quake, unsettling, peevishness, cerebral pain, burdensome responses, syncope, sleep deprivation, and seizures
Gastrointestinal
Sickness, retching, and epigastric uneasiness.
Hematologic
Leukopenia, pancytopenia
Medication Interactions
Medication INTERACTIONS
CNS Depressants
The calming impacts of SOMA and other CNS depressants (e.g., liquor, benzodiazepines, narcotics, tricyclic antidepressants) might be added substance. Thusly, alert ought to be practiced with patients who take more than one of these CNS depressants all the while. Associative utilization of SOMA and meprobamate, a metabolite of SOMA, isn't suggested
CYP2C19 Inhibitors And Inducers
Carisoprodol is processed in the liver by CYP2C19 to frame meprobamate [see CLINICAL PHARMACOLOGY]. Co-organization of CYP2C19 inhibitors, like omeprazole or fluvoxamine, with SOMA could bring about expanded openness of carisoprodol and diminished openness of meprobamate. Co-organization of CYP2C19 inducers, like rifampin or St. John's Wort, with SOMA could bring about diminished openness of carisoprodol and expanded openness of meprobamate. Low portion anti-inflamatory medicine likewise showed an enlistment impact on CYP2C19. The full pharmacological effect of these expected adjustments of openings as far as one or the other viability or security of SOMA is obscure.
Chronic drug use And Dependence
Soma contains carisoprodol, a Schedule IV controlled substance. Carisoprodol has been liable to manhandle, abuse, and criminal redirection for nontherapeutic use [see WARNINGS AND PRECAUTIONS].
Misuse
Maltreatment of carisoprodol represents a danger of overdosage which might prompt passing, CNS and respiratory melancholy, hypotension, seizures and different issues [see WARNINGS AND PRECAUTIONS and OVERDOSAGE]. Patients at high danger of SOMA misuse might incorporate those with delayed utilization of carisoprodol, with a background marked by substance addiction, or the individuals who use SOMA in blend with other manhandled drugs.
Physician recommended substance addiction is the purposeful non-helpful utilization of a medication, even once, for its remunerating mental impacts. Illicit drug use, which creates after rehashed substance addiction, is described by a powerful urge to take a medication notwithstanding hurtful results, trouble in controlling its utilization, giving a higher need to tranquilize use than to commitments, expanded resistance, and now and again actual withdrawal. Illicit drug use a lot enslavement are independent and unmistakable from actual reliance and capacity to bear (model, misuse or habit may not be joined by resilience or actual reliance).
Reliance
Resilience is the point at which a patient's response to a particular dose and focus is dynamically diminished without illness movement, requiring an increment in the measurement to keep up with the equivalent. Actual reliance is described by withdrawal indications after sudden stopping or a critical portion decrease of a medication. Both resistance and actual reliance have been accounted for with the drawn out utilization of SOMA. Detailed withdrawal manifestations with SOMA incorporate a sleeping disorder, retching, stomach cramps, migraine, quakes, muscle jerking, tension, ataxia, mental trips, and psychosis. Teach patients taking huge portions of SOMA or those taking the medication for a drawn out time frame to not suddenly stop SOMA
Other Pills
Vilafinil 200
Artvigil 150
0 notes
Link
Custom Kitchen Styles with Forevermark Kitchen Cabinets
0 notes
Link
0 notes
Link
Power BI has a really good analysis engine, you can analyze & calculate data to meet your needs. You can model data, add measured or estimated columns. And build a star schema on your own using Power BI’s In-Memory super quick engine.
0 notes
Link
#NurseryschoolfranchiseinIndia#playschoolsetupcost#montessorifranchise#internationalpreschoolfranchise
0 notes
Link
0 notes
Text
Palonosetron and Postmarketing Experience
Palonosetron and Postmarketing Experience
In this article, we will discuss Palonosetron and Postmarketing Experience. So, let’s get started. Palonosetron and Postmarketing Experience The following adverse reactions have been identified during postapproval use of another intravenous formulation of palonosetron HCl. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably…
View On WordPress
0 notes
Link
0 notes
Text
COVID19 Updates: 11/18/2020
Switzerland: All intensive care beds are now occupied in Switzerland.
Japan: Japan's daily coronavirus infections reaches 2,189, highest ever -NHK Ruling Party Lawmaker: Strongly Concerned About New Cases Of Coronavirus Now
Japan: Tokyo has just reported 493 new #COVID19 cases in one day. That´s the highest count ever.
South Korea: SEOUL, Nov 18 (Reuters) - South Korea reported 313 new daily COVID-19 cases on Wednesday, the highest number since August, as cluster infections continued to emerge from offices, medical facilities and small gatherings, prompting authorities to tighten social distancing rules.
South Dakota: Some COVID-positive patients don’t believe virus is real, even as they’re dying, SD nurse says LINK
Ethiopia: Knowledge and perceptions of COVID-19 among government employees in Ethiopia LINK
US: Track Santa 2020: NORAD making adjustments to live tracker due to COVID-19 LINK
Mississippi: State Health Officer warns of possible “explosive outbreak” of COVID-19 after holidays LINK
Texas: Our COVID-19 approach isn’t working. Here’s what Texas, Tarrant leaders must change LINK
Texas: Raging virus cases in Texas strain state health care system LINK
Texas: Pictures show doctors on frontline of Covid battle in Texas as state becomes new epicentre LINK
Texas: Thousands Line Up In Dallas For North Texas Food Bank’s ‘Largest Mobile Food Distribution Ever’ LINK
World: Acute kidney injury with COVID-19 shows potential for ‘lifetime’ impact LINK
North Dakota: I’m a contact tracer in North Dakota. The virus is so rampant that we gave up LINK
US: States ranked by COVID-19 test positivity rates: Nov. 18 LINK
World: Carnival Cruise Line cancels all sailings through January LINK
World: Postlicensure Evaluation of COVID-19 Vaccines LINK
World: Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US LINK
NYC: Covid: schools in New York will close from tomorrow. New York will close schools starting tomorrow following the increase in Covid-19 cases. Mayor Bill de Blasio announces it. For New York, which has the largest public school district in the United States, this is a blow in the context of the reopening decided in recent months
Texas: The last county free of COVID-19 on the US mainland now has cases. It’s in Texas LINK
US: Hospitals across US lack beds or equipment to treat COVID-19 patients LINK
World: COVID-19 Event Risk Assessment Planning Tool LINK
U.S.: COVID update: Daily death toll at highest level since May as number in ICU sets new record - New cases: 163,975 - Positivity rate: 10.6% (+0.2) - In hospital: 79,410 (+2,580) - In ICU: 15,350 (+499) - New deaths: 1,869
US: Texas, Florida and South Dakota governors refuse lockdowns as coronavirus resurges LINK
World: Facebook accused of forcing staff back to offices LINK
Australia: Far Deadlier Strain Of Coronavirus Discovered In South Australia LINK
Australia: COVID-19 strain in South Australia feared to be especially lethal LINK
0 notes