#Technical Pre-Feasibility Study
Text
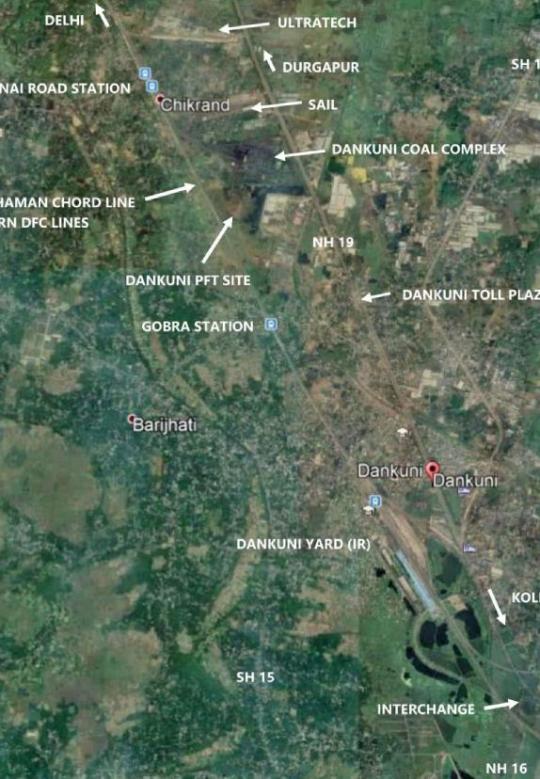
Technical Pre-Feasibility Study for Dankuni Private Freight Terminal
Read More: https://www.ekainfra.com/technical-pre-feasibility-study-for-private-freight-terminal/
0 notes
Text
With the Alberta government announcing a ban on gender-affirming care until 16 years old, let’s take a minute to correct some misinformation using peer-reviewed publications. A thread.🧵
“High quality evidence doesn’t support gender-affirming care.”
‘High quality evidence’ is a technical term that essentially just means ’no randomized controlled trials.’ RCTs are not scientifically feasible for trans youth care and would be unethical (link).
The evidence-base for gender-affirming care is quite robust and is at least as good at the evidence base for comparable interventions like abortion and birth control. For an overview of available studies, albeit already few years outdated, see page 144 onwards (link).
“Over 80% of kids grow out of being trans.”
That’s just not true. The claim is based on old, poor-quality studies that included tons of kids who never claimed to be trans (link).
But even if we took the percentage at face value, it would be irrelevant since it’s based on pre-pubertal data and virtually all the so-called ‘desistance’ occurred before puberty, when gender-affirming care becomes available (link).
More recent, better studies suggest that only around 2.5% have ‘grown out of it’ after 5 years (link).
“Kids falsely believe that they are trans because of social contagion.”
There is no evidence for that claim. It’s based on the reports of transphobic parents who were surprised that their kid came out ‘out of the blue’ and happened to have trans friends, as trans kids tend to do. For a careful explanation of why the claim is completely unsupported by evidence, see this (link).
Studies of trans youth that used clinical data to look into the claim have also failed to find any evidence of epidemic or large-scale social contagion (link).
“We need a years-long diagnostic process to make sure kids are ‘truly’ trans before they transition.”
There is no evidence that gender assessments fare any better than self-report at predicting future outcomes, as we explain in our recent review (link).
“Gender-exploratory therapy can help identify the trauma that made these kids gender dysphoric.”
Gender-exploratory therapy is extremely difficult to distinguish from classic conversion therapy, which also starts from the premise that ‘trauma’ makes people LGBTQ2S+ (link).
Since conversion therapy is known to be harmful, we have reasons to believe that gender-exploratory therapy would be as well.
Self-directed exploration is good. Forced exploration rooted in suspicion towards trans identities isn’t. If you’re starting from the belief that trans identities are inherently suspicious, you’re not doing therapy, you’re doing transphobia.
Any more myths about gender-affirming care you’d like me to bust, Tumblr?
#transgender#trans#lgbtq#lgbtqia#queer#lgbt#gender affirming care#gender affirming healthcare#trans healthcare#science#misinformation
254 notes
·
View notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Information Today Online
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183808&_unique_id=66d22149a5271
#GLOBAL - BLOGGER
BLOGGER
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types
of tests (for example, in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Pest Management Regulatory Agency 29 August 2024 ISSN: 1925-0940 (PDF version) Catalogue number: H113-25/2024-9E-PDF (PDF version) Digital Products
Table of contents Under the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans … Read More
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Information Today Online - BLOGGER
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183802&_unique_id=66d22142d1748
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types of tests (for example,
in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
BLOGGER - #GLOBAL
Pest Management Regulatory Agency 29 August 2024 ISSN: 1925-0940 (PDF version) Catalogue number: H113-25/2024-9E-PDF (PDF version)
Digital Products Table of contents Under the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans … Read More
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Information Today Online
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183796&_unique_id=66d2201b66e71
#GLOBAL - BLOGGER
BLOGGER
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types
of tests (for example, in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Pest Management Regulatory Agency 29 August 2024 ISSN: 1925-0940 (PDF version) Catalogue number: H113-25/2024-9E-PDF (PDF version) Digital Products
Table of contents Under the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans … Read More
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet - BLOGGER
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183807&_unique_id=66d22148851e1
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types of tests (for example,
in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet - #GLOBAL
BLOGGER - #GLOBAL
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183805&_unique_id=66d2214641be7
#GLOBAL - BLOGGER
BLOGGER
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types
of tests (for example, in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Pest Management Regulatory Agency 29 August 2024 ISSN: 1925-0940 (PDF version) Catalogue number: H113-25/2024-9E-PDF (PDF version) Digital Products
Table of contents Under the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans … Read More
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet - BLOGGER
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183795&_unique_id=66d2201a3f598
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types of tests (for example,
in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet - #GLOBAL
BLOGGER - #GLOBAL
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183793&_unique_id=66d220180d478
#GLOBAL - BLOGGER
BLOGGER
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types
of tests (for example, in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
Pest Management Regulatory Agency 29 August 2024 ISSN: 1925-0940 (PDF version) Catalogue number: H113-25/2024-9E-PDF (PDF version) Digital Products
Table of contents Under the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans … Read More
0 notes
Text
Registration Decision RD2024-09, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E - Notice Global Internet - #GLOBAL
https://www.merchant-business.com/registration-decision-rd2024-09-didecyl-dimethyl-ammonium-chloride-ddac-acticide-ddq-50-e/?feed_id=183791&_unique_id=66d2201662080
Pest Management Regulatory Agency29 August 2024ISSN: 1925-0940 (PDF version)Catalogue number: H113-25/2024-9E-PDF (PDF version)Digital Products Table of contentsUnder the authority of the Pest Control Products Act, pesticides must be assessed before they are sold or used in Canada in order to determine that they do not pose unacceptable risks to humans or the environment and have value when used according to the label instructions. The pre-market assessment considers available data and informationFootnote 1 from pesticide registrants, published scientific reports, other governments, and international regulatory agencies, as well as written comments if received during public consultations. Health Canada applies internationally accepted current risk assessment methods as well as risk management approaches and policies. More details, on the legislative requirements, risk assessment and risk management approach, are provided under the Evaluation approach Section.Registration decision statementFootnote 2 for didecyl dimethyl ammonium chloride (DDAC)Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide DDQ 50-E containing the technical grade active ingredient didecyl dimethyl ammonium chloride (DDAC) for use as a material preservative in polymers.The Proposed Registration Decision PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E, containing the detailed evaluation of the information submitted in support of this registration, underwent a 45 day consultation period ending on 15 July 2024. The evaluation found that, under the approved conditions of use, the health and environmental risks and the value of the pest control product is acceptable. Health Canada received a written comment relating to the health assessment during the public consultation period conducted in accordance with section 28 of the Pest Control Products Act.Comment and responseComment on the toxicology assessmentA member of the public asked Health Canada to end the practice of testing wood preservatives and other pesticide products on animals, and stated that forcing animals to inhale and eat these chemicals is not necessary.Health Canada responseHealth Canada requires information on the potential toxic effects of pesticides to determine the potential hazards and risk to human health and the environment from pesticide exposure. Toxicity information typically includes, in part, animal testing data generated by pesticide manufacturers. These studies are conducted according to international testing protocols, which include requirements to ensure protection of the welfare of laboratory animals.While animal toxicity testing currently plays a critical role in assessing human health and environmental risks from exposure to pesticides, Health Canada supports the reduction of unnecessary animal testing where scientifically justified. To this end, Health Canada does consider requests from pesticide manufacturers to waive requirements for animal studies or to consider validated non-animal alternatives in hazard assessment when feasible and supported scientifically. Health Canada issued guidance for industry on the waiving of mammalian acute toxicity studies in 2013.Health Canada is also an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health and the environment. Continued analysis of international trends and approaches is important to ensure continued alignment and harmonization.While non-animal alternatives exist for certain types of tests (for example,
in-vitro tests for irritation), animal testing continues to provide a more accurate assessment of a variety of other potential effects, and more importantly, at what dose level effects may occur, so that this information can then be used to protect human health and the environment.Other informationThe relevant confidential test data on which the decision is based (as referenced in PRD2024-06, Didecyl Dimethyl Ammonium Chloride (DDAC), Acticide DDQ 50-E) are available for public inspection, upon application, in the PMRA’s Reading Room. For more information, please contact the PMRA’s Pest Management Information Service.Any person may file a notice of objectionFootnote 3 regarding this registration decision within 60 days from the date of publication of this Registration Decision. For more information regarding the basis for objecting (which must be based on scientific grounds), please refer to the Pesticides and pest management portion of the Health Canada’s website (Public Engagement Portal – Public Engagement Forms – Notice of Objection) or contact the PMRA’s Pest Management Information Service.Evaluation approachLegislative frameworkThe Minister of Health’s primary objective under the Pest Control Products Act subsection 4(1) is to prevent unacceptable risks to individuals and the environment from the use of pest control products.As noted in the preamble of the Act, it is in the national interest that the attainment of the objectives of the federal regulatory system continue to be pursued through a scientifically-based national registration system that addresses risks to human health, the environment and value both before and after registration and applies to the regulation of pest control products throughout Canada; and that pest control products with acceptable risk and value be registered for use only if it is shown that their use would be efficacious and if there is acceptable risk to human health and the environment, taking into account the conditions of registration.For the purposes of the Act, the health or environmental risks of a pest control product are acceptable if there is reasonable certainty that no harm to human health, future generations or the environment will result from exposure to or use of the product, taking into account its conditions of registration as per subsection 2(2) of the Pest Control Products Act.Risk for the human health and environment, and value are defined under the Act subsection 2(1) as follows:Health risk, in respect of a pest control product, means the possibility of harm to human health resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Environmental risk, in respect of a pest control product, means the possibility of harm to the environment, including its biological diversity, resulting from exposure to or use of the product, taking into account its conditions or proposed conditions of registration.Value, in respect of a pest control product, means the product’s actual or potential contribution to pest management, taking into account its conditions or proposed conditions of registration, and includes the product’s (a) efficacy; (b) effect on host organisms in connection with which it is intended to be used; and (c) health, safety and environmental benefits and social and economic impact.When evaluating the health and environmental risks of a pesticide and determining whether those risks are acceptable, subsection 19(2) of the Pest Control Products Act requires Health Canada to apply a scientifically-based approach. The science-based approach to assessing pesticides considers both the toxicity and the level of exposure of a pesticide in order to fully characterize risk.Pre-market assessments are based on a required set of scientific data that must be provided by the applicants for pesticide registrations. Additional information from published scientific reports, other government departments and international regulatory agencies are also considered.
Footnote 4Risk and value assessment frameworkHealth Canada uses a comprehensive body of modern scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pesticides. This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies, consistent with the purpose described in the preambular text set out above.Health Canada’s approach to risk and value assessment is outlined in A Framework for Risk Assessment and Risk Management of Pest Control Products.Footnote 5 A high-level overview is provided below.i) Assessing potential health risksWith respect to the evaluation and management of potential health risks, Health Canada’s risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6The evaluation of potential health risks begins with a consideration of the toxicological profile of a pesticide to establish reference doses at which no adverse effect is expected and against which the expected exposure is assessed. This includes, where appropriate, the use of uncertainty (protection) factors to provide additional protection that accounts for the variation in sensitivity among members of human population and the uncertainty in extrapolating animal test data to humans. Under certain conditions, the Pest Control Products Act requires the use of another factor to provide additional protection to pregnant women, infants, and children. Other uncertainty factors, such as a database deficiency factor, are considered in specific cases. More details related to the application of the uncertainty factors are provided in SPN2008-01.Footnote 7Assessments estimate potential health risks to defined populationsFootnote 8 under specific exposure conditions. They are conducted in the context of the proposed or registered conditions of use, such as the use of a pesticide on a particular field crop using specified application rates, methods and equipment. Potential exposure scenarios consider exposures during and after application of the pesticide in occupational or residential settings, food and drinking water exposure, or exposure when interacting with treated pets. Also considered are the anticipated durations (short-, intermediate- or long-term) and routes of exposure (oral, inhalation, or skin contact). In addition, an assessment of health risks must consider available information on aggregate exposure and cumulative effects.ii) Assessing risks to the environmentWith respect to the evaluation of environmental risks, Health Canada’s environmental risk assessments follow a structured, tiered approach to determine the likelihood that exposure to a pesticide can cause adverse effects on individual organisms, populations, or ecological systems. This involves screening assessments starting with simple methods, conservative exposure scenarios and sensitive toxicity effects metrics, then moving on, where required, to more refined assessments that can include exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods.The environmental assessment considers both the exposure (environmental fate, chemistry, and behaviour, along with the application rates and methods) and hazard (toxic effects on organisms) of a pesticide. The exposure assessment examines the movement of the pesticide in soil, water, sediments and air, as well as the potential for uptake by plants or animals and transfer through the food web. The possibility for the pesticide to move into sensitive environmental compartments such as groundwater or lakes and rivers, as well as the potential for atmospheric transport, is also examined. The hazard assessment examines effects on a large number of internationally recognized indicator species of plants
and animals (terrestrial organisms include invertebrates such as bees, beneficial arthropods, and earthworms, birds, mammals, plants; aquatic organisms include invertebrates, amphibians, fish, plants and algae), and includes considering effects on biodiversity and the food chain. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide.The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk.iii) Value assessmentValue assessments consist of two components: an assessment of the performance of a pest control product and its benefits.Assessing pesticide performance involves an evaluation of the pesticide’s efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.In many cases, proof of performance alone is sufficient to establish the value of the pesticide, so that an in-depth or extensive evaluation of benefits may not be required. However, a more thorough assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.Risk managementThe outcomes of the assessments of risks to human health and the environment, and the assessment of value, form the basis for identifying risk management strategies. These include appropriate risk mitigation measures and are a key part of decision-making on whether health and environmental risks are acceptable. The development of risk management strategies take place within the context of the pesticide’s conditions of registration. Conditions can relate to, among other things, the specific use (for example, application rates, timing and frequency of application, and method of application), personal protective equipment, pre-harvest intervals, restricted entry intervals, buffer zones, spray drift and runoff mitigation measures, handling, manufacture, storage or distribution of a pesticide. If feasible conditions of use that have acceptable risk and value cannot be identified, the pesticide use will not be eligible for registration.The selected risk management strategy is then implemented as part of the registration decision. The pesticide registration conditions include legally-binding use directions on the label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.Following a decision, continuous oversight activities such as post-market assessments, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability of risks and value of registered pesticides.“Health Canada’s Pest Management Regulatory Agency (PMRA), under the authority of the Pest Control Products Act, is granting registration for the sale and use of Acticide DDQ 80-F, and Acticide…”Source Link: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2024/didecyl-dimethyl-ammonium-chloride.html
http://109.70.148.72/~merchant29/6network/wp-content/uploads/2024/08/gc679829beb8c8b87b0253963dd91ae5de583c334ada5c601dfc143d9a5be2478a2a3a8de8b3376b3776f24144c33f2890c0.png
BLOGGER - #GLOBAL
0 notes
Text
"Challenges and Best Practices in Implementing Lighting for Game Levels"
Implementing effective lighting in game levels presents numerous challenges, from technical constraints to artistic considerations. This post discusses common challenges in game lighting and offers best practices for overcoming these obstacles to create visually compelling and functional game environments.
Challenges in Game Lighting - One of the main challenges in game lighting is balancing realism with performance. Realistic lighting, particularly with techniques like ray tracing, can be computationally expensive, leading to potential performance issues on lower-end hardware (Lee & Reinhard, 2018). Another challenge is ensuring that lighting remains consistent across different platforms, which can have varying graphical capabilities.
Artistic vs. Technical Considerations - Game designers often face a tension between artistic vision and technical feasibility. While dynamic lighting can enhance realism, it can also introduce issues like excessive shadows or inconsistent lighting that detracts from the player experience (Lindell & Jakobsson, 2015). Balancing these elements requires careful planning and a deep understanding of both the artistic goals and the technical limitations of the game.
Best Practices for Effective Lighting Design - To overcome these challenges, designers can follow several best practices:
Pre-Visualization: Using tools like lighting mockups and pre-visualization software to experiment with different lighting setups before implementation can save time and resources (Smith & Wright, 2020).
Performance Optimization: Techniques like light baking, where lighting information is pre-computed and stored, can help optimize performance without sacrificing visual quality.
Cross-Platform Testing: Regular testing on different hardware platforms ensures that lighting remains consistent and performs well across all supported devices.
Case Study: "Control" The game "Control" by Remedy Entertainment is a prime example of effective lighting implementation. The game utilizes ray tracing to create stunning visuals while maintaining solid performance. The developers used a combination of real-time and baked lighting techniques to achieve a balance between realism and playability, making "Control" a benchmark in modern lighting design (Remedy Entertainment, 2019).
Conclusion -Implementing lighting in game levels is a complex task that requires balancing artistic vision with technical constraints. By understanding the challenges and following best practices, game designers can create environments that are not only visually impressive but also enhance the overall gameplay experience.
References
Lee, J., & Reinhard, E. (2018). Balancing Performance and Realism in Game Lighting. Journal of Graphics Techniques, 22(3), 56-68.
Lindell, D. B., & Jakobsson, H. (2015). Artistic and Technical Balance in Video Game Lighting. Proceedings of the International Symposium on Computer Graphics and Interactive Techniques.
Remedy Entertainment. (2019). Control: The Art and Science of Ray Tracing. Developer Diary.
Smith, A., & Wright, T. (2020). Pre-Visualization Techniques in Video Game Design. Game Art & Design Journal, 13(1), 99-114.
0 notes
Text
Elevate Your Construction Efficiency with these BIM Services in Texas

Building Information Modeling (BIM) has transformed the construction industry by providing a digital platform to capture the physical and functional aspects of a structure. Project success, cost-effectiveness, and efficiency can all be considerably improved in Texas when building projects use BIM services.
This article explores the many advantages of BIM services, with a specific focus on how they can benefit the construction industry in Texas.
Technical Specifications of BIM
Building Information Modeling (BIM) is a collaborative approach that enables various stakeholders and professionals to develop, oversee, and visualize digital depictions of both the physical and functional aspects of a construction venture. This includes all of the information needed to plan, design, develop, and effectively manage infrastructure projects. It also includes information on building materials, components, dimensions, and performance standards.
Applications of BIM in Texas Construction
BIM finds application throughout different stages of construction projects in Texas, spanning planning, design, construction, operation, and maintenance. Its applications include project visualization, collaboration, and simulation—all of which boost a project’s overall efficiency.
Planning Phase

Site Analysis: BIM helps in analyzing site conditions and constraints.
Feasibility Studies: Assists in evaluating the feasibility of project designs
Pre-construction Planning: Facilitates detailed planning and scheduling.
Design Phase

Design Coordination: Enhance cooperation amongst stakeholders, engineers, and architects.
Visualization: Provides 3D visualizations of project designs.
Energy Analysis: Conducts energy performance simulations.
Construction Phase

Construction Sequencing: Simulates construction sequences.
Cost Estimation: Provides accurate cost estimations and budget management.
Clash Detection: Identify and fix issues before the building even starts.
Benefits of BIM Services for Texas-Based Construction Projects
Greater Project Collaboration
By providing a common digital model that is available to all project stakeholders, BIM promotes collaboration. This cooperative approach reduces miscommunications and mistakes, which leads to more efficient project management.
Improved Design Quality
Engineers and architects may create more complex and exact designs with BIM. The capacity to see projects in 3D and the early detection of possible problems during the design stage.
Cost Savings
Through improved accuracy in both design and construction, BIM significantly reduces the possibility of damaging errors and costly rework. Important cost savings are also a result of accurate cost forecasts and efficient resource management.
Efficient Project Management
BIM supports improved project planning, scheduling, and resource allocation. The capacity to simulate construction sequences and forecast project timelines aids in delivering projects punctually and within budget.
Clash Detection

A key advantage of BIM is clash detection, which helps identify and resolve conflicts between various building systems prior to construction commencement. This feature minimizes expensive delays and rework, enhancing overall project efficiency.
Sustainable Building Practices

Through the facilitation of energy performance simulations and environmental impact evaluations, BIM contributes to the promotion of sustainable building practices. This guarantees that building projects in Texas will adhere to environmental laws and lessen their carbon footprint.
Challenges and Limitations of BIM
Although BIM offers various advantages, its implementation poses challenges such as high initial costs, the necessity for skilled professionals, and the requirement for thorough training.
The potential of BIM in Texas Construction
BIM promises to simplify procedures, foster better teamwork, and enable data-driven decision-making in the construction industry in Texas, opening the door to an inventive and efficient future.
Comparative Analysis with Traditional Methods
Compared to conventional construction techniques, BIM offers better accuracy, productivity, and teamwork. Conventional methods frequently rely on manual procedures and 2D drawings, which raises the risk of mistakes and inefficiencies.
User Manuals for Implementing BIM
Building information modeling (BIM) user guides provide comprehensive guidance and support for implementing BIM in construction projects.
0 notes
Text
Understanding Commercial General Contracting: A Comprehensive Guide

Commercial general contracting is a cornerstone of the construction industry, encompassing the comprehensive management and execution of building projects for businesses and institutions. This field requires a blend of technical expertise, managerial skills, and a deep understanding of construction processes to ensure that projects are completed on time, within budget, and to the highest standards. This guide will delve into the various aspects of commercial general contracting, offering insights into its complexities and essential components.
The Role of a Commercial General Contractor
A commercial general contractor (GC) is responsible for overseeing all aspects of a construction project from inception to completion. Their duties include planning, coordinating, budgeting, and supervising the construction process. They serve as the central point of contact for clients, architects, engineers, subcontractors, and suppliers, ensuring that all parties are aligned and working towards the common goal of successfully completing the project.
Key Responsibilities
1.Project Planning and Management : This involves creating a detailed plan that outlines the project scope, timeline, and budget. The GC must also anticipate potential challenges and develop contingency plans.
2. Budgeting and Cost Control : A critical aspect of the GC’s role is to develop an accurate budget and ensure that the project stays within financial constraints. This includes negotiating contracts with subcontractors and suppliers to secure the best possible prices.
3. Coordination and Communication: Effective communication is crucial in managing the various parties involved in a project. The GC must coordinate activities between architects, engineers, subcontractors, and clients to ensure that everyone is on the same page.
4. Quality Control and Safety: Ensuring that the construction meets all quality standards and regulatory requirements is paramount. The GC is responsible for implementing safety protocols to protect workers and prevent accidents on the job site.
5. Permits and Inspections: The GC must obtain all necessary permits before construction begins and ensure that the project complies with local building codes. Regular inspections are conducted to ensure adherence to these codes and to maintain quality standards.
Phases of a Commercial Construction Project
Commercial construction projects typically follow a series of phases, each with its specific tasks and milestones.
Pre-Construction Phase
1. Project Feasibility and Site Selection: This involves assessing the viability of the project, selecting a suitable site, and conducting a feasibility study to determine potential challenges and opportunities.
2. Design and Planning: Architects and engineers work together to develop detailed plans and specifications for the project. The GC plays a key role in this phase by providing input on constructability and cost estimates.
3. Permitting and Approvals: Obtaining the necessary permits and approvals from local authorities is a crucial step before construction can begin.
Construction Phase
1. Mobilization: This involves setting up the construction site, including temporary facilities, fencing, and signage.
2. Site Work and Foundation: Site preparation includes clearing the land, grading, and laying the foundation. This phase is critical as it sets the stage for the structural integrity of the building.
3. Structural Work: Erecting the framework of the building, which includes walls, floors, and roofs. This phase requires meticulous attention to detail to ensure the building’s stability and safety.
4. Exterior and Interior Finishes: Installing exterior walls, windows, doors, and roofing, followed by interior finishes such as drywall, flooring, and fixtures.
5. MEP Systems: Installation of mechanical, electrical, and plumbing systems is essential for the functionality of the building. This includes HVAC systems, electrical wiring, and plumbing infrastructure.
Post-Construction Phase
1. Inspection and Commissioning: Once construction is complete, the GC coordinates final inspections to ensure that the building meets all regulatory and quality standards. Commissioning involves testing all systems to verify they are operating correctly.
2. Handover and Training: The completed project is handed over to the client, and training is provided on the operation and maintenance of the building’s systems.
3. Closeout and Documentation: The GC compiles all project documentation, including as-built drawings, warranties, and maintenance manuals, and provides them to the client.
Challenges in Commercial General Contracting
Commercial general contracting is fraught with challenges that require careful management and strategic planning.
Budget Overruns
One of the most common challenges is managing the budget. Unexpected costs can arise from design changes, unforeseen site conditions, or price fluctuations in materials. Effective cost control measures and contingency planning are essential to mitigate these risks.
Scheduling Delays
Delays can occur due to various factors such as inclement weather, supply chain disruptions, or labor shortages. The GC must develop a realistic schedule and implement strategies to address potential delays.
Quality Control
Maintaining high-quality standards is critical, but it can be challenging with multiple subcontractors and suppliers involved. The GC must implement rigorous quality control measures and regular inspections to ensure compliance with specifications.
Safety Concerns
Construction sites are inherently hazardous environments. Ensuring the safety of all workers is a top priority. The GC must enforce strict safety protocols and provide ongoing training to minimize the risk of accidents.
Trends and Innovations in Commercial General Contracting
The construction industry is continuously evolving, with new technologies and methodologies transforming the way projects are managed and executed.
Building Information Modeling (BIM)
BIM is a digital representation of the physical and functional characteristics of a building. It enables better collaboration, improved visualization, and more accurate cost and time estimates. BIM is becoming increasingly integral in commercial construction projects.
Sustainable Construction
There is a growing emphasis on sustainability in construction. Green building practices, such as using eco-friendly materials, energy-efficient systems, and waste reduction techniques, are becoming standard. LEED certification is a sought-after designation for sustainable buildings.
Prefabrication and Modular Construction
Prefabrication involves manufacturing building components off-site and assembling them on-site. This method can significantly reduce construction time and costs while improving quality control. Modular construction, a subset of prefabrication, involves constructing entire sections of a building off-site.
Advanced Project Management Software
Modern project management software offers tools for scheduling, budgeting, communication, and documentation. These platforms enable real-time collaboration and data sharing, improving efficiency and transparency in project management.Commercial general contracting is a complex and dynamic field that requires a blend of technical knowledge, managerial acumen, and strategic planning. From project inception to completion, the GC plays a pivotal role in ensuring that construction projects are delivered on time, within budget, and to the highest standards of quality and safety. As the industry continues to evolve, embracing new technologies and sustainable practices will be key to meeting the challenges of the future and driving success in commercial construction projects.
0 notes
Text
Ultimate USA Master's Programme Guide 2025

Missed the Fall 2024 application deadlines? Not sure what to do next? You’re in the right place! While Fall semester applications are more common, Spring 2025 semester admissions are equally feasible and offer ample opportunities, and we’re here to guide you through the application process. Let’s break it down step by step.
Why Apply for Spring 2025?
Opting for the Spring semester doesn’t just offer flexibility; it unlocks doors to a range of academic opportunities. Numerous universities offer admissions for both Fall and Spring semesters, allowing students to select the timeline that perfectly fits their needs. Choosing Spring 2025 isn’t just about avoiding a year-long wait due to a missed Fall deadline; it’s about seizing the chance to align your academic aspirations with your preferred start date, ensuring a smooth transition into your Master’s program.
Application Timeline
To help you navigate the application process smoothly, let’s break down the timeline:
Pre-requisite Tests: February-June 2024
Before diving into the application process, it’s essential to complete the necessary tests:
GRE and TOEFL/IELTS:
Aim to complete these exams by June 30, 2024. You can explore the comprehensive study options at Dilip Oak’s Academy here: GRE Classroom Coaching, TOEFL Classroom Coaching, and IELTS Classroom Coaching.
Gather Necessary Documents: June-July 2024
Assemble the required documents for your applications:
Statement of Purpose (SOP):
The SOP is a crucial document that offers insight into not only your reasons for choosing the course and university, but also your personality, field experience, and long-term goals. It should be concise, compelling, grammatically correct, and technically sound, spanning approximately 500 to 800 words.
Transcripts:
Obtain official transcripts from your previous institutions. Apply for transcripts well in advance, as some colleges or universities may require more time to process them.
Recommendation Letters:
Recommendation letters are critical documents that vouch for your qualities, background, and achievements as a candidate for the master’s programs. Virtually every university requires applicants to submit three recommendation letters. Choose your recommenders (college professors, project guides, or employers) carefully as these letters are meant to provide a comprehensive view of your suitability for the academic program.
Selection of Universities for Final Application: July-September 2024
Once you’ve completed your tests, it’s time to research and shortlist universities. Consider the following factors:
Specializations:
Not every university provides all specializations. To identify the right fit, explore each university’s course structure, programs, and research areas of professors, aligning them with your academic interests and career goals.
GRE Score:
Usually, universities don’t specify the minimum required score for applications. The score needed varies based on the university’s rank and reputation, information that’s often not available on their website. To gauge the score required, you can refer to the database of previous students admitted to these universities. Dilip Oak’s Academy maintains an extensive database of over 32,000 students who enrolled in various American universities since 1996, including those universities that offer a GRE waiver post COVID. Thus, our extensive database can help you gauge the scores accepted by various institutions.
TOEFL/IELTS Score:
Students must achieve the minimum qualifying score set by the university. Typically, most universities require a TOEFL score of 80, but some may ask for a higher score, up to 100. Similarly, for IELTS, a band score of 6.5 is commonly required, although some universities may seek a higher band, up to 7.5.
Academic Record:
Your academic record from your bachelor’s degree plays an important role in the application process. A strong year-wise GPA is necessary for admission to reputable universities. It’s advisable for students to avoid backlogs or year gaps.
Co-curricular Activities:
Projects, internships, paper presentations, publications, and seminar participations will strengthen your profile, increasing your chances of acceptance into better universities.
Budget:
Tuition fees and living expenses vary among universities, with state universities generally being more affordable than private ones. Students should carefully consider their financial situation when selecting universities. Additionally, students can explore education loans offered by various financial institutions to help cover costs.
Sending applications to universities: July-September 2024
Select 6 to 8 Universities:
Based on the above mentioned criteria, narrow down your choices to 6 to 8 universities for your final applications.
Complete Online Applications:
Make sure to finish the online application process before the university’s specified deadlines. Some institutions may require additional documents via courier alongside the online submission.
Forward Test Scores:
Request ETS to send your GRE and TOEFL scores to the selected universities (scores typically take a minimum of 2 weeks to arrive). The additional score reporting fee for GRE is $27 and TOEFL is $25.
After Sending Applications: Await Decisions and Prepare for Visa
Admission Decisions:
Anticipate admission decisions around September/October 2024. Once you receive an offer, promptly accept it. You’re allowed to accept multiple admissions before finalizing your choice for visa application.
Obtain I-20:
Upon acceptance, fulfill the necessary documentation requirements. The university will then issue you the I-20, a crucial immigration document for obtaining a visa.
Prepare for Visa:
Verify the accuracy of the information on the I-20 and gather the required financial documentation to apply for your visa.
Visa Application Process:
Schedule a visa interview date upon receiving your I-20. You can apply for an F-1 visa (student visa) within 360 days from the course commencement date mentioned on the I-20 form. Once you secure the visa date, proceed with the interview and complete the remaining formalities leading up to your departure.
Ready to dive into your Master’s journey for Spring 2025? Step up your game by gearing up for your GRE and TOEFL/IELTS exams with us! Already aced those exams? Reach out today to kick start your application process with confidence!
As India’s leading Study Abroad Consultant, Dilip Oak’s Academy offers a comprehensive suite of admission counseling services that can guide you through the entire process from Shortlisting Universities to Visa Counseling. With our expertise, we have successfully sent 32,000 students to various prestigious American universities like MIT, Stanford, Cornell, and Carnegie Mellon. We also offer classroom and online coaching for GRE, TOEFL, and IELTS, as well as GRE Self Prep. To explore our services, book a free consultation or call us at 91–20–67444222.
dispensable tool on the path to success. With its comprehensive content, personalized approach, and unwavering commitment to student excellence, this course equips learners with the skills and confidence needed to excel in one of the most challenging standardized tests worldwide.
Contact Details Of Dilip Oak’s Academy:
www.dilipoakacademy.com
Phone Number:
+91–20–67444222
+91–8007878496
+91–8007878497
Email Id: [email protected]
0 notes
Text
Benchmarking Quality: The Significance of Medical Device Certification
Regulatory requirements and standards ensure that medical devices undergo extensive testing and certification before reaching patients. From conception to market approval, medical technologies must navigate a rigorous process to validate performance and confirm safety. This article discusses the key aspects of medical device testing and certification market.
Pre-Clinical Testing
The development of a new medical device begins in the lab, where designers conduct extensive pre-clinical testing to evaluate safety and effectiveness. Device prototypes are subjected to a variety of tests to simulate real-world use over extended periods. Bench tests examine components for durability, biocompatibility testing explores interaction with living tissue, and animal studies provide early safety data. This stage aims to identify and address any design flaws or safety issues before moving to clinical trials. The goal is to have a device ready for human use with all major bugs and risks addressed.
Clinical Evaluations
Once pre-clinical testing is complete, developers can begin clinical evaluations to collect data on human subjects. Institutional review boards closely oversee clinical investigations to protect participants. Initial feasibility and pilot studies use small sample sizes to generate preliminary performance and safety results. Pivotal clinical trials employ randomized, controlled testing on larger patient populations to gather definitive data. Rigorous documentation and oversight ensure risks are minimized and participants provide informed consent. Clinical evaluations aim to demonstrate a device's treatment benefits outweigh any potential harms.
Regulatory Review and Approval
With clinical data in hand, developers submit applications to regulatory agencies like the FDA in the US or EU MDR in Europe seeking marketing authorization. Regulators conduct a thorough review of all pre-clinical and clinical data along with the manufacturer's quality systems and labeling. Their role is to independently validate whether a device is sufficiently safe and effective to be marketed for its intended use. The submission is evaluated against applicable standards and regulations. If approved, the device receives market clearance along with any performance limitations or post-market conditions.
Conformity Assessment
For many devices, regulatory approval is just the beginning. Many jurisdictions also require third-party evaluation and certification of quality management systems and technical documentation. Notified bodies conduct audits and inspections to verify compliance with essential requirements. Product testing examines all attributes like biocompatibility, software validation, electromagnetic compatibility and more. Conformity assessment provides objective confirmation a device and its manufacturer adhere to prescribed standards. This offers assurance to health providers and patients regarding safety, reliability and performance claims.
Post-Market Monitoring
Even after market release, testing and evaluation continue. Manufacturers must monitor real-world performance through post-market surveillance programs. Any issues or adverse incidents are reported to regulators who may require corrective or preventative actions. Long-term studies may also be mandated. If problems emerge indicating the risk-benefit profile has changed, a device can be recalled or restricted for certain uses. Post-market monitoring plays a key role in safeguarding patients as technologies are used more widely outside of clinical settings.
Strict regulations, standards, guidelines and independent oversight ensure medical technologies undergo extensive testing and validation at every stage - from concept to ongoing performance monitoring. While a rigorous process, this framework provides confidence that approved devices are sufficiently safe and effective for their intended medical purpose. Patients can have faith innovative therapies meet high criteria before entering routine clinical care. North America currently dominates the Medical Device Testing and certification market attributed to strong presence of device manufacturers and frequent new product launches in the region.
0 notes
Text
Navigating the Blueprint: The Art and Science of Construction Management
Introduction:
In the realm of architecture and infrastructure, the role of construction management stands as the cornerstone of successful project execution. As the intricate dance between creativity and practicality, construction management is the art and science of bringing dreams from blueprints to reality. In this blog post, we delve into the essential components that define effective construction management, exploring the challenges, strategies, and innovations that shape this dynamic field.

1. The Foundation: Planning and Pre-construction:
Before the first brick is laid, meticulous planning and pre-construction activities set the stage for success. Construction managers are the architects of the project timeline, budget, and risk assessment. This phase involves feasibility studies, site assessments, and the development of a comprehensive project plan. Through careful consideration and collaboration with architects, engineers, and stakeholders, construction managers lay the groundwork for a seamless construction process.
2. Communication is Key: Stakeholder Engagement:
A successful construction project hinges on effective communication among all stakeholders. Construction managers play a pivotal role in ensuring that architects, contractors, subcontractors, and clients are on the same page. Regular updates, transparent reporting, and swift issue resolution contribute to a harmonious and efficient construction process. Stakeholder engagement extends beyond the immediate project team to include local communities, regulatory bodies, and environmental agencies.
3. Building Blocks: Construction Execution:
As the project moves into the construction phase, construction managers take charge of coordinating the diverse elements involved in the build. Managing the construction schedule, ensuring quality control, and addressing unexpected challenges are all part of the daily routine. Construction managers must be adept at resource allocation, problem-solving, and adapting to changing conditions to keep the project on track.
4. Safety First: Health and Safety Compliance:
Construction sites are dynamic environments with inherent risks. Safety is paramount, and construction managers are responsible for implementing and enforcing strict safety protocols. This includes regular safety training, inspections, and the use of advanced technologies such as drones and sensors to monitor and enhance safety on-site. Prioritizing the well-being of the construction team and adhering to health and safety regulations are non-negotiable aspects of construction management.
5. Technology Integration: The Digital Construction Manager:
In the digital age, construction management is increasingly reliant on innovative technologies. Building Information Modeling (BIM), project management software, and real-time collaboration tools empower construction managers to streamline processes, enhance efficiency, and mitigate risks. Embracing technology is not just a trend; it is a necessity for staying competitive and delivering projects with precision.
6. Adapting to Sustainable Practices: Green Construction Management:
Sustainability is a defining theme in modern construction management. As environmental awareness grows, construction managers are tasked with integrating sustainable practices into every phase of the project. From eco-friendly materials to energy-efficient design, construction managers play a crucial role in shaping the industry towards a more sustainable future.

Conclusion:
Construction management is a complex and multifaceted discipline that requires a blend of leadership, technical expertise, and adaptability. As the construction industry continues to evolve, so too must the strategies and practices of construction managers. By prioritizing collaboration, communication, safety, technology, and sustainability, construction managers can navigate the blueprint of any project, turning visions into tangible, lasting structures that stand as testaments to their skill and dedication.
#contruction#construction management#project management#top project management firm in nyc#project manager#succession#construction project management
0 notes