#QualityManagement
Text

ICV Assessments Pvt. Ltd. provided ISO certification which builds more trust for the people and improves the quality of your company.
We offer-
✅ ISO 9001:2015
✅ ISO 14001:2015
✅ ISO 45001:2018
✅ ISO 22000:2018
✅ ISO 27001:2013
Contact to our team 👇
☎️: +91 8826777664
🌐: www.icvassessments.com
.
#icvcertified #RepublicDay #iso #isocertification #certification #training #certificationbody #business #ISO9001 #innovation #improvement #assessment #qualitymanagement #assessor #neverstopimproving #isocertificationbody #isoquotes #haccp #quality #productivitytips #isocertified #ISO2200 #ISO_GMP_Approved #ISO45001 #ISO14001 #isoaudit #ISO27001 #compliance #foodmansgement #qualityassurance
#icvcertified#RepublicDay#iso#isocertification#certification#training#certificationbody#business#ISO9001#innovation#improvement#assessment#qualitymanagement#assessor#neverstopimproving#isocertificationbody#isoquotes#haccp#quality#productivitytips#isocertified#ISO2200#ISO_GMP_Approved#ISO45001#ISO14001#isoaudit#ISO27001#compliance#foodmansgement#qualityassurance
2 notes
·
View notes
Text

#quality#qualitymanager#qualityassurance#qualitycontrol#qualitymanagement#manufacturing#QA#QC#QAQC#procurement#qualityimprovement#course#certification#training#meritphase#saudiarabia#oman#qatar#dubai#kuwait#bahrain#kenya#dammam#riyadh#jeddah#mascat#doha#teaching
8 notes
·
View notes
Text
ISO Standard 9001 is still essential for businesses in 2024 since it guarantees effective quality management systems. Putting ISO 9001 into practice increases consumer trust, which boosts satisfaction and loyalty. Enhanced productivity, optimized procedures, and a competitive advantage in the marketplace are all advantageous to businesses.
#ISO9001#QualityManagement#Efficiency#CustomerSatisfaction#Productivity#CompetitiveEdge#QualityAssurance#ISOStandards#BusinessPerformance#2024Trends#ISO Expert#ISO Consulting#ISO Certification In Nepal#ISO Standards#ISO Audits
0 notes
Text
Quality & Recycling Signs.

Quality and recycling signs have different purposes but both aim to inform and guide individuals in their actions. Quality signs are often used to indicate the quality standards of products or services. they are symbols of reliability and customer satisfaction. Quality signs help consumers make informed decisions and build trust in the products they are purchasing.
Recycling signs display a list of recyclable materials. These signs play a vital role in encouraging environmental sustainability by promoting responsible waste management practices.
Banner House is a leading supplier of quality & recycling signs across Australia and also provides delivery to major locations such as Perth, Sydney, Brisbane, Darwin, Hobart, Gold Coast, and other cities.
#quality & recycling signs#productquality#qualitymanagement#recyclingprogram#ecofriendly#gogreen#greenliving#recyclingstation
0 notes
Text
Plan-Do-Check-Act (PDCA)
Plan-Do-Check-Act (PDCA) is a continuous process that doesn’t stop with successful implementation. Your improved product or process will become the new baseline, and you can apply PDCA to new ideas in a constant loop of improvement and adjustment.
The PDCA/PDSA framework works well in all types of organizations. It’s specifically helpful for implementing Total Quality Management or Six Sigma initiatives.

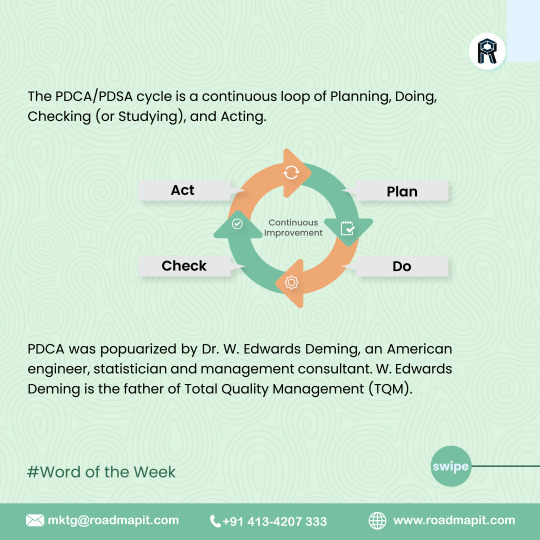



#continuousimprovement#qualitymanagement#totalqualitymanagement#PDCAcycle#PDSA#PDCAinTQM hashtag#deming hashtag#ISO9001#processimprovement#projectmanagement#strategicplanning#wordoftheweek#roadmaperp#softwarrservices#erpsoftwareinchennai#erpsoftwareinbangalore
0 notes
Text
Exploring Efficient Root Cause Analysis
Root cause analysis (RCA) is a pivotal process in problem-solving methodologies across various industries. From manufacturing to healthcare, understanding the root cause of an issue is essential for implementing effective solutions and preventing recurrence. In this blog post, we delve into some widely used root cause analysis methods, shedding light on their principles and applications. If you're eager to enhance your problem-solving skills, consider enrolling in a Six Sigma course to master these techniques and drive impactful change within your organization.
Understanding Root Cause Analysis
Before delving into specific methodologies, it's crucial to grasp the fundamental concept of root cause analysis. At its core, RCA aims to identify the underlying reason(s) behind a problem or undesired outcome. Instead of merely addressing symptoms, RCA digs deeper to uncover the systemic issues that contribute to the manifestation of problems. By understanding these root causes, organizations can implement targeted interventions to rectify issues and prevent their recurrence.
Fishbone Diagram (Ishikawa Diagram)
One of the most widely used tools in root cause analysis is the Fishbone Diagram, also known as the Ishikawa Diagram. This visual representation organizes potential causes of a problem into categories, such as people, processes, equipment, materials, environment, and management. By systematically dissecting the contributing factors, teams can gain insights into the root causes of an issue.
What is Quality
youtube
5 Whys Technique
The 5 Whys technique, rooted in the principles of Six Sigma methodology and bolstered by Six Sigma certification, is a simple yet powerful approach to root cause analysis. It involves iteratively asking "why" questions to drill down to the fundamental cause of a problem. By challenging assumptions and probing deeper with each iteration, teams can uncover the underlying issues driving surface-level symptoms. Incorporating this technique into your problem-solving arsenal can lead to more effective and sustainable solutions.
Failure Mode and Effects Analysis (FMEA)
Failure Mode and Effects Analysis (FMEA) is a proactive approach to identifying and mitigating potential failures within a system, product, or process. It systematically evaluates failure modes, their causes, and the potential effects on system performance or end-users. By quantifying the severity, occurrence, and detectability of failure modes, teams can prioritize mitigation efforts and allocate resources efficiently. Enrolling in a Six Sigma training course can provide you with the necessary skills to conduct FMEA effectively and drive continuous improvement initiatives.
Pareto Analysis
Pareto Analysis, named after economist Vilfredo Pareto, is based on the principle that a small number of causes (the "vital few") are responsible for the majority of problems or defects. This technique involves identifying and prioritizing the most significant contributors to an issue, allowing teams to focus their efforts where they will have the greatest impact. By targeting the root causes that yield the most significant improvements, organizations can optimize their resources and streamline problem-solving processes.
Root Cause Analysis in Action
To illustrate the practical application of root cause analysis methodologies, let's consider a hypothetical scenario in a manufacturing setting. Suppose a company experiences a recurring issue with product defects on the production line, resulting in increased rework and customer complaints.
Upon conducting a Fishbone Diagram analysis, the team identifies potential causes across various categories, including equipment malfunction, inadequate training, and suboptimal quality control procedures. Utilizing the 5 Whys technique, they delve deeper into each potential cause, ultimately pinpointing a lack of preventive maintenance as the root cause of equipment failures.
By implementing a proactive maintenance schedule and providing comprehensive training to operators, the company successfully reduces the occurrence of equipment-related defects, leading to improved product quality and customer satisfaction. This real-world example underscores the importance of root cause analysis in driving tangible outcomes and fostering a culture of continuous improvement, including Six Sigma training for employees.
Root cause analysis is a cornerstone of effective problem-solving, enabling organizations to identify and address the underlying issues that impact performance and quality. By leveraging methodologies such as the Fishbone Diagram, 5 Whys technique, FMEA, and Pareto Analysis, teams can uncover root causes, prioritize interventions, and drive sustainable improvements. If you're eager to sharpen your problem-solving skills and enhance your organization's performance, consider enrolling in a training course offered by a reputable Six Sigma institute to master these invaluable techniques. Embrace the power of root cause analysis and unlock new opportunities for efficiency and excellence in your endeavors.
What is Six Sigma
youtube
Six Sigma Green Belt Training Define Phase
youtube
Six Sigma Black Belt Training DMAIC
youtube
#sixsigmacourse#sixsigmatraining#six sigmacertification#sixsigmagreenblet#sixsigmablackbelt#qulaitymanagers#qualitymanagement#Youtube
0 notes
Text
How to do a Kaizen Event?
Read more on how to conduct a Kaizen event and see how they foster a continuous improvement mindset: https://tcard.leantransitionsolutions.com/software-blog/how-to-do-kaizen-event

#lean#lts#leantransitionsolutions#continuousimprovement#industry4.0#leanmanufacturing#visualmanagement#kaizen#processimprovement#qualitymanagement#kaizen5s#kaizenmethod#kaizenblitz#kaizenimplementation#kaizenexamples#10kaizenprinciples#gembakaizen#kaizenphilosophy#kaizenevent#kaizenprocess#leankaizen#kaizenmethodology#kaizenandlean#kaizenproject#kaizenleanproduction#implementationofkaizen#improvementkaizen#kaizenhistory#kaizentools#kaizensystem
0 notes
Text
Confused about ISO? Learn how it can improve your business's reputation and results!
0 notes
Text

Get your business ISO certified today with us!
ICV Assessments Pvt. Ltd.
(ISO Certification Body)
We offer-
✅ ISO 9001:2015
✅ ISO 14001:2015
✅ ISO 45001:2018
✅ ISO 22000:2018
✅ ISO 27001:2013
For more information contact us👇
☎️: +91 8826777664
🌐: www.icvassessments.com
#icvcertified#iso#isocertification#certification#training#certificationbody#sertifikasiiso#business#ISO9001#innovation#improvement#assessment#qualitymanagement#assessor#neverstopimproving#isocertificationbody#isoquotes#haccp#quality#productivitytips#isocertified#ISO2200#ISO_GMP_Approved#ISO45001#ISO14001#isoaudit#ISO27001#compliance#foodmansgement#qualityassurance
3 notes
·
View notes
Text
A management standard called ISO 9001 certification emphasizes quality management, making sure businesses exceed client expectations and boost satisfaction. This accreditation lowers errors, boosts productivity, and streamlines procedures—all of which save money. Additionally, it increases trust and credibility, which leads to new business prospects. The methodical strategy guarantees ongoing enhancement, enabling establishments to adjust to evolving market requirements. Check out this blog for more information.

#ISO9001#QualityManagement#QualityAssurance#ISOStandards#QMS#ISO9001Audits.#ISO 9001 Services#ISO 9001 certification in Nepal
0 notes
Text

Skilled Quality and Production Managers with Six Sigma Certification | Stamping and Plastic Process Experts Available
Discover highly qualified candidates for your QA and production management roles. Our pool includes Six Sigma Black Belt certified QA managers, Green Belt certified QA engineers, Plastic Process Management (PPM) trained production managers, and stamping department managers with extensive experience in all stamping processes.
For more Information :
Visit : @ https://www.crescendointernational.uk/
#QAManager#QAEngineer#SixSigmaBlackBeltCertified#SixSigmaGreenBeltCertified#ProductionManager#PlasticProcessManagement(PPM)#StampingDepartmentManager#StampingProcessExpertise#QualityAssurance#ProductionManagement#ManufacturingLeadership#ProcessImprovement#QualityControl#PlasticManufacturing#StampingIndustry#OperationalExcellence#LeanManufacturing#ContinuousImprovement#QualityManagement#ProcessOptimization
0 notes
Text
Plan-Do-Check-Act
Plan-Do-Check-Act (PDCA) is a continuous process that doesn't stop with successful implementation. Your improved product or process will become the new baseline, and you can apply PDCA to new ideas in a constant loop of improvement and adjustment.
The PDCA/PDSA framework works well in all types of organizations. It's specifically helpful for implementing Total Quality Management or Six Sigma initiatives.

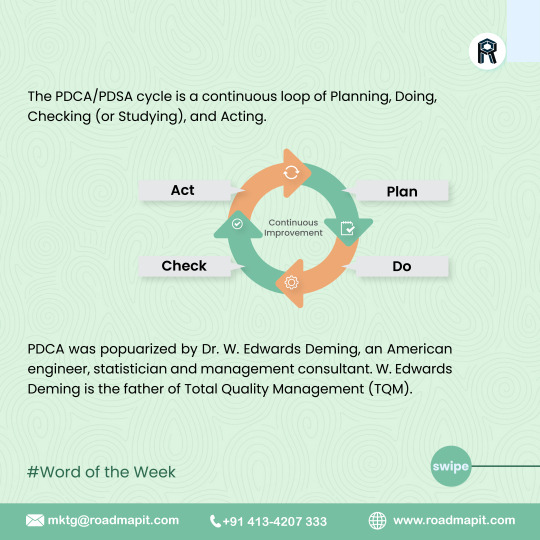



#continuousimprovement#qualitymanagement#totalqualitymanagement#PDCAcycle#softwarrservices#erpsoftware#erp#roadmapitsolutions#erpsoftwareinchennai#erpsoftwareinbangalore
0 notes
Text
youtube
Get ISO Certification Online | आईएसओ प्रमाणन ऑनलाइन प्राप्त करें
#isocertification#qualitymanagement#iso9001#iso14001#iso27001#iso45001#iso22000#iso50001#iso20000#iso13485#iso17025#iso22301#iso31000#iso18001#iso10002#iso26000#iso19011#iso19770#iso31010#iso3103#certification#registration#iso#businessregistration#certified#legalservices#privatelimitedcompany#coding#isocertificationregistration#isocertified
1 note
·
View note
Text
0 notes
Text
Navigating Excellence: Unveiling MakroCare's Comprehensive Medical Device Audit Services

In the intricate landscape of medical devices, adherence to rigorous quality standards is paramount. Explore how MakroCare's audit services, detailed on its dedicated Audits page, are instrumental in ensuring regulatory compliance, product quality, and organizational excellence.
Understanding MakroCare's Audit Expertise:
Regulatory Compliance Assurance: Dive into how MakroCare's audit services provide a meticulous examination of regulatory compliance, ensuring that medical devices meet the stringent standards set by global regulatory bodies.
Quality Management Evaluation: Explore the comprehensive approach taken by MakroCare in evaluating and enhancing Quality Management Systems (QMS) within organizations, fostering a culture of continuous improvement.
Risk Mitigation Strategies: Delve into the risk assessment methodologies employed by MakroCare, crucial for identifying and mitigating potential risks associated with medical device manufacturing and compliance.
Why Choose MakroCare for Audits?
Industry-Experienced Auditors: Discover the advantage of having auditors with deep industry knowledge, ensuring audits are conducted with a thorough understanding of the complexities within the medical device sector.
Tailored Audit Solutions: Learn about MakroCare's commitment to providing customized audit solutions, recognizing the unique requirements of each client and adapting audit processes accordingly.
Comprehensive Audit Reports: Explore how MakroCare's audit reports go beyond compliance checks, offering actionable insights and recommendations to drive continuous improvement within organizations.
Conclusion
In a sector where precision and compliance are paramount, MakroCare's audit services stand as a beacon for organizations seeking excellence in medical device manufacturing. Find out more about these services here, and empower your organization with the assurance of quality, compliance, and continuous improvement.
#MedicalDeviceAudits#RegulatoryCompliance#QualityManagement#AuditExcellence#RiskAssessment#MedicalDeviceQuality#AuditingServices#ComplianceAssurance#ContinuousImprovement#MakroCareAudits#AuditExperts#QualityAssurance#ManufacturingExcellence#AuditReports#IndustryStandards#AuditSolutions#QualityControl#HealthcareAudits#RiskMitigation#AuditInsights#regulatorychallenges#medical devices
0 notes