#Quality Management System in Manufacturing
Explore tagged Tumblr posts
Text
How a Quality Management System in Manufacturing Reduces Defects and Downtime
Introduction: The Growing Need for Efficient Manufacturing Systems
In the modern manufacturing landscape, minimizing defects and reducing downtime are two of the biggest challenges that manufacturers face. To stay competitive and meet regulatory requirements, manufacturers must adopt efficient and reliable solutions that enhance product quality, streamline operations, and reduce the risk of costly delays. One such solution is the implementation of a Quality Management System in Manufacturing, which plays a pivotal role in addressing these challenges.
A quality management system (QMS) in manufacturing enables companies to systematically monitor, control, and improve their production processes. With the help of QMS system software, manufacturers can identify inefficiencies, enhance quality control measures, and prevent defects, ultimately leading to reduced downtime and greater operational efficiency. This blog will explore how a quality management system in manufacturing reduces defects and downtime, ensuring higher productivity and greater customer satisfaction.
Understanding the Impact of Defects and Downtime in Manufacturing
Manufacturers are constantly striving to improve the efficiency of their operations while reducing the risks of defects and downtime. Product defects can result in higher rejection rates, customer dissatisfaction, increased rework, and potential legal and financial liabilities. On the other hand, downtime can lead to lost production time, missed deadlines, and wasted resources, all of which impact the company’s bottom line.
The implementation of a quality management system can significantly mitigate these risks. By providing structured workflows for quality assurance, monitoring, and continuous improvement, a QMS helps identify issues early, ensures compliance, and streamlines production processes. This ultimately leads to improved product quality, reduced operational costs, and more consistent production schedules.
How a Quality Management System in Manufacturing Reduces Defects
A quality management system in manufacturing plays a key role in reducing defects by providing the necessary framework for Quality Control and continuous improvement. Through effective monitoring, testing, and auditing, a QMS system ensures that any potential issues are identified and addressed early in the production process.
Enhanced Quality Control with QMS System Software
Quality control is a critical element of any manufacturing process, as it ensures that products meet established specifications and are free from defects. By implementing QMS System Software, manufacturers can automate quality control procedures, making it easier to track product quality, identify nonconformances, and initiate corrective actions. This leads to fewer defects and higher quality standards across production lines.
Automated quality control checks, such as real-time inspections, automated measurements, and data logging, allow manufacturers to detect and address defects before they reach the customer. By continuously monitoring product quality at every stage of production, manufacturers can reduce the risk of defective products leaving the facility.
Standardizing Processes to Prevent Defects
A QMS system in manufacturing helps standardize production processes by establishing clear guidelines for each stage of production. Standardized procedures ensure that all employees follow the same best practices, reducing variability and the likelihood of defects. This uniformity enhances product quality and minimizes the chances of human error, which is often a primary cause of defects in manufacturing.
Additionally, a QMS ensures that all raw materials, components, and finished products are tested against predefined quality standards, ensuring that they meet the necessary criteria before moving on to the next stage of production.
Reducing Downtime with Quality Management System
Downtime in manufacturing is expensive, both in terms of lost productivity and increased operational costs. In industries such as automotive, aerospace, and medical devices, where precision and efficiency are critical, unplanned downtime can have significant consequences. A quality management system in manufacturing helps reduce downtime by identifying bottlenecks, streamlining processes, and ensuring that equipment and processes are running at optimal efficiency.
Real-Time Monitoring and Preventive Maintenance
One of the most effective ways to reduce downtime is by using quality management system software to monitor equipment performance in real-time. Preventive maintenance, which is built into a QMS, ensures that machines are regularly inspected, maintained, and repaired before they fail. By detecting early signs of equipment malfunction and addressing them promptly, manufacturers can avoid unplanned downtime and extend the lifespan of machinery.
A QMS system also allows manufacturers to schedule maintenance and repairs at the most convenient times, minimizing production disruptions and reducing the likelihood of costly shutdowns.
Streamlined CAPA (Corrective and Preventive Actions)
When issues arise, a QMS system helps manage corrective and preventive actions (CAPA). If a defect is identified, the system tracks the root cause and implements corrective actions to fix the issue. By documenting these actions and preventing future occurrences, manufacturers can minimize disruptions and maintain consistent production schedules. This not only reduces downtime but also improves the overall quality and reliability of products.
Reducing Delays and Improving Workflow Efficiency
A QMS system in manufacturing improves workflow efficiency by automating tasks, simplifying reporting, and ensuring that all production processes are tracked in real-time. By streamlining these processes, a QMS reduces bottlenecks and improves communication between departments, ensuring that production flows smoothly without unnecessary delays.
With integrated workflows, manufacturers can also prioritize tasks, allocate resources more effectively, and resolve issues quickly, preventing downtime caused by miscommunication or disorganization.
Leveraging QMS Software for Continuous Improvement
A key component of a quality management system is its focus on continuous improvement. By collecting and analyzing data from various production processes, QMS system software helps manufacturers identify opportunities for improvement. Whether it’s optimizing a particular process, improving equipment efficiency, or enhancing workforce training, the ability to make informed, data-driven decisions leads to reduced defects and downtime over time.
Analyzing Data to Identify Areas for Improvement
QMS software collects and stores valuable data, such as defect rates, production speed, and machine performance, which can be used to identify areas where improvements are needed. By analyzing this data, manufacturers can pinpoint recurring issues, trends, and inefficiencies that contribute to downtime and defects. This proactive approach enables continuous improvement and helps optimize production processes to minimize defects and downtime.
Promoting a Culture of Quality and Accountability
Implementing a QMS system fosters a culture of quality and accountability throughout the organization. By promoting continuous improvement and empowering employees to take ownership of their tasks, manufacturers can ensure that everyone is invested in maintaining high-quality standards and reducing downtime. This collaborative approach helps build a more efficient and productive work environment.
Integrating QMS Software with Other Enterprise Systems
Integrating a quality management system with other enterprise systems, such as Enterprise Resource Planning (ERP) and Manufacturing Execution Systems (MES), further enhances its effectiveness in reducing defects and downtime. A unified system provides a seamless flow of information across departments, improving visibility, tracking, and coordination. This integration allows manufacturers to make data-driven decisions, optimize workflows, and respond quickly to quality issues.
Optimizing Resource Allocation and Planning
By integrating QMS software with other systems, manufacturers can optimize resource allocation, improve scheduling, and plan production activities more effectively. The integration ensures that resources, such as personnel and equipment, are allocated efficiently, minimizing idle time and reducing production delays.
Conclusion: The Future of Manufacturing with a Robust QMS
In today’s competitive manufacturing landscape, reducing defects and downtime is critical for maintaining profitability and meeting customer expectations. A quality management system in manufacturing provides the tools and processes needed to address these challenges head-on. By leveraging QMS system software, manufacturers can automate quality control processes, streamline workflows, and continuously improve their operations, leading to higher product quality, reduced downtime, and greater efficiency.
As the manufacturing industry continues to evolve, adopting an advanced quality management system like ComplianceQuest’s QMS software will be essential for staying competitive and compliant. In 2025, manufacturers who invest in cutting-edge QMS solutions will be better positioned to meet customer demands, reduce operational costs, and maintain high standards of quality and efficiency.
0 notes
Text
#ISO 9001 for manufacturing#ISO 9001 certification 2025#benefits of ISO 9001 in manufacturing#quality management system for manufacturers#ISO 9001 implementation guide
1 note
·
View note
Text
🐢 The Turtle Diagram in IATF 16949: More Than a Drawing—It’s Process Intelligence
🔍 What is a Turtle Diagram? A Turtle Diagram is a visual tool used in process-based thinking, required under IATF 16949 and ISO 9001. It’s designed to help understand, control, and improve a process by analyzing: Inputs & outputs Responsibilities (who) Methods (how) Resources (with what) Performance (KPIs) Risks It’s called a “turtle” because the layout resembles a turtle shell, with the…

View On WordPress
#automotive quality#Continuous Improvement#cross-functional alignment#department linkage#IATF 16949#inputs and outputs#Internal Audit#ISO 9001#KPIs#manufacturing processes#operational control#process approach#process mapping#process ownership#QMS#quality assurance#quality management#quality system#Risk-Based Thinking#SOP#third-party audit#Turtle Diagram
0 notes
Text
Exploring the Growth and Excellence of the Pharmaceutical Industry in India
Exploring the Growth and Excellence of the Pharmaceutical Industry in India
The pharmaceutical industry in India has emerged as a key global player, recognized for its cost-effective production, advanced pharmaceutical development and technology, and strong presence in international markets. With a growing focus on healthcare R&D, innovation in drug research and development, and robust pharmaceutical manufacturing, India continues to be a trusted supplier of pharmaceutical products and medicines across the globe.
The Rise of India's Pharmaceutical Sector
India holds a prominent position in the global pharmaceutical landscape, being among the largest pharmaceutical companies contributors to generic drug supply and vaccine production. The Indian government’s support, combined with private sector innovation, has made the country a leader in pharmaceutical manufacturing companies, with exports reaching over 200 countries.
This growth is driven by the country's commitment to high standards in quality control in pharmaceuticals, investment in pharma healthcare, and a vision to lead in global healthcare solutions.
Pharmaceutical Manufacturing and Innovation
India’s pharma manufacturing companies operate under stringent international regulations and practices, including GLP (Good Laboratory Practices), WHO-GMP certifications, and advanced automation systems. These companies are constantly innovating through strong R&D departments in pharmaceutical industry, focusing on developing new therapies, enhancing drug delivery methods, and ensuring quality throughout the supply chain.
Such innovation has fueled the creation of effective, accessible pharmaceutical drugs that have transformed public health outcomes both in India and abroad.

Key Attributes of Leading Pharma Companies
The top pharmaceutical manufacturing companies in India are recognized not just for their production volumes, but for their dedication to quality assurance, ethical standards, and technological advancement. Many of them rank among the top pharma companies worldwide thanks to their continuous delivery of new pharma products, global reach, and patient-focused approaches.
In this elite group, Zuventus Healthcare holds a distinguished place, known for its excellence in innovation, affordability, and trust.
About Zuventus Healthcare
Zuventus Healthcare Ltd. is a reputed name in the Indian pharmaceutical landscape. Based in Mumbai, Zuventus has steadily climbed the ranks to be recognized as a top pharmaceutical company in Mumbai and is counted among the top 10 pharmaceutical companies in India.
With a diverse portfolio of Zuventus healthcare products, the company serves multiple therapeutic areas, delivering effective and high-quality treatments. Its flagship brands, including Zuventus Gromaxx and Zuventus Odenea, highlight the company’s capability in creating targeted, result-driven medications that meet real-world healthcare demands.
Zuventus' Commitment to Quality and Ethics
What sets Zuventus Healthcare apart from other pharmaceutical companies in India is its unwavering focus on ethical practices, innovation, and operational excellence. The company follows robust quality management systems, with a strong emphasis on quality control methods to ensure that every product released meets stringent safety and efficacy benchmarks.
Its facilities are equipped to meet global manufacturing standards, making Zuventus a trusted name not only within India but also in the international health care market.
Driving Healthcare Through Vision and Values
Zuventus believes that a strong foundation lies in strong values. The company’s core corporate values—integrity, teamwork, innovation, and accountability—drive every aspect of its operations. Its organization's vision is to contribute meaningfully to healthcare by being a partner in wellness, committed to improving lives through quality and affordable medication.
With a clear teamwork vision, Zuventus cultivates a collaborative culture that encourages innovation, learning, and shared responsibility—making it not only a leading pharmaceutical company but also a great place to work and grow.
The Future of Indian Pharma: Opportunities and Challenges
The Indian pharmaceutical industry is poised for even greater expansion, driven by increasing global demand, a maturing domestic healthcare system, and rapid adoption of digital technologies. Challenges such as regulatory complexities and pricing pressures remain, but with continued investments in research and development in the pharmaceutical industry, the outlook remains highly optimistic.
Companies like Zuventus, with their blend of innovation, ethical focus, and manufacturing strength, are well-positioned to lead this next phase of growth. Their continuous contribution to drug research and development, focus on pharma manufacturing excellence, and strong market presence reflect the future of Indian pharma.
Final Thoughts
India’s pharma industry is a shining example of how science, innovation, and purpose can come together to serve humanity. From groundbreaking Zuventus medicines to world-class pharmaceutical manufacturers in India, the nation stands tall on the global healthcare stage.
Zuventus Healthcare, with its strong legacy, state-of-the-art infrastructure, and a values-driven mission, embodies the promise and potential of the Indian pharmaceutical sector. As the company continues to grow, launch new pharma products, and expand its impact, it remains a beacon of trust, quality, and healthcare advancement in both local and global markets.
#quality standards#top pharmaceutical manufacturing companies in india#quality control and quality assurance#quality control methods#quality management system#glp good lab practice#quality control in pharmaceuticals#pharmaceutical quality assurance#good manufacturing practices#pharmaceutical compliance#GMP certification#drug quality testing#pharmaceutical manufacturing process#regulatory standards in pharmaceuticals#pharmaceutical testing labs#pharmaceutical industry standards#medicine manufacturing companies in india#pharmaceutical product quality#pharma regulatory compliance#pharma quality guidelines#pharmaceutical quality control techniques#research and development#R&D#R&D pharma#pharma industry
0 notes
Text
Numerical Relays - Adlite Electricals

Enhance Power System Efficiency with CGI 14N 75-250VDC Relay
For reliable electrical system performance, a high-quality auxiliary relay is essential. The CGI 14N 75-250VDC Relay, available at Adlite Electricals, is designed for superior performance in industrial, commercial, and power utility applications. With its voltage range of 75-250VDC, it ensures stable and efficient operation in electrical protection and automation systems.
What is the CGI 14N 75-250VDC Relay?
The CGI 14N 75-250VDC Relay is an advanced auxiliary relay used in control and protection circuits. It processes electrical signals efficiently and enables precise switching for power management.
Key Features of CGI 14N 75-250VDC Relay
This relay offers exceptional advantages, making it an ideal choice for power system applications:
Wide Voltage Compatibility: Operates efficiently between 75-250VDC, making it suitable for diverse electrical systems.
High-Speed Response: Ensures rapid activation to prevent faults and enhance system safety.
Rugged and Durable Design: Built for long-term use in demanding industrial environments.
Compact and Easy Installation: Allows seamless integration into various electrical setups.
Reliable Contact Multiplication: Enhances control circuit performance and dependability.
Applications of CGI 14N 75-250VDC Relay
The CGI 14N 75-250VDC Relay is widely used in multiple industries due to its high reliability and efficiency, including:
Power Plants: Assists in relay protection and circuit breaker operations.
Industrial Automation: Enables precise switching in manufacturing processes.
Substations: Supports stable grid management and fault isolation.
Renewable Energy Systems: Facilitates integration in solar and wind energy projects for efficient power control.
Why Choose CGI 14N 75-250VDC Relay from Adlite Electricals?
When it comes to sourcing top-quality electrical protection devices, Adlite Electricals is your trusted provider. Here’s why:
Genuine and Certified Products: Ensuring superior quality and reliability.
Affordable Prices: Get the best value for high-performance electrical components.
Hassle-Free Online Shopping: A seamless purchasing experience with expert support.
Fast and Secure Delivery: Ensuring timely arrival of your relay in perfect condition.
Conclusion
The CGI 14N 75-250VDC Relay is a must-have for industries that require a dependable, high-speed, and durable relay solution. Its wide voltage range and compact design make it ideal for numerous electrical applications.
Order your CGI 14N 75-250VDC Relay today from Adlite Electricals and enhance your system’s efficiency and safety!
Related Products
#CGI 110VDC Master Trip Relay
#CGI 14C 18-52VDC Relay
#CGI 14C 75-250VDC Relay
#CGI 14N 18-52VDC Relay
#CGI 14S 230VAC Relay
#CGI 24C 18-52VDC Relay
#CGI 24C 75-250VDC Relay
#CGXH1 3 Element Aux 110 VDC Relay
#Crompton TCSR Unit 110 VDC Relay
#Megawin M140c Relay
#Megawin MB 140c (Breaker Manager Relay)
#Enhance Power System Efficiency with CGI 14N 75-250VDC Relay#For reliable electrical system performance#a high-quality auxiliary relay is essential. The CGI 14N 75-250VDC Relay#available at Adlite Electricals#is designed for superior performance in industrial#commercial#and power utility applications. With its voltage range of 75-250VDC#it ensures stable and efficient operation in electrical protection and automation systems.#What is the CGI 14N 75-250VDC Relay?#The CGI 14N 75-250VDC Relay is an advanced auxiliary relay used in control and protection circuits. It processes electrical signals efficie#Key Features of CGI 14N 75-250VDC Relay#This relay offers exceptional advantages#making it an ideal choice for power system applications:#•#Wide Voltage Compatibility: Operates efficiently between 75-250VDC#making it suitable for diverse electrical systems.#High-Speed Response: Ensures rapid activation to prevent faults and enhance system safety.#Rugged and Durable Design: Built for long-term use in demanding industrial environments.#Compact and Easy Installation: Allows seamless integration into various electrical setups.#Reliable Contact Multiplication: Enhances control circuit performance and dependability.#Applications of CGI 14N 75-250VDC Relay#The CGI 14N 75-250VDC Relay is widely used in multiple industries due to its high reliability and efficiency#including:#Power Plants: Assists in relay protection and circuit breaker operations.#Industrial Automation: Enables precise switching in manufacturing processes.#Substations: Supports stable grid management and fault isolation.#Renewable Energy Systems: Facilitates integration in solar and wind energy projects for efficient power control.#Why Choose CGI 14N 75-250VDC Relay from Adlite Electricals?#When it comes to sourcing top-quality electrical protection devices#Adlite Electricals is your trusted provider. Here’s why:
0 notes
Text
Hyper-Hypothermia Unit manufacturer and supplier - Mercury Healthcare.

Mercury Healthcare is a leading manufacturer and supplier of Hyper-Hypothermia Units in India. These advanced medical devices play a crucial role in temperature management, particularly during surgeries or critical care where body temperature control is essential.
What are Hyper-Hypothermia Units?
Hyper-hypothermia units are specialized devices designed to regulate body temperature, crucial in various medical procedures like cardiac surgeries, traumatic injuries, and intensive care units (ICUs). These devices can either induce hypothermia (lowering body temperature) or hyperthermia (raising body temperature), depending on the medical requirement.
Features of Mercury Healthcare’s Hyper-Hypothermia Units
Mercury Healthcare manufactures high-quality, reliable hyper-hypothermia units built with advanced technology and safety features. These units ensure precise temperature control and are designed for use in a range of clinical environments.
Key features include:
Accurate temperature control for maintaining and adjusting the patient's body temperature.
User-friendly interface allowing healthcare professionals to monitor and manage settings easily.
Energy-efficient systems designed to reduce power consumption while maintaining operational efficiency.
Durability and mobility, with lightweight materials and easy-to-move structures to support their use across different medical departments.
Why Choose Mercury Healthcare?
Mercury Healthcare has earned a reputation as one of the top suppliers and manufacturers of medical equipment, particularly hyper-hypothermia units, across India. The company's commitment to quality, innovation, and reliability makes it a preferred choice for hospitals and medical centers. Some of the key reasons why healthcare providers choose Mercury Healthcare include:
State-of-the-art Technology: Mercury Healthcare employs cutting-edge technology to ensure its devices meet the stringent demands of modern medicine.
Comprehensive Service Support: Offering after-sales service, maintenance, and on-site training ensures that healthcare facilities maximize the use of the hyper-hypothermia units.
Custom Solutions: Mercury Healthcare works closely with healthcare providers to customize solutions that meet their specific clinical needs.
Compliance with Medical Standards: The company's products are compliant with international safety and medical standards, ensuring quality and safety during critical procedures.
Application of Hyper-Hypothermia Units
Mercury Healthcare’s hyper-hypothermia units are widely used in:
Cardiac surgeries: For regulating body temperature during procedures like coronary artery bypass.
Trauma cases: For managing temperature in critically injured patients.
Neurological surgeries: These units help in reducing brain injury by controlling temperature during operations.
Critical care: Used in ICUs to manage patient temperature for recovery from severe infections or fevers.
Mercury Healthcare: Trusted for Innovation and Quality
With years of experience and a commitment to innovation, Mercury Healthcare continues to be a top player in manufacturing hyper-hypothermia units in India. Whether it's for use in advanced medical facilities or routine hospital care, their units offer unmatched quality and performance.
If you are looking for a reliable hyper-hypothermia unit supplier, Mercury Healthcare offers a range of devices that are designed to meet the needs of healthcare professionals across various specialties.
#Hyper-Hypothermia Unit manufacturer in India#Best Hyper-Hypothermia Unit suppliers#Hyper-Hypothermia device for hospitals#Medical temperature control systems India#Advanced hyper-hypothermia units for ICU#Cardiac surgery temperature regulation devices#Critical care temperature management unit#Hyperthermia and Hypothermia medical device suppliers#Body temperature regulation devices for hospitals#Top manufacturer of hyper-hypothermia units#Affordable Hyper-Hypothermia equipment India#Hospital temperature control system manufacturers#High-quality hyper-hypothermia units for surgery#Portable medical hyper-hypothermia units#Hypothermia control device suppliers India
0 notes
Text
Global Pharmaceutical Leaders in India and the USA | Chemxpert Database
Pharmaceutical companies in India and the top pharmaceutical companies in the USA are at the forefront of the global pharma industry. These industry leaders drive pharmaceutical product development, contributing to innovative healthcare solutions worldwide. The largest pharmaceutical companies continue to expand their influence, while medical companies strive for breakthroughs. For up-to-date insights on these key players, Chemxpert Database offers comprehensive data on global pharma, helping you navigate the competitive landscape of the medical industry with ease.

#process validation in pharmaceutical industry#pharmaceutical manufacturers UK#pharmaceutical chemistry#pharma quality management system#tablet manufacturing process
1 note
·
View note
Text

Quality defects? Not on our watch! We're committed to providing accurate insights, leading to ZERO DEFECTS with qDataOps, our Total Quality 4.0 Software.
#quality control software for manufacturing#Quality Control processes#quality management system in manufacturing industry
0 notes
Text
A Comprehensive Analysis on Eric Harris, 3.
Disclaimer: This analysis/psychoanalysis is limited only to analysis as a means to reflect and understand the people involved. It is strictly informative. Just like all of my posts, I am detached from the media I write about and solely focus on the people to understand their psychology, for others to gain insight. There is no room for me to romanticize or glorify anything I write because I am only here to explain. I understand and research, but I do not condone. Thank you.
note: This is all solely based on independent research. If I may be wrong with the medical aspects of this post, please correct me. I would appreciate it a lot.
Fluvoxamine Maleate

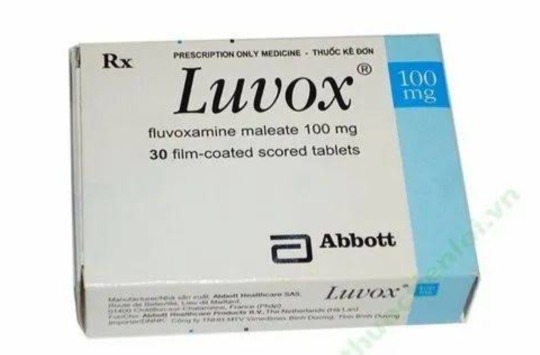
Just a few days before the shooting, Eric was promptly rejected from his application to serve in the marines. Despite his desirable qualities, he was rejected for the sole reason of being tested positive for consuming a prescripted drug after rejecting that he was off any medication. With a trace of Luvox in his system, theories speculate that the drug's side effects played a role on the events that have transpired. Luvox is a medication prescription drug that is used to treat mental illnesses such as Obsessive Compulsive Disorder (OCD), anxiety, depression, and others. While there are positive effects, the downside are heightened sensations of dread, irritation, depression, and arising violent behaviors. It also has a chance to increase manic symptoms, especially if taken in particularly large doses.
Anti-depressant usage, effects of overdose, and detoxification
When taken over the prescribed dosage, Luvox can lead to increased agression and suicidal ideation and tendencies. Eric was in anger management therapy after being on probation alongside Dylan for attempting to steal a vehicle containing thousands worth of valuable electronics and tools. After getting caught, Eric openly expressed his difficulties in managing his anxiety and explosive anger issues that his counselor said, "he frequently blew up and often cried." Revealing that this influences his homicidal and suicidal thoughts, so he was prescribed Fluvoxamine, which is a selective serotonin reuptake inhibitor (SSRIs). These drugs increase the capacity of the brain to receive serotonin.
According to an interview with Eric's friends, they speculate that Eric was off his medication completely for sometime. Abruptly stopping medication can lead to the development of a more violent response. For some people, drugs are able to fix their chemical imbalances, but it can also lead to withdrawal symptoms of taken for over the duration of 6 weeks. Abruptly discontinuing anti-depressants can lead to rebound depression or relapse. Symptoms may return stronger than before. Furthermore, SSRI's such as Luvox and Prozac take about 5 days to linger in one's system before subsequently washing off.
In a period of 11 months, Eric and Dylan have been under a juvenile detention program at Jefferson County district — this was in 1998, just a year before the shooting. They were allowed to leave the program by early February 1999. After finishing, Eric wrote a letter contained in his juvenile diversion program file. He states, "I learned that thousands of suggestions are worthless if you still believe in violence."
After public demands for stricter regulations on SSRIs after the tragic shooting, Luvox was temporarily banned in 2002. By 2008, drug manufacturers had reformulated Luvox to a controlled-release version specifically designed to treat OCD, excluding indications for depression or anxiety. Subsequently, the FDA approved Luvox CR for the treatment of OCD. The label does not explicitly prohibit prescribing it to the pediatric population, it notes that the smallest dose "may not be appropriate" for children, without providing further explanation.
Eric was about 17 at the time of taking the medication, which means he is part of the pediatric population. Though it's still unclear if he was taking normal doses for a long period of time or taken more than what was prescribed, it is clear that there was negligence with prescribing a child a SSRI that has the posibility of worsening their already apparent symptoms.
Columbine was really a case that opened the public's eyes into the dysfunctional aspects of society and institutions, transcending even to the medical field.
434 notes
·
View notes
Text
The Role of Automation in Manufacturing Quality Management
In today’s competitive manufacturing landscape, achieving operational efficiency while maintaining high product quality is paramount. A robust Quality Management System in Manufacturing is essential for ensuring compliance, reducing defects, and improving productivity. However, traditional quality management processes often involve manual inspections, data entry, and error-prone reporting, leading to inefficiencies and quality inconsistencies.
Automation is transforming manufacturing quality management by streamlining quality control processes, reducing human errors, and enhancing regulatory compliance. By integrating qms system software with automated tools, manufacturers can significantly improve their ability to maintain quality standards, drive continuous improvement, and meet industry regulations.
How Automation is Reshaping Quality Management in Manufacturing
The Shift from Manual to Automated Quality Control
Historically, manufacturing relied on manual inspections and human intervention to detect and correct defects. However, these methods are often inconsistent and prone to human errors. Automation eliminates these inefficiencies by leveraging machine learning, artificial intelligence (AI), and real-time monitoring to ensure accuracy in Quality Control processes.
With an advanced quality management system in manufacturing, companies can integrate automated inspection systems that detect defects in real-time, reducing the need for rework and preventing defective products from reaching the market. This shift significantly enhances production efficiency while maintaining high-quality standards.
The Role of AI and Machine Learning in Quality Management
AI-powered qms system software enables predictive quality analytics, helping manufacturers identify trends and potential issues before they escalate. Machine learning algorithms analyze historical data to detect patterns, enabling proactive quality control strategies.
By integrating AI into a quality management system, manufacturers can:
Predict potential defects and take preventive actions.
Reduce downtime by identifying inefficiencies in production processes.
Enhance compliance tracking by automating regulatory documentation.
This data-driven approach to quality management improves decision-making and ensures continuous process optimization.
Enhancing Compliance with Automated QMS System Software
Meeting Regulatory Standards with Automation
Manufacturers across industries must comply with stringent regulations such as ISO 9001, FDA guidelines, and GMP requirements. Manual compliance tracking is inefficient and increases the risk of non-conformances.
Automation within a quality management system in manufacturing ensures real-time compliance tracking, automated audit trails, and digital record-keeping. This reduces compliance risks and enhances transparency across manufacturing operations.
Digital Documentation and Audit Readiness
One of the major challenges in quality management is maintaining accurate and accessible documentation. Traditional paper-based systems are inefficient and increase the likelihood of misplaced records.
Automated qms system software enables manufacturers to:
Store and retrieve compliance records instantly.
Maintain a centralized database for regulatory audits.
Automate document version control and approvals.
With digital documentation, manufacturers can ensure quick and efficient responses during regulatory inspections, avoiding compliance penalties.
Improving Production Efficiency Through Automation
Real-Time Quality Monitoring and Process Optimization
A fully integrated quality management system in manufacturing enables real-time monitoring of production processes. Automation tools such as IoT sensors and AI-driven analytics provide real-time insights into quality deviations, allowing for immediate corrective actions.
By integrating automation with qms system software, manufacturers can:
Reduce cycle times by eliminating production bottlenecks.
Minimize scrap and rework by detecting defects early.
Optimize resource allocation for improved operational efficiency.
This real-time approach enhances process stability and ensures consistent product quality.
Automated Defect Detection and Corrective Actions
Traditional defect detection methods rely on manual inspections, which are prone to inconsistencies. Automated defect detection systems, powered by AI and computer vision, can identify flaws with greater accuracy and speed.
By integrating automated corrective action workflows, manufacturers can:
Ensure non-conforming products are immediately flagged and addressed.
Implement root cause analysis to prevent recurring defects.
Streamline communication between quality control teams and production units.
This automated approach significantly reduces waste and improves first-pass yield rates.
Leveraging Automation for Supplier Quality Management
Strengthening Supplier Collaboration with Digital QMS
Managing supplier quality is a critical aspect of maintaining overall product quality. Traditional supplier management processes involve extensive paperwork, manual approvals, and lengthy response times.
Automating supplier quality management within a qms system software framework provides:
Real-time supplier performance tracking.
Automated supplier audits and qualification processes.
Digital scorecards for supplier risk assessments.
This ensures that only high-quality materials enter the production line, reducing the risk of defects and delays.
Predictive Risk Management for Supplier Compliance
By integrating predictive analytics with quality management, manufacturers can assess supplier risks before issues arise. Automated risk assessment tools analyze historical supplier data to:
Identify suppliers prone to quality inconsistencies.
Monitor compliance trends in real-time.
Recommend alternative suppliers based on performance history.
Predictive risk management enables manufacturers to proactively address supplier-related quality challenges, reducing disruptions in the supply chain.
The Future of Automation in Manufacturing Quality Management
Integrating Smart Manufacturing with QMS
The evolution of smart manufacturing is driving the need for more sophisticated automation tools within Quality Management Systems. Industry 4.0 technologies, such as IoT, blockchain, and digital twins, are further enhancing quality control processes.
Future-ready quality management system in manufacturing will include:
AI-driven predictive maintenance to prevent equipment failures.
Blockchain-enabled traceability for complete product lifecycle tracking.
Autonomous quality inspections using robotic process automation (RPA).
By embracing smart manufacturing principles, companies can stay ahead of quality challenges and drive innovation.
Continuous Improvement Through Automated Analytics
Quality management is not a one-time process but a continuous cycle of improvement. Automation provides real-time feedback loops that help manufacturers refine their processes continuously.
Advanced analytics in QMS System Software enable:
Ongoing process optimization through AI-driven recommendations.
Dynamic quality control adjustments based on real-time production data.
Automated performance benchmarking to identify areas of improvement.
This continuous improvement model ensures that manufacturers maintain compliance, reduce costs, and enhance product quality over time.
Why ComplianceQuest is Essential for Business in 2025
As manufacturing evolves, the integration of automation in quality management is no longer optional but a necessity. ComplianceQuest’s quality management system in manufacturing offers a comprehensive, automated approach to quality control, compliance tracking, and process optimization.
By implementing ComplianceQuest qms system software, manufacturers can:
Achieve real-time visibility into quality metrics.
Ensure seamless regulatory compliance.
Leverage AI-driven analytics for smarter decision-making.
In 2025, businesses that embrace automation in quality management will have a competitive edge in operational efficiency, compliance, and overall product excellence.
0 notes
Text
youtube
Moises Santillan draws attention to the pervasive problem of microplastics, which are becoming an increasingly serious environmental and health risk. Microplastics, or minute plastic pieces, are not just found in water but appear to be prevalent almost everywhere. Moises notes that they may be found in a variety of daily plastic goods and in locations excessively contaminated with plastic garbage. The widespread prevalence of microplastics is an increasing concern since these particles infiltrate both our environment and our bodies, as scientific investigations show.
The health consequences of microplastics are especially concerning. Moises emphasizes that enhanced microscopy and other technologies have enabled researchers to detect microplastic particles within the human body. Considerably more troubling is the potential for microplastics to break down into smaller particles known as nanoplastics, which are considerably more widespread. These nanoplastics can enter the circulation, spread throughout the body, and potentially disrupt numerous biological activities. This compounding effect, in which plastics degrade into increasingly minute particles, raises major concerns about their influence on human health.
Microplastic pollution of the environment is also a significant concern. Moises notes that microplastics may be discovered anywhere plastic debris collects, contributing to the overall pollution catastrophe. Their persistence in the environment and tendency to disintegrate into smaller particles make them incredibly difficult to control. Pollution not only endangers animals, but it may also impact land, water, and food quality. As microplastics permeate many ecosystems, the long-term environmental consequences might be severe, threatening biodiversity and altering ecological equilibrium.
Moises emphasizes the urgent need for study and action to understand and reduce the impact of microplastics. The presence of these particles in the human body, their metamorphosis into nanoplastics, and their widespread environmental distribution all necessitate further scientific investigation. Potential solutions include lowering plastic manufacturing, improving waste management systems, and developing creative methods to break down these polymers safely. Through collaborative effort and ongoing study, humanity can address the threats posed by microplastics and move toward a healthier, more sustainable future.
27 notes
·
View notes
Text
#IATF 16949:2024 Certification#Automotive Quality Management System#Electric Vehicle Manufacturing Standards#IATF 16949 Risk-Based Approach#Supplier Management for EV Industry#EV Manufacturing Compliance
1 note
·
View note
Text
🔄 Process Approach Across ISO & IATF Standards with 🚗 OEM Requirements in 2025
✅ Introduction: Why the Process Approach Matters in 2025 The process approach isn’t just a buzzword from ISO standards—it’s the engine behind operational excellence. As we step into 2025, OEMs (Original Equipment Manufacturers) demand more than compliance—they expect traceability, zero-defect culture, and real-time performance. In this guide, you’ll learn: 📌 How the process approach is defined…

View On WordPress
#5W2H model ISO#Automotive manufacturing#automotive quality system#clause 4.4 explained#EHS process compliance#energy management ISO 50001#environmental compliance ISO 14001#IATF 16949 process control#ISO digital integration#ISO standards 2025#lean manufacturing#occupational health ISO 45001#OEM compliance 2025#PFMEA process improvement#process approach ISO#process mapping ISO 9001#process ownership in QMS#quality management ISO 9001#risk-based thinking ISO#SIPOC diagram example#smart factory process control#Turtle diagram QMS
0 notes
Text
Exploring the Pharmaceutical Industry in India: A Closer Look at Leading Companies like Zuventus Healthcare
Exploring the Pharmaceutical Industry in India: A Closer Look at Leading Companies like Zuventus Healthcare
The pharmaceutical industry in India has evolved into one of the largest and most dynamic sectors of the economy. With increasing investments in pharmaceutical manufacturing, innovations in pharmaceutical products, and strict adherence to quality control and quality assurance, India has become a global hub for healthcare solutions. Among the top pharmaceutical manufacturing companies in India, Zuventus Healthcare stands out for its commitment to quality, innovation, and excellence.
About Zuventus Healthcare: A Leading Name in Pharmaceuticals
Established in 2002, Zuventus Healthcare has emerged as one of the top pharma companies in India, driven by its mission to bring "Joy of Good Health" to all. Headquartered in Mumbai, Zuventus is recognized as a leading pharma company in Mumbai and has steadily built its reputation as one of the best pharma companies in India.
With a diverse portfolio that includes specialized divisions like Zuventus Gromaxx and Zuventus Odenea, the company caters to a wide range of therapeutic areas. From Zuventus medicine for pain management to respiratory care, the brand’s commitment to healthcare innovation is evident across all its products.
Pharmaceutical Manufacturing and Quality Control Excellence
At the heart of India's thriving pharma industry is the focus on advanced pharmaceutical manufacturing practices. Pharmaceutical manufacturers in India must meet rigorous global standards, and companies like Zuventus lead the way with their adherence to GLP (Good Laboratory Practices), robust quality control methods, and a comprehensive quality management system.
Zuventus, a prominent player among pharmaceutical manufacturing companies, emphasizes stringent quality control in pharmaceuticals to ensure patient safety and product efficacy. Their manufacturing facilities are a testament to best practices in pharmaceutical manufacturing, ensuring compliance with international standards.
Zuventus Healthcare Products: A Diverse and Dynamic Portfolio
Zuventus Healthcare products cover a wide range of therapeutic areas, including anti-infectives, cardiovascular medicines, respiratory care, and nutritional supplements. Their offerings reflect the company's deep understanding of the evolving needs of healthcare practitioners and patients alike.
In the competitive world of drugs pharmaceuticals, Zuventus has managed to carve a niche for itself, becoming synonymous with trust and reliability.
Zuventus Among the Top Pharmaceutical Companies in India
Being consistently ranked among the leading pharmaceutical companies in India and the top 10 pharmaceutical companies, Zuventus has built a strong reputation based on its focus on quality, innovation, and affordability. It competes fiercely with some of the largest pharmaceutical companies in India, thanks to its superior product development, ethical practices, and efficient marketing strategies.
Whether you’re evaluating pharma manufacturing companies or looking at the best performers in the pharmaceutical company in India listings, Zuventus remains a brand to watch.
The Importance of Core Values in the Pharmaceutical Industry
Every successful organization is driven by its core corporate values. Zuventus believes in integrity, leadership, innovation, and teamwork vision. These values shape its organization's vision and ensure that its mission is aligned with delivering better healthcare outcomes.
Having good core values for a company is crucial, especially in sectors like healthcare where trust is paramount. Zuventus's mission vision values reflect its commitment to quality, patient safety, and continuous innovation.
The Future of India's Pharmaceutical Industry
With a booming demand for healthcare solutions and increasing emphasis on global quality standards, the pharmaceutical industry in India is poised for unprecedented growth. Companies like Zuventus, with their focus on pharmaceutical manufacturing, innovation, and customer-centric approaches, are expected to lead the way.
By maintaining the highest standards of quality control and quality assurance and by investing heavily in research and development, Zuventus exemplifies what it means to be among the top pharmaceutical manufacturing companies in India.
Final Thoughts
If you're looking for trusted names in the pharmaceutical industry in India, Zuventus Healthcare stands as a beacon of reliability, innovation, and excellence. With its wide range of pharmaceutical products, focus on quality control in pharmaceuticals, and dedication to strong company core values, Zuventus continues to solidify its place among the best pharma companies in India.
#about zuventus healthcare#best pharma company in india#largest pharmaceutical companies#pharmaceutical manufacturing#pharmaceutical products#top 10 pharmaceutical companies#pharma company in mumbai#pharmaceutical industry in india#top pharma companies in india#zuventus medicine#pharmaceutical manufacturing companies#Zuventus healthcare products#pharmaceutical company in india#pharma industry#Zuventus Gromaxx#Zuventus Odenea#pharmaceutical manufacturers in india#drugs pharmaceuticals#leading pharmaceutical companies in india#top pharmaceutical manufacturing companies in india#quality control and quality assurance#quality control methods#quality management system#glp good lab practice#quality control in pharmaceuticals#company core values#good core values for a company#team work vision#core corporate values#organization's vision
0 notes
Text
Common Electrical Issues That a High-Quality Current Transformer Can Prevent

In today’s fast-paced industrial world, electrical reliability is more crucial than ever. A small error in current measurement can lead to serious system failures, downtime, and costly repairs. This is where high-quality current transformers (CTs) make a huge difference. But what exactly can a superior CT prevent? Let’s dive in.
What Is a Current Transformer?
A current transformer (CT) is an essential device used to measure alternating current (AC) by producing a scaled-down, manageable current for meters, relays, and other instruments. It enables safe monitoring and accurate metering in high-voltage environments, protecting both equipment and personnel.
Common Electrical Problems a High-Quality CT Can Prevent
1. Overloading and Equipment Failure
Problem: Without accurate current measurement, systems can easily become overloaded, causing motors, transformers, and cables to overheat.
How a CT Helps: A precision CT ensures real-time, reliable current monitoring. It detects overcurrent conditions immediately, allowing protective relays to trip and prevent expensive equipment damage.
2. Inaccurate Energy Billing
Problem: Incorrect current readings can lead to wrong billing, causing businesses to either overpay for energy or face disputes with utilities.
How a CT Helps: High-accuracy CTs provide precise energy data for billing and cost allocation, especially critical in commercial complexes, factories, and power plants.
3. Protection Relay Malfunction
Problem: If a CT delivers incorrect signals, protection relays may not operate during faults, leading to extended damage and system blackouts.
How a CT Helps: Reliable CTs ensure protection relays receive the correct fault current levels, enabling fast and accurate circuit isolation.
4. Short Circuits Going Undetected
Problem: A minor fault can escalate into a full-blown short circuit if the protection system doesn’t detect it early.
How a CT Helps: Quality CTs capture even small fault currents, triggering alarms or shutdowns before damage spirals out of control.
5. Phase Imbalance Issues
Problem: Imbalanced phases cause excessive heating, motor inefficiency, and damage to sensitive equipment.
How a CT Helps: High-precision CTs monitor each phase accurately, enabling detection of phase unbalance conditions early and preventing system inefficiencies.
6. Harmonic Distortions and Power Quality Problems
Problem: Harmonic distortions interfere with the performance of sensitive equipment and reduce the overall power quality.
How a CT Helps: Specialized CTs can detect abnormal waveform distortions, enabling corrective action through harmonic filtering or load balancing.
Why Invest in a High-Quality Current Transformer?
Accuracy: Achieve metering-class precision essential for both billing and protection. Durability: Longer lifespan even in harsh industrial environments. Safety: Better insulation, thermal stability, and overload capacity. Compliance: Meets international standards like IEC and ANSI.
How Enza Electric Ensures CT Excellence
At Enza Electric, we specialize in manufacturing current transformers built with precision, reliability, and global standards compliance. Whether you need CTs for commercial metering, industrial protection, or utility-scale power distribution, our solutions guarantee unmatched performance.
Customizable options for various ratings High dielectric strength for safety Long service life even in extreme conditions
Explore our Current Transformer Range
Final Thoughts
A high-quality current transformer isn’t just a tool — it’s a first line of defense for your electrical system. Investing in precision-engineered CTs prevents common electrical issues, boosts system longevity, ensures accurate billing, and improves overall operational safety.
If you’re serious about protecting your infrastructure and optimizing performance, choosing Enza Electric’s current transformers is a smart move.
9 notes
·
View notes
Text
Cost vs. Quality: What to Consider When Investing in Switchgear

In today’s energy-intensive world, switchgear plays a critical role in managing power distribution safely and efficiently. Whether you’re upgrading your industrial facility, building a commercial plant, or powering a large infrastructure project, choosing the right switchgear is not just a technical decision — it’s a strategic investment. One of the most common dilemmas buyers face is balancing cost vs. quality. So, how do you decide?
Understanding Switchgear: The Heart of Electrical Safety
Switchgear is a combination of electrical disconnect switches, fuses, or circuit breakers used to control, protect, and isolate electrical equipment. Its primary role is to ensure the reliability and safety of your power system.
Types of switchgear include:
· Low-voltage switchgear (for commercial and residential use)
· Medium-voltage switchgear (typically for industrial applications)
· High-voltage switchgear (used in power transmission)
Investing in the right switchgear directly impacts operational continuity, personnel safety, and overall infrastructure reliability.
The True Cost of Cheap Switchgear:
While it’s tempting to opt for budget-friendly solutions, low-cost switchgear often comes with hidden risks and long-term expenses.
Inferior Material Quality
Cheaper models often use substandard materials that degrade faster, leading to frequent maintenance or early replacement.
Safety Hazards
Low-quality switchgear can result in arc faults, insulation failure, or overheating — putting workers and equipment at risk.
Increased Lifecycle Costs
Although the initial price may be low, the total cost of ownership (including downtime, repair, and energy inefficiency) is usually higher.
Limited Scalability and Customization
Budget systems are often rigid and harder to scale as your facility grows or needs change.
Why Quality Switchgear Pays Off
When you invest in premium switchgear, you’re not just buying a product — you’re buying peace of mind.
Enhanced Reliability
High-quality switchgear is engineered to perform in extreme conditions and handle high fault levels without compromising performance.
Superior Safety Standards
Reputable brands comply with international standards such as IEC, ANSI, or UL, reducing liability and improving workplace safety.
Ease of Maintenance
Well-built switchgear is modular and user-friendly, simplifying diagnostics and minimizing downtime during maintenance.
Energy Efficiency & Smart Capabilities
Modern switchgear includes IoT sensors, real-time monitoring, and predictive maintenance features, ensuring optimal energy use and proactive problem resolution.
Key Factors to Consider When Choosing Switchgear
When evaluating switchgear options, balance cost and quality by focusing on the following:
1. Application Requirements
Understand your voltage class, load types, and fault current ratings. Quality should match your operational demands.
2. Brand Reputation & Certification
Look for trusted brands with certifications like ISO 9001, CE, or IEC 62271. Positive reviews and case studies add credibility.
3. Lifecycle Costs
Don’t just compare sticker prices — consider maintenance, service availability, spare part costs, and expected lifespan.
4. Customization & Flexibility
Choose systems that can evolve with your operation. Modular designs support upgrades and expansions more efficiently.
5. Support and Service
Ensure the manufacturer provides robust after-sales support, technical training, and warranty services.
Cost vs. Quality: The Bottom Line
When it comes to switchgear, cheap is rarely cheerful. Cutting corners today can lead to outages, hazards, and hefty repair bills tomorrow. On the other hand, investing in high-quality switchgear ensures operational resilience, safety, and long-term savings.
The smartest strategy? Aim for value, not just price. Evaluate switchgear as a long-term asset, not just a one-time purchase.
Trending Tip: Think Smart and Sustainable
With rising energy demands and climate-conscious regulations, smart and sustainable switchgear is trending. Look for:
· Eco-friendly insulation (like SF₆-free switchgear)
· Energy management features
· Digital monitoring systems
Investing in such features not only future-proofs your infrastructure but can also help you qualify for green certifications and incentives.
Final Thoughts
Balancing cost and quality in switchgear selection is about understanding your long-term operational goals. By focusing on durability, safety, and lifecycle value, you can make a decision that protects both your budget and your business.
8 notes
·
View notes